Abstract
CD40 ligation has been shown to induce antitumor effects in mice and cancer patients. Most of the studies have focused on the ability of an agonistic anti-CD40 mAb to either directly kill CD40-positive tumor cells or activate T-cell immune responses. In this review the authors focus on the ability of CD40 ligation to activate antitumor effector mechanisms of the cells of innate immunity such as macrophages and NK cells.
Keywords: anti-CD40, macrophages, NK cells
CD40 is a member of the tumor necrosis factor receptor (TNFR) family. It is expressed on B cells, dendritic cells, macrophages, fibroblasts, endothelial cells, and some granulocytes. Initial studies on the role of CD40 were conducted on B cells and then on dendritic cells; more historical background on CD40 is reviewed in [1]. In cancer immunology and immunotherapy studies, because CD40 molecules are expressed on certain tumors, such as B-cell lymphomas, initial antitumor applications of CD40 ligation were tested for direct targeting of these tumors. In addition to killing CD40+ tumor cells directly, CD40 ligation can also activate host cells to mediate antitumor effects, either directly or indirectly. This second approach was prompted by studies showing that the interaction between the CD40 molecule expressed on antigen-presenting cells (APC), such as dendritic cells and macrophages, and CD40 ligand (CD40L), expressed on T cells, plays an important role in activation of adaptive immunity [2–4]. The requirement for CD4+ T cells in this interaction can be replaced by agonistic anti-CD40 antibody (anti-CD40) [2, 5]. When stimulated by CD40–CD40L interaction, APC produce IL-12 and co-stimulatory molecules B7-1 and B7-2, which are necessary for activating effector CD8 T cells. By this approach, tumor-specific T cells can be activated by certain tumor antigens used as vaccines in concert with CD40–CD40L interaction [6]. This induction of immunity has prompted efforts to activate antitumor immune T cells in tumor-bearing hosts by means of CD40 ligation; thus, most research on the role of CD40 in cancer therapy has been focused on activating T cells [5–11]. This review addresses T-cell-independent approaches of tumor therapy by CD40 ligation that involve different effector cells and mechanisms summarized in Table 1. Analyses of the published data suggest that macrophages could be the key cells responding to the CD40 ligation to induce antitumor effects either directly or indirectly by activating the cells of adaptive and innate immunity as shown in Figure 1.
TABLE 1.
Cells involved in the CD40 ligation-induced antitumor effects
| Target cells | Mechanism of antitumor effect | Reference |
|---|---|---|
| CD40+ tumors | ADCC | [17, 18] |
| DC | T-cell activation | [27, 49, 82] |
| Macrophages | NK activation | [11, 39] |
| Apoptosis | [55, 60] | |
| NO and TNF | [56] | |
| B cells | Complement-dependent cytotoxicity | [74] |
| ADCC | [75] | |
| Endothelial cells | T-cell activation | [79] |
| Not determined | Neutrophil activation | [77] |
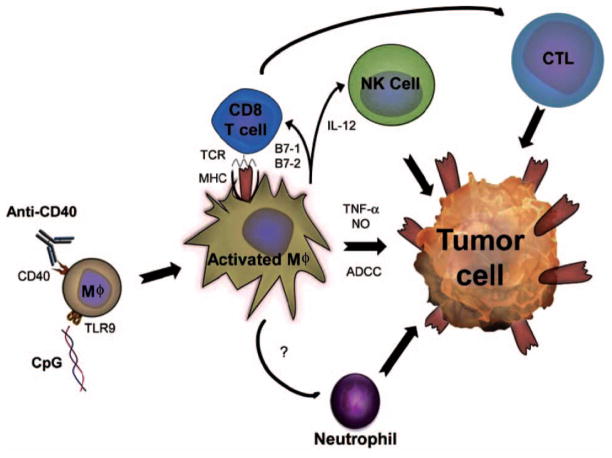
FIGURE 1.
Role of macrophages in anti-CD40 - induced antitumor effects. Agonistic anti-CD40 interacts with the CD40 molecule expressed on the surface of APC (e.g. macrophages, Mφ), which leads to the regulation of co-stimulatory molecules B7-1 and B7-2 and secretion of cytokines including TNF-α, NO and IL-12. Anti-CD40 synergizes with the TLR9 ligand CpG in macrophage activation. Interaction of the co-stimulatory molecules APC with their ligands on T cells in conjunction with the interaction of MHC-tumor antigen with the T cell receptor (TLR) leads to CD8+ T cell activation and following tumor cell killing by cytotoxic T lymphocytes (CTL). IL-12 produced by macrophages activates NK cells to kill the tumor. Macrophages activated via CD40 ligation also can kill tumor cells via antibody-dependent cell-mediated cytotoxicity (ADCC), cytotoxic molecules (TNF-α, NO) or directly. In addition, other effector cells such as neutrophils are induced in anti-CD40-treated mice via an unknow mechanism which may involve cytokines or chemokines secreted by macrophages.
Activation through CD40 can be achieved using agonist anti-CD40 mAbs (anti-CD40). The CD40 can also be activated by using soluble CD40L [12], or can be blocked with CD40 antagonist antibodies [13]. However, for this review we focus on activation of the CD40 pathway using agonist mAbs, of which there are several clones available for both mouse CD40 and human CD40 (Table 2). Most anti-CD40 clones were tested for their agonistic strength by their ability to stimulate B-cell proliferation in vitro [14, 15]. However, other groups have evaluated anti-CD40-mediated activation of B cells by looking at cytokine secretion and upregulation of co-stimulatory molecules [16]; these studies can show clear agonistic activity, but make it difficult to compare agonistic strength to other mAbs using B-cell proliferation.
TABLE 2.
Agonist anti-CD40
| Target species | Target molecule | Function | Clone/name | Description | Strength | Citation |
|---|---|---|---|---|---|---|
| Mouse | CD40 | Agonist | 3/23 | Rat IgG2b | Strong | [30] |
| Mouse | CD40 | Agonist | FGK45 | Rat IgG2b | Strong | [31] |
| Human | CD40 | Agonist | CP-870,893 | Human IgG2 | * | [16, 22] |
| Human | CD40 | Agonist | SGN-40 | Humanized IgG1 | Weak | [21] |
| Human | CD40 | Agonist | Chi lob 7/4 | Chimeric IgG1 | Minimal Info. | [32] |
Difficult to compare to other agonists due to a difference in the assays used to determine activity.
TARGETING CD40+ TUMORS
Engagement of CD40 on the surface of some tumors has demonstrated antitumor benefits. The mechanisms through which CD40 ligation by agonist mAb produces antitumor effects is likely multifaceted [12, 17]. Antibody-dependent cell-mediated cytotoxicity (ADCC) [18, 19] and inhibition of proliferation are both potentially important mechanisms of action of agonist anti-CD40. Apoptosis of human tumor cells by CD40 ligation in vitro has been demonstrated by multiple groups [17, 20, 21], and at least one clinical study has suggested a dose-dependent effect of agonist anti-CD40 on B-cell activation, correlating with B-cell depletion in the peripheral blood [22]. The sensitivity of B-cell lymphoma to CD40 therapy can be determined by mRNA expression signature of CD40 pathway elements [23]. However, the direct antitumor activity of anti-CD40 is not observed in all tumors that express CD40 on the cell surface. Melanoma is one example of a CD40+ tumor that is not sensitive to the direct effects of anti-CD40 [24], demonstrating that the expression of CD40 by a tumor does not assure that the tumor will respond well to anti-CD40 therapy.
Some studies have reported ADCC and antibody-dependent cellular phagocytosis (ADCP) as mechanisms through which anti-CD40 mediates anti-lymphoma responses [18, 19]. Engineering of the Fc end of the anti-CD40 for increased FcγR binding resulted in up to 150-fold increased ADCC and ADCP relative to the parent anti-CD40, when tested against B-lymphoma, leukemia, and multiple myeloma cell lines [18]. Such observations have led to combinatorial approaches between anti-CD40 and other mAbs targeting the same tumor cell. This approach has been very successful in preclinical studies. Anti-human CD40 (dacetuzumab) and anti-human CD20 (rituximab) had additive to synergistic antitumor effects in vitro and in a xenograft non-Hodgkin lymphoma model via distinct apoptotic mechanisms [25]. Another potential application of anti-CD40 is to attach toxic compounds to the tumor cell surface. Antitumor effects in mouse models were achieved against CD40-expressing tumors in vivo by linking anti-CD40 to an immunotoxin causing direct tumor cell toxicity [26]. However, such studies were only completed using tumor lines that respond to anti-CD40 therapy, and the efficacy of this approach in CD40-resistant tumor lines was not reported.
The ligation of CD40 on the B-cell surface can act as a prosurvival or mitogenic signal, leading some to question the usefulness of CD40 agonists as a cancer therapeutic. Mouse models of spontaneous mammary carcinogenesis show delayed tumorigenesis with blockade of CD40L or in HER2/neu transgenic mice lacking CD40 [27]. However, studies using human tonsular B cells and transformed B-cell lines have suggested that both primary and transformed cells are susceptible to CD40-mediated inhibition, correlating with an upregulation of the TNF-related apoptosis receptor Fas [28, 29]. The discrepancy in these observations may be due to dose-dependent effects of CD40 ligation as has been described in clinical trial patients [22]. However, one important consideration is the wide expression of CD40 on immune cells and how CD40-mediated immune activation can be used as a therapeutic. Therefore, the use of agonistic anti-CD40 has a broader application for the activation of immune effector cells in models of tumor immunotherapy.
ROLE OF NK CELLS
The CD40–CD40L pathway can activate NK cells both directly through CD40L on the NK cell, or in an indirect manner, after activation of APCs. Nakajima et al. were the first to report an indirect activation of NK cells following CD40-mediated APC activation using P815 cells expressing CD40L [11]. In this model, CD40L-expressing P815 cells directly activated macrophages through the ligation of CD40 on the macrophages; this resulted in macrophage production of IL-12, thereby activating the NK cells [11]. Antitumor effects by CD40L expressing tumor cells have been observed in nude mice [11], and with tumors lacking appreciable expression of MHC class I [33]; both observations suggest that indirect activation of NK cells through CD40 can produce T-cell-independent antitumor responses. Transduced expression of CD40L on APCs is another way to indirectly activate NK cells [34]. Cis activation of APCs expressing both CD40 and CD40L results in subsequent activation of NK cells in a contact-independent manner. NK-cell activation in this system likely occurs through the production of cytokines; the resultant antitumor effects are, in part, the result of increased surface expression of TNF-related apoptosis-inducing ligand (TRAIL) on the activated NK cells [34].
The CD40–CD40L pathway can directly activate NK cells through CD40L. Carbone et al. first described CD40L activation of NK cells after observing an upregulation of CD40L on the surface of human NK cells after in vitro activation with IL-2 [35]. Co-incubation of P815 cells either transduced to express CD40 or coated with an anti-CD40L mAb showed that triggering of NK-cell surface-bound CD40L can elicit target cell cytolysis [35]. In vivo functionality of CD40L on the surface of NK cells was subsequently demonstrated in a rat adenocarcinoma model [36]. In this model, NK cells lysed CD40+ adenocarcinoma cells in a contact dependent manner and this lysis was abrogated by blockade of the CD40–CD40L interaction. While few tumor immunotherapy models use CD40L agonists to activate NK cells, triggering of CD40L on the surface of NK cells by CD40 on APCs results in NK-cell proliferation [37, 38]. Therefore, approaches that activate and expand NK cells in vivo through CD40L may be worth pursuing as potential tumor immunotherapy regimens.
Our group has observed NK-cell activation and subsequent antitumor activity in vivo following administration of an agonist anti-CD40 in murine models of melanoma and adenocarcinoma. NK cells activated as a result of anti-CD40 therapy in our model mediated antitumor effects in the absence of T cells. NK-cell activation was likely the result of increased levels of IL-12 detected in the sera of anti-CD40 treated mice [39] and produced by activated APC, including macrophages (Figure 1). Furthermore, anti-CD40 administration induced NK-cell activation and tumor lysis in knockout mice lacking CD40L, indicating that NK activation was indirect, as direct NK-cell activation (via CD40L) was not playing a role. Even though these studies demonstrated a role for NK cells, when NK cells were depleted in anti-CD40 treated mice bearing subcutaneous B16 tumors, the antitumor effects were reduced, but not eliminated [39]. These data suggest that cells other than T cells and NK cells may also contribute to the antitumor effects of anti-CD40.
ROLE OF MACROPHAGES
Macrophages can be generally divided into classically activated, antitumor effector macrophages (M1) and alternatively activated, pro-tumor macrophages (M2) [40]. Classical activation of M1 macrophages requires two signals. First, macrophages respond to “priming” by IFN-γ usually secreted by activated NK and T cells [41, 42]. This priming results in activation of certain Toll-like receptors (TLR), e.g., TLR4, TLR2, TLR9 [43] and activation of signal transducing molecules (MyD88, NF-κB, etc.) that facilitate responsiveness to a second signal. The second or “triggering” signal, typically provided by lipopolysaccharide (LPS) or other microbial derivatives [44], is induced via a TLR pathway and evokes a spectrum of biological responses attributable to cytotoxic macrophages. The main effector molecules secreted by activated macrophages include TNFα [45] NO [46], and TRAIL [47].
Because APC, including macrophages, express CD40 molecules and can be activated by CD40 ligation to induce T-cell-dependent immune responses [2–4], most attention regarding CD40 on macrophages has been focused on CD40-mediated maturation or activation of APC functions and their role in enhancing T-cell responses [27, 48–51]. In contrast, less attention has been placed on the role of CD40 ligation in effector functions of macrophages [52]. Alderson et al. were the first to describe the ability of CD40L-expressing tumor cells to activate tumoricidal rather than antigen-presenting functions of human monocytes in vitro [53]. Imaizumi et al. confirmed these findings in a mouse model of lung cancer by demonstrating the induction of tumoricidal activity of alveolar macrophages in vitro via CD40–CD40 ligand interactions [54].
In agreement with these in vitro studies, our group showed that in vivo treatment with anti-CD40 activated peritoneal macrophages to produce elevated levels of NO and mediate cytostatic effects against tumor target cells in vitro [39]. Macrophages activated by anti-CD40 produced IFN-γ, IL-12, and NO, and mediated destruction of tumor targets via apoptosis in vitro [55]. These antitumor effects were greatly enhanced by LPS and observed against various murine and human tumor cell lines. Macrophages obtained either from C57BL/6 mice depleted of T cells with anti-CD4 and anti-CD8 or from SCID/beige mice were still activated by anti-CD40 to mediate cytostatic activity, indicating that in this setting the observed anti-CD40-induced antitumor effects were T-cell independent [55]. The tumoristatic effects of anti-CD40-activated macrophages in this setting involved NO and TNF-α [56].
These ex vivo results were confirmed and extended in vivo to show that anti-CD40 induced suppression of tumor growth in A/J mice bearing NXS2 neuroblastomas and in C57Bl/6 mice bearing B16 melanomas [57]. These antitumor effects were obtained in the absence of T and NK cells, but were inhibited by silica treatment, indicating a role for macrophages [57]. Moreover, anti-CD40 was able to induce reduction of tumor growth in the absence of T cells even against highly immunogenic tumors that are normally suppressed by T-cell responses [58].
CD40 ligation alone does not seem to be very effective in activating macrophages ex vivo: a second signal, such as LPS, is needed to achieve consistent activation. To enhance the antitumor effect of anti-CD40, it was combined with the Toll-like receptor (TLR) 9 agonist, CpG, which shares some immunostimulatory properties with LPS, but is much less toxic in vivo [59]. Activation of macrophages with anti-CD40 and LPS or CpG is similar to the “classical” activation of macrophages with IFN-γ and LPS, where anti-CD40 or IFN-γ serves as a priming signal and CpG or LPS serves as a triggering signal. Thus, anti-CD40 priming of macrophages requires IFN-γ [55, 60], and the synergy between anti-CD40 and CpG was observed only when CpG followed CD40 ligation [60]. Treatment with class B CpG 1826 alone induced macrophage-mediated antitumor effects [61], but a combination of anti-CD40 and CpG was synergistic in production of IFN-γ, IL-12, TNF-α, and NO by macrophages, as well as in their ability to mediate cytostatic and apoptotic effects against tumor cells in vitro [60] as shown in Figure 1. In addition, anti-CD40 and CpG synergized in vivo in suppressing tumor growth. This antitumor effect persisted in T- and NK-cell-deficient mice, but was reduced by silica treatment, implicating macrophages as effector cells [60]. Stone and colleagues confirmed the involvement of macrophages following CD40 ligation by showing that a triple combination of pSP-D-CD40L, CpG, and poly(I:C) in tumor-bearing mice led to measurable antitumor effects along with dramatic increases in F4/80+ macrophages [62, 63].
The ability of anti-CD40 with and without CpG to activate innate immune cells in the absence of T cells suggests that CD40 ligation might be a viable approach for treatment of cancer patients whose adaptive immune systems may be suppressed by other treatments, such as chemotherapy, surgery, or radiotherapy. Indeed, chemotherapy inhibits adaptive immune function (including T-cell immunity) much more than it inhibits innate immune function (including NK cells and macrophages) [64, 65], suggesting that the approaches directed to macrophage activation in tumor-bearing hosts may be beneficial in combination with chemotherapy. Indeed, treatment of B16 melanoma-bearing mice with cyclophosphamide (CY) and anti-CD40 + CpG resulted in a significant reduction in tumor growth in immunocompetent mice compared with either CY alone or anti-CD40 + CpG. This antitumor effect involved macrophages as it was reduced in mice treated with clodronate liposomes to partially deplete macrophages [66]. Even multidrug chemotherapy consisting of vincristine, CY, and doxorubicin suppressed the functions of T cells and NK cells, but primed macrophages to secrete NO, IFN-γ, and IL-12. Furthermore, the combination of vincristine, CY, and doxorubicin synergized with anti-CD40/CpG to induce tumor growth suppression in C57BL/6 mice with established B16 melanoma or 9464D neuroblastoma [67].
Interestingly, a combination of CY and anti-CD40 therapy can be beneficial when the antitumor effect is mediated by innate immune cells [66], and detrimental when the antitumor effect of CD40 ligation is T-cell mediated [68]. Synergy between gemcitabine and anti-CD40 was observed in a tumor model where the antitumor effect was CD8 T-cell mediated [69]. However, in a pancreatic cancer model where CD40 ligation activated macrophages, addition of gemcitabine to anti-CD40 did not enhance its antitumor effect [70].
Tumor-associated macrophages (TAM) are often functionally inhibited, mediate immunosuppression, and promote tumor growth [71]. These TAM have been categorized as alternatively activated M2 macrophages due to the influence of tumor-derived factors. Tumor-induced suppression of macrophage function can be reversed by appropriate strategies, converting M2 macrophages to M1 effector cells. Thus, disruption of the immunosuppressive IL-10 pathway in combination with the macrophage-activating agents CpG and LEC/CCL16, influenced TAM to become potent antitumor effectors [72]. Similarly, anti-CD40 in combination with CpG and chemotherapy caused a marked decrease in expression of M2 characteristics (surface antigens [IL-4Rα and B7-H1] and cytokines [IL-4 and IL-10]), and augmented the expression of M1 characteristics (antigens [CD80, CD86, MHC class II], and cytokines [IFN-γ, TNF-α, and IL-12]) in TAM [67]. The clinical potential of CD40 ligation combined with chemotherapy has been recently demonstrated; Robert Vonderheide and his colleagues have shown regression of pancreatic carcinoma in 4 of 21 patients treated with anti-CD40 and gemcitabine [70]. They confirmed a role for macrophages in an animal model of this therapy by using a genetically engineered mouse model of pancreatic ductal adenocarcinoma [70].
In addition to CD40 ligation activating macrophages to induce apoptotic effects against tumors, anti-CD40 can engage macrophages to become antitumor effector cells against CD40+ tumors via ADCC. Thus, anti-CD40 (IgG1) genetically engineered to express Fc with the better binding to activating FcγR facilitated better ADCC by NK cells and macrophages than nonmodified anti-CD40 against B lymphoma, leukemia, and multiple myeloma cell lines [18]. It has been recently shown that the type of FcγR that binds to anti-CD40 can influence whether this Ab will mediate ADCC or induce antitumor immune response against CD40-expressing tumors [73].
ROLE OF OTHER CELLS
Purified B cells from the tumor-draining lymph nodes of mice bearing the 4T1 mammary tumor were activated in vitro with anti-CD40 and LPS; this activation enabled them to kill tumor cells in vitro and mediate anti-metastatic effects in vivo [74]. A role for B cells in CD40-induced antitumor effects was also observed when anti-CD40 was injected locally into a murine mesothelioma [75]. The mechanism of B-cell-dependent antitumor effect after CD40 stimulation is not clear, but may involve secretion of antibodies directed against tumors followed by complement-mediated lysis, as was shown in vitro [74], or ADCC involving macrophages.
Granulocytes are another effector cell type that may be activated by CD40 ligation (Figure 1). Thus, it was shown that neutrophils can become dendritic cells and respond to activation with CD40 ligand [76]. In a separate model, direct injection of anti-CD40 and IL-2 into the tumor resulted in complete elimination of the injected tumor and also resulted in systemic immunity effective against distant tumors; both CD8 cells and neutrophils were involved in this effect [77]. The mechanism of granulocyte-mediated tumor-cell killing following CD40 ligation is not known.
Differences in CD40 expression by endothelial cells have been reported between mice and humans, suggesting that while CD40 is found on human endothelial cells, endothelial cells from mice lack surface expression of CD40 [78]. However, Hamzah et al. have reported an upregulation of CD40 on the surface of tumor-associated endothelial cells [79]. The importance of CD40 expression in tumor vasculature was demonstrated using a combinatorial approach of an agonist anti-CD40 targeted to the tumor vasculature and interleukin-2. In a transgenic mouse model of spontaneously arising insulinoma, triggering of CD40 on tumor-associated endothelial cells induced an inflammatory response of the vessel wall and facilitated T effector cell accumulation in the tumor parenchyma. Subsequent administration of IL-2 in this model promoted the persistence of antigen-specific immune cells. Together, these reagents led to a persistent antitumor response and increased overall survival more than systemic administration of αCD40 and IL-2 [79].
It is evident that CD40 ligation can activate various cells of innate immunity (NK cells, macrophages, granulocytes) and adaptive immunity (T cells, B cells). Because of that, and because several different cellular mechanisms can be simultaneously involved in the induction of in vivo antitumor activity, the results of experiments depleting single cell populations should be interpreted with caution. For example, in a model of spontaneous tumor regression using mice with a phenotype that enables rejection of primary tumor challenges, NK cells, macrophages, and neutrophils were each independently involved in the resistance, as determined in vitro. In vivo depletion of one or two these cell subsets did not reduce tumor resistance, whereas it was abolished by depleting all tumor-infiltrating leukocytes [80].
CONCLUDING REMARKS
As much as CD40 ligation can be an attractive immunotherapeutic modality, its antitumor efficacy when used alone is limited. The approaches combining anti-CD40 with certain TLR agonists showed synergistic activation of innate immunity [60] or T-cell immunity [81, 82]. It is likely that combining CD40 ligation with agents that simultaneously induce activation of both innate immunity and adaptive immunity will help achieve better therapeutic effects. Thus, a combination of CCL16 chemokine (to induce accumulation of macrophages and dendritic cells in the tumor), CpG (to activate both APC and innate effector functions), and anti-IL-10 (to block one pathway of tumor-induced immune suppression) resulted in powerful antitumor effects due to both tumor necrosis initiated by activated M1 macrophages and CTL priming [72]. Similarly, it has been recently shown that greater activation of both innate and adoptive immunity can be achieved by using several TLR agonists with a tumor vaccine, resulting in regression of established weakly immunogeneic B16-OVA tumors [83]. Synergistic antitumor effects against mouse tumors were achieved by the triple antibody therapy with anti-CD40, anti-DR5, and anti-CD137 [84], by a triple combination of anti-CD40, anti-CD137, and IL-12 [85], or by a triple combination of CD40L, CpG, and poly (I:C) injected into the tumor [63].
Another important consideration for using CD40 ligation in the clinic is the dose-dependent toxicity of anti-CD40 due to cytokine release syndrome experienced by some patients in the clinical trials [22, 86, 87]. Local delivery of anti-CD40 into the tumor microenvironment has been effective in mouse models and allowed to reduce the therapeutic dose of anti-CD40 and its toxicity [75]. Local delivery of anti-CD40 in a slow-release formulation had a similar beneficial effect [88], suggesting that these local approaches, alone or in combination, may be effective in patients with accessible tumors.
As both CD40L [89] and anti-CD40 [22, 70, 86, 87] are being tested in clinical trials with promising results, the combinatory approaches showing preclinical efficacy and involving activation of innate immunity as well as adaptive immunity provide future hope for improved cancer immunotherapy.
Acknowledgments
This work was supported by NIH-NCI grants CA87025 and CA32685, a grant from the Midwest Athletes Against Childhood Cancer (MACC) Fund, and support from The Crawdaddy Foundation. We thank Tyler Van De Voort for the artistic work.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21(5):265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett SR, Carbone FR, Karamalis F, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 3.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 4.Schoenberger SP, Toes RE, van der Voort EI, et al. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 5.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5(7):780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 6.Mackey MF, Gunn JR, Ting PP, et al. Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997;57(13):2569–2574. [PubMed] [Google Scholar]
- 7.Diehl L, den Boer AT, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5(7):774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 8.Fonsatti E, Maio M, Altomonte M, et al. Biology and clinical applications of CD40 in cancer treatment. Semin Oncol. 2010;37(5):517–523. doi: 10.1053/j.seminoncol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.French RR, Chan HT, Tutt AL, et al. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5(5):548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 10.Gurunathan S, Irvine KR, Wu CY, et al. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998;161(9):4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima A, Kodama T, Morimoto S, et al. Antitumor effect of CD40 ligand: elicitation of local and systemic antitumor responses by IL-12 and B7. J Immunol. 1998;161(4):1901–1907. [PubMed] [Google Scholar]
- 12.Khalil M, Vonderheide RH. Anti-CD40 agonist antibodies: preclinical and clinical experience. Update Cancer Ther. 2007;2(2):61–65. doi: 10.1016/j.uct.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai YT, Li X, Tong X, et al. Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer Res. 2005;65(13):5898–5906. doi: 10.1158/0008-5472.CAN-04-4125. [DOI] [PubMed] [Google Scholar]
- 14.Malmborg Hager AC, Ellmark P, Borrebaeck CA, et al. Affinity and epitope profiling of mouse anti-CD40 monoclonal antibodies. Scand J Immunol. 2003;57(6):517–524. doi: 10.1046/j.1365-3083.2003.01271.x. [DOI] [PubMed] [Google Scholar]
- 15.Pound JD, Challa A, Holder MJ, et al. Minimal cross-linking and epitope requirements for CD40-dependent suppression of apoptosis contrast with those for promotion of the cell cycle and homotypic adhesions in human B cells. Int Immunol. 1999;11(1):11–20. doi: 10.1093/intimm/11.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter EL, Mick R, Ruter J, et al. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. J Transl Med. 2009;7:93. doi: 10.1186/1479-5876-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funakoshi S, Longo DL, Murphy WJ. Differential in vitro and in vivo antitumor effects mediated by anti-CD40 and anti-CD20 monoclonal antibodies against human B-cell lymphomas. J Immunother Emphasis Tumor Immunol. 1996;19(2):93–101. doi: 10.1097/00002371-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Horton HM, Bernett MJ, Peipp M, et al. Fc-engineered anti-CD40 antibody enhances multiple effector functions and exhibits potent in vitro and in vivo antitumor activity against hematologic malignancies. Blood. 2010;116(16):3004–3012. doi: 10.1182/blood-2010-01-265280. [DOI] [PubMed] [Google Scholar]
- 19.Oflazoglu E, Stone IJ, Brown L, et al. Macrophages and Fc-receptor interactions contribute to the antitumour activities of the anti-CD40 antibody SGN-40. Br J Cancer. 2009;100(1):113–117. doi: 10.1038/sj.bjc.6604812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jundi M, Nadiri A, Al-Zoobi L, et al. CD40-mediated cell death requires TRAF6 recruitment. Immunobiology. 2011;217(3):375–383. doi: 10.1016/j.imbio.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Law CL, Gordon KA, Collier J, et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65(18):8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 22.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 23.Burington B, Yue P, Shi X, et al. CD40 pathway activation status predicts response to CD40 therapy in diffuse large B cell lymphoma. Sci Transl Med. 2011;3(74):74ra22. doi: 10.1126/scitranslmed.3001620. [DOI] [PubMed] [Google Scholar]
- 24.Kalbasi A, Fonsatti E, Natali PG, et al. CD40 expression by human melanocytic lesions and melanoma cell lines and direct CD40 targeting with the therapeutic anti-CD40 antibody CP-870,893. J Immunother. 2010;33(8):810–816. doi: 10.1097/CJI.0b013e3181ee73a7. [DOI] [PubMed] [Google Scholar]
- 25.Lewis TS, McCormick RS, Emmerton K, et al. Distinct apoptotic signaling characteristics of the anti-CD40 monoclonal antibody dacetuzumab and rituximab produce enhanced antitumor activity in non-Hodgkin lymphoma. Clin Cancer Res. 2011;17(14):4672–4681. doi: 10.1158/1078-0432.CCR-11-0479. [DOI] [PubMed] [Google Scholar]
- 26.Francisco JA, Schreiber GJ, Comereski CR, et al. In vivo efficacy and toxicity of a single-chain immunotoxin targeted to CD40. Blood. 1997;89(12):4493–4500. [PubMed] [Google Scholar]
- 27.Chiodoni C, Paglia P, Stoppacciaro A, et al. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190(1):125–133. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schattner EJ, Elkon KB, Yoo DH, et al. CD40 ligation induces Apo-1/Fas expression on human B lymphocytes and facilitates apoptosis through the Apo-1/Fas pathway. J Exp Med. 1995;182(5):1557–1565. doi: 10.1084/jem.182.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai YT, Catley LP, Mitsiades CS, et al. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004;64(8):2846–2852. doi: 10.1158/0008-5472.can-03-3630. [DOI] [PubMed] [Google Scholar]
- 30.Hasbold J, Johnson-Leger C, Atkins CJ, et al. Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur J Immunol. 1994;24(8):1835–1842. doi: 10.1002/eji.1830240817. [DOI] [PubMed] [Google Scholar]
- 31.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5(4):319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 32.Johnson PWSN, Chowdhury F, et al. A Cancer Research UK phase I study evaluating safety, tolerability, and biological effects of chimeric anti-CD40 monoclonal antibody (MAb) Chi Lob 7/4. J Clin Oncol. 2010;28(15s) suppl; abstr 2507. [Google Scholar]
- 33.Grangeon C, Cormary C, Douin-Echinard V, et al. In vivo induction of antitumor immunity and protection against tumor growth by injection of CD154-expressing tumor cells. Cancer Gene Ther. 2002;9(3):282–288. doi: 10.1038/sj.cgt.7700439. [DOI] [PubMed] [Google Scholar]
- 34.Tomihara K, Kato K, Masuta Y, et al. Gene transfer of the CD40-ligand to human dendritic cells induces NK-mediated antitumor effects against human carcinoma cells. Int J Cancer. 2007;120(7):1491–1498. doi: 10.1002/ijc.22518. [DOI] [PubMed] [Google Scholar]
- 35.Carbone E, Ruggiero G, Terrazzano G, et al. A new mechanism of NK cell cytotoxicity activation: the CD40-CD40 ligand interaction. J Exp Med. 1997;185(12):2053–2060. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jyothi MD, Khar A. Regulation of CD40L expression on natural killer cells by interleukin-12 and interferon gamma: its role in the elicitation of an effective antitumor immune response. Cancer Immunol Immunother. 2000;49(10):563–572. doi: 10.1007/s002620000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amakata Y, Fujiyama Y, Andoh A, et al. Mechanism of NK cell activation induced by coculture with dendritic cells derived from peripheral blood monocytes. Clin Exp Immunol. 2001;124(2):214–222. doi: 10.1046/j.1365-2249.2001.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atochina O, Harn D. LNFPIII/LeX-stimulated macrophages activate natural killer cells via CD40-CD40L interaction. Clin Diagn Lab Immunol. 2005;12(9):1041–1049. doi: 10.1128/CDLI.12.9.1041-1049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner JG, Rakhmilevich AL, Burdelya L, et al. Anti-CD40 antibody induces antitumor and antimetastatic effects: the role of NK cells. J Immunol. 2001;166(1):89–94. doi: 10.4049/jimmunol.166.1.89. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Dalton DK, Pitts-Meek S, Keshav S, et al. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 42.Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 43.Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35(9):555–562. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- 44.Klimp AH, de Vries EG, Scherphof GL, et al. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002;44(2):143–161. doi: 10.1016/s1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- 45.Urban JL, Shepard HM, Rothstein JL, et al. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci U S A. 1986;83(14):5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hibbs JB, Jr, Taintor RR, Vavrin Z, et al. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 47.Griffith TS, Wiley SR, Kubin MZ, et al. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189(8):1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labeur MS, Roters B, Pers B, et al. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162(1):168–175. [PubMed] [Google Scholar]
- 49.Mackey MF, Gunn JR, Maliszewsky C, et al. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161(5):2094–2098. [PubMed] [Google Scholar]
- 50.Morse MA, Lyerly HK, Gilboa E, et al. Optimization of the sequence of antigen loading and CD40-ligand-induced maturation of dendritic cells. Cancer Res. 1998;58(14):2965–2968. [PubMed] [Google Scholar]
- 51.Tschoep K, Manning TC, Harlin H, et al. Disparate functions of immature and mature human myeloid dendritic cells: implications for dendritic cell-based vaccines. J Leukoc Biol. 2003;74(1):69–80. doi: 10.1189/jlb.0702352. [DOI] [PubMed] [Google Scholar]
- 52.DeKruyff RH, Gieni RS, Umetsu DT. Antigen-driven but not lipopolysaccharide-driven IL-12 production in macrophages requires triggering of CD40. J Immunol. 1997;158(1):359–366. [PubMed] [Google Scholar]
- 53.Alderson MR, Armitage RJ, Tough TW, et al. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178(2):669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imaizumi K, Kawabe T, Ichiyama S, et al. Enhancement of tumoricidal activity of alveolar macrophages via CD40-CD40 ligand interaction. Am J Physiol. 1999;277(1 Pt 1):L49–L57. doi: 10.1152/ajplung.1999.277.1.L49. [DOI] [PubMed] [Google Scholar]
- 55.Buhtoiarov IN, Lum H, Berke G, et al. CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J Immunol. 2005;174(10):6013–6022. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 56.Lum HD, Buhtoiarov IN, Schmidt BE, et al. Tumoristatic effects of anti-CD40 mAb-activated macrophages involve nitric oxide and tumour necrosis factor-alpha. Immunology. 2006;118(2):261–270. doi: 10.1111/j.1365-2567.2006.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lum HD, Buhtoiarov IN, Schmidt BE, et al. In vivo CD40 ligation can induce T-cell-independent antitumor effects that involve macrophages. J Leukoc Biol. 2006;79(6):1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 58.Rakhmilevich AL, Buhtoiarov IN, Malkovsky M, et al. CD40 ligation in vivo can induce T cell independent antitumor effects even against immunogenic tumors. Cancer Immunol Immunother. 2008;57(8):1151–1160. doi: 10.1007/s00262-007-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao JJ, Diesl V, Wittmann T, et al. Bacterial LPS and CpG DNA differentially induce gene expression profiles in mouse macrophages. J Endotoxin Res. 2003;9(4):237–243. doi: 10.1179/096805103225001431. [DOI] [PubMed] [Google Scholar]
- 60.Buhtoiarov IN, Lum HD, Berke G, et al. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176(1):309–318. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- 61.Buhtoiarov IN, Sondel PM, Eickhoff JC, et al. Macrophages are essential for antitumour effects against weakly immunogenic murine tumours induced by class B CpG-oligodeoxynucleotides. Immunology. 2007;120(3):412–423. doi: 10.1111/j.1365-2567.2006.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stone GW, Barzee S, Snarsky V, et al. Regression of established AB1 murine mesothelioma induced by peritumoral injections of CpG oligodeoxynucleotide either alone or in combination with poly(I:C) and CD40 ligand plasmid DNA. J Thorac Oncol. 2009;4(7):802–808. doi: 10.1097/JTO.0b013e3181a8634d. [DOI] [PubMed] [Google Scholar]
- 63.Stone GW, Barzee S, Snarsky V, et al. Nanoparticle-delivered multimeric soluble CD40L DNA combined with Toll-like receptor agonists as a treatment for melanoma. PLoS One. 2009;4(10):e7334. doi: 10.1371/journal.pone.0007334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dye ES, North RJ. Macrophage accumulation in murine ascites tumors, I: cytoxan-induced dominance of macrophages over tumor cells and the anti-tumor effect of endotoxin. J Immunol. 1980;125(4):1650–1657. [PubMed] [Google Scholar]
- 65.Solomayer EF, Feuerer M, Bai L, et al. Influence of adjuvant hormone therapy and chemotherapy on the immune system analysed in the bone marrow of patients with breast cancer. Clin Cancer Res. 2003;9(1):174–180. [PubMed] [Google Scholar]
- 66.Johnson EE, Buhtoiarov IN, Baldeshwiler MJ, et al. Enhanced T-cell-independent antitumor effect of cyclophosphamide combined with anti-CD40 mAb and CpG in mice. J Immunother. 2011;34(1):76–84. doi: 10.1097/CJI.0b013e318200b28a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buhtoiarov IN, Sondel PM, Wigginton JM, et al. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology. 2011;132(2):226–239. doi: 10.1111/j.1365-2567.2010.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Honeychurch J, Glennie MJ, Illidge TM. Cyclophosphamide inhibition of anti-CD40 monoclonal antibody-based therapy of B cell lymphoma is dependent on CD11b+ cells. Cancer Res. 2005;65(16):7493–7501. doi: 10.1158/0008-5472.CAN-04-3808. [DOI] [PubMed] [Google Scholar]
- 69.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63(15):4490–4496. [PubMed] [Google Scholar]
- 70.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40(11):1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 72.Guiducci C, Vicari AP, Sangaletti S, et al. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65(8):3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 73.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333(6045):1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q, Lao X, Pan Q, et al. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17(15):4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jackaman C, Cornwall S, Graham PT, et al. CD40-activated B cells contribute to mesothelioma tumor regression. Immunol Cell Biol. 2011;89(2):255–267. doi: 10.1038/icb.2010.88. [DOI] [PubMed] [Google Scholar]
- 76.Oehler L, Majdic O, Pickl WF, et al. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J Exp Med. 1998;187(7):1019–1028. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackaman C, Lew AM, Zhan Y, et al. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol. 2008;20(11):1467–1479. doi: 10.1093/intimm/dxn104. [DOI] [PubMed] [Google Scholar]
- 78.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 79.Hamzah J, Nelson D, Moldenhauer G, et al. Vascular targeting of anti-CD40 antibodies and IL-2 into autochthonous tumors enhances immunotherapy in mice. J Clin Invest. 2008;118(5):1691–1699. doi: 10.1172/JCI33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hicks AM, Riedlinger G, Willingham MC, et al. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. Proc Natl Acad Sci U S A. 2006;103(20):7753–7758. doi: 10.1073/pnas.0602382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Broomfield SA, van der Most RG, Prosser AC, et al. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009;182(9):5217–5224. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- 82.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, et al. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69(18):7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aranda F, Llopiz D, Diaz-Valdes N, et al. Adjuvant combination and antigen targeting as a strategy to induce polyfunctional and high-avidity T-cell responses against poorly immunogenic tumors. Cancer Res. 2011;71(9):3214–3224. doi: 10.1158/0008-5472.CAN-10-3259. [DOI] [PubMed] [Google Scholar]
- 84.Uno T, Takeda K, Kojima Y, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12(6):693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 85.Pan PY, Ma G, Weber KJ, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70(1):99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Advani R, Forero-Torres A, Furman RR, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(26):4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 87.Hussein M, Berenson JR, Niesvizky R, et al. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010;95(5):845–848. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fransen MF, Sluijter M, Morreau H, et al. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17(8):2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 89.Malmstrom PU, Loskog AS, Lindqvist CA, et al. AdCD40L immunogene therapy for bladder carcinoma—the first phase I/IIa trial. Clin Cancer Res. 2010;16(12):3279–3287. doi: 10.1158/1078-0432.CCR-10-0385. [DOI] [PubMed] [Google Scholar]