Abstract
Objective
T cell large granular lymphocyte leukemia (T-LGL) is a chronic clonal lymphoproliferation of cytotoxic T cells (CTL) often complicated by cytopenias. Because the outcomes of splenectomy in patients with T-LGL have been only sporadically reported we objectively assessed the outcomes of splenectomy.
Patients and Methods
When a cohort of 56 T-LGL patients was analyzed, patients with splenomegaly (n=34) and had higher frequency of bi- and pancytopenia than patients with no splenomegaly (70% vs. 27%; p=.001). We identified 15 patients who, in their clinical course, underwent splenectomy and studied their hematological and clinical outcomes.
Results
Indications for splenectomy included symptomatic splenomegaly and/or severe refractory cytopenias. Median spleen weight was 1300g, consistent with the diagnosis of splenomegaly; TCR-γ rearrangement and typical T-LGL were detected by immunophenotype in all specimens. There was no surgery-related mortality, with the median follow up and survival of 719 and 498 days, respectively. Two patients died due to causes possibly related to the splenectomized state and/or primary disease. All patients showed lineage-specific hematologic response and achieved transfusion independence; however, precise molecular analysis of TCR and Vβ flow cytometry showed persistence of the LGL clones.
Conclusion
We conclude that splenectomy constitutes a viable and safe therapeutic option for patients with T-LGL, splenomegaly and refractory cytopenias.
Keywords: LGL, Splenectomy, CTL
INTRODUCTION
T cell large granular lymphocytic leukemia (T-LGL) is a chronic clonal lymphoproliferation of cytotoxic T lymphocytes (CTL) often associated with immune cytopenias[1,2]. Clinically, T-LGL overlaps with less polarized clonal expansions of CTL and polyclonal reactive processes occurring in the context of viral infections and autoimmune diseases[3,4]. In a significant proportion of patients T-LGL may be asymptomatic. Similarly, benign clonal expansions can be encountered in apparently healthy elderly individuals, a condition often referred to as monoclonal (T cell) clonopathy of unclear significance (MCUS, TCUS)[3,5,6]. The proliferation of CTL is not totally autonomous and may be a reflection of an exaggerated immune response possibly sustained by a persistent antigenic drive[3]. Alternative pathogenic mechanisms may include clonal acquisition of resistance in the context of polyclonal responses.
Diagnostic criteria for T-LGL include the presence of characteristic LGL on blood smear although the absolute LGL count has not been consistently set (either >.400 or >2000/uL of blood), evidence for flow cytometric population of abnormal CD3+,CD16+,CD28−,CD57+. CD8+ T cell population, and clonal T cell receptor (TCR)-γ rearrangement studies [2,7–10]. More than 1/3 of these patients present clinically with cytopenias, recurrent bacterial infections, and/or splenomegaly. Most common hematologic complications, lineage-restricted cytopenias, may be either a result of direct clone–mediated specific cytotoxicity directed against corresponding committed progenitors (erythroid or myeloid precursors in red cell aplasia or neutropenia, respectively) or due to cytokine-mediated proapoptotic effects [11,12]. Some patients may present also with hemolytic anemia. Moreover, cytopenias seen in T-LGL may also be a result of splenic sequestration.
Historically, splenectomy in T-LGL has been sporadically reported and improvement of counts has been reported following procedure[13]. Improvement of immune-mediated cytopenias has been reported following removal of an enlarged spleen [14,15]. Splenectomy can be effective also in immune thrombocytopenic purpura[16,17]. Its potential utility has also been demonstrated in various hematologic malignancies[18–21]. In Felty’s syndrome (FS), a condition closely associated with T-LGL, splenectomy was an important component of treatment before the advent of its modern management[22]. Among 118 cases reported of FS treated by surgical splenectomy, immediate hematological resolution of neutropenia was reported in 100% of the patients. However, the response was not persistent, with 20% of patients relapsing within first 6 months [23]. Clinically, splenectomy is beneficial in relieving the gastrointestinal related symptoms of fullness, nausea, early satiety and pain related to splenomegaly.
To objectively assess the outcomes of splenectomy in patients with T-LGL we retrospectively analyzed a cohort of patients with T-LGL who underwent splenectomy for various clinical indications.
MATERIALS AND METHODS
Patients
Informed consent for the study of the patients’ records and for the blood sample collection for the laboratory studies was obtained from the Institutional Review Board of the Cleveland Clinic Foundation according to the established procedures. For the purpose of this study, we used modified diagnostic criteria as previously reported[7,24,25]. Diagnostic criteria included 1) presence of T cell receptor (TCR) γ-chain rearrangement, 2) detection of an expanded discrete cell population characterized by expression of CD3, CD8, CD16 and CD57 markers, 3) morphologic detection of LGL on blood smear (>.400/μL of blood) and/or 4) restricted usage of TCR variable chain β (Vβ) within T cell repertoire[3]. We analyzed samples from a total of 56 patients diagnosed with T-LGL leukemia between 2002 to 2007 (Table 1). Splenomegaly was identified by palpation or clinical suspicion and confirmed by ultrasound or CT scan. We identified 15 patients with T-LGL who, in their clinical course, underwent elective splenectomy (Table 2). Median follow up was 719 days. No patients were lost to follow up. All splenectomized patients received pneumococcal, meningococcal and haemophilus influenzae vaccination before surgery. Patients underwent either open (5/15) or laparoscopic splenectomy (10/15) depending on surgical conditions such as size of the spleen and prior surgical history.
Table 1.
Clinical characteristics of patients with T-LGL
| T-LGL Leukemia Cohort | Splenomegaly | No Splenomegaly | p-Value |
|---|---|---|---|
| Total Number in Cohort (Female) | 34 (16) | 22 (8) | 0.61 |
| Median Age at Diagnosis in Years (Range) | 64 (28–81) | 64 (31–78) | 0.88 |
| Single Lineage Cytopenia | 10/34 (29%) | 15/22 (68%) | <0.01 |
| Anemia (Hgb<10g/dL) | 8/34 (24%) | 9/22 (41%) | 0.17 |
| Neutropenia | 1/34 (3%) | 5/22 (23%) | 0.02 |
| Thrombocytopenia | 1/34 (3%) | 1/22 (5%) | 0.75 |
| Bicytopenia | 16/34 (47%) | 3/22 (14%) | <0.01 |
| Pancytopenia | 8/34 (24%) | 3/22 (14%) | 0.36 |
| Median LGL Count (Range) | 1800 (100–20575) | 1130 (100–7410) | 0.41 |
| Median CD4:CD8 (Range) | 0.35 (0.03–3.00) | 0.31 (0.04–1.39) | 0.51 |
| Median % Vβ expansion (Range) | 63 (19–98) | 64 (11–90) | 0.88 |
Splenomegaly was identified by palpation or clinical suspicion and confirmed by ultrasound or CT scan.
Table 2.
Clinical Features of Splenectomized T-Cell patients
| Pt | Age | Sex | Presentation | Prior Therapy | Reason for Splenectomy | Spleen Size | Ref.Dx | Conf.Dx | Associated Conditions |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | F | GI symptoms, Anemia | Cytoxan, | Transfusion-dependant anemia | N/a | LGL | T-LGL | MGUS, Thyroid ca |
| 2 | 43 | M | Weight loss, Splenomegaly, night sweats | CHOP | Diagnostic | 500 g | LGL | T-LGL | Sarcoidosis, CMV IgG Ab + |
| 3 | 74 | M | Pancytopenia, fatigue, GI symptoms | Cyclosporine | Pancytopenia with normal BM and high retic count | 515 g | MDS | T-LGL | Hypothyroidism |
| 4 | 52 | M | Neutropenia, thrombocytopenia, splenomegaly | G-CSF | Severe thrombocytopenia, splenomegaly | 5800 g | LGL | T-LGL | Multiple Myleoma |
| 5 | 33 | M | Pancytopenia | Erythropoeitin, Transfusions | Pancytopenia | 936 g | MDS | T-LGL | Graves, Gilbert’s Disease |
| 6 | 62 | F | Neutropenia, lymphocytosis | G-CSF | Neutropenia with hypercellular bone marrow | 500 g | LGL | T-LGL | RA |
| 7 | 53 | M | Neutropenia, anemia | G-CSF | Transfusion-dependant anemia | 804 g | CLL | T-LGL | |
| 8 | 70 | M | Neutropenia, splenomegaly | G-CSF | Neutropenia | N/a | LGL | T-LGL | RA |
| 9 | 39 | F | Pancytopenia | G-CSF | Pancytopenia with hypercellular bone marrow | 1668 g | LGL | T-LGL | Autoimmune hepatitis |
| 10 | 58 | M | Anemia | CHOP, Erythropoeitin, Transfusions | Transfusion-dependant Anemia | 1000 g | LGL | T-LGL | Rectal Carcinoma |
| 11 | 65 | M | Leukopenia, thrombocytopenia | Transfusions | Thrombocytopenia, h/o splenic bleeding | 570 g | LGL | T-LGL | |
| 12 | 52 | M | Pancytopenia | G-CSF | GI symptoms | 570 g | LGL | T-LGL | EBV viremia, Parvovirus B19 IgG+ |
| 13 | 80 | F | Neutropenia | G-CSF | Neutropenia with normal BM, GI symptoms | 186 g | Neutropenia | T-LGL | RA, melanoma, BCC |
| 14 | 34 | M | Pancytopenia | Erythropoeitin, Transfusions | Pancytopenia with splenomegaly | 3700 g | Pancytopenia | T-LGL | |
| 15 | 58 | M | Pancytopenia | Cyclosporine, Splenectomy | Thrombocytopenia | 340 g | ITP/CLL | T-LGL |
N/a: Not available; RA: Rheumatoid Arthritis;CLL: Chronic Lymphocytic Leukemia; BCC: Basal Cell Carcinoma; ITP: Idiopathic Thrombocytopenia Purpura; Ref. Dx: Referral Diagnosis; Conf.Dx: Confirmatory diagnosis; Diagnosis of MDS based on WHO classification.
Laboratory parameters studied
The patients were evaluated on the basis of pre-post absolute lymphocyte count (ALC), absolute neutrophil count (ANC), hemoglobin, and platelet count. Pancytopenia was defined as a deficiency in all three blood cell lineages. Anemia was defined as absolute reticulocyte count < 40,000/μL and hemoglobin <10g/μL. Neutropenia was defined as ANC < 1,000/μL; with severe neutropenia as ANC < 500/μL. Thromobcytopenia was defined as platelet count < 100,000/μL. Thrombocytosis was defined as platelet count > 750,000/μL.
Flow cytometric immunophenotyping and Vβ typing
Flow cytometric analysis of Vβ utilization was employed to quantify the size of T-LGL clone as previously described[26]. The results were expressed as the percentage of α/β CD4+ or CD8+ cells. Fresh peripheral blood was stained for Vβ flow cytometry according to manufacturer’s instructions (IOTest Beta Mark kit; Beckman-Coulter, Fullerton, CA, USA). Due to incomplete coverage of the entire Vβ spectrum by the Vβ mAb set, further analysis was done and Vβ polymerase chain reaction (PCR) for Vβ families 6, 15 and 24 was performed. Clonotypic sequences of expanded Vβ and Jβ families were determined, and expanded CDR3 clonotypes were also detected.[26]
Immunophenotyping and Genotyping
HLA and killer immunoglobulin-like receptor (KIR) genotyping, KIR and KIR-L assignments, and genotyping of various cytokine polymorphisms were performed as previously described including TGF-β1 (codons 10 C/T, 25 G/C), TNF-α (−308G/A), interleukin-6 (IL-6) (−174 G/C), interleukin-10 (IL-10) (−1082 G/A, −819 C/T, and −592 C/A), IFN-γ (+874 T/A), CTLA4 (+45 A/G), FcγRIIIa(158V/F) and CD45 (77 C/G) [27].
RESULTS
Clinical characteristics of T-LGL patients
We have analyzed our cohort of 56 T-LGL patients with regard to several clinical parameters (Table 1). At the time of diagnosis 1/34 patient has been previously splenectomized for the initially presumed idiopathic thrombocytopenic purpura. At the time of referral we have verified that the proper diagnosis was T-LGL.
We dichotomized patients into two groups with respect to presence (n=34) or absence of splenomegaly (n=22). When these 2 groups were compared with regard to the types and severity of associated cytopenias, we have noted that patients with splenomegaly have more often bi- and pancytopenia (70.5%) than seen in patients lacking splenomegaly (27.2%; p=.001). The median age in both the groups was 64 years. The median LGL count in the splenomegaly group was 1800/μL vs. 1130/μL in the group of patients with no splenomegaly (p=.410).
Associated autoimmune conditions included ulcerative colitis (n=1), rheumatoid arthritis (RA) (n=15), multiple sclerosis (MS) (n=1), systemic lupus erythematosus (SLE) (n=2), idiopathic pulmonary hypertension (n=1), and auto-immune thyroiditis (n=2). Interestingly several patients had a history of malignancies. The majority of the CTL populations expressed CD3, CD8, CD16, and CD57 antigens. Using Vβ flow cytometry, Vβ family expansion was detected in 54/56 patients. On average, Vβ expansions in constituted 64%± 30% of the Vβ repertoire within a given Vβ family A total of 86 immunodominant LGL clonotypes were identified (in some patients 2 co-dominant clones can be found). There was no difference in the Vβ clone size between splenomegalic patients (63%) and those without splenomegaly (64%; p=.882).
We also investigated HLA, KIR/KIR-ligand, and cytokine/cytokine receptor genotypes as previously described [27]. Comparison of patients with and without splenomegaly showed no difference in the KIR/KIR-ligand profile, HLA type, or frequency of cytokine and immunoregulatory receptor studied, including TGF-β1 (codons 10 C/T, 25 G/C), TNF-α (−308G/A), interleukin-6 (IL-6) (−174 G/C), interleukin-10 (IL-10) (−1082 G/A, −819 C/T, and −592 C/A), IFN-γ (+874 T/A), CTLA4 (+45 A/G), FcγRIIIa(158V/F) and CD45 (77 C/G)[27].
Clinical characteristics of splenectomized patients and outcomes
We further identified 15 patients who in the course of their disease underwent splenectomy; hematological and clinical outcomes were studied (Table 2). Diagnosis was established in all cases using immunophenotypic and flow cytometric evidence of Vβ expansions and TCR-γ rearrangement. Median age at splenectomy was 58 years and male/female ratio was 11/4. Associated conditions in these patients included RA, Grave’s disease, FS, thyroid cancer, rectal carcinoma, melanoma, basal cell carcinoma, MDS, CLL, and monoclonal gammopathy of unknown significance (MGUS). The frequent association of T-LGL with B cell dyscrasias has been reported [28]. Due to the limited number of cases, no association was found between splenectomy and improvement in any co-associated condition. The indications for the splenectomy included symptomatic splenomegaly (5/15) and/or severe refractory cytopenias (pancytopenia, n=6; bicytopenia, n=3; single lineage cytopenias, n=5). Prior therapies were mostly symptomatic and supportive with granulocyte-colony stimulating factor (G-CSF), erythropoietin, or red cell transfusions. Two patients each received prior CHOP chemotherapy or cyclosporine, and 1 received oral cytoxan chemotherapy. Median spleen size was 1300 g and all patients showed clonal TCR-γ rearrangement in resected material; immunophenotype included positivity for CD3, CD5, CD8, CD57, CD16 and CD56. The majority of the patients underwent laparoscopic splenectomy (67%) with the remainder having a standard open procedure (33%). The presence of T-LGL was established/confirmed in resected spleens by histological methods and immunophenotyping.
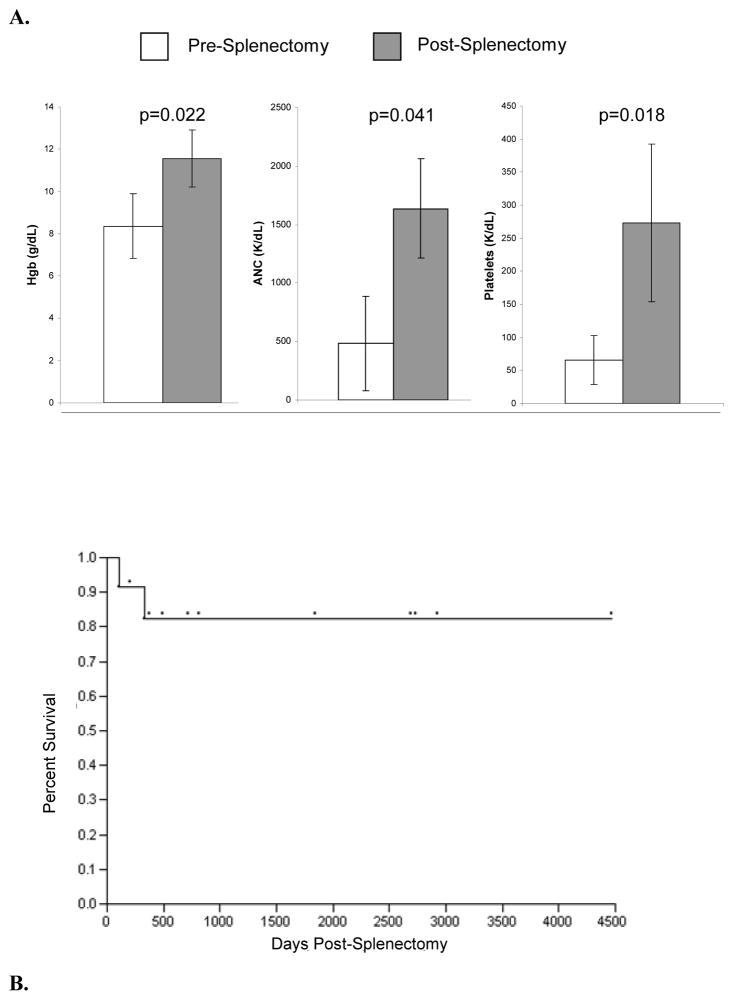
There was no surgery related mortality in this cohort of T-LGL patients. Median follow- up of 719 days and the median survival time was 498 days (Figure 1A). Two patients died due to causes possibly related to splenectomised state and/or cytopenias (one of Gram negative sepsis and the other of EBV viremia with multiorgan dysfunction). Patient survival and length of follow-up is shown on the Kaplan Meier curve (Figure 1B). Blood parameter values for 10 of 11 patients showed improved counts following splenectomy and did not require further treatment for cytopenias, including achieving transfusion-independence. (Hgb, p=0.022, ANC, p=0.041 Plt, p=0.018; Figure 1). Molecular analysis of TCR-γ utilization spectrum and Vβ flow cytometry showed persistence of the LGL clone in all patients tested (n=12) but no further expansion was observed.
Figure 1. Effects of splenectomy on hematologic parameters affected by T-LGL.
Hemoglobin (Hgb); Absolute Neutrophil Count (ANC) and platelets are depicted with standard error of the mean values pre and post splenectomy. B. Survival of patients with T-LGL who underwent splenectomy with median follow up 719 days the median survival time was 498 days, maximum survival at the end of this study was 4436 days for patient who received splenectomy at outside hospital.
DISCUSSION
To date, this is the largest reported study of splenectomized patients with T-LGL. Due to its low incidence, the therapies of T-LGL have often been empiric or based on case reports and retrospective single institution studies[11,29,30]. Most common indications for treatment include recurrent or life threatening infections, severe neutropenia, symptomatic anemia, thrombocytopenia or severe B symptoms. Although a therapeutic algorithm has been suggested[11,29], the role of several therapies has not been well defined and in many instances recommendations are empirically driven. Often cytopenias or systemic symptoms do not correlate with number of circulating T-LGL or bone marrow infiltration [11,31].
Inciting or sustaining signals of CTL clonal expansions in the etiology of T-LGL remain unknown. Its clinical course may be variable. Although, the involvement of the spleen is almost universal in T-LGL, its clinical significance remains uncertain. Splenectomy has been occasionally used as a therapeutic option in T-LGL patients whose counts remain refractory to medical measures e.g., in an attempt to decrease the transfusion-dependence. Systematic studies on this subject are not available; the largest series included 4 patients[13] and most of the reports contain individual cases [32–34].
Our investigations revealed that in most of the patients, splenectomy was necessitated either by refractory cytopenias requiring treatment and/or splenomegaly-related gastrointestinal complaints. Splenectomy in our cohort of T-LGL patients resulted in improvement in affected blood counts in all three lineages. None of the patients proceeded to the aggressive variant of T- LGL, with persistence of chronic disease, but clearly the procedure was not curative. The observed mortality included septic death and overwhelming EBV viremia can be either attributed to the primary disease or to splenectomized state of the patients.
Pathophysiologically, in addition to inherent hypersplenism, splenectomy could have a favorable impact on cytopenia as evidenced by increased frequency of bi- or pancytopenias in splenomegalic T-LGL[35]. Precise analysis of clonal size in the clinical course showed persistence of the malignant CTL clone post-splenectomy also suggesting that elimination of hypersplenism, rather than reduction in LGL-clone size (leukemic burden), contributes to the improvement of blood counts. Alternatively, removal of the spleen could result in a decreased disease burden (e.g., number of malignant LGLs) including associated autoimmune mechanisms not primarily related to LGL itself, such as autoantibodies or complement.
Significant limitations of our report are its retrospective nature, referral-bias and short follow up. However, prospective studies of splenectomy in T-LGL are unlikely possible in this rare condition. While the relatively short follow-up period post-splenectomy does not allow conclusions with regard to the long-term outcomes in this disease, the more immediate impact of the procedure on hematologic response proves to be favorable. Of note, cases referred for splenectomy were biased by clinical severity of symptoms and their refractoriness to conservative therapies.
In sum, the improvement in counts and low morbidity are encouraging to offer splenectomy as viable option to refractory and symptomatic cytopenias in T-LGL patients with splenomegaly.
Table 3.
Pre-splenectomy and Post-splenectomy laboratory cell counts of various blood parameters tested.
| Pre-Splenectomy Counts | Post-Splenectomy Counts | |||||||
|---|---|---|---|---|---|---|---|---|
| Pt # | ALC | ANC | Hgb | Plt | ALC | ANC | Hgb | Plt |
| 1 | 330 | 122 | 8 | 403 | 1390 | 1770 | 13.1 | 273 |
| 2 | 197 | 234 | 15.7 | 285 | 2760 | 4110 | 14.3 | 285 |
| 3 | 610 | 1200 | 10.2 | 120 | 3300 | 930 | 10.5 | 434 |
| 4 | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a |
| 5 | N/a | N/a | N/a | N/a | 3200 | 1760 | 14.7 | 250 |
| 6 | 3460 | 89 | 13.2 | 297 | 2840 | 1770 | 13.2 | 247 |
| 7 | 4180 | 620 | 8.6 | 175 | 17380 | 1800 | 10.7 | 444 |
| 8 | N/a | N/a | N/a | N/a | 4510 | 340 | 12.8 | 181 |
| 9 | 750 | 579 | 9.3 | 57 | 7490 | 2120 | 13.3 | 400 |
| 10* | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a |
| 11 | 2100 | 240 | 11.1 | 83 | 2800 | 680 | 12 | 174 |
| 12* | 610 | 380 | 11.8 | 51 | 1530 | 1790 | 13.8 | 12 |
| 13 | 1390 | 450 | 11.8 | 154 | 4330 | 1041 | 12.2 | 328 |
| 14 | 900 | 99 | 5.7 | 63 | 3480 | 1953 | 10.2 | 156 |
| 15 | 550 | 740 | 8.7 | 7 | 5930 | 7710 | 15.1 | 202 |
ALC, Absolute lymphocyte count; ANC Absolute neutrophil count; Hgb, Hemoglobin; Plt, Platelet count;
deceased;
N/a, Not available
Table 4.
Literature review of reports of splenectomized T-Cell LGL patients
| Citation | T-LGL | Splenomegaly | Country | n | Reason for Splenectomy | Findings |
|---|---|---|---|---|---|---|
| Loughran et al 1987[13] | Y | Y | USA | 4 | Neutropenia | Increase in neutrophil counts, 2/4 sustained, 1 patient died due to aggressive disease |
| Furukawa et al. 1991[32] | Y | Y | Japan | 1 | Thrombocytopenia | Platelets returned to nl after splenectomy, 2 years later patient died secondary to sepsis -DIC |
| Coad JE et al. 1993[36] | Y | Y | UK | 4 | Therapy-resistant disease and therapeutic splenectomy | Retrospective study of 70 heterogenous patients with chronic lymphoproliferative disorders. |
| Gentile TC et al 1996[33] | Y | Y | USA | 1 | AIHA | Increase in Hb to normal level |
| Brinkman K et al 1998[34] | Y | Y | Netherlands | 1 | N/A | Treatment failure with splenectomy and remission with cyclosporin A |
| Nowakowski et al 2005[35] | Y | Y | USA | 4 | Therapeutic for Splenomegaly | Pts. in KIR/HLA-I mismatch group 4 pts. required therapeutic splenectomy. Outcomes not reported |
| CCTCI* | Y | Y | USA | 15 | Symptomatic cytopenias, Splenomegaly | 2/15 died. 13/15 sustained response |
CCTCI-Cleveland Clinic Taussig Cancer Institute series;
Y-Yes ;N/A- Not available
Acknowledgments
Acknowledgements and Grant support:
Supported in part by a grant from R01 655365071402, U54 – 655365070704, K24 655365071503, to JPM.A grant from the Aplastic Anemia and MDS International Foundation (J.P.M.); and a generous gift from the Trotter family. Aaron D. Viny is a Howard Hughes Medical Institute Medical Research Training Fellow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanchan K, Loughran TP., Jr Antigen-driven clonal T cell expansion in disorders of hematopoiesis. Leuk Res. 2003;27:291–292. doi: 10.1016/s0145-2126(02)00270-9. [DOI] [PubMed] [Google Scholar]
- 2.Lamy T, Loughran TP., Jr Clinical features of large granular lymphocyte leukemia. Semin Hematol. 2003;40:185–195. doi: 10.1016/s0037-1963(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 3.Wlodarski MW, Schade AE, Maciejewski JP. T-large granular lymphocyte leukemia: current molecular concepts. Hematology. 2006;11:245–256. doi: 10.1080/10245330600774793. [DOI] [PubMed] [Google Scholar]
- 4.Lazaro E, Caubet O, Menard F, Pellegrin JL, Viallard JF. Presse Med. 2007 doi: 10.1016/j.lpm.2007.06.002. [Large granular lymphocyte leukemia.] [DOI] [PubMed] [Google Scholar]
- 5.Dhodapkar MV, Li CY, Lust JA, Tefferi A, Phyliky RL. Clinical spectrum of clonal proliferations of T-large granular lymphocytes: a T-cell clonopathy of undetermined significance? Blood. 1994;84:1620–1627. [PubMed] [Google Scholar]
- 6.Sabnani I, Tsang P. Are clonal T-cell large granular lymphocytes to blame for unexplained haematological abnormalities? Br J Haematol. 2007;136:30–37. doi: 10.1111/j.1365-2141.2006.06374.x. [DOI] [PubMed] [Google Scholar]
- 7.Semenzato G, Zambello R, Starkebaum G, Oshimi K, Loughran TP., Jr The lymphoproliferative disease of granular lymphocytes: updated criteria for diagnosis. Blood. 1997;89:256–260. [PubMed] [Google Scholar]
- 8.Lima M, Almeida J, Santos AH, dos Anjos Teixeira M, Alguero MC, Queiros ML, Balanzategui A, Justica B, Gonzalez M, San Miguel JF, Orfao A. Immunophenotypic analysis of the TCR-Vbeta repertoire in 98 persistent expansions of CD3(+)/TCR-alphabeta(+) large granular lymphocytes: utility in assessing clonality and insights into the pathogenesis of the disease. Am J Pathol. 2001;159:1861–1868. doi: 10.1016/s0002-9440(10)63032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamy T, Loughran TP., Jr Current concepts: large granular lymphocyte leukemia. Blood Rev. 1999;13:230–240. doi: 10.1054/blre.1999.0118. [DOI] [PubMed] [Google Scholar]
- 10.Melenhorst JJ, Eniafe R, Follmann D, Molldrem J, Kirby M, El Ouriaghli F, Barrett AJ. T-cell large granular lymphocyte leukemia is characterized by massive TCRBV-restricted clonal CD8 expansion and a generalized overexpression of the effector cell marker CD57. Hematol J. 2003;4:18–25. doi: 10.1038/sj.thj.6200212. [DOI] [PubMed] [Google Scholar]
- 11.Alekshun TJ, Sokol L. Diseases of large granular lymphocytes. Cancer Control. 2007;14:141–150. doi: 10.1177/107327480701400207. [DOI] [PubMed] [Google Scholar]
- 12.O’Malley DP. T-cell large granular leukemia and related proliferations. Am J Clin Pathol. 2007;127:850–859. doi: 10.1309/A8FHDA0VVRJ05GJP. [DOI] [PubMed] [Google Scholar]
- 13.Loughran TP, Jr, Starkebaum G, Clark E, Wallace P, Kadin ME. Evaluation of splenectomy in large granular lymphocyte leukaemia. Br J Haematol. 1987;67:135–140. doi: 10.1111/j.1365-2141.1987.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 14.Lanzi S, Lancini GP, Piardi T, Biasca F, Ottaviani GM, Rossi G, Pizzoccaro C, Pouche A. Splenectomy in immune thrombocytopenia and other hematological diseases. G Chir. 1999;20:479–486. [PubMed] [Google Scholar]
- 15.Font J, Jimenez S, Cervera R, Garcia-Carrasco M, Ramos-Casals M, Campdelacreu J, Ingelmo M. Splenectomy for refractory Evans’ syndrome associated with antiphospholipid antibodies: report of two cases. Ann Rheum Dis. 2000;59:920–923. doi: 10.1136/ard.59.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 17.Bromberg ME. Immune thrombocytopenic purpura--the changing therapeutic landscape. N Engl J Med. 2006;355:1643–1645. doi: 10.1056/NEJMp068169. [DOI] [PubMed] [Google Scholar]
- 18.Vevon PA, Ellison EC, Carey LC. Splenectomy for hematologic disease. Adv Surg. 1989;22:105–139. [PubMed] [Google Scholar]
- 19.Seymour JF, Cusack JD, Lerner SA, Pollock RE, Keating MJ. Case/control study of the role of splenectomy in chronic lymphocytic leukemia. J Clin Oncol. 1997;15:52–60. doi: 10.1200/JCO.1997.15.1.52. [DOI] [PubMed] [Google Scholar]
- 20.Bouvet M, Babiera GV, Termuhlen PM, Hester JP, Kantarjian HM, Pollock RE. Splenectomy in the accelerated or blastic phase of chronic myelogenous leukemia: a single-institution, 25-year experience. Surgery. 1997;122:20–25. doi: 10.1016/s0039-6060(97)90259-2. [DOI] [PubMed] [Google Scholar]
- 21.Berman RS, Feig BW, Hunt KK, Mansfield PF, Pollock RE. Platelet kinetics and decreased transfusion requirements after splenectomy for hematologic malignancy. Ann Surg. 2004;240:852–857. doi: 10.1097/01.sla.0000143303.10884.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burks EJ, Loughran TP., Jr Pathogenesis of neutropenia in large granular lymphocyte leukemia and Felty syndrome. Blood Rev. 2006;20:245–266. doi: 10.1016/j.blre.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Rashba EJ, Rowe JM, Packman CH. Treatment of the neutropenia of Felty syndrome. Blood Rev. 1996;10:177–184. doi: 10.1016/s0268-960x(96)90024-7. [DOI] [PubMed] [Google Scholar]
- 24.Berliner N, Duby AD, Linch DC, Murre C, Quertermous T, Knott LJ, Azin T, Newland AC, Lewis DL, Galvin MC, et al. T cell receptor gene rearrangements define a monoclonal T cell proliferation in patients with T cell lymphocytosis and cytopenia. Blood. 1986;67:914–918. [PubMed] [Google Scholar]
- 25.Herling M, Khoury JD, Washington LT, Duvic M, Keating MJ, Jones D. A systematic approach to diagnosis of mature T-cell leukemias reveals heterogeneity among WHO categories. Blood. 2004;104:328–335. doi: 10.1182/blood-2004-01-0002. [DOI] [PubMed] [Google Scholar]
- 26.Wlodarski MW, O’Keefe C, Howe EC, Risitano AM, Rodriguez A, Warshawsky I, Loughran TP, Jr, Maciejewski JP. Pathologic clonal cytotoxic T-cell responses: nonrandom nature of the T-cell-receptor restriction in large granular lymphocyte leukemia. Blood. 2005;106:2769–2780. doi: 10.1182/blood-2004-10-4045. [DOI] [PubMed] [Google Scholar]
- 27.Nearman ZP, Wlodarski M, Jankowska AM, Howe E, Narvaez Y, Ball E, Maciejewski JP. Immunogenetic factors determining the evolution of T-cell large granular lymphocyte leukaemia and associated cytopenias. Br J Haematol. 2007;136:237–248. doi: 10.1111/j.1365-2141.2006.06429.x. [DOI] [PubMed] [Google Scholar]
- 28.Viny ADLA, Pohlman B, Loughran TP, Maciejewski JP. Chronic B-cell Dyscrasias are an Important Clinical Feature of LGL Leukemia. Leukemia & Lymphoma. 2008 doi: 10.1080/10428190801932635. In Press. [DOI] [PubMed] [Google Scholar]
- 29.Sokol L, Loughran TP., Jr Large granular lymphocyte leukemia. Oncologist. 2006;11:263–273. doi: 10.1634/theoncologist.11-3-263. [DOI] [PubMed] [Google Scholar]
- 30.Osuji N, Matutes E, Tjonnfjord G, Grech H, Del Giudice I, Wotherspoon A, Swansbury JG, Catovsky D. T-cell large granular lymphocyte leukemia: A report on the treatment of 29 patients and a review of the literature. Cancer. 2006;107:570–578. doi: 10.1002/cncr.22032. [DOI] [PubMed] [Google Scholar]
- 31.Sood R, Stewart CC, Aplan PD, Murai H, Ward P, Barcos M, Baer MR. Neutropenia associated with T-cell large granular lymphocyte leukemia: long-term response to cyclosporine therapy despite persistence of abnormal cells. Blood. 1998;91:3372–3378. [PubMed] [Google Scholar]
- 32.Furukawa Y, Tanaka K, Hasuike T, Hirai M, Masuzawa K, Ohira H, Ota K, Yasui Y, Nakao Y, Inoue T, et al. Chronic lymphocytic leukemia with peripheral T lymphocytes expressing CD 2+, CD 3+, CD 4−, CD 8−, CD 16+, and CD 56+ and lymph-node lymphocytes expressing CD 2+, CD 3−, CD 4−, CD 8−, CD 16+, CD 38+, and CD 56+ Rinsho Byori. 1991;39:557–561. [PubMed] [Google Scholar]
- 33.Gentile TC, Loughran TP., Jr Resolution of autoimmune hemolytic anemia following splenectomy in CD3+ large granular lymphocyte leukemia. Leuk Lymphoma. 1996;23:405–408. doi: 10.3109/10428199609054846. [DOI] [PubMed] [Google Scholar]
- 34.Brinkman K, van Dongen JJ, van Lom K, Groeneveld K, Misere JF, van der Heul C. Induction of clinical remission in T-large granular lymphocyte leukemia with cyclosporin A, monitored by use of immunophenotyping with Vbeta antibodies. Leukemia. 1998;12:150–154. doi: 10.1038/sj.leu.2400907. [DOI] [PubMed] [Google Scholar]
- 35.Nowakowski GS, Morice WG, Phyliky RL, Li CY, Tefferi A. Human leucocyte antigen class I and killer immunoglobulin-like receptor expression patterns in T-cell large granular lymphocyte leukaemia. Br J Haematol. 2005;128:490–492. doi: 10.1111/j.1365-2141.2004.05341.x. [DOI] [PubMed] [Google Scholar]
- 36.Coad JE, Matutes E, Catovsky D. Splenectomy in lymphoproliferative disorders: a report on 70 cases and review of the literature. Leuk Lymphoma. 1993;10:245–264. doi: 10.3109/10428199309148547. [DOI] [PubMed] [Google Scholar]