Abstract
Spectrin is a large, flexible protein that stabilizes membranes and organizes proteins and lipids into microdomains in intracellular organelles and at the plasma membrane. Alternative splicing occurs in spectrins, but it is not yet clear if these small variations in structure alter spectrin’s functions. Three alternative splice sites have been identified previously for αII-spectrin. Here we describe a new alternative splice site, a 21 amino acid sequence in the 21st spectrin repeat that is only expressed in significant amounts in cardiac muscle (GenBank GQ502182). The insert, which we term αII-cardi+, results in an insertion within the high affinity nucleation site for binding of α-spectrins to β-spectrins. To assess the developmental regulation of the αII-cardi+ isoform, we used qRT-PCR and quantitative immunoblotting methods to measure the levels of this form and the αII-cardi− form in the cardiac muscles of rats, from embryonic day 16 (E16) through adulthood. The αII-cardi+ isoform constituted ~26% of the total αII-spectrin in E16 hearts, but decreased to ~6% of the total after 3 weeks of age. We used long-range RT-PCR and southern blot hybridization to examine possible linkage of the αII-cardi+ alternatively spliced sequence with alternatively spliced sequences of αII-spectrin that had been previously reported. We identified two new isoforms of αII-spectrin containing the cardi+ insert. These were named αIIΣ9 and αIIΣ10 in accordance with the spectrin naming conventions. In vitro studies of recombinant αII-spectrin polypeptides representing the two splice variants of αII-spectrin, αII-cardi+ and αII-cardi−, revealed that the αII-cardi+ subunit has lower affinity for the complementary site in repeats 1-4 of βII-spectrin, with a KD value of ~1 nM, as measured by surface plasmon resonance (SPR). In addition, the αII-cardi+ form showed 1.8-fold lower levels of binding to its site on βII-spectrin than the αII-cardi− form, both by SPR and blot overlay. This suggests that the 21-amino acid insert prevented some of the αII-cardi+ form from interacting with βII-spectrin. Fusion proteins expressing the αII-cardi+ sequence within the two terminal spectrin repeats of αII-spectrin were insoluble in solution and aggregated in neonatal myocytes, consistent with the possibility that this insert removes a significant portion of the protein from the population that can bind β subunits. Neonatal rat cardiomyocytes infected with adenovirus encoding GFP-fusion proteins of repeats 18-21 of αII-spectrin with the cardi+ insert formed many new processes. These processes were only rarely seen in myocytes expressing the fusion protein lacking the insert or in controls expressing only GFP. Our results suggest that the embryonic mammalian heart expresses a significant amount of αII-spectrin with a reduced avidity for β-spectrin and the ability to promote myocyte growth.
Introduction
The spectrins are a superfamily of actin binding proteins composed of at least two alpha and five beta subunits [1–4]. The most common form of this protein is a heterodimer composed of αII and βII spectrin subunits, which together form an elongated dimer. Typically, two such dimers self-associate head-to-head, forming heterotetramers [5, 6]. αII-Spectrin is expressed in most tissues, including heart, whereas αI-spectrin is found principally in erythrocytes [3, 7]. β-Spectrins have complex patterns of expression, but striated muscles are known to have a βI isoform at the plasma membrane and isoforms of βII associated with intracellular membranes [3, 4, 8]. At the plasma membranes of striated muscle, spectrin is organized in a lattice-like network at costameres, which lie principally over Z-discs [9]. Costameres are sites of transmembrane linkage between the extracellular matrix and the internal cytoskeleton [9, 10]. Although the functions of spectrins in the heart are not well understood, it seems likely that they are involved in organizing and stabilizing the surface and internal membranes against the stresses associated with contraction and in organizing them into distinct domains and compartments [4, 5]. Spectrin has also been found to play an essential role in the development of excitable cells, in cell cycle regulation and in actin organization [11–14]. Immunofluorescence studies with antibodies to αII-spectrin in cryosections of adult mouse heart show a strong signal surrounding the myofibrils at Z-discs, as well as at the plasma membrane of cardiomyocytes [2, 4, 5]. Immunogold analysis at the ultrastructural level shows αII-spectrin within myocytes near the edges of the Z-discs and between Z-discs and the plasma membrane [9].
Alternative splicing is a major source of proteomic diversity in mammals and, according to large-scale genomics studies, it may occur in 40 to 60% of human genes [1]. Pre-mRNA splicing combined with alternative promoter usage is a mechanism commonly used by genes encoding components of the spectrin-based cytoskeleton to increase functional diversity and to regulate expression in a tissue specific manner [1, 3]. Alternative splicing of spectrins has been well documented [1], including in cardiomyocytes, in which 3 splice variants of αII-spectrin have been identified [1]. (i) A 20 amino acid insert, located in the 10th spectrin repeat, or motif, just after the SH3 domain, controls the Ca2+-dependent cleavage of spectrin and its ability to bind particular proteins [15]. The insert (TRITKEAGSVSLRMKQVEEL), which we call “SH3i+” [16], contains two potential sites of phosphorylation by protein kinases A and C, suggesting that the biological function of this region of αII-spectrin is regulated by physiological stimuli. (ii) A 5 amino acid insert, found in the 15th spectrin motif, has an exposed peptide loop with opposed hydrophobic and charged faces, reminiscent of the structure of highly antigenic epitopes and of the binding site on p53 for the ankyrin-like p53 binding protein [16–19]. (iii) A 6 amino acid insert in motif 21, of unknown function, immediately N-terminal to the site of the insert that we describe below, has also been reported [2, 20]. These alternatively spliced variants were found in erythrocytes, brain, kidney, and skeletal muscle.
In this study, we present evidence for a novel alternatively spliced product of αII-spectrin, found at significant levels only in heart muscle. This 21-amino acid insert, located just after insert (iii), near the C-terminus of αII-spectrin, is designated as αII-cardi+. This unique sequence occurs within the high affinity nucleation site for binding of αII-spectrin to β-spectrin [6, 21, 22]. Its expression in cardiac muscle is developmentally regulated and may influence cell growth and differentiation.
Materials and Methods
Antibodies
Antibodies recognizing multiple isoforms of αII-spectrin were prepared in rabbits, with αII-spectrin purified from bovine brain as the immunogen. The antibodies were affinity-purified and cross-adsorbed to generate antibodies specific for each immunogen, as previously described [23], and used at a concentration of 2 μg/ml for immunofluorescence experiments and 100 ng/ml for immunoblotting. Peptide-specific antibodies to the αII-cardi+ form of spectrin were prepared as previously described [24] by immunizing rabbits against the peptide specific for the αII-cardi+ sequence, NH2-IAYRRVIRVYQYEVGDDLSGR-COOH, synthesized as a MAP-peptide complex and affinity-purified with ImmunoPure Gentle Ag/Ab Elution Buffer (Pierce, Rockford, IL). Mouse monoclonal antibodies to αII-spectrin were purchased from Chemicon (Billerica, MA), to βII-spectrin from BD Biosciences (San Jose, CA), and to GST from Amersham Bioscience (Piscataway, NJ). Secondary antibodies included Alexa Fluor 488 or 568 goat anti-rabbit IgG and Alexa Fluor 488 or 568 goat anti-mouse IgG (Molecular probes, Inc., Eugene, OR), alkaline phosphatase-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies (Jackson Immunoresearch Laboratories, Inc., West Chester, PA).
RNA preparation
All experiments were carried out with the approval of the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine. Adult rats and timed pregnant rats of the Sprague-Dawley strain were obtained from Harlan Laboratories (Indianapolis, IN). The animals were anesthetized by intraperitoneally injecting a combination of ketamine (100 mg/kg) (LLOYD Laboratories, Shenandoah, Iowa, USA) and xylazine (6 mg/kg) (Ben Venue Laboratories, Bedford, Ohio, USA). The brain, kidney, skeletal muscle and cardiac tissues were collected from fetuses at embryonic days 16 (E16) and 19 (E19) and from rats at postnatal days 1 (D1), 3 (D3), 7 (D7), and 21 (D21), as well as from adult rats at 6 months of age (M6). The tissues were stored frozen at −80 °C prior to RNA extraction. Total RNA was prepared from the tissues with Trizol (Invitrogen, Carlsbad, CA) following the manufacturer’s directions.
RT-PCR
Aliquots containing 2 μg total RNA were used in a first strand cDNA synthesis (Bio-Rad, Hercules, CA). Primer sets used to amplify the various constructs for this study are listed in Supplementary Table 1. Primer set I [αII-cardi+ and αII-card-] was designed to amplify the sequence that includes the αII-cardi+ insertion. PCR was performed for 35 cycles at 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, followed by a final extension at 72°C for 10 min. PCR products were separated on 2% agarose gels and stained with 0.5 μg/ml ethidium bromide.
Real-time RT-PCR
Quantitation of specific mRNAs were performed by real-time PCR with the DNA Engine Opticon Continuous Fluorescence Detector (MJ Research, Waltham, MA). The real-time PCR reaction mixture consisted of the cDNA samples, RNase free water, SYBR green qPCR master mix (F-400S, Finnzymes, New England BioLabs, Ipswich, MA) and 60 nM of specific primer set II [αII-spectrin splice F1 (specific for cardi+ isoform)] and primer set III [αII-spectrin splice F2 (specific for cardi− isoform)] (Supplementary Table 1). As a control, we used primers specific to 18S rRNA [25]. We did not detect any amplified αII-cardi+ products using αII-spectrin splice F2 primers and vice versa (data not shown) [26]. A stock of cDNA generated by the reverse transcription of day 3 rat heart tissue was used to construct a standard curve in every assay. The results were expressed as ratios of αII-cardi− or αII-cardi+ to total mRNA levels and compared at each developmental time point.
Long-range RT-PCR and southern blot hybridization
We used RT-PCR with several different primer sets to amplify an approximately 3.7 kb product from total RNA, using 35 cycles at 94 °C for 1 min, 55 °C for 1 min, 72 °C for 4 min, followed by a final extension at 72 °C for 10 min. Primer set IV [αII-spectrin motif 9-21 (M)] (Supplementary Table 1) has 9 bp on either side of the junctions formed when the SH3 insert and cardi insert are absent, and only amplifies the fragment without insertions (i) and (iv); Primer set V [αII-spectrin repeat 9-21 (I)] (Supplementary Table 1) crosses the alternatively spliced junctions, with 9 bp on either side, formed when SH3 insert or cardi insert are present; it only amplifies the fragment with insertions (i) and (iv); Primer set VI [αII-spectrin repeat 9-21 (F)] (Supplementary Table 1) contains 18 bp inside of the insertions (i) and (iv) and only amplifies the fragment with both insertions (i) and (iv). All PCR products were sub-cloned into the Zero Blunt vector (Invitrogen, Carlsbad, CA) and analyzed by southern blot and DNA sequencing.
Southern blot hybridization was performed with end-labeled probes. Aliquots containing 200 ng of plasmid DNA encoding each clone were placed onto Nybond-N+ membrane (Ambion, Austin, TX). The fixed membranes were hybridized with [γ-32P]-ATP end-labeled probes overnight at 65°C with hybridization buffer (5XSSC, 5XDenhardt’s, 0.5% SDS, 2 mg of Salmon DNA) and washed twice for 10 min with 1 × SSC buffer (0.15 M NaCl, 0.15 M Sodium Citrate, 0.1 % SDS, pH 7.0). The hybridization probe for αII-cardi− was prepared by PCR amplification using the primer set IX [αII-card-probe] (Supplementary Table 1). For SH3+ and αII-cardi+ detections, the probes were synthesized oligonucleotides of 60 bp and 63 bp inside of the SH3+ insertion (i) and Cardi+ insertion (iv).
Immunoblotting
Homogenates of tissues from rats at embryonic day 19 (E19) and at 6 months (M6) of age were prepared from brain, kidney, skeletal muscle and heart tissues, as described [13]. Briefly, tissues were homogenized with a Brinkmann Polytron Homogenizer (Switzerland) at 1x PBS containing 1% NP-40 with protease inhibitors pH 7.2 (Complete Protease Inhibitor Cocktail Tablets, Roche Diagnostics, Indianapolis, IN). The homogenates were incubated on ice for 1 hr before centrifugation at 12,000 × g and collection of the supernatant. Protein concentrations were measured with the BCA method (Bio-Rad, Hercules, CA) with BSA as the standard. Immunoblots were prepared from 12 μg of these supernatants.
For immunoblotting, the samples were heated at 70 °C for 10 min in SDS-PAGE sample buffer (Invitrogen, Carlsbad, CA) and loaded onto 4–12% SDS gels. After electrophoresis and transfer to nitrocellulose (Protan, Schleicher & Schuell, Village of Cleves, OH) in MOPS buffer (Invitrogen, Carlsbad, CA), membranes were saturated in 3% non-fat-milk, 0.05% Tween 20, PBS, pH 7.4. After transfer, the membranes were stained with ponsour as an loading control. The membranes were incubated overnight at room temperature with anti-βII-spectrin antibody (mouse), anti-αII-spectrin antibody (9052, rabbit) or anti-αII-cardi+ antibody (rabbit). After washing, the blots were incubated for 1 hour at room temperature with secondary goat anti-rabbit IgG or anti-mouse IgG coupled to alkaline phosphatase (1:6500) (Jackson Immunoresearch Laboratories, West Grove., PA). The colorimetric reaction was developed with ECL reagents (TROPIX, Bedford, MA) and scanned densitometrically. The chemiluminescence was quantified using the ImageJ software (NIH, Bethesda, MD).
Immunofluorescence Labelling
Enzymatically dissociated adult rat cardiomyocytes and skeletal myofibers isolated from the flexor digitorum brevis muscle (FDB). FDB myofibers were prepared as described [27], fixed in suspension in ice-cold ethanol, then rehydrated in PBS with BSA (1mg/ml), followed by incubation in 5% normal goat serum/3% BSA in PBS [7, 28–30], to inhibit non-specific binding. Cells were allowed to settle by gravity for each change of solution. Neonatal rat cardiomyocytes prepared as described [13], were washed in ice-cold PBS, fixed in 2% paraformaldehyde, permeabilized with 0.5% Triton X-100 and incubated for 1 hour in PBS containing 0.1% BSA. Dissociated cells were incubated in suspension with primary antibodies overnight at 4 °C, washed in PBS with BSA (0.01%) and incubated for 1 hr in secondary antibody at room temperature (ALEXA 568-goat anti-rabbit; Molecular Probes, Eugene, OR). Cells were washed and mounted on slides with Vectashield (Vector Laboratories, Burlingame, CA). Samples were imaged by confocal laser scanning microscopy in a LSM 410 (Zeiss, Peabody, MA), with pinholes set at 18.
Recombinant polypeptides
To create MBP fusion proteins, mRNA encoding repeats 18-21 of αII-spectrin was amplified by RT-PCR using primer set VII [αII-spectrin repeats18-21] (Supplementary Table 1). The resulting PCR products (αII-cardi− and αII-cardi+) were digested with EcoRI and subcloned into the bacterial expression vector, pMal-c2x (New England Biolabs, Beverly, MA). To create GST fusion proteins, mRNA encoding repeats 1-4 of βII-spectrin was amplified by RT-PCR using primer set VIII [βII-spectrin repeats 1-4] (Supplementary Table 1). The resulting PCR product was digested with BamH I and subcloned into the bacterial expression vector, pGEX-4T (Amersham Bioscience, Piscataway, NJ). To control for non-specific effects of the fusion partners, the same primers were used to sub-clone each of the spectrin sequences into both pMal and pGex-4T. The RT-PCR products of βII-1-4 were subcloned into pMal-c2x, to encode the MBP-βII-1-4 fusion protein. The RT-PCR products of αII-18-21 with or without the cardi insert were subcloned into pGEX-4T, to generate GST-αII-cardi+ and GST-αII-cardi−.
Recombinant polypeptides were expressed in E. coli strain BL21 (Invitrogen, Carlsbad, CA). Expression was induced with isopropyl beta-D-thiogalactoside (IPTG; 1 mM for GST-fusion proteins and 0.3 mM for MBP-fusion proteins), and bacteria were lysed by sonication. Recombinant proteins were purified from the soluble proteins in bacterial cell lysates by affinity chromatography on glutathione-Sepharose (for GST-fusion protein; Amersham Bioscience, Piscataway, NJ) or agarose (for MBP-fusion protein; New England Biolabs, Beverly, MA) columns.
Adenoviral Constructs
For adenoviral constructs, the same primers used to sub-clone repeats 18-21 of αII-spectrin, with or without the cardi+ insert, into bacterial expression vectors were used for subcloning these sequences into the pEGFP-C1 vector (Clontech, Mountain View, CA). Recombinant adenovirus were created and purified as previously described [13, 24]. All adenoviral constructs were used at a multiplicity of infection of 50–100 particles per cell, as described (31). Cells were incubated for 48 hrs after infection followed by immunofluorescence labeling with anti-α-actinin or by harvesting with lysis buffer (1XPBS containing 1% NP40 with protease inhibitor, pH7.2).
Overlay assay
MBP-αII-cardi+ and MBP-αII-cardi− fusion proteins (2 μg each) were heated at 70 C for 5 min in SDS-PAGE sample buffer and loaded on 4–12% acrylamide gels with 1×MOPS buffer. MBP (0.5 μg) was used as a control. After electrophoresis and transfer to nitrocellulose, the blots were probed with 6.6 nM GST-βII-1-4 overnight at 4 C in 3% non-fat milk, 0.05% tween-20, 1XPBS. The membrane was washed and probed with primary mouse anti-GST antibody (1:1000, mouse), and secondary goat anti-mouse IgG coupled to alkaline phosphatase antibody (1:6500) with 1XPBS 0.05% Tween 20, pH 7.4. The colorimetric reaction was developed with ECL reagents (TROPIX, Bedford, MA) and scanned densitometrically. As an internal control, the membrane was also probed with mouse anti-MBP antibody (1:1000, mouse) followed by anti-mouse Ig, and developed as above. To confirm non-specific effects of the fusion partners, the same amounts of GST-αII-cardi+ and GST-αII-cardi− fusion proteins were heated at 70 C for 5 min in SDS-PAGE sample buffer and loaded on 4–12% acrylamide gels with 1×MOPS buffer. GST (0.5 μg) was used as a control.
In other experiments, Aliquots containing 50 μg of protein from homogenates of rat heart were heated at 70 C for 5 min in SDS-PAGE sample buffer and loaded onto 3–8% Tris-Acetate gels. After electrophoresis and transfer to nitrocellulose, the membrane was probed with 6.6 nM of the GST fusion proteins of spectrin repeats 18-21 with (GST-αII-cardi+) or without (GST-αII-cardi−) the cardiac-specific insert, or with GST alone. Incubation was overnight at 4 °C in 3% non-fat milk, 0.05% Tween 20, 1XPBS. After washing (1XPBS, 0.05% Tween 20), the blots were probed with mouse anti-GST antibody (1:1000, mouse), and secondary goat anti-mouse IgG coupled to alkaline phosphatase antibody (1:6500) in 3% non-fat milk, 0.05% Tween 20, 1XPBS, pH 7.4, and developed as above.
Surface plasmon resonance analysis
Surface preparation
Binding reactions were done in HBS-EP buffer (10 mM Hepes, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% P-20) from Biacore (GE Healthcare, Piscataway, New Jersey), which was filtered through 0.2 μM filters and degassed before use. A monoclonal antibody against GST was bound to the surface of a BIAcore CM5 sensor chip of a Biacore 3000 surface plasmon resonanance unit as follows. The carboxymethyl-dextran surface of the chip (flow cells 1 and 2) was activated with a 35 μl injection of a mixture of 0.1 M NHS and 0.1 M EDC in water. A monoclonal antibody against GST was diluted with 10 mM sodium acetate buffer, pH 5.0. An aliquot of 50 μl (10 μg/ml) of the antibody solution was injected into flow cells 1 and 2, sufficient to immobilize the equivalent of 1.2 × 104 resonance units (RU). Any remaining activated residues on the dextran surface were blocked with 35 μl 1M ethanolamine, pH 8.2, and washed at 100 μl/min with two 25 μl pulses of 10 mM glycine, pH 2.2.
Capture of ligand
Purified GST-fusion proteins were captured on the anti-GST surfaces of flow cell 2 and flow cell 1, used as a reference, by injecting an aliquot of 0.5 μM or 0.1 μM solution of proteins in HBS-EP buffer at the rate of 10 μl/min, until a total change of 100 RU was registered.
Kinetics analysis of binding
In order to minimize mass transport effects, the binding analyses were performed at a flow rate of 30 μl per minute at 25°C. The analytes (60 μl aliquots of MBP-αII-cardi+ and MBP-αII-cardi−, at concentrations ranging from 0–20 nM in HBS-EP buffer, were injected into flow cells 1 and 2 and the association reaction was recorded. The surface was then washed with buffer for 10 min and the dissociation of analyte-ligand complexes was followed over time. The surfaces of the flow cells were regenerated by injecting 50 μl 10 mM glycine, pH 2.2, then re-introducing antibodies, etc.
Data analysis
Sensorgrams were analyzed using BIAeval 3.2 software (Biacore). Values from the reference flow cell were subtracted to obtain the values for specific binding. Data were globally fitted to the Langmuir model for a 1:1 binding.
Statistics
Data were expressed as mean±S.E.M. The statistical significance of the differences between groups was assessed by Student’s t-test. Statistical differences of mRNA levels in the developmental time points and the binding activities between αII-cardi+ and αII-cardi− were assessed by one-way ANOVA. Differences were considered significant at p<0.05.
Results
Expression of αII-Cardi+ in Rat Heart Tissue
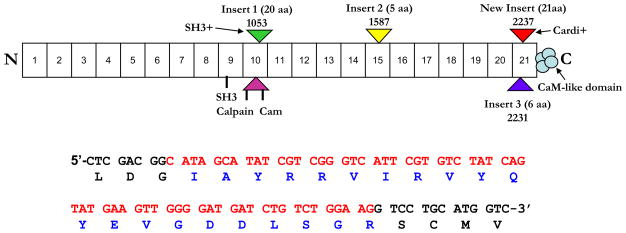
As described previously, repeat 21 of αII-spectrin contains an alternatively spliced region that occurs after amino acid 2230 and encodes an additional 6 amino acids [1]. A similar alternatively spliced region in αI-spectrin decreases the binding affinity to βI-spectrin, which destabilizes the erythrocyte membrane [7]. Our studies of αII-spectrin in the heart revealed the presence of a second alternatively spliced product just adjacent to the 6-amino acid insert (Fig. 1 and Fig. 2A).
Figure 1. An alternatively spliced sequence of 63 nucleotides in spectrin repeat 21, near the C-terminus of cardiac αII-spectrin.
This figure diagrams the structure of αII-spectrin with the position of the cardi+ insert in repeat 21 marked. Below the cartoon is the sequence of the 63 nucleotides (in red) present in the cDNA, and the amino acids (in blue) of the cardi+ insert.
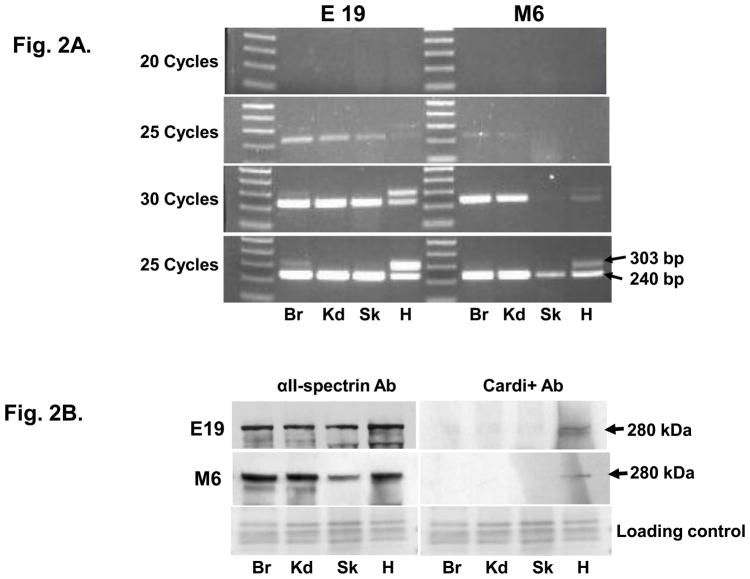
Figure 2. The αII-cardi+ splice variant is expressed in rat cardiac muscle.
A. RT-PCR was performed on mRNA extracted from brain (Br), kidney (Kd), skeletal muscle (Sk) and cardiac muscle (H) of embryonic day 19 (E19) and 6 month old (M6) rats. The sequence encoding the αII-cardi+ splice variant was detected in brain and heart tissues from E19 rat, but only in heart from M6 rat. B. Immunoblotting with antibodies specific for the αII-cardi+ epitope show that it is expressed in significant amounts in heart tissue. Both RT-PCR and immunoblotting suggests that αII-cardi+ is much more prominent in late embryonic than in adult heart.
We isolated total RNA from rat brain, kidney, skeletal and cardiac muscle at embryonic day 19 (E19) and at 6 months (M6) and performed RT-PCR with specific primers (primer set I, Supplementary Table 1) to amplify a region of cDNA of 240 bp that encodes most of repeat 21. This product was obtained from each of the embryonic and adult tissues we examined (Fig. 2A), and it included the 6 amino acid insert [1]. We also found a second larger product (303 bp) in all tissues at E19. This product was barely detectable in brain, kidney and skeletal muscle but it was prominent in heart. At M6, the larger PCR product (303 bp) was only seen slightly in brain and again more prominently in heart. DNA sequencing revealed that an extra 63 bp of nucleotides was present just adjacent to the 18 nucleotides that encode the 6-amino acid insert of repeat 21 [1] and resulted in the addition of a 21-amino acid peptide (IAYRRVIRVYQYEVGDDLSGR) in this region. Because this alternatively spliced product is expressed preferentially in the heart, we call it αII-cardi+.
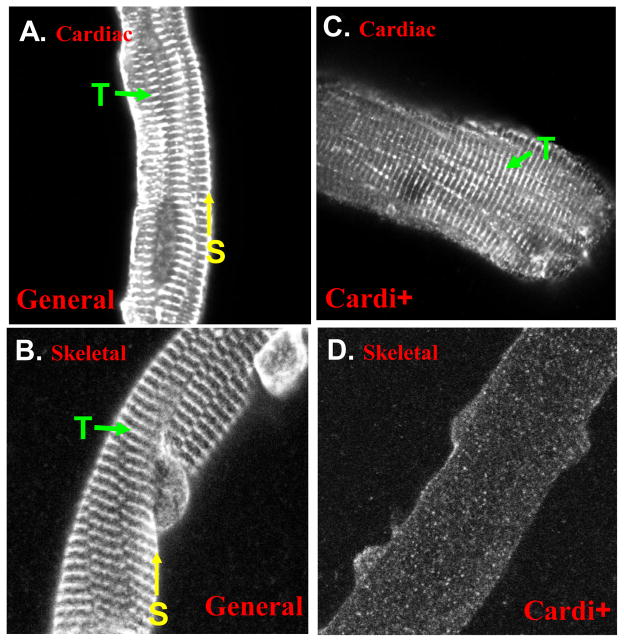
We prepared antibodies to this 21-amino acid sequence in rabbits and used them to confirm the expression of this alternatively spliced form of αII-spectrin. Western blots showed αII-spectrin in all tissues tested, but the αII-cardi+ isoform was only detected in heart tissue in both E19 and M6 samples, with less expressed at M6 compared to E19 (Fig. 2B). We obtained similar results in immunofluorescence studies of cardiac muscle and skeletal muscle from day 21 rats. Antibodies specific to αII-spectrin labeled both the sarcolemma (yellow arrow) and structures present at the level of the Z-disks (T-tubule, green aroow) in both cardiac muscle (Fig. 3A) and skeletal muscle (Fig. 3B), whereas the peptide-specific antibody to the αII-cardi+ isoform only labeled internal structures at the level of Z-disks (T-tubule, green arrow) and only in cardiac muscle (Fig. 3C), not skeletal muscle (Fig. 3D. These results indicate that αII-cardi+ is selectively expressed at significant levels in rat cardiac muscle.
Figure 3. The αII-cardi+ splice variant concentrates intracellularly at the level of the Z-line in cardiac muscle, but not skeletal muscle.
Enzymatically dissociated adult rat cardiomyocytes and skeletal muscle fibers were fixed and stained with antibodies to all forms of αII-spectrin or specific for the αII-cardi+ epitope. Immunofluorecence shows that general antibodies to αII-spectrin label both the sarcolemma (S, yellow arrow) and structures present at the level of the Z-disks (T-tubule, T, green arrow) in cardiac muscle (A) and skeletal muscle (B), whereas the antibody specific for αII-cardi+ immunolabels only at the level of Z-disks in cardiac muscle (C) and fails to label any distinctive structures in skeletal muscle (D).
Developmental Changes in Cardi+ Expression
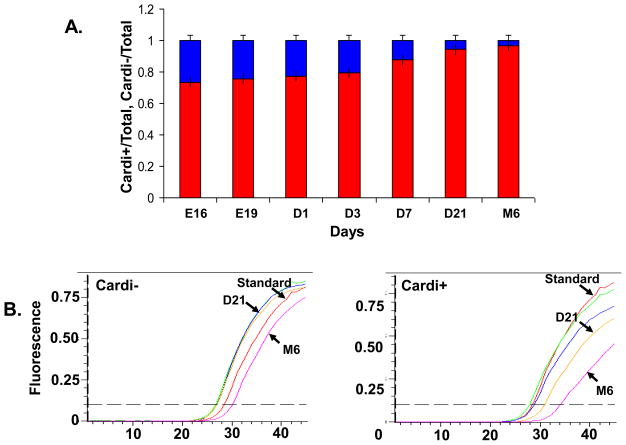
The results depicted in Figures 2A and 2B indicate the expression of the αII-cardi+ isoform of αII-spectrin is developmentally regulated. We used real-time, quantitative RT-PCR to determine the abundance of the αII-cardi− and αII-cardi+ isoforms in rat heart tissues from E16 through adulthood. A standard curve was used to calculate relative amounts of each product. Specific primers selectively amplified the mRNA encoding αII-cardi− and αII-cardi+ from cDNA samples (see Supplementary Table 1). Consistent with our qualitative results (Fig. 2), quantitative RT-PCR showed that the mRNA levels encoding the αII-cardi+ splice form decreased with postnatal age, compared to the αII-cardi− isoform. mRNA coding for the αII-cardi+ isoform comprised ~26% of the total αII-spectrin in E16 hearts, but decreased to ~6% of the total after 3 weeks of age (Fig. 4A), due to a reduction in the mRNA levels encoding this alternatively spliced product. Over the time period we studied, the levels of mRNA for αII-cardi− did not change significantly (Fig. 4B left). The mRNA level from M6 rat shifted slightly to the right over the time period studied, but, after normalization with 18S, the changes have been minimized. These results show that αII-spectrin containing the cardi+ insert is expressed at significant levels in the heart late in embryogenesis and at diminished but still detectable levels in the adult heart.
Figure 4. Real-time RT-PCR of mRNA encoding αII-cardi− and αII-cardi+.
mRNAs were isolated from rat heart tissues at E16, E19, D1, D3, D7, D21 and M6 of age. Quantitative RT-PCR was performed with primers specific for αII-cardi− and αII-cardi+ (see Materials and Methods, Supplementary Table 1). A. mRNA for αII-cardi− (red) and αII-cardi+ (blue), expressed as % total mRNA encoding the two isoforms (n=4). The differences with development were highly significant (p<0.001). B. mRNA encoding αII-cardi+ decreases in amount between E16 and M6, but those encoding αII-cardi− do not (after normalizing 18S RNA, data not shown).
Expression of the αII-Cardi+ Variant is Independent of Other Splice Products
We used long range RT-PCR and southern blot hybridization to determine the relationship of the cardi+ alternative splice form, inserted into repeat 21 of αII-spectrin, to splicing events at two other of αII-spectrin, the SH3+ insert in repeat 10, and the 5-amino acid insert in repeat 15. As mentioned above, all cDNAs isolated from the heart for this study included the sequence coding for the 6 amino acid insert in repeat 21, just upstream of the cardi+ insert. Long-range RT-PCR with primers designed to detect all possible combinations of alternatively spliced sequences (see Materials and Methods and Supplementary Table 1) produced 8 different products of ~3.7 kb, covering the region from repeat 10 to repeat 21 of αII-spectrin. The PCR products were subsequently subcloned into the Zero Blunt vector and either sequenced or used in Southern blots.
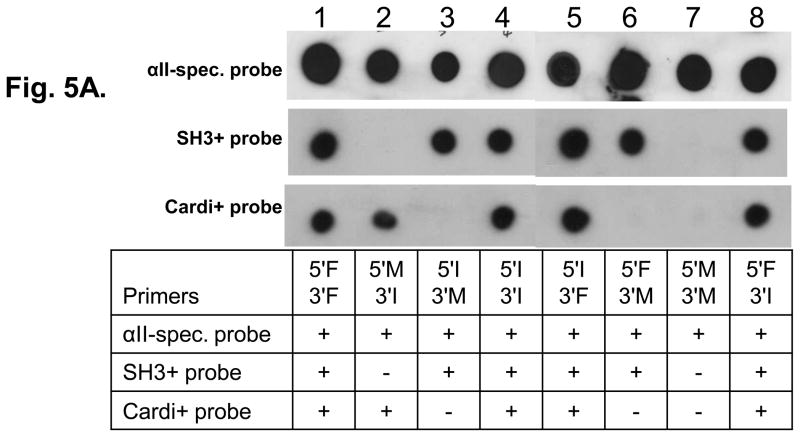
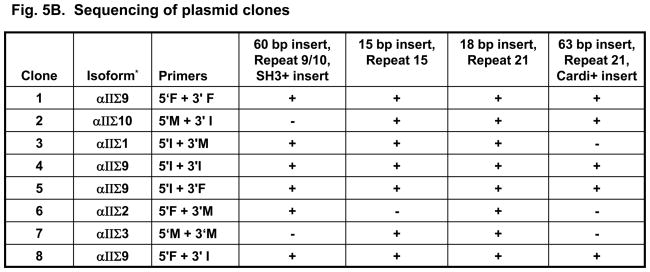
Southern blots were prepared from the 8 plasmid DNAs described above and probed with DNA probes specific for αII-spectrin and for the alternatively spliced sequences coding for cardi+ and SH3+. (A probe specific to the 5-amino acid insert in repeat 15 was also designed and used in southern blot analysis, but the size of the probe was too small to be specific). Hybridization of the probe for all forms of αII-spectrin was effective for all 8 clones tested (Fig. 5A). The SH3+ probe failed to hybridize with clones #2 (created with primers 5′M and 3′I) and #7 (created with primers 5′M and 3′M) (Fig. 5B), indicating that these two clones did not contain the SH3+ sequence. The cardi+ probe failed to label clones #3 (primers 5′I and 3′M), #6 (primers 5′F and 3′M) and #7 (primers 5′M and 3′M) (Fig. 5B) indicating that these three clones did not encode the cardi+ sequence. DNA sequencing analysis confirmed the results of the Southern blots and showed the presence of the 60 bp SH3+ insert in clones #1,3,4,5,6 and 8 (Fig. 5B), the 15 bp insertion at repeat 15 in clones #1,2,3,4,5,7 and 8 (Fig. 5B) and the 63 bp insertion (αII-cardi+) in repeat 21 in clones #1,2,4,5 and 8 (Fig. 5B). These experiments suggest that each of these alternatively spliced sequences are randomly expressed in rat heart tissue, and that the expression of the αII-cardi+ insert in particular is independent of the presence or absence of inserts 1 or 2. Insert 3, however, was present in all clones sequenced. We identify αIIΣ9 and αIIΣ10 as new cardi+ isoforms of αII-spectrin (Fig. 5B, Column 2), and name the cardi− isoforms we cloned in accordance to those previously identified [1].
Figure 5. Three alternatively spliced variants of αII-spectrin are expressed independently in rat heart.
A. Eight plasmid DNAs containing 3.7 kb of RT-PCR products, amplified by the primers listed in the table 1, were placed onto Nybond-N+ membrane and hybridized to the αII-spectrin probe, which was shared by all variants (A, top row) and showed positive in all clones. The membrane was then stripped and rehybridized with probes specific to the SH3+ (A, middle row) and Cardi+ (A, bottom row) variants. The probes used, and the scores for labeling of each plasmid, are summarized in the table below the blots. For information on the probes used, please see Supplementary Table 1. B. Additional sequencing of all RT-PCR products was performed. These experiments confirmed the results of Southern blots and also showed the presence or absence of the 5 amino acid insert in repeat 15 (insert 2) and the presence of the 6 amino acid insert (insert 3) in repeat 21 in all sequenced clones, which could not be assayed by blotting. The results indicate that the expression of mRNAs encoding the SH3+ insert (insert 1), the 5 amino acid insert in repeat 15 (insert 2), and the cardi+ insert in repeat 21 (cardiac-selective) are not linked. *The conventional isoform name is given in column 2, identifying αIIΣ9 and αIIΣ10 as new isoforms of αII-spectrin, according to previously identified isoforms [1].
Analysis of αII-cardi+ binding to βII-spectrin
As alternative splicing in the 21st repeat of αII-spectrin affects its affinity for β-spectrin [7, 31], we studied the effects of the αII-cardi+ insertion on binding by overlay assays and surface plasmon resonance. We performed overlay assays on both endogenous βII-spectrin, separated by SDS-PAGE from adult rat heart tissue. Blots were probed with GST-αII-18-21 with or without the 21-amino acid cardi+ insert, or with GST alone, followed by anti-GST antibody. These studies revealed that GST fusion proteins of αII-cardi+ and αII-cardi− bind similarly to the two endogenous isoforms, “long” and “short”, of βII-spectrin (Supplementary Fig. 1) [32]. We did not detect reliable differences between αII-cardi+ and αII-cardi− in their ability to bind to the endogenous βII-spectrin bands. By contrast, overlay experiments of fusion proteins of MBP linked to repeats 18-21 of αII-spectrin, which contain the high affinity binding site for βII-spectrin showed that MBP-αII-cardi− showed more avid binding to GST-βII-spectrin-1-4 than MBP-αII-cardi+. Quantitations of these differences showed that the αII-cardi+ fusion product bound half as well as the αII-cardi− protein (Fig. 6A, B). Internal controls did not reveal significant differences in protein loading (Fig. 6A, B, right), these results suggest that αII-cardi− binds more avidly to its binding site on βII-spectrin than αII-cardi+. We obtained similar results when the fusion partners were switched, and blots were probed with MBP fusion proteins (data not shown).
Figure 6. Binding of αII-cardi+ to β-spectrins in blot overlay.
A. MBP-αII-cardi+ and MBP- αII-cardi− fusion proteins (2 μg each) were separated by SDS-PAGE, blotted with GST-βII-spectrin-1-4 (6.6 nM) and then with antibodies to GST (left) or to MBP (right, loading control). The results reveal that MBP-αII-cardi− binds more avidly than MBP-αII-cardi+ to GST-βII-spectrin-1-4 (6A. left). Similar amounts were present on the blot, as indicated by antibodies to MBP (6A right). B. Quantitations of blot overlays in A (n=4). The differences in binding of the αII-cardi− and αII-cardi+ fusion proteins are significant (*, p<0.05).
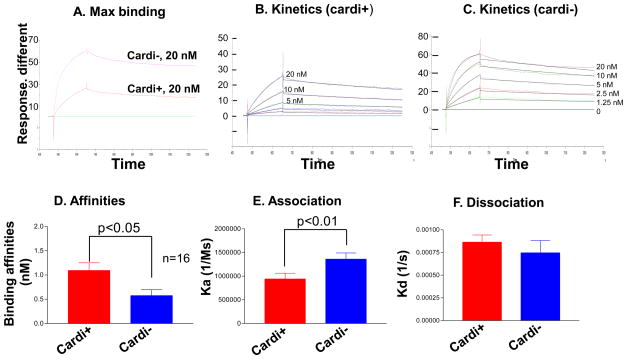
We studied binding in more detail by surface plasmon resonance (SPR) on a Biacore 3000. Using the same fusion proteins, MBP-αII-cardi− or MBP-αII-cardi+ with GST-βII-spectrin repeats 1-4, we again found 2-fold lower binding of MBP-αII-cardi+ compared to MBP-αII-cardi− (Fig. 7A). The kinetics analysis confirmed a 2-fold difference in affinity for the complementary site on βII-spectrin, with KD values of ~0.57 nM for αII-cardi− and ~1.09 nM for αII-cardi+ (Fig. 7D). This change in affinity is due primarily to the faster association (ka) kinetics of MBP-αII-cardi− binding to GST-βII-spectrin 1-4 (Fig. 7E, F), and is consistent with our overlay data (Fig. 6) showing greater binding of αII-cardi− to βII-spectrin.
Figure 7. Binding kinetics measured by surface plasmon resonance.
A. Binding of MBP-αII-cardi+ and MBP-αII-cardi− to GST-βII-1-4 at saturation, in response units. The differences were highly significant (p<0.001, n= 4). B, C. Binding at different concentrations, from 0 to 20 nM, of αII-cardi+ and αII-cardi− fusion proteins was measured (colored lines, from top to bottom, 20 nM, 10 nM, 5 nM, 2.5 nM 1.25 nM and 0) and fit by Michaelis-Menten kinetics (black lines). D-F. Values for binding affinities, and association and dissociation rates for αII-cardi+ and αII-cardi−. The results show that αII-cardi− (KD=0.57 nM) binds to βII-1-4 more avidly than αII-cardi+ (KD=1.09 nM), consistent with results from blot overlays, and that its higher avidity is due to a faster association rate, which leads to tighter binding.
Overexpression of GFP-αII-Cardi+ in Neonatal Rat Cardiomyocytes
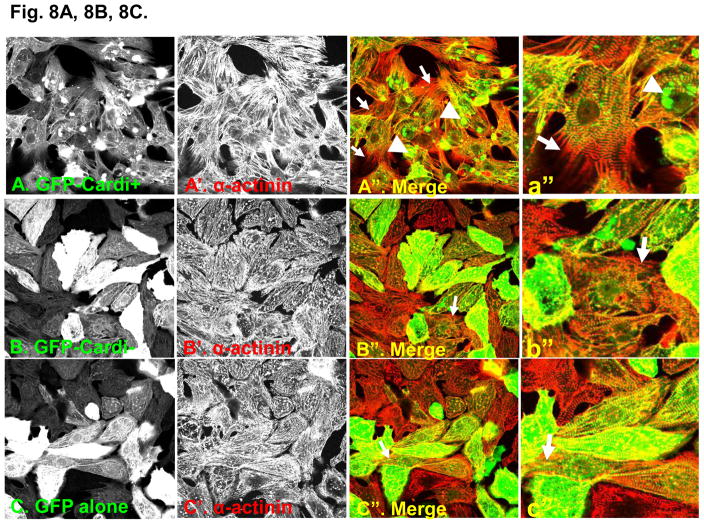
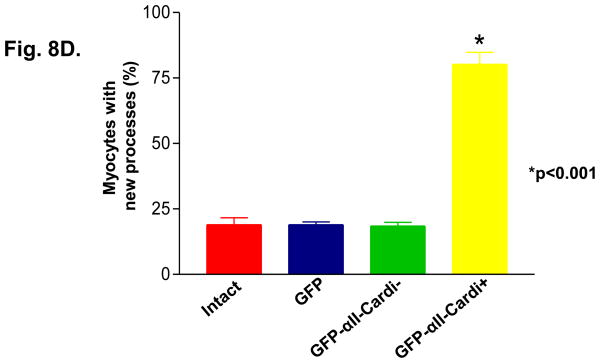
We used adenoviral constructs to express GFP fusion proteins of αII-spectrin either with or without the cardi+ insert (GFP-αII-cardi+ and GFP-αII-cardi−, respectively) in cultured neonatal rat cardiomyocytes. The infected cultures were labeled with antibodies to α-actinin prior to visualization under confocal optics. Almost half (44%, white arrow) of the myocytes expressing GFP-αII-cardi+ contained aggregates of the protein of different sizes (Fig. 8A, 8A″, 8C), whereas nearly all (82%, white arrowhead) of the infected cells had elongated processes with gaps between the myofibrils and the sarcolemma, typical of cells at early stages of hypertrophy [33]. When labeled with antibodies to α-actinin, these processes contained sarcomeres at different stages of assembly (Fig. 8A, 8A′, 8A″). By contrast, the GFP-αII-cardi− fusion protein was diffusely distributed throughout the cytoplasm and did not aggregate. Only a small number (17%, white arrow) of myocytes expressing GFP-αII-cardi− had elongated processes with sarcomeres at different stages of assembly (Fig 8B, 8B″). Control cardiomyocytes that were not infected with adenovirus (not shown), and cells infected with virus expressing only GFP (Fig. 8C), were morphologically similar to those expressing GFP-αII-cardi−. These results suggest that overexpression of the GFP-αII-cardi+ fusion protein induced changes in cell shape and organization, similar to those seen with hypertophic cell growth.
Figure 8. Overexpression of αII-cardi+ in cardiomyocytes promotes cell growth.
A, B, C. Neonatal rat cardiomyocytes were infected with adenovirus encoding either GFP-αII-cardi+ (A, green) or GFP-αII-cardi− (B, green) or GFP alone (C, green) and co-labeled with antibodies to α-actinin (A′, A″, B′ B″ and C′ C″ red). Areas of overlapping label are shown in yellow. White arrowhead indicates aggregation (A″, a″) and white arrow shows new processes (A″, B″, C″ and a″, b″, c″). D. Cells were examined for the presence of newly forming processes and aggregates of the GFP fusion proteins, and the numbers were quantitated. Controls included cells that were infected with GFP alone (C, GFP), or not infected (intact. Differences between cells expressing GFP-αII-cardi+ and othercells were highly significant (p<0.001).
Discussion
Alternatively spliced isoforms of αII-spectrin have been the subject of research for nearly two decades. The presence of the various isoforms of αII-spectrin in different cell types and sometimes within the same cell suggests distinct functions for each [1, 21, 34–36]. In this study, we identify and characterize a new heart-sselective alternatively spliced insert of 21-amino acids in repeat 21 of αII-spectrin. A search of the Entrez Nucleotide database revealed the presence of this nucleotide sequence in the rat, mouse and human genomes but we found no specific report on this isoform of αII-spectrin. We term the novel isoforms containing this insert “αII-cardi+” based on the abundant and selective expression in heart tissue. By conventional spectrin nomenclature, the two new isoforms that contain this insert are αIIΣ9 and αIIΣ10. We show that, in addition to its reduced affinity for β-spectrin compared to isoforms lacking the cardi+ insert, it is selectively concentrated at structures associated with Z-disks, and it is linked to the growth of cardiomyocytes both developmentally and when it is overexpressed in myocytes in culture.
We combined a variety of cell and molecular biological methods to show that, in cardiac muscle, the presence or absence of a novel 63 bp alternatively spliced sequence in αII-spectrin’s 21st repetitive unit gives rise to transcripts encoding αII-cardi+ or αII-cardi−, respectively, and then studied its expression, subcellular distribution and biological activities. We used peptide-specific antibodies to the 21-amino acid cardi+ insert for some of these studies. As the αII-cardi− region flanking the insert was a poor immunogen, we were obliged to use subunit specific antibodies to αII-spectrin to examine the distribution of all forms of this cytoskeletal protein, which includes the αII-cardi− and αII-cardi+ isoforms. Immunofluorescent studies using the peptide-specific antibodies to the αII-cardi+ isoform localized this protein to the structures present at the level of the Z-disk in adult cardiac muscle cells (Fig. 3C) but failed to detect it in skeletal muscle (Fig. 3D). By contrast, antibodies specific to full-length αII-spectrin label both the sarcolemma and structures at the Z-disk in both cardiac and skeletal muscle (Fig. 3A and B, respectively). Thus, the distribution of the αII-cardi− and αII-cardi+ isoforms clearly differs between cardiac and skeletal muscle. Additional microscopy studies [4, 33, 37] have localized αII-spectrin to t-tubules, which align with Z-disks in the heart. The αII-cardi+ variant may therefore have specific functions at the t-tubules of cardiac muscle, but, because other structures, such as the terminal cisternae of the sarcoplasmic reticulum, are also present at the level of Z-disks, it may have other roles as well.
The αII-cardi+ alternative splice form of αII-spectrin is only one of several isoforms of this protein characterized so far. Cianci et. al. [1] identified αII-spectrin transcripts that included a 60 bp insertion (insert 1) in repeat 10 and an 18 bp insertion (insert 3) within repeat 21 in all tissues tested [1]. Transcripts containing a 15 bp insertion (insert 2) within repeat 15 were only expressed in brain, heart, skeletal muscle and embryonic tissues. These three inserts appear to be expressed independently of each other, resulting in a total of eight different transcripts of αII-spectrin [1]. The new alternative splice product reported here, αII-cardi+ (insert 4), is a 63 bp insertion within the coding region of repeat 21 of αII-spectrin and is located adjacent to insert 3. It is only expressed in significant amounts in heart, especially prenatally. Like the other inserts, this newly identified splice variant occurs independently of other alternatively spliced sequences (Fig. 5A, 5B). As the 5 amino acid insert is present in cardiac mRNAs encoding αII-spectrin, the cardi+ insert is therefore the fourth identified in the cardiac forms of this protein. With the characterization of this new insert, we now have a total of ten possible transcripts of αII-spectrin in rat heart tissue (see Fig. 5B). Although specific roles for each of these alternatively spliced sequences, expressed alone or in combination, are still not defined, our studies of developing heart and cardiomyocytes in culture suggest that, in addition to a role at internal membranes (see above), the αII-cardi+ insert is expressed in developing heart muscle cells, where it may be linked to growth.
Our quantitative RT-PCR experiments, as well as our immunoblotting studies, show that the level of αII-cardi+ mRNA and protein is significant (26%) late in embryogenesis in the rat heart, but that it decreases after birth. Thus, it is more likely to have important functions during cardiac development. This role is likely to be specific, as αII-cardi− forms of αII-spectrin (Fig. 4), and forms of αII-spectrin containing the SH3+ insert (our unpublished results), do not change significantly in rats in the weeks after birth. Although the binding of αII-cardi+ to β-spectrin is not greatly altered compared to αII-cardi−, the presence of this alternatively spliced sequence may reduce the solubility of αII-spectrins in which it is expressed. Aggregation of αII spectrin has been reported in lymphocytes and is likely to be physiological [38–41].
The fact that the presence of the cardi+ insert within repeats 18-21 of αII-spectrin, which contains the “nucleation” site for formation of the αβ-spectrin heterodimer [5], appears to have only a 2-fold effect on its affinity for the complementary region of β-spectrin, containing its first 4 repeats, is surprising. Indeed, the presence of a 6 amino acid insert, just adjacent to cardi+, has been shown to diminish the affinity of αI-spectrin in erythrocytes for its β-spectrin partner, which in turn destabilizes the red cell membrane [7]. The cardi+ insert is a larger insert, however, and occurs near the end of the first helix in the triple helical structure of repeat 21. It is therefore possible that this insert forms a structure apart from the triple helix that does not interfere with the binding interface between αII- and βII-spectrin.
Alternatively, the 21 amino acid cardi+ insert may be exposed on a surface of the 21st repeat that is not involved in binding β-spectrin. This in turn would allow it to remain free in the spectrin network to bind other proteins, perhaps including proteins that regulate cell growth, Ca2+ homeostasis, or myofibril formation. Although the formation of aggregates of GFP-αII-cardi+ complicates the interpretation of our data, our results clearly indicate that these are not directly linked to the formation of new myofibrils or processes, as many cardiomyocytes show the latter without the former. It therefore seems more likely that the αII-cardi+ form of αII-spectrin binds proteins that regulate cell size, abnormalities of which have been linked to hypertrophic cardiomyopathies [33]. Further experiments will be needed to test this possibility.
Supplementary Material
Acknowledgments
We thank Shirley Gaa and Aristide Chikando for sharing their preparations of neonatal cardiomyocytes, Dawn Catino for her help in surface plasmon resonance analysis, Diana Ford-Speelman for sharing her preparation of flexor digitorum brevis muscle fibers and John Strong for his help in confocal microscopy. Our research was supported by grants from the NIH (RO1 HL075106 to R.J.B.), PO1 HL HL70709 to R.J.B. (T. Rogers, P.I.) and T32 HL 072751 to Y.Z. (T. Pallone, P.I.) and a grant from the American Heart Association, Mid-Atlantic Affiliate to J.A.U.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cianci CD, Zhang Z, Pradhan D, Morrow JS. Brain and muscle express a unique alternative transcript of alphaII spectrin. Biochemistry. 1999;38:15721–30. doi: 10.1021/bi991458k. [DOI] [PubMed] [Google Scholar]
- 2.Du A, Sanger JM, Sanger JW. Cardiac myofibrillogenesis inside intact embryonic hearts. Dev Biol. 2008;318:236–46. doi: 10.1016/j.ydbio.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubreuil RR. Functional links between membrane transport and the spectrin cytoskeleton. J Membr Biol. 2006;211:151–61. doi: 10.1007/s00232-006-0863-y. [DOI] [PubMed] [Google Scholar]
- 4.Bennett PM, Baines AJ, Lecomte MC, Maggs AM, Pinder JC. Not just a plasma membrane protein: in cardiac muscle cells alpha-II spectrin also shows a close association with myofibrils. J Muscle Res Cell Motil. 2004;25:119–26. doi: 10.1023/b:jure.0000035892.77399.51. [DOI] [PubMed] [Google Scholar]
- 5.Baines AJ, Pinder JC. The spectrin-associated cytoskeleton in mammalian heart. Front Biosci. 2005;10:3020–33. doi: 10.2741/1759. [DOI] [PubMed] [Google Scholar]
- 6.Bignone PA, Baines AJ. Spectrin alpha II and beta II isoforms interact with high affinity at the tetramerization site. Biochem J. 2003;374:613–24. doi: 10.1042/BJ20030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ursitti JA, Kotula L, DeSilva TM, Curtis PJ, Speicher DW. Mapping the human erythrocyte beta-spectrin dimer initiation site using recombinant peptides and correlation of its phasing with the alpha-actinin dimer site. J Biol Chem. 1996;271:6636–44. doi: 10.1074/jbc.271.12.6636. [DOI] [PubMed] [Google Scholar]
- 8.Bignone PA, King MD, Pinder JC, Baines AJ. Phosphorylation of a threonine unique to the short C-terminal isoform of betaII-spectrin links regulation of alpha-beta spectrin interaction to neuritogenesis. J Biol Chem. 2007;282:888–96. doi: 10.1074/jbc.M605920200. [DOI] [PubMed] [Google Scholar]
- 9.Bennett PM, Maggs AM, Baines AJ, Pinder JC. The transitional junction: a new functional subcellular domain at the intercalated disc. Mol Biol Cell. 2006;17:2091–100. doi: 10.1091/mbc.E05-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 11.Hammarlund M, Davis WS, Jorgensen EM. Mutations in beta-spectrin disrupt axon outgrowth and sarcomere structure. J Cell Biol. 2000;149:931–42. doi: 10.1083/jcb.149.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sridharan DM, McMahon LW, Lambert MW. alphaII-Spectrin interacts with five groups of functionally important proteins in the nucleus. Cell Biol Int. 2006;30:866–78. doi: 10.1016/j.cellbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Ursitti JA, Petrich BG, Lee PC, Resneck WG, Ye X, Yang J, et al. Role of an alternatively spliced form of alphaII-spectrin in localization of connexin 43 in cardiomyocytes and regulation by stress-activated protein kinase. J Mol Cell Cardiol. 2007;42:572–81. doi: 10.1016/j.yjmcc.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte MC. AlphaII-spectrin is critical for cell adhesion and cell cycle. J Biol Chem. 2009;284:2409–18. doi: 10.1074/jbc.M801324200. [DOI] [PubMed] [Google Scholar]
- 15.Nedrelow JH, Cianci CD, Morrow JS. c-Src binds alpha II spectrin’s Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176. J Biol Chem. 2003;278:7735–41. doi: 10.1074/jbc.M210988200. [DOI] [PubMed] [Google Scholar]
- 16.Davis LH, Davis JQ, Bennett V. Ankyrin regulation: an alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. J Biol Chem. 1992;267:18966–72. [PubMed] [Google Scholar]
- 17.Hopitzan AA, Baines AJ, Ludosky MA, Recouvreur M, Kordeli E. Ankyrin-G in skeletal muscle: tissue-specific alternative splicing contributes to the complexity of the sarcolemmal cytoskeleton. Exp Cell Res. 2005;309:86–98. doi: 10.1016/j.yexcr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy SP, Warren SL, Forget BG, Morrow JS. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991;115:267–77. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohler PJ, Yoon W, Bennett V. Ankyrin-B targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–93. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 20.Moorthy S, Chen L, Bennett V. Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J Cell Biol. 2000;149:915–30. doi: 10.1083/jcb.149.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon RT, McMahon AP. Generation of diversity in nonerythroid spectrins. Multiple polypeptides are predicted by sequence analysis of cDNAs encompassing the coding region of human nonerythroid alpha-spectrin. J Biol Chem. 1990;265:4427–33. [PubMed] [Google Scholar]
- 22.Williams MW, Resneck WG, Kaysser T, Ursitti JA, Birkenmeier CS, Barker JE, et al. Na, K-ATPase in skeletal muscle: two populations of beta-spectrin control localization in the sarcolemma but not partitioning between the sarcolemma and the transverse tubules. J Cell Sci. 2001;114:751–62. doi: 10.1242/jcs.114.4.751. [DOI] [PubMed] [Google Scholar]
- 23.Zhou D, Ursitti JA, Bloch RJ. Developmental expression of spectrins in rat skeletal muscle. Mol Biol Cell. 1998;9:47–61. doi: 10.1091/mbc.9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ursitti JA, Lee PC, Resneck WG, McNally MM, Bowman AL, O’Neill A, et al. Cloning and characterization of cytokeratins 8 and 19 in adult rat striated muscle. Interaction with the dystrophin glycoprotein complex. J Biol Chem. 2004;279:41830–8. doi: 10.1074/jbc.M400128200. [DOI] [PubMed] [Google Scholar]
- 25.Kazi AA, Jones JM, Koos RD. Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor alpha and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol Endocrinol. 2005;19:2006–19. doi: 10.1210/me.2004-0388. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Ji H, Fabucci ME, Falconetti C, Zheng W, Sandberg K. Translational control of the rat angiotensin type 1a receptor by alternative splicing. Gene. 2004;341:93–100. doi: 10.1016/j.gene.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Brown LD, Schneider MF. Delayed dedifferentiation and retention of properties in dissociated adult skeletal muscle fibers in vitro. In Vitro Cell Dev Biol Anim. 2002;38:411–22. doi: 10.1290/1071-2690(2002)038<0411:DDAROP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Williams MW, Bloch RJ. Differential distribution of dystrophin and beta-spectrin at the sarcolemma of fast twitch skeletal muscle fibers. J Muscle Res Cell Motil. 1999;20:383–93. doi: 10.1023/a:1005512217552. [DOI] [PubMed] [Google Scholar]
- 29.Williams MW, Bloch RJ. Extensive but coordinated reorganization of the membrane skeleton in myofibers of dystrophic (mdx) mice. J Cell Biol. 1999;144:1259–70. doi: 10.1083/jcb.144.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilmotte R, Harper SL, Ursitti JA, Marechal J, Delaunay J, Speicher DW. The exon 46-encoded sequence is essential for stability of human erythroid alpha-spectrin and heterodimer formation. Blood. 1997;90:4188–96. [PubMed] [Google Scholar]
- 31.Viel A, Branton D. Interchain binding at the tail end of the Drosophila spectrin molecule. Proc Natl Acad Sci U S A. 1994;91:10839–43. doi: 10.1073/pnas.91.23.10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes NV, Scott C, Heerkens E, Ohanian V, Maggs AM, Pinder JC, et al. Identification of a novel C-terminal variant of beta II spectrin: two isoforms of beta II spectrin have distinct intracellular locations and activities. J Cell Sci. 2000;113 ( Pt 11):2023–34. doi: 10.1242/jcs.113.11.2023. [DOI] [PubMed] [Google Scholar]
- 33.Messina DA, Lemanski LF. Studies of hamster cardiac myofibrillogenesis in vivo with antibodies to spectrin, desmin, and alpha-actinin. Am J Anat. 1991;191:85–94. doi: 10.1002/aja.1001910109. [DOI] [PubMed] [Google Scholar]
- 34.Bloch RJ, Morrow JS. An unusual beta-spectrin associated with clustered acetylcholine receptors. J Cell Biol. 1989;108:481–93. doi: 10.1083/jcb.108.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain Res Bull. 1995;36:593–606. doi: 10.1016/0361-9230(94)00264-2. [DOI] [PubMed] [Google Scholar]
- 36.McMahon LW, Sangerman J, Goodman SR, Kumaresan K, Lambert MW. Human alpha spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links. Biochemistry. 2001;40:7025–34. doi: 10.1021/bi002917g. [DOI] [PubMed] [Google Scholar]
- 37.Messina DA, Lemanski LF. Immunocytochemical studies of spectrin in hamster cardiac tissue. Cell Motil Cytoskeleton. 1989;12:139–49. doi: 10.1002/cm.970120303. [DOI] [PubMed] [Google Scholar]
- 38.Black JD, Koury ST, Bankert RB, Repasky EA. Heterogeneity in lymphocyte spectrin distribution: ultrastructural identification of a new spectrin-rich cytoplasmic structure. J Cell Biol. 1988;106:97–109. doi: 10.1083/jcb.106.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del Buono BJ, Williamson PL, Schlegel RA. Relation between the organization of spectrin and of membrane lipids in lymphocytes. J Cell Biol. 1988;106:697–703. doi: 10.1083/jcb.106.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes CS, Repasky EA, Bankert RB, Johnson RJ, Subjeck JR. Effects of hyperthermia on spectrin expression patterns of murine lymphocytes. Radiat Res. 1987;112:116–23. [PubMed] [Google Scholar]
- 41.Pauly JL, Bankert RB, Repasky EA. Immunofluorescent patterns of spectrin in lymphocyte cell lines. J Immunol. 1986;136:246–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.