SUMMARY
Cytoplasmic Ags derived from viruses, cytosolic bacteria, tumours and allografts are presented to T cells by MHC class I or class II molecules. In the case of class II-restricted Ags, professional Ag-presenting cells acquire them during uptake of dead, class II-negative cells and present them via a process called indirect presentation. It is generally assumed that the cytosolic Ag-processing machinery—which supplies peptides for presentation by class I molecules—plays very little role in indirect presentation of class II-restricted, cytoplasmic Ags. Remarkably, upon testing this assumption, we found that proteasomes, TAP and ERAAP, but not tapasin, partially destroyed or removed cytoplasmic, class II-restricted Ags such that their inhibition or deficiency led to dramatically increased TH cell responses to allograft (HY) and microbial (Listeria monocytogenes) Ags, both of which are indirectly presented. This effect was neither due to enhanced ER-associated degradation nor competition for Ag between class I and class II molecules. From these findings a novel model emerges in which the cytosolic Ag-processing machinery regulates the quantity of cytoplasmic peptides available for presentation by class II molecules, and hence modulates TH cell responses.
INTRODUCTION
T cell responses are primed when the innate immune response culminates in the display of processed Ags by Ag-presenting cells (APC) in the context of Major histocompatibility complex-encoded class I and class II molecules (1, 2). Class I molecules, which regulate cytotoxic CD8+ T lymphocyte (CTL) functions, present endogenous/cytoplasmic Ags, whilst class II molecules, which control CD4+ T helper (TH) cell functions, present exogenous/extracellular Ags (3, 4). Notwithstanding, CTL responses can also be primed by class I-restricted extracellular Ags by a well-defined process termed cross presentation (4, 5). Likewise, studies of TH cell responses to allografts, cancers, viruses and cytosolic bacteria have suggested that class II molecules can also present cytoplasmic Ags (6–18). Furthermore, biochemical analyses have revealed that a substantial proportion of naturally processed self peptides associated with class II molecules are derived from nuclear and cytoplasmic proteins (19–21). Because such proteins are exposed to the cytosolic Ag-processing (CAP) pathway, one would predict that this pathway might impact the repertoire of cytoplasmic Ags presented by class II molecules.
Class II-restricted presentation of exogenous Ags requires the endolysosomal processing pathway (22). Thus, extracellular pathogens or derived proteins and toxins are delivered to the endolysosomes, wherein the action of the disulphide reductase–γ-interferon induced lysosomal thiol reductase (GILT)—and cysteine and asparate proteases generate peptides for assembly with peptide-receptive class II-CLIP complexes in a process catalysed by the class II-like molecule DM. Thence, stably assembled class II-peptide complexes egress to the cell surface for an appraisal by TH cells (3, 5, 22).
In contrast to extracellular Ags, class II-restricted cytoplasmic Ags (8–13, 15–17) can take two routes towards the class II-containing endolysosomes. If Ags are presented by the same cell, then macroautophagy, microautophagy and chaperone (HSP70-LAMP2a)-mediated autophagy (12–14, 23, 24) delivers them from the cytosol into the endolysosomes. If Ags are synthesised by class II-negative cells, then they need to be presented by a professional APC. In such cases, the Ag is delivered to the endolysosomes by endocytic or phagocytic uptake by APCs. For alloantigens this process is called indirect presentation (6, 25, 26), a term used herein for all class II-restricted Ags donated from infected cells to APCs. In both cases, prior to reaching the lysosomes, cytoplasmic Ags are exposed to the CAP machinery—which is predominantly thought to assist in Ag processing and presentation by class I molecules. This machinery consists of the proteasomes, transporter associated with Ag-processing (TAP), tapasin and ER-associated aminopeptidase associated with Ag processing (ERAAP). They function to proteolytically process nucleo-cytoplasmic proteins, transport the emerging peptides into the ER and trim such peptides to a length conducive for binding to class I molecules or to degrade those that are incompatible with class I binding (27–30). Therefore, one would predict that all cytoplasmic proteins—including those from which class II-restricted Ags are generated—could become substrates for degradation by the CAP machinery. If such cytoplasmic degradation occurs, it would regulate the quantity of cytoplasmic proteins reaching the endolysosomes for presentation by class II molecules.
Notwithstanding the above regulatory mechanism, none of the numerous studies focused on determining whether the proteasomes and TAP regulate class II Ag presentation have thus far indicated a dramatic role for the CAP machinery in this process. For example, a few reports have demonstrated a positive role for the proteasome and TAP in generating class II-restricted Ags (14, 31–35), whilst the majority of such studies have indicated that the CAP machinery exerts no influence on class II-restricted Ag presentation (11, 36–39). It should be noted however, that all of these studies were focused on direct presentation of cytoplasmic Ags by class II molecules. Additionally, many such studies were performed using in vitro models. However, most infected, tumour and allogeneic cells do not express class II molecules themselves, and hence indirect class II-restricted presentation is a major pathway for their recognition by TH cells. Hence, how the CAP machinery impacts indirect presentation of class II-restricted, cytoplasmic Ags in vivo remains a critical unanswered question of fundamental import.
To acquire Ags for indirect presentation, APC are thought to phagocytose dying cells from which derived Ags are presented by class II molecules (3, 5, 40); It is thus generally assumed that indirect presentation of class II-restricted Ags—including those of donor cytoplasmic origin—follows the same principles as direct presentation because it involves phagocytosis of exogenous Ags from apoptotic cells and direct delivery of cargo to the endolysosomal processing pathway (3).
We sought to gain insights into the role of the CAP machinery in sculpting the repertoire of cytoplasmic Ags indirectly presented by class II molecules and its impact on TH cell responses. Toward this goal, we defined the cellular and molecular bases for class II-restricted indirect presentation of cytosolic Ags derived from the HY alloantigen and Listeria monocytogenes. Remarkably, our findings revealed that proteasomes, TAP and ERAAP played destructive roles, thereby regulating the quantity of cytoplasmic Ags indirectly presented by class II molecules. Such alteration in Ag presentation modulated the magnitude of TH cell responses to cytoplasmic Ags in vivo.
MATERIALS AND METHODS
Mice
All mouse strains, their histocompatibility genotype and sources are described in Table S1. All mice were bred, maintained and used in experiments in compliance with Vanderbilt University Institutional Animal Care and Use Committee regulations and approval.
Cell lines
Wild type K41 and calreticulin-null K42 MEF (41) as well as Hsf1-null MEF (42) were maintained in RPMI-1640 (Invitrogen) supplemented with 5% foetal calf serum (FCS; Hyclone), L-glutamine, HEPES and antibiotics (Invitrogen). These MEFs were transfected with Dby cDNA (43) and selected with 0.5 mg/ml G418 for ~4 weeks to express the HY alloantigen. Dby expression was verified by RT-PCR using forward (GGTCTGGAAAAACTGCTGC) and reverse (TTGGTGGCATTGTGTCCTGC) primers (43).
Preparation of donor cells
In some experiments, donor splenocytes were treated with PBS or the irreversible proteasome inhibitor epoxomicin or protein glycosylation inhibitor/ER stress inducer (Sigma) for 2 or 3hrs, respectively, at 37°C. In other experiments, donor splenocytes were starved for 2hrs in Hanks balanced-salt solution (Cellgro) or maintained in DMEM containing 10% foetal calf serum, penicillin, streptomycin, L-glutamine, sodium bicarbonate and HEPES buffer. Cells were washed thoroughly, resuspended at ~2×108 cells/ml and used for immunisation.
Peptides
All peptides used in this study (Table S2) were synthesized using Fmoc chemistry and determined to be >90% pure by MALDI-MS analysis (The Pennsylvania State University College of Medicine, Hershey, PA). Peptide stocks and working dilutions were prepared as described (44).
Immunisation and ELISpot assay
Recipient mice were immunised i.p. with 2×107 donor splenocytes. After seven days, splenocytes were prepared and used in ELISpot assay. For this, Immobilon-P plates (Millipore) were activated and coated with 1—2μg/mL IFNγ capture monoclonal antibody (mAb; AN18; eBiosciences) overnight. Excess mAb was washed and blocked with 10% FCS in RPMI-1640. Meanwhile, 2.5—3×105 red blood cell-free immune splenocytes were stimulated with the indicated concentrations of peptides (see Table 2) in triplicate. After 48hrs, plates were washed first with Ca2+- and Mg2+-free PBS and then with PBS containing 1% FBS and 0.05% Tween-20. Cytokine spots were detected with 1μg/mL IFNγ-specific biotinylated mAb (R4-6A2; eBiosciences). After ~3hrs at room temperature, excess mAb was washed away and Vectastain ABC peroxidase (Vector Laboratories) was added to each well. Spots were visualised by reacting 2.2-dimethyl-formamide and 3-amino-9-ethylcarbazole with 30% hydrogen peroxide (Sigma). Spots were counted using CTL ImmunoSpot analyzer and CTL ImmunoSpot software, version 3.2 (Cellular Technology).
The response of H3ba-specific CD4 T cell clones, LPa/B10-B6 and LPa/B10-line, was determined by stimulating ~105 cells with increasing numbers of splenocytes isolated from the indicated mouse strains at 1:1; 1:2.5; 1:5; and 1:10 ratio of responder to stimulator. After 48hrs, IFNγ-secreting cells were detected by ELISpot assay as described above.
DC depletion
Vehicle (PBS) or diphteria toxin (DT) (Sigma) was administered i.p. to hemizygous hDTRtg mice at 4ng/g body weight as previously described. After 18—24hrs, vehicle- or DT-treated mice were used either as recipients or to isolate donor splenocytes for immunisation. Flow cytometry analysis in pilot experiments and of donor hDTRtg splenocytes indicated that DT-treated mice were depleted of ≥90% splenic CD11c+ cells within 18hrs and remained in this state for ~72hrs (45).
L. monocytogenes infection
To elicit primary CD4+ T cell responses, mice were inoculated retro-orbitally with ~5×104 cfu L. monocytogenes. After 12—14d, the response of 0.5—1×106 immune splenocytes to L. monocytogenes-derived peptide epitopes was determined by ELISpot assay as described above. To determine secondary CD4+ T cell responses, mice were inoculated i.p. with ~103 cfu L. monocytogenes in 0.2 ml PBS or with PBS alone. After 14 days, mice were boosted i.p. with ~106 cfu and analyzed 14d later by ELISpot assay. For this, 0.5—1×106 non-immune and immune splenocytes were stimulated with a panel of class II-restricted L. monocytogenes-derived peptide epitopes or negative control peptides (see Table S2).
RESULTS
Indirect presentation of HY alloantigen primes TH cells in vivo
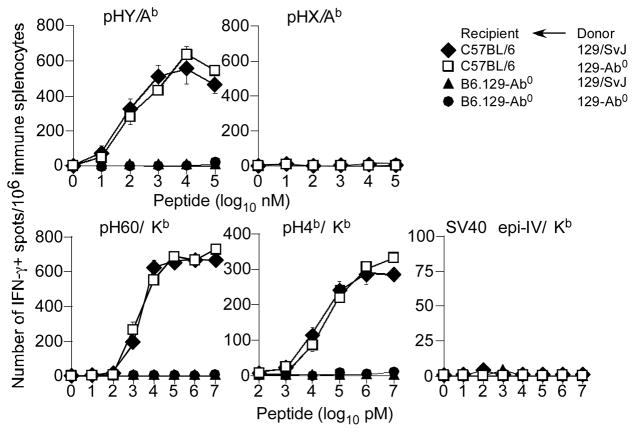
In order to study the mechanism(s) underlying indirect presentation of cytosolic MHC class II-restricted Ags, we first determined how the male H2Ab-restricted HY minor histocompatibility Ag (mHAg) is presented to TH cells. The alloantigenic HY peptide (pHY) is derived from RNA helicase, a ubiquitously expressed nucleo-cytoplasmic protein encoded by the evolutionarily conserved Dby gene located on the Y-chromosome (6, 43). No other H2b-restricted T cell epitopes are derived from this helicase (46). Thus, female C57BL/6 (B6) and B6.129-Ab0 mice were immunized with H2b-compatible but mHAg-incompatible (Table S1) male 129 donor splenocytes. After 7d, the ability of mHAg-reactive TH cells and CTL to produce interferon-γ (IFNγ) was determined by ELISpot assay.
Immunisation of B6 mice resulted in IFNγ-producing splenic TH cells to pHY but not to the control Dbx-encoded self HX peptide (pHX; Fig. 1) expressed by both males and females. This response was specific because pHY did not elicit any IFNγ response from immune B6.129-Ab0 mice (Fig. 1). Moreover, female B6 mice immunized with male 129-Ab0 splenocytes also primed pHY-reactive TH cells (Fig. 1). Therefore, we conclude that the H2Ab-restricted HY Ag is indirectly presented to TH cells in vivo.
Figure 1. Indirect presentation of the male HY alloantigen.
B6 and B6.129-IAb−/− female mice were immunised with either male donor 129/SvJ or 129-Ab0 splenocytes. After 7d, IFNγ response by TH cells to pHY/Ab or negative control pHX/Ab was assessed by ex vivo ELISpot assay. At the same time, IFNγ response by CTL to pH60/Kb, pH4b/Kb and negative control SV40 epi IV/Kb was similarly determined. Data represent 6 similar experiments using ~4 recipient mice per group per experiment; ± sem (standard error of mean).
In the same experiment described above, the role of pHY-specific TH response in eliciting CTL responses to class I-restricted mHAgs was determined. We found IFNγ-producing CTL responses to the immunodominant H2Kb-restricted, H60 and H4b alloantigens but not to control H2Kb-restricted, SV40 TAg (TAg) epitope-IV (epi-IV) in B6 mice immunized with either male 129 or 129-Ab0 splenocytes (Fig. 1). Nonetheless, B6.129-Ab0 recipients did not elicit CTL responses to class I-restricted pH60 and pH4b (Fig. 1). Furthermore, TH and CTL responses similar to those described above were obtained using Ii-deficient recipients (data not shown). These data together suggest that the primary CTL response to mHAgs is entirely dependent on CD4 help.
Indirect presentation of pHY requires CD8+ dendritic cells
Because Dby is broadly expressed (47), it was important to determine which donor cell type donates and which recipient APC type presents the alloantigen. For this, we took advantage of the hDTRtg mouse–in which the human DT receptor transgene expression is regulated by the Cd11c enhancer/promotor (48). Thus, DT administration renders hDTRtg mice conditionally deficient in CD11c+ myeloid cells including DCs and splenic sub-capsular macrophages (48, 49). We previously reported that DT-treated B6.FVB-hDTRtg mice became DC-deficient within ~18hrs and remained so for 72hrs (45).
To determine which APC type presents donor mHAg, we treated B6.FVB-hDTRtg mice with PBS or DT and immunized them ~18 hrs later with male splenocytes from 129.FVB-hDTRtg mice that received PBS ~18 hrs earlier. On d7, pHY-specific TH cell responses were monitored. Depletion of recipients’ CD11c+ cells dramatically tempered TH cell responses to pHY compared to that observed in mice containing CD11c+ cells (Fig. 2a). Similarly, depletion of donor CD11c+ cells resulted in poor TH cell responses to pHY (Fig. 2b) indicating a significant role for CD11c+ cells in donating alloantigens for indirect presentation. As expected, depletion of both recipient and donor CD11c+ cells resulted in no TH cell response to pHY (Fig. 2a). Additional data revealed that both donor and recipient CD11c+ cells were required to prime class I-restricted pH60 and pH4b-CTL responses in vivo (unpublished data). Because DCs express high levels of CD11c, constitute the majority of CD11c+ splenocytes and are critical for priming naïve T cells, the above data suggest that DCs are responsible for indirect presentation.
Figure 2. Indirect presentation of the class II-restricted HY alloantigen requires CD8+ DCs.
(a) Female recipient B6.FVB-hDTRtg mice treated with vehicle (PBS) or DT and immunised 24 hrs later with male donor 129.FVB-hDTRtg splenocytes from mice that were either PBS- or DT-treated 24 hrs earlier. After 7d, IFNγ response by TH cells to pHY/Ab or negative control pHX/Ab was assessed by ex vivo ELISpot assay. Data represent 6 similar experiments using ~3 recipient mice per group per experiment; ± sem. (b) Male donor 129.FVB-hDTRtg mice treated with vehicle or DT and used 24 hrs later to immunise female B6.FVB-hDTRtg mice. After 7d, IFNγ response by TH cells to pHY/Ab and pHX/Ab was determined by ex vivo ELISpot assay. Data represent 6 similar experiments using ~3 recipient mice per group per experiment; ± sem. (c) 129/SvJ and 129-Batf30 female recipients were immunized with male C57BL/6 donor splenocytes. After 7d, IFNγ response by TH cells to pHY/Ab and pHX/Ab was determined by ex vivo ELIspot assay. Data represents 2 similar experiments using ~2—4 recipient per group per experiment; ± sem. (d) B6 female recipients were immunized with male 129/SvJ or 129-Batf30 donor splenocytes. After 7d, IFNγ response by TH cells to pHY/Ab and pHX/Ab was determined by ex vivo ELIspot assay. Data represents 2 similar experiments using ~2—4 recipient per group per experiment; ± sem. (e) 129/SvJ and 129-Batf30 female recipients were immunized with either male 129/SvJ or 129-Batf30 donor splenocytes. After 7d, IFN-γ response by TH cells to pHY/Ab and pHX/Ab was determined by ex vivo ELIspot assay. Data represents 2 similar experiments using ~2—4 recipient per group per experiment; ± sem.
To firm the contribution of DCs in indirect presentation and to determine which DC subset is responsible, we used the recently reported 129-Batf30 mice, which are deficient in splenic CD8+ DCs (50). Female 129 and 129-Batf30 mice were immunized with male B6, 129 or 129-Batf30 splenocytes and HY-specific TH cell response was monitored 7d later. The data revealed that the lack of splenic CD8+ DCs in the recipient dramatically reduced the TH cell response to HY (Fig. 2c). Similarly, the lack of donor CD8+ DCs resulted in much tempered TH cell response to HY (Fig. 2d), which was completely lost upon immunising CD8+ DC-deficient female recipients with male donor splenocytes lacking CD8+ DCs (Fig. 2e).
We also monitored pH60-speicific CTL responses in the experiment described above. The data revealed a requirement for donor and recipient CD8+ DCs for cross-priming CTL responses to pH60 (unpublished data). Together, these data suggest that CD8+ DC play important roles in donating and indirectly presenting the HY alloantigen.
TAP and ERAAP regulate TH cell responses to pHY
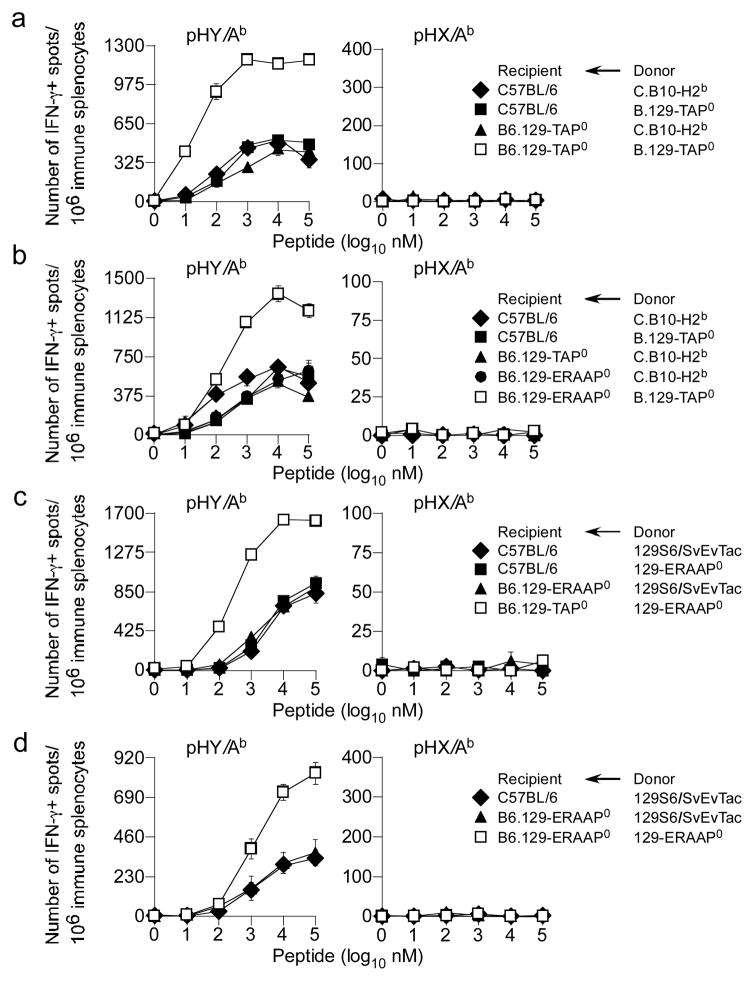
Because pHY is derived from nucleo-cytoplasmic RNA helicase, we predicted that components of the CAP machinery might have access to HY and potentially regulate its availability for indirect presentation. Therefore, we next determined whether TAP had any role in indirect presentation of pHY. Immunisation of female B6 mice with male splenocytes derived from H2b-compatible but mHAg-incompatible C.B10-H2b (BALB.B; Table S1) or B.129-TAP0 (B stands for BALB.B) mice generated comparable pHY-specific TH cell response (Fig. 3a, b). Similarly, B6.129-TAP0 female mice immunised with C.B10-H2b male splenocytes elicited comparable pHY-specific TH cell responses (Fig. 3a, b). Surprisingly, however, when B6.129-TAP0 female recipients were immunised with B.129-TAP0 male donor splenocytes, 2—3-fold increased TH cell response against H2Ab-restricted pHY was observed (Fig. 3a). Thus, TAP function in both donor and recipient cells had a detrimental effect on the indirect presentation of class II-restricted cytoplasmic Ag that tempered the TH cell response.
Figure 3. ERAAP and TAP impact indirect presentation of class II-restricted HY alloantigen.
(a) B6 and B6.129-TAP0 female mice were immunised with either male donor C.B10-H2b or B.129-TAP0 splenocytes. IFNγ response by TH cells to pHY/Ab and pHX/Ab was determined by ex vivo ELISpot assay after 7d. Data represent 8 similar experiments using ~3—4 recipient mice per group per experiment; ± sem. (b) B6, B6.129-TAP0 and B6.129-ERAAP0 female mice were immunised with either male donor C.B10-H2b or B.129-TAP0 splenocytes and IFNγ response by TH cells to pHY/Ab and pHX/Ab was assessed 7d later by ex vivo ELISpot assay. Data represent 7 similar experiments using ~4 recipient mice per group per experiment; ± sem. (c) B6, B6.129-TAP0 and B6.129-ERAAP0 female mice were immunised with either male donor 129S6/SvEvTac or 129-ERAAP0 splenocytes. IFNγ response by TH cells to pHY/Ab and pHX/Ab was determined 7d later by ex vivo ELISpot assay. Data represent 7 similar experiments using ~2—3 recipient mice per group per mice; ± sem. (d) B6 and B6.129-ERAAP0 female mice were immunized with male donor 129S6/SvEvTac or 129-ERAAP0 splenocytes. After 7d, IFNγ response by TH cells to pHY/Ab and pHX/Ab was determined by ex vivo ELISpot assay. Data represent 3 similar experiments using ~2—3 recipient mice per group per experiment; ± sem.
We considered the possibility that peptides translocated by TAP into the ER might become substrates for destruction by ERAAP, and hence unavailable for presentation. To test this possibility, B6, B6.129-TAP0 and B6.129-ERAAP0 female mice were immunised with C.B10-H2b, B.129-TAP0 or 129-ERAAP0 male splenocytes. As with B6 and B6.129-TAP0 mice, B6.129-ERAAP0 female mice immunised with wt male splenocytes elicited similar pHY-specific TH cell responses (Fig. 3b—d). In striking contrast, immunisation of B6.129-ERAAP0 female mice with B.129-TAP0 male splenocytes resulted in two-fold increases pHY-specific TH cell responses (Fig. 3b). Similarly, immunisation of B6.129-TAP0 or B6.129-ERAAP0 mice with 129-ERAAP0 male splenocytes resulted in a 2—3-fold increase in pHY-specific TH cell response (Fig. 3c, d).
As a control for the above experiments, the monitoring of CTL response in wt mice immunised with male wt or TAP-deficient donor splenocytes revealed an identical CTL response to pH60 and pH4b, suggesting that the two class I-restricted mHAgs are cross-presented (Fig. S1a). As expected, TAP-deficient recipient did not respond to class I-restricted mHAgs as they are devoid of CD8+ T cells (Fig. S1a). We therefore, conclude that a pool of cytoplasmic class II-restricted Ags is pumped into the ER by TAP, thence destroyed by ERAAP.
TAP and ERAAP regulate indirect presentation of class II-restricted bacterial Ags in vivo
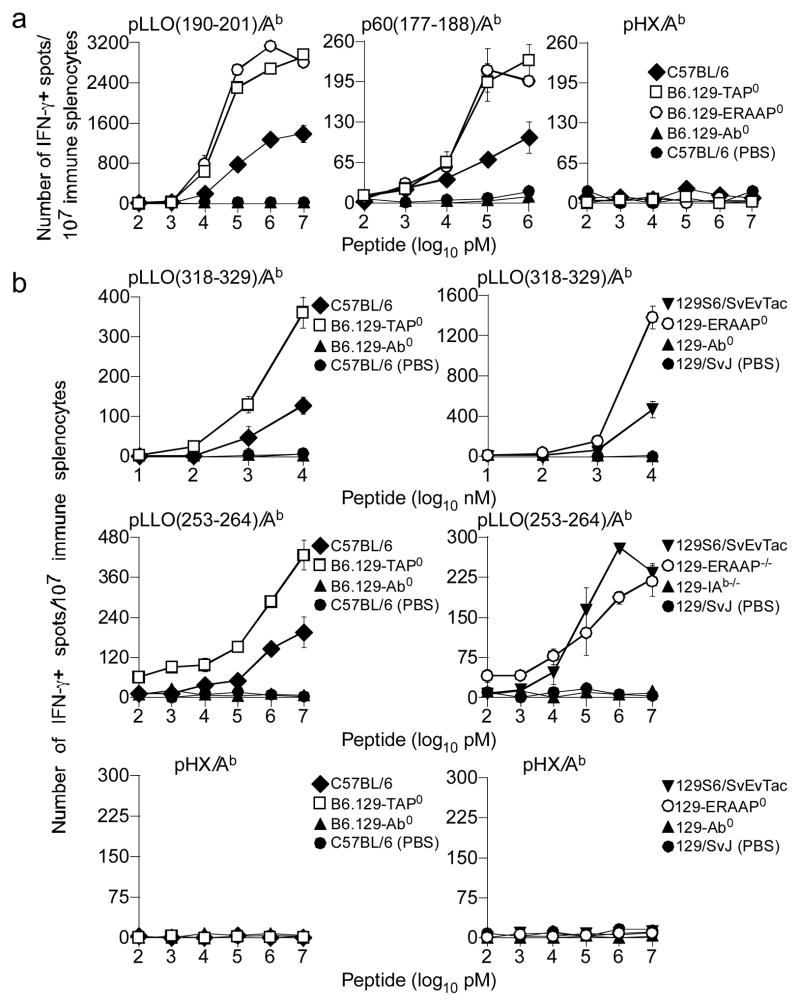
To determine the generality of TAP’s and ERAAP’s role in indirect Ag presentation, we tested whether the CAP pathway impacts indirect presentation of L. monocytogenes-derived class II-restricted Ags. L. monocytogenes listerolysin O (LLO) disrupts the phagolysosome to permit entry of the organism into the cytoplasm for its growth, and multiplication. Therefore, the priming of TH cell responses against listerial Ags requires indirect presentation (51–53). Thus, B6, B6.129-TAP0, B6.129-ERAAP0 and B6.129-Ab0 as well as 129S6/SvEvTac, 129-ERAAP0, B6.129-Ab0 and 129-Ab0 mice were inoculated i.p. with bacteria, boosted 14d later and secondary TH cell response to known H2Ab-restricted epitopes were monitored after an additional 14d. PBS-treated B6 and 129 mice served as negative controls. We observed a 2—5-fold increase in the secondary TH cell response to H2Ab-restricted pLLO(190–201), p60(177–188), pLLO(318–329), and pLLO(253–264) in B6.129-TAP0 mice compared to B6 mice (Fig. 4a, b). A similar pattern of increased TH cell reactivity to pLLO(190–201), p60(177–188) and pLLO(318–329) was observed in ERAAP-deficient mice compared to B6 mice (Fig. 4a, b). In contrast, the response to pLLO(253–264) was indistinguishable between wt and ERAAP-deficient mice (Fig 4b). As expected, neither Listeria-inoculated H2Ab0 nor PBS-treated wt mice responded to the three listerial peptides; none of the mice responded to irrelevant peptides (Fig. 4). In additional experiments, we also found that the primary TH response to L. monocytogenes Ags—elicited by retro-orbital bacterial inoculation—yielded similar results as above (Fig. S2). Thus, the TAP and ERAAP effect on indirect presentation of cytoplasmic class II-restricted Ags appears to be a general principle as they impact TH cell responses to mHAgs and bacterial Ags similarly.
Figure 4. TAP and ERAAP regulate class II-restricted listerial Ag presentation.
(a) B6, B6.129-TAP0, B6.129-ERAAP0 or B6-129-Ab0 mice were inoculated with L. monocytogenes or delivered PBS i.p. and boosted 2 weeks later. Two weeks after boost, immune splenocytes were stimulated with the indicated peptides for 48hrs and IFN-γ response by TH cells was monitored by ELIspot assay. (b) 129S6/SvEvTac, B.129-TAP0, 129-ERAAP0 or 129-Ab0 mice were primed and boosted as in (a). Two weeks after boost, immune splenocytes were stimulated with the indicated peptides for 48hrs and IFN-γ was monitored by ELIspot assay. Data represents 2 similar experiments using 3—4 mice/group; ± sem.
Proteasomes regulate indirect presentation of HY mHAg
Several mechanisms can potentially explain the above finding: (a) competition between class I and class II molecules for Ag; (b) competition between CD4+ and CD8+ T cells; (c) enhanced autophagy and/or enhanced ER-associated degradation (ERAD); and (d) quantitative differences in the Ag(s) presented.
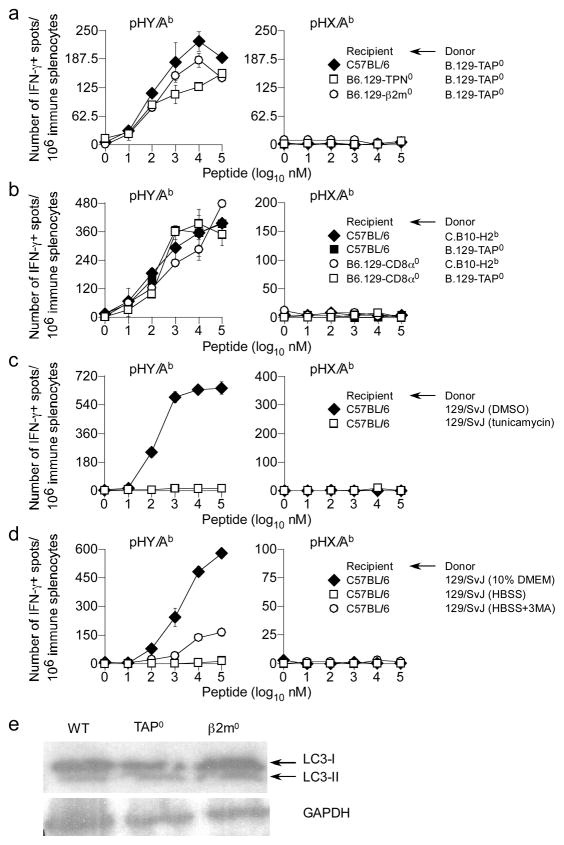
To test whether competition for Ag played a role, TH response of female B6, B6.129-β2m−/− and B6.129-Tpn−/− mice—which, akin to TAP deficiency, lack functional class I-assembly complex due to β2m and tapasin deficiency—was assessed after immunising with male C.B10-H2b or B.129-TAP0 splenocytes. All three recipients elicited similar pHY-specific TH cell responses (Fig. 5a), suggesting that simply lacking class I does not ‘free up’ more cytoplasmic Ags for presentation by class II molecules. In conjunction with the fact that no known CTL epitopes are derived from Dby-encoded helicase (46), competition for Ag is a less likely explanation for our finding.
Figure 5. Neither Ag competition, T cell competition, ERAD nor enhanced autophagy explain increased TH cell response to pHY in TAP0 mice.
(a) B6, B6.129-TPN0 and B6.129-β2m0 female mice were immunised with male donor B.129-TAP0 splenocytes and IFN-γ response by CD4 T cells to pHY/Ab and pHX/Ab was assessed 7d later by ex vivo ELIspot assay. Data represents 3 similar experiments using 27 recipient mice; ± sem. (b) B6 and B6.129-CD8α0 female mice were immunised with either male donor C.B10-H2b, or B.129-TAP0 splenocytes. IFN-γ response by CD4 T cells to pHY/Ab and pHX/Ab was determined by ex vivo ELIspot assay 7d later. Data represents 4 similar experiments using 24 recipient mice; ± sem. (c) Female B6 mice were immunised with male donor 129 splenocytes treated with either DMSO or tunicamycin for ~2hrs. IFN-γ response by TH cells to pHY/Ab and pHX/Ab was determined 7d later as described above. Data represents 2 similar experiments using 12 recipient mice; ± sem. (d) Female B6 mice were immunised with male donor 129 splenocytes incubated in either nutrition-rich medium or HBSS for ~3hrs and IFN-γ response by TH cells to pHY/Ab and pHX/Ab was assessed 7d later as above. Data represent 2 similar experiments using 12 recipient mice; ± sem. (e) Protein extracts from SV40 TAg-transformed wt, TAP0 and β2m0 kidney fibroblast lines were separated by SDS-PAGE, transferred onto PVDF membrane and probed with LC3- and GADPH-specific mAbs.
To ascertain whether the increased TH cell responses was a compensatory effect caused by the absence of recipient CTL, B6.129-CD8α−/− female mice were immunised with either C.B10-H2b or B.129-TAP0 male splenocytes. The resulting TH cell response to pHY was comparable in both wt and CD8+ T cell-deficient mice (Fig. 5b). As expected, female B6.129-β2m0, B6.129-Tpn0 and B6.129-CD8α0 recipients did not elicit IFNγ response to class I-restricted mHAgs (Fig. S1b, c). Hence, competition between CD4+ and CD8+ T cells is unlikely to explain the increased TH response in the absence of TAP or ERAAP.
TAP andβ2m deficiency enhances ERAD (54). ERAD can enhance autophagy (55), which is required for class II-restricted cytoplasmic Ag presentation (11–14, 23). Nevertheless, we found that immunisation of female B6 mice with male 129 splenocytes treated with tunicamycin—which induces ERAD due to stress from accumulating unfolded proteins—completely abrogated TH response to pHY whilst DMSO-treated donor cells responded as expected (Fig. 5c). Similarly, induction of autophagy—by maintaining donor male splenocytes in nutrition-free conditions prior to immununisation of female B6 mice—did not enhance, but instead abrogated the TH response to pHY (Fig. 5d). Additionally, constitutive autophagy was not enhanced in TAP0 (TAPTAg) or β2m0 (β2mTAg) TAg-transformed fibroblast lines compared to similarly transformed wt fibroblasts (wtTAg; (56) as similar levels of LC3-I and LC3-II were detected in immunoblots of proteins extracted from wt and mutant lines (Fig. 5e). Together, these data discount a role for enhanced ERAD and autophagy in explaining the impact of TAP and ERAAP on indirect presentation of cytosolic Ags. If anything, the data argue that if autophagy is enhanced by TAP or ERAAP deficiency, it would destroy and not protect cytoplasmic Ags for indirect presentation by class II molecules.
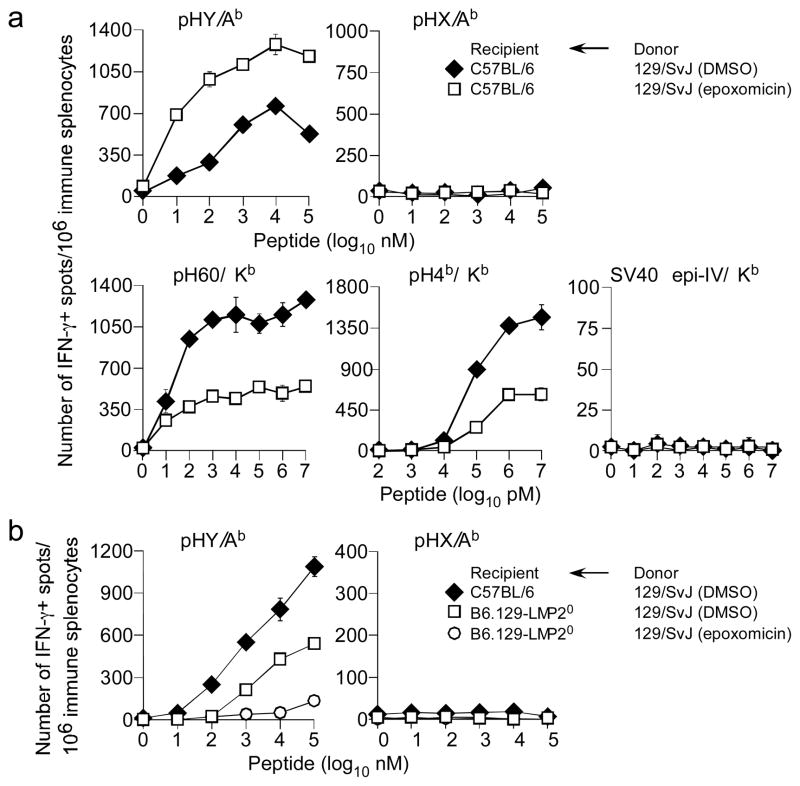
As proteasomal degradation is enhanced by ERAD (55), we tested whether proteolysis within the cytosol of donor cells impacted indirect Ag presentation. If enhanced ERAD was the cause for the phenotype then proteasome inhibition should abrogate TH cell response to HY. Conversely, if cytosolic degradation, rather than ERAD, was the mechanism, then proteasome inhibition should recapitulate the TAP and ERAAP effect. Thus, B6 mice were immunized with male 129 splenocytes that were treated for 2hr with either DMSO or the selective proteasome inhibitor epoxomicin (57, 58) and TH cell responses were monitored. Surprisingly, in contrast to the negative outcome of immunisation with tunicamycin-treated cells, we found that irreversible proteasome inhibition of donor cells resulted in a two-fold increase in TH cell responses to pHY when compared to that elicited by donor cells containing functional proteasomes (Fig. 6a). Thus, proteasomes negatively impact indirect presentation and the intact form of the HY alloantigen is perhaps donated to recipient CD8+ DCs for indirect presentation.
Figure 6. Proteasomes regulate indirect presentation of the class II-restricted pHY alloantigen.
(a) B6 female mice were immunised with either DMSO (vehicle)- or epoxomicin-treated male donor 129/SvJ splenocytes. After 7d, IFNγ response by TH cells to pHY/Ab and pHX/Ab and by CTL to pH60/Kb, pH4b/Kb and SV40 epi IV/Kb was determined by ex vivo ELISpot assay. Data represent 5 similar experiments using ~2—3 recipient mice per group per experiment; ± sem. (b) 129/SvJ male donor splenocytes were treated for 2 hours with either DMSO (vehicle) or epoxomicin. B6 and B6.129-LMP20 female mice were immunised with extensively washed male donor 129/SvJ splenocytes. After 7d, IFNγ response TH cells to pHY/Ab and pHX/Ab was assessed by ex vivo ELISpot assay. Data represent 2 similar experiments using ~3 recipient mice per group per experiment; ± sem.
If intact antigen is donated for indirect presentation, then it may require processing within recipient DCs. Because recipient TAP and ERAAP influenced indirect presentation of pHY, we reasoned that the recipient’s proteasomes may be involved. Thus, immunisation of female B6.129-LMP2−/− mice with male 129 splenocytes resulted in tempered TH response to pHY/Ab (Fig. 6b). Surprisingly however, the TH response to pHY/Ab was completely lost if the donor splenocytes were treated with epoxomicin and then transferred into LMP2-deficient recipient (Fig. 6b). Consistent with this result is the finding that altered pH balance of the phagolysosome caused by a deficiency in donor and/or recipient gp91PHOX did not affect TH cell response to pHY/Ab (data not shown). These data suggest that the increased donation of intact HY Ag upon proteasomal inhibition of donor cells requires cytosolic processing within the recipient DCs.
Role for chaperones in indirect presentation of HY alloantigen
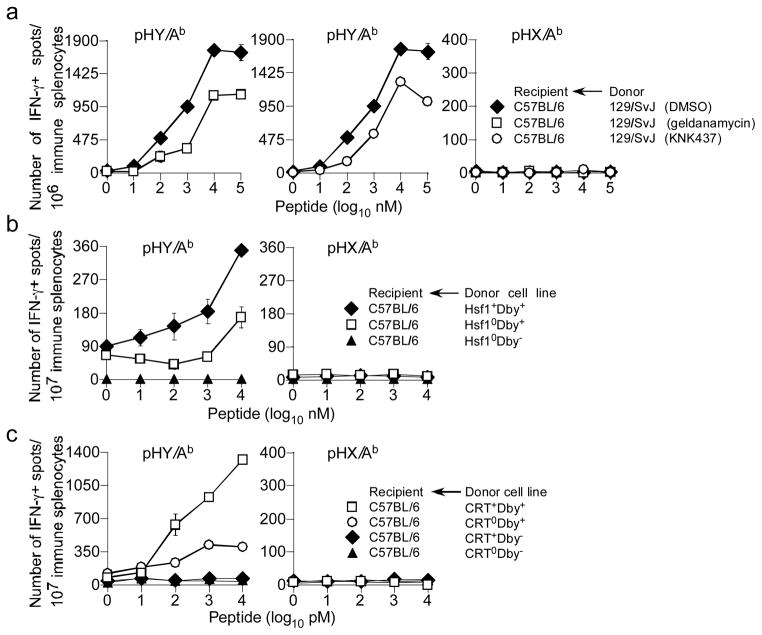
Cross-presentation of class I-restricted antigens require heat shock proteins (HSP) (59, 60). Because the HY alloantigen is a nucleo-cytoplasmic protein that is degraded by donor proteasomes (Fig. 6), we reasoned that donor HSP may play a role in indirect presentation of this antigen. This possibility was addressed in two ways: In the first approach, male 129 splenocytes were treated with pharmacologic HSP inhibitors, geldanamycin and KNK437, or DMSO for 4hrs and used to immunise B6 mice. Inhibition of HSP90 with either geldanamycin or KNK437 tempered TH cell responses against pHY (Fig. 7a). This result suggested a role for HSP90 in indirect presentation of HY alloantigen.
Figure 7. Donor HSP90, HSP70 and calreticulin facilitate indirect presentation of class II-restricted HY alloantigen.
(a) B6 female mice were immunised with Hsf10Dby+, Hsf1+Dby+ or Hsf10Dby− MEF. After 7d, IFNγ response by Th cells to pHY/Ab and pHX/Ab was determined by ex vivo ELISpot assay. Data represent 2 similar experiments using ~2—3 recipient mice per group per experiment; ± sem. (b) Donor 129/SvJ splenocytes were treated with pharmacological inhibitors for 3 hours and next extensively washed with PBS. B6 female mice were immunised with either DMSO (vehicle), geldanamycin (HSP90 inhibitor) or KNK437 (HSP70 inhibitor) treated male donor 129/SvJ splenocytes. After 7d, IFNγ response Th cells to pHY/Ab and pHX/Ab was assessed by ex vivo ELISpot assay. Data represent 3 similar experiments using ~2—3 recipient mice per group per experiment; ± sem. (c) Female B6 mice were immunised with CRT+Dby+, CRT0Dby+, CRT+Dby− or CRT0Dby− MEF. After 7d, IFNγ response by TH cells to pHY/Ab and pHX/Ab was determined as above. Data represent 3 similar experiments using ~3—4 recipient mice per group per experiment; ± sem.
To firm a role for HSP90, in the second approach, we employed mouse embryonic fibroblasts (MEF) deficient in heat shock factor protein 1 (Hsf1), a transcription factor that regulates the expression of members of the HSP90 family of heat shock proteins (42). We first generated HY+Hsf10 and wt MEF by Dby cDNA transfer because these cells do not otherwise express HY mHAg (data not shown). Immunisation of B6 mice with HY+Hsf10 MEF resulted in tempered TH cell responses to HY compared to mice immunized with HY+ wt MEF (Fig. 7b). These results imply a critical role for HSP90 in efficient indirect presentation of the HY alloantigen.
Calreticulin (CRT), an ER-resident chaperone, is implicated in cross-presentation of class I-restricted antigens (61). Therefore, we determined whether CRT expression by donor APC was essential for indirect presentation of HY alloantigen. For this, we first generated HY+CRT0 and HY+CRT+ MEF by Dby cDNA transfer. Immunisation of B6 mice with HY+CRT0 MEF resulted in tempered TH cell responses to HY compared to mice immunized with HY+CRT+ MEF (Fig. 7c). These results imply a critical role for CRT in efficient indirect presentation of the HY alloantigen.
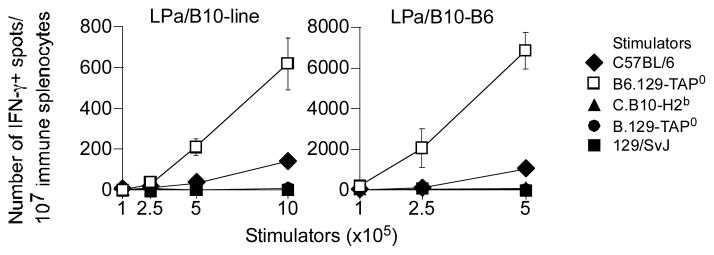
TAP regulates the quantity of class II-restricted Ags displayed
To test the idea that TAP and ERAAP regulate the quantitative aspects of class II-restricted Ag presentation, we determined the response of two distinct H3ba mHAg-specific TH cell lines, LPa/B10-B6 and LPa/B10-line. Akin to HY, the H3ba mHAg is also derived from a cytoplasmic protein, ribosome binding protein-1 (RRBP1; AC Brown, GJC & DCR, in preparation). Moreover, the H3ba-reactive T cell lines allowed us to address the direct role of TAP and ERAAP in class II-restricted cytosolic Ag presentation independently of any potential indirect effect TAP and ERAAP might have on responder T cells in the intact mouse. Thus, LPa/B10-B6 and LPa/B10-line were stimulated with B6, B6.129-TAP0, C.B10-H2b, B.129-TAP−/− or 129/SvJ splenocytes for 48 hrs and the number of IFNγ+ spots determined. The data revealed that B6.129-TAP0 splenocytes, compared to B6 splenocytes, induced 7—8-fold greater number of IFNγ+ spots from the two H3ba-specific TH clones compared to B6 splenocytes (Fig. 8). This response was Ag-specific because the TH cell clones did not respond to negative control C.B10-H2b, B.129-TAP0 and 129/SvJ splenocytes (Fig. 8). Thus, TAP and ERAAP regulate the quantity of class II-restricted Ag presentation.
Figure 8. TAP affects the quantitative aspects of the direct presentation of class II-restricted H3ba mHAg.
H3ba-specific T helper clones LPa/B10-line and LPa/B10-B6 were directly stimulated with the indicated numbers of B6, B6.129-TAP0, C.B10-H2b, B.129-TAP0 or 129/SvJ splenocytes. After 48hrs, IFN-γ response by the TH clones was determined by ELISpot assay. Data represent 2 similar experiments; ± sem.
DISCUSSION
Despite the recognition that class II molecules present cytoplasmic Ags directly and indirectly, the principles underlying indirect presentation are poorly defined. Understanding this process is highly significant because TH cells regulate antibody- and CTL-mediated adaptive immunity to pathogens, cancers, allografts and autoantigens. Such an understanding is especially important because many tumour and virus infected cells down regulate TAP gene expression to evade CTL-mediated immune surveillance. Furthermore, our findings will impact how we understand T cell responses in individuals that express TAP null and ERAAP variants (62–64), especially those that inhibit or alter peptide processing within the ER.
We have shown here that indirect presentation of class II-restricted Ags requires CD8+ donor and recipient DCs. Within these cells, proteasomes, TAP and ERAAP—key components of the cytoplasmic Ag-processing pathway—regulate indirect presentation of class II-restricted Ags thereby impacting the magnitude of TH cell responses to cytoplasmic alloantigens (HY and H3b) and bacterial (L. monocytogenes) Ags. Because these effects were observed with two distinct models, we suggest that the impact of the CAP machinery on indirect presentation of cytoplasmic Ags might be a general regulatory process, one that is of significant immunologic import.
TAP deficiency is known to alter NK cell development and function in both mice and humans (62, 63, 65). The altered NK cell function was previously shown to indirectly regulate CD4+ T cell priming in a Toxoplasma gondii infection model (66). Hence, it was possible that the several fold increased TH cell response to the HY alloantigen and listerial Ags in TAP-null recipients were indirectly regulated by NK cells. Therefore, we immunised both wt and NK cell-deficient IL-150 mice with male donor cells and found that the pHY/Ab-specific TH response was similar in both recipients (data not shown). Thus, we conclude that NK cells contributed very little to the TH cell response to HY.
Although TAP and β2-m deficiencies are known to cause ERAD (67), and ERAD enhances autophagy (55), we systematically ruled out a role for these degradative processes as mechanisms underlying our central observations. Note that we do not claim that autophagy per se is not involved. But we claim that because TAP-deficiency does not enhance autophagy, the increased class II-restricted Ag presentation in the absence of peptide transport to the ER is not due to overt autophagy. Furthermore, neither β2m nor tapasin deficiencies altered indirect Ag presentation. Their absence, akin to TAP deficiency, renders class I molecules unstable and also results in mice that lack CD8+ T cells. Hence, competition between class I and class II molecules as well as competition between TH cells and CTL for the same Ag is a most unlikely mechanism by which TAP and ERAAP deficiencies alter indirect presentation of cytosolic Ags.
Our data suggests that TAP and ERAAP are acting directly on class II-restricted cytoplasmic Ags. Such Ags are perhaps processed by the proteasome in the cytoplasm and transported to the ER lumen. Thus, TAP and ERAAP deficiency would prevent transport of processed cytoplasmic peptides into the ER lumen and their subsequent degradation. Such a process would then quantitatively increase the cytoplasmic Ag pool making it available for indirect presentation. Indeed, our data favours this role for TAP and ERAAP in indirect Ag presentation as observed with the increased presentation of the H3ba mHAg by TAP-deficient splenocytes.
Curiously, the effect of TAP and ERAAP on indirect presentation was only observed when both the donor and recipient APC were deficient in the CAP components. Therefore, one possibility is that the donated Ag escapes into the cytoplasm of the recipient APC upon donation by donor allogeneic cells. That such escape might occur is consistent with the need for recipient proteasome for indirect presentation of pHY and the lack of a role for gp91PHOX for indirect presentation of the same Ag. The escape of Ags from the phagosome to the cytoplasm has been observed with several model and microbial Ags used for mechanistic studies of class I-restricted Ag cross-presentation (68–70). Thus, the CAP pathway can sculpt the repertoire of class II-restricted cytoplasmic Ags in both donor and recipient APC.
We view the data obtained with LMP2-deficient mice with caution as prior studies have shown that alterations in the immunoproteasomes can impact CTL repertoire as well as T cell activation (71, 72). We reported herein that LMP2 deficiency resulted in ~50% reduction in TH cell response to pHY/Ab. This result could be explained entirely by deficiencies in T cell repertoire and/or activation in the LMP2-null recipients as suggested in previous reports (71, 72). Notwithstanding, the TH response to pHY/Ab was completely lost when the LMP2-null recipients were immunised with donor cells in which the proteasomes were irreversibly inhibited. If processing of the donated Ag occurred independent of the recipient’s proteasome, then one would have expected the same level of TH cell response to pHY/Ab when LMP20 mice were immunised with untreated or epoxomicin-treated donor cells. But instead, the response to the latter was completely lost. Hence, we suggest that the HY alloantigen is donated as an intact protein, which is then processed by the recipient immunoproteasomes for indirect presentation. Furthermore, this finding suggests that the donor HY alloantigen accesses the recipient’s cytoplasm as has been reported for HIV nef and HSV-1 glycoprotein B (68, 70). This is perhaps why TAP and ERAAP impact indirect presentation of the donated Ags by class II molecules.
It is noteworthy that the inhibition of constitutive and induced HSP90 function in the donor cells disrupted indirect presentation of class II-restricted Ags, and so did the absence of calreticulin. Both HSP90 and calreticulin are implicated as chaperonins for the donation of cross-presented Ags to presenting APC (59–61). Therefore, HSP90 and calreticulin may work together to chaperone Ags for indirect presentation of class II-restricted Ags as well. Although calreticulin deficiency could induce/enhance ERAD/autophagy, for afore discussed reasons, these processes do not explain the need for the two chaperonins in indirect presentation. Moreover, calreticulin is also known to act as an “eat me” signal for apoptotic cells, which express the otherwise ER-resident protein at the plasma membrane (73, 74). Therefore, calreticulin-deficiency may have resulted in poor phagocytosis of the allogeneic donor cells, thereby severely impeding indirect presentation.
Taken together, the model that emerges from the data presented herein is that proteasomes, TAP and ERAAP regulate the quantity of the class II-associated self (mHAgs) and non-self (listerial) peptide repertoire. The increased self-peptide presentation could alter the CD4+ T cell repertoire in recipient cells. Nonetheless, current serological data indicates that the CD4+ T cell repertoire is very similar between wt and TAP-deficient mice (54). Altered cytosolic Ag pool within donating cells coupled with altered Ag presentation by the APC could explain how the CAP machinery regulates TH cell responses to indirectly presented cytosolic Ags.
Supplementary Material
Acknowledgments
We thank S Roopenian for help with growth and maintenance of T cell clones; Dr. K Murphy for providing Batf30 mice; and Dr I Benjamin for providing Hsf-10 cell line.
Footnotes
Supported by NIH grants HL54977 and AI40079 to SJ; AI70305 and HL89667 to LVK; and AI28802 to DCR. The US Army supported TH.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004;16:21–25. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 4.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson NS, Villadangos JA. Regulation of antigen presentation and cross-presentation in the dendritic cell network: facts, hypothesis, and immunological implications. Adv Immunol. 2005;86:241–305. doi: 10.1016/S0065-2776(04)86007-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Demir Y, Valujskikh A, Heeger PS. The male minor transplantation antigen preferentially activates recipient CD4+ T cells through the indirect presentation pathway in vivo. J Immunol. 2003;171:6510–6518. doi: 10.4049/jimmunol.171.12.6510. [DOI] [PubMed] [Google Scholar]
- 7.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 8.Russmann H, Gerdemann U, Igwe EI, Panthel K, Heesemann J, Garbom S, Wolf-Watz H, Geginat G. Attenuated Yersinia pseudotuberculosis carrier vaccine for simultaneous antigen-specific CD4 and CD8 T-cell induction. Infect Immun. 2003;71:3463–3472. doi: 10.1128/IAI.71.6.3463-3472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentino MD, Hensley LL, Skrombolas D, McPherson PL, Woolard MD, Kawula TH, Frelinger JA, Frelinger JG. Identification of a dominant CD4 T cell epitope in the membrane lipoprotein Tul4 from Francisella tularensis LVS. Mol Immunol. 2009;46:1830–1838. doi: 10.1016/j.molimm.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matyszak MK, Young JL, Gaston JS. Uptake and processing of Chlamydia trachomatis by human dendritic cells. Eur J Immunol. 2002;32:742–751. doi: 10.1002/1521-4141(200203)32:3<742::AID-IMMU742>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Dissanayake SK, Tuera N, Ostrand-Rosenberg S. Presentation of endogenously synthesized MHC class II-restricted epitopes by MHC class II cancer vaccines is independent of transporter associated with Ag processing and the proteasome. J Immunol. 2005;174:1811–1819. doi: 10.4049/jimmunol.174.4.1811. [DOI] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, Mautner J. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 13.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson S, Sekaly RP, Jacobson CL, McFarland HF, Long EO. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989;63:1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaraquemada D, Marti M, Long EO. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med. 1990;172:947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang RF, Wang X, Atwood AC, Topalian SL, Rosenberg SA. Cloning genes encoding MHC class II-restricted antigens: mutated CDC27 as a tumor antigen. Science. 1999;284:1351–1354. doi: 10.1126/science.284.5418.1351. [DOI] [PubMed] [Google Scholar]
- 18.Brooks A, Hartley S, Kjer-Nielsen L, Perera J, Goodnow CC, Basten A, McCluskey J. Class II-restricted presentation of an endogenously derived immunodominant T-cell determinant of hen egg lysozyme. Proc Natl Acad Sci U S A. 1991;88:3290–3294. doi: 10.1073/pnas.88.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dongre AR, Kovats S, deRoos P, McCormack AL, Nakagawa T, Paharkova-Vatchkova V, Eng J, Caldwell H, Yates JR, 3rd, Rudensky AY. In vivo MHC class II presentation of cytosolic proteins revealed by rapid automated tandem mass spectrometry and functional analyses. Eur J Immunol. 2001;31:1485–1494. doi: 10.1002/1521-4141(200105)31:5<1485::AID-IMMU1485>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Rudensky A, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 22.Bird PI, Trapani JA, Villadangos JA. Endolysosomal proteases and their inhibitors in immunity. Nat Rev Immunol. 2009;9:871–882. doi: 10.1038/nri2671. [DOI] [PubMed] [Google Scholar]
- 23.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2011;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michalek MT, Benacerraf B, Rock KL. The class II MHC-restricted presentation of endogenously synthesized ovalbumin displays clonal variation, requires endosomal/lysosomal processing, and is up-regulated by heat shock. J Immunol. 1992;148:1016–1024. [PubMed] [Google Scholar]
- 25.Reed AJ, Noorchashm H, Rostami SY, Zarrabi Y, Perate AR, Jeganathan AN, Caton AJ, Naji A. Alloreactive CD4 T cell activation in vivo: an autonomous function of the indirect pathway of alloantigen presentation. J Immunol. 2003;171:6502–6509. doi: 10.4049/jimmunol.171.12.6502. [DOI] [PubMed] [Google Scholar]
- 26.Richards DM, Dalheimer SL, Ehst BD, Vanasek TL, Jenkins MK, Hertz MI, Mueller DL. Indirect minor histocompatibility antigen presentation by allograft recipient cells in the draining lymph node leads to the activation and clonal expansion of CD4+ T cells that cause obliterative airways disease. J Immunol. 2004;172:3469–3479. doi: 10.4049/jimmunol.172.6.3469. [DOI] [PubMed] [Google Scholar]
- 27.Hammer GE, Gonzalez F, Champsaur M, Cado D, Shastri N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat Immunol. 2006;7:103–112. doi: 10.1038/ni1286. [DOI] [PubMed] [Google Scholar]
- 28.Hammer GE, Gonzalez F, James E, Nolla H, Shastri N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat Immunol. 2007;8:101–108. doi: 10.1038/ni1409. [DOI] [PubMed] [Google Scholar]
- 29.Yan J, V, Parekh V, Mendez-Fernandez Y, Olivares-Villagomez D, Dragovic S, Hill T, Roopenian DC, Joyce S, Van Kaer L. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J Exp Med. 2006;203:647–659. doi: 10.1084/jem.20052271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci U S A. 2006;103:9202–9207. doi: 10.1073/pnas.0603095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med. 2000;191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tewari MK, Sinnathamby G, Rajagopal D, Eisenlohr LC. A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nat Immunol. 2005;6:287–294. doi: 10.1038/ni1171. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee P, Dani A, Bhatia S, Singh N, Rudensky AY, George A, Bal V, Mayor S, Rath S. Efficient presentation of both cytosolic and endogenous transmembrane protein antigens on MHC class II is dependent on cytoplasmic proteolysis. J Immunol. 2001;167:2632–2641. doi: 10.4049/jimmunol.167.5.2632. [DOI] [PubMed] [Google Scholar]
- 34.Testa JS, Apcher GS, Comber JD, Eisenlohr LC. Exosome-driven antigen transfer for MHC class II presentation facilitated by the receptor binding activity of influenza hemagglutinin. J Immunol. 2011;185:6608–6616. doi: 10.4049/jimmunol.1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malnati MS, Marti M, LaVaute T, Jaraquemada D, Biddison W, DeMars R, Long EO. Processing pathways for presentation of cytosolic antigen to MHC class II-restricted T cells. Nature. 1992;357:702–704. doi: 10.1038/357702a0. [DOI] [PubMed] [Google Scholar]
- 36.Dani A, Chaudhry A, Mukherjee P, Rajagopal D, Bhatia S, George A, Bal V, Rath S, Mayor S. The pathway for MHCII-mediated presentation of endogenous proteins involves peptide transport to the endo-lysosomal compartment. J Cell Sci. 2004;117:4219–4230. doi: 10.1242/jcs.01288. [DOI] [PubMed] [Google Scholar]
- 37.Gueguen M, Long EO. Presentation of a cytosolic antigen by major histocompatibility complex class II molecules requires a long-lived form of the antigen. Proc Natl Acad Sci U S A. 1996;93:14692–14697. doi: 10.1073/pnas.93.25.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oxenius A, Bachmann MF, Ashton-Rickardt PG, Tonegawa S, Zinkernagel RM, Hengartner H. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur J Immunol. 1995;25:3402–3411. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- 39.Loss GE, Jr, Elias CG, Fields PE, Ribaudo RK, McKisic M, Sant AJ. Major histocompatibility complex class II-restricted presentation of an internally synthesized antigen displays cell-type variability and segregates from the exogenous class II and endogenous class I presentation pathways. J Exp Med. 1993;178:73–85. doi: 10.1084/jem.178.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 41.Gao B, Adhikari R, Howarth M, Nakamura K, Gold MC, Hill AB, Knee R, Michalak M, Elliott T. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity. 2002;16:99–109. doi: 10.1016/s1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- 42.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. Embo J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott D, Addey C, Ellis P, James E, Mitchell MJ, Saut N, Jurcevic S, Simpson E. Dendritic cells permit identification of genes encoding MHC class II-restricted epitopes of transplantation antigens. Immunity. 2000;12:711–720. doi: 10.1016/s1074-7613(00)80221-6. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura Y, Yadav R, Christianson GJ, Ajayi WU, Roopenian DC, Joyce S. Duration of alloantigen presentation and avidity of T cell antigen recognition correlate with immunodominance of CTL response to minor histocompatibility antigens. J Immunol. 2004;172:6666–6674. doi: 10.4049/jimmunol.172.11.6666. [DOI] [PubMed] [Google Scholar]
- 45.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 46.Spencer CT, Gilchuk P, Dragovic SM, Joyce S. Minor histocompatibility antigens: presentation principles, recognition logic and the potential for a healing hand. Curr Opin Organ Transplant. 2010;15:512–525. doi: 10.1097/MOT.0b013e32833c1552. [DOI] [PubMed] [Google Scholar]
- 47.Mazeyrat S, Saut N, Sargent CA, Grimmond S, Longepied G, Ehrmann IE, Ellis PS, Greenfield A, Affara NA, Mitchell MJ. The mouse Y chromosome interval necessary for spermatogonial proliferation is gene dense with syntenic homology to the human AZFa region. Hum Mol Genet. 1998;7:1713–1724. doi: 10.1093/hmg/7.11.1713. [DOI] [PubMed] [Google Scholar]
- 48.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aoshi T, Zinselmeyer BH, Konjufca V, Lynch JN, Zhang X, Koide Y, Miller MJ. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity. 2008;29:476–486. doi: 10.1016/j.immuni.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skoberne M, Schenk S, Hof H, Geginat G. Cross-presentation of Listeria monocytogenes-derived CD4 T cell epitopes. J Immunol. 2002;169:1410–1418. doi: 10.4049/jimmunol.169.3.1410. [DOI] [PubMed] [Google Scholar]
- 52.Lara-Tejero M, Pamer EG. T cell responses to Listeria monocytogenes. Curr Opin Microbiol. 2004;7:45–50. doi: 10.1016/j.mib.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Geginat G, Schenk S, Skoberne M, Goebel W, Hof H. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J Immunol. 2001;166:1877–1884. doi: 10.4049/jimmunol.166.3.1877. [DOI] [PubMed] [Google Scholar]
- 54.Tourne S, van Santen HM, van Roon M, Berns A, Benoist C, Mathis D, Ploegh H. Biosynthesis of major histocompatibility complex molecules and generation of T cells in Ii TAP1 double-mutant mice. Proc Natl Acad Sci U S A. 1996;93:1464–1469. doi: 10.1073/pnas.93.4.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kroeger H, Miranda E, MacLeod I, Perez J, Crowther DC, Marciniak SJ, Lomas DA. Endoplasmic reticulum-associated degradation (ERAD) and autophagy cooperate to degrade polymerogenic mutant serpins. J Biol Chem. 2009;284:22793–22802. doi: 10.1074/jbc.M109.027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mylin LM, Schell TD, Roberts D, Epler M, Boesteanu A, Collins EJ, Frelinger JA, Joyce S, Tevethia SS. Quantitation of CD8(+) T-lymphocyte responses to multiple epitopes from simian virus 40 (SV40) large T antigen in C57BL/6 mice immunized with SV40, SV40 T-antigen-transformed cells, or vaccinia virus recombinants expressing full-length T antigen or epitope minigenes. J Virol. 2000;74:6922–6934. doi: 10.1128/jvi.74.15.6922-6934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarz K, de Giuli R, Schmidtke G, Kostka S, van den Broek M, Kim KB, Crews CM, Kraft R, Groettrup M. The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up-or down-regulate antigen presentation at nontoxic doses. J Immunol. 2000;164:6147–6157. doi: 10.4049/jimmunol.164.12.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lev A, Takeda K, Zanker D, Maynard JC, Dimberu P, Waffarn E, Gibbs J, Netzer N, Princiotta MF, Neckers L, Picard D, Nicchitta CV, Chen W, Reiter Y, Bennink JR, Yewdell JW. The exception that reinforces the rule: crosspriming by cytosolic peptides that escape degradation. Immunity. 2008;28:787–798. doi: 10.1016/j.immuni.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 61.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. Embo J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de la Salle H, Hanau D, Fricker D, Urlacher A, Kelly A, Salamero J, Powis SH, Donato L, Bausinger H, Laforet M, et al. Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science. 1994;265:237–241. doi: 10.1126/science.7517574. [DOI] [PubMed] [Google Scholar]
- 63.Matamoros N, Mila J, Llano M, Balas A, Vicario JL, Pons J, Crespi C, Martinez N, Iglesias-Alzueta J, Lopez-Botet M. Molecular studies and NK cell function of a new case of TAP2 homozygous human deficiency. Clin Exp Immunol. 2001;125:274–282. doi: 10.1046/j.1365-2249.2001.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andres AM, Dennis MY, Kretzschmar WW, Cannons JL, Lee-Lin SQ, Hurle B, Schwartzberg PL, Williamson SH, Bustamante CD, Nielsen R, Clark AG, Green ED. Balancing selection maintains a form of ERAP2 that undergoes nonsense-mediated decay and affects antigen presentation. PLoS Genet. 2010;6:e1001157. doi: 10.1371/journal.pgen.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ljunggren HG, Van Kaer L, Ploegh HL, Tonegawa S. Altered natural killer cell repertoire in Tap-1 mutant mice. Proc Natl Acad Sci U S A. 1994;91:6520–6524. doi: 10.1073/pnas.91.14.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldszmid RS, Bafica A, Jankovic D, Feng CG, Caspar P, Winkler-Pickett R, Trinchieri G, Sher A. TAP-1 indirectly regulates CD4+ T cell priming in Toxoplasma gondii infection by controlling NK cell IFN-gamma production. J Exp Med. 2007;204:2591–2602. doi: 10.1084/jem.20070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raposo G, van Santen HM, Leijendekker R, Geuze HJ, Ploegh HL. Misfolded major histocompatibility complex class I molecules accumulate in an expanded ER-Golgi intermediate compartment. J Cell Biol. 1995;131:1403–1419. doi: 10.1083/jcb.131.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 69.Singh R, Jamieson A, Cresswell P. GILT is a critical host factor for Listeria monocytogenes infection. Nature. 2008;455:1244–1247. doi: 10.1038/nature07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh R, Cresswell P. Defective cross-presentation of viral antigens in GILT-free mice. Science. 2010;328:1394–1398. doi: 10.1126/science.1189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen W, Norbury CC, Cho Y, Yewdell JW, Bennink JR. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J Exp Med. 2001;193:1319–1326. doi: 10.1084/jem.193.11.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hensley SE, Zanker D, Dolan BP, David A, Hickman HD, Embry AC, Skon CN, Grebe KM, Griffin TA, Chen W, Bennink JR, Yewdell JW. Unexpected role for the immunoproteasome subunit LMP2 in antiviral humoral and innate immune responses. J Immunol. 2011;184:4115–4122. doi: 10.4049/jimmunol.0903003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 74.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.