Abstract
Simian immunodeficiency virus (SIV) challenge of rhesus macaques provides a relevant model for the assessment of human immunodeficiency virus (HIV) vaccine strategies. To ensure that all macaques become infected, the vaccinees and controls are exposed to large doses of pathogenic SIV. These nonphysiological high-dose challenges may adversely affect vaccine evaluation by overwhelming potentially efficacious vaccine responses. To determine whether a more physiologically relevant low-dose challenge can initiate infection and cause disease in Indian rhesus macaques, we used a repeated low-dose challenge strategy designed to reduce the viral inoculum to more physiologically relevant doses. In an attempt to more closely mimic challenge with HIV, we administered repeated mucosal challenges with 30, 300, and 3,000 50% tissue culture infective doses (TCID50) of pathogenic SIVmac239 to six animals in three groups. Infection was assessed by sensitive quantitative reverse transcription-PCR and was achieved following a mean of 8, 5.5, and 1 challenge(s) in the 30, 300, and 3,000 TCID50 groups, respectively. Mortality, humoral immune responses, and peak plasma viral kinetics were similar in five of six animals, regardless of challenge dose. Interestingly, macaques challenged with lower doses of SIVmac239 developed broad T-cell immune responses as assessed by ELISPOT assay. This low-dose repeated challenge may be a valuable tool in the evaluation of potential vaccine regimes and offers a more physiologically relevant regimen for pathogenic SIVmac239 challenge experiments.
Worldwide, there are an estimated 42 million people who are currently living with human immunodeficiency virus (HIV). Heterosexual transmission is the predominant route of viral infection, particularly in Asia and sub-Saharan Africa where more than 35 million people are currently infected (29). The risk of HIV infection is affected by multiple factors that include transmission route, frequency of sexual contact, genetic predisposition, and immunocompetence of the individual (6, 9, 20). The frequency of HIV infection, particularly among women, has risen steadily, and there are twice as many young women (aged 15 to 24 years) as men that are currently infected with HIV in sub-Saharan Africa (29). According to the Joint United Nations Programme on HIV/AIDS, approximately 58% of HIV-infected individuals in sub-Saharan Africa are women and 9% are children (29). Both sexual and perinatal transmission of HIV are associated with a high plasma viral load (10, 14, 20, 23, 25, 27, 28).
Access to new and effective antiretroviral drugs is limited, and 5 million more people were infected during 2002 (29). Development of an effective vaccine strategy is therefore paramount. The majority of HIV vaccines in current clinical trials target cytotoxic T lymphocytes (CTL) because the generation of broadly neutralizing antibody response has been difficult to achieve (13, 22). Vaccines that specifically induce CTL have been tested in vaccinated macaques that were challenged with high doses of either simian-human immunodeficiency virus (SHIV) or simian immunodeficiency virus (SIV) (1, 4, 31). However, to evaluate such vaccines in the macaque model, a clinically relevant challenge is crucial to vaccine development. To date, even though amelioration of the disease course has been observed after challenge with the chimeric SHIV89.6P virus (3, 4, 8, 21, 24), few vaccination strategies have managed to significantly curtail the progression to simian AIDS (SAIDS) in animals challenged with highly pathogenic SIVs (239, 251, or E660) (5, 7). However, we and other groups have used SIVmac239 at doses of 103 to 105 50% tissue culture infective doses (TCID50) when challenging animals for the evaluation of potential vaccines (2, 18). These high-dose challenges ensure that all control animals become infected after a single exposure. However, SIV challenge following administration of a potential vaccine should ideally be at a dose that most accurately reflects challenge with HIV. The actual dose of HIV transmitted via sexual contact has been investigated but has proved to be dependent upon the type of model used (6, 10, 26). A study in sub-Saharan Africa showed a correlation between plasma viral loads in excess of 35,000 copies/ml and transmission to HIV-negative partners. Conversely, individuals with fewer than 1,500 copies/ml were less likely to transmit the virus (10, 20). Therefore, it is likely that the rate of transmission depends upon the concentration of the virus in the inoculum. Unfortunately, the recovery and detection of virus in semen has proved difficult, and concentrations ranging from <103 to >105 HIV RNA copies/ml of seminal plasma have been reported previously (6, 30). The routine mucosal challenge inoculum used in nonhuman primate SIV challenge studies far exceeds the amount of HIV in semen and can be in excess of 8 × 107 SIV RNA copies/ml. Here we investigate whether a more relevant low-dose viral challenge can infect and cause disease in Indian rhesus macaques.
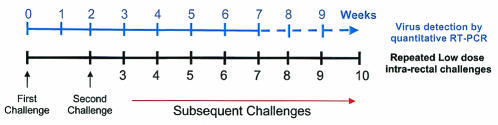
To achieve a low-inoculum dose, we repeatedly challenged six animals intrarectally according to the strategy depicted in Fig. 1. Animals 1941 and 96107 received 30 TCID50, AJ10 and AJ11 received 300 TCID50, and 97009 and 98019 received 3,000 TCID50. Challenge was repeated after 2 weeks and then at weekly intervals thereafter until infection was detected. The plasma virus concentration was tested prior to the next weekly dose; if positive, the challenges for that animal were terminated. From the time of the initial challenge, the animals were screened regularly for humoral and cellular immune responses by enzyme-linked immunosorbent assay and whole-proteome ELISPOT assay, respectively.
FIG. 1.
Schedule for the repeated challenging with low-dose SIVmac239. The initial challenge was followed 2 weeks later with weekly challenges until infection was detected.
Repeated low-dose challenge results in SIVmac239 infection.
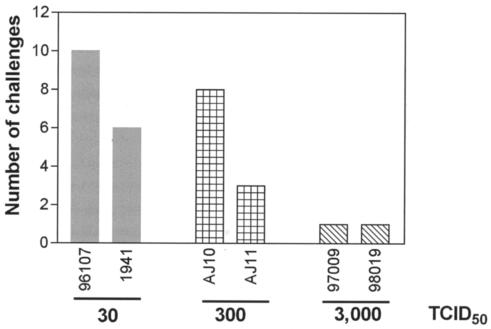
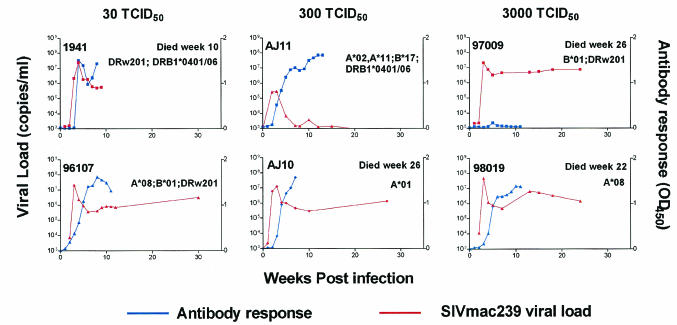
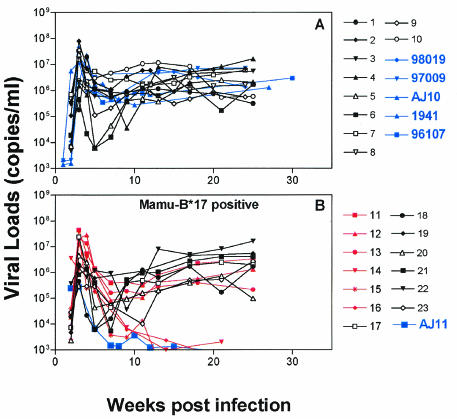
Quantitative reverse transcription-PCR (RT-PCR) was used for early virus detection and determination of plasma viral concentration as described previously. Viral RNA was extracted from 800 μl of plasma after the pelleted virus was digested with 1 mg of proteinase per ml, and viral RNA was precipitated. Plasma viral RNA concentrations were determined by quantitative RT-PCR using the Roche LightCycler (Roche Diagnostics Corporation, Columbus, Ind.). The forward primer was SIV-61F (5′-CCACCTACCATTAAGCCCGA-3′), the reverse primer was SIV-143R (5′-CTGGCACTACTTCTGCTCCAAA-3′), and the probe was SIV-84T (FAM reporter, TAMRA quencher) [5′-CATTAAATGCCTGGGTAAAATTGATAGAGGA(G/A)AAGAA-3′]. Cycling conditions were as follows: 61°C for 15 min, 95°C for 30 s, 45 cycles at 95°C for 2 s, and 60°C for 12 s. Data were collected at the end of the extension phase only. As expected, SIVmac239 was detected in the plasma from animals 97009 and 98019 following a single challenge with 3,000 TCID50 of SIVmac239 (Fig. 2). However, AJ10 and AJ11 were challenged eight and three times, respectively, (mean, 5.5 challenges) with 300 TCID50 before they became infected. The lowest dose of 30 TCID50 was administered 10 and 6 times (mean of eight challenges) to animals 1941 and 96107 to establish SIVmac239 infection (Fig. 2). Longitudinal analysis of the plasma viral concentration from all groups revealed similar kinetics regardless of inoculum or number of infections the animals received (Fig. 3.). The plasma viral concentration peaks were in the expected range of 107 to 108 copies/ml, which subsequently reduced to approximately 106 copies/ml—a finding typical for this virus (Fig. 3) (19). The plasma viral concentration produced by low-dose challenge showed no difference when compared to that of 10 Mamu-B*17-negative animals exposed to a high-dose challenge (Fig. 4A). However, one animal, AJ11, was challenged three times with 300 TCID50 of SIVmac239 and had a peak viral load of only 105 copies/ml, a plasma viral concentration 10-fold lower than that of the other animals that received low doses of SIVmac239. Indeed, following this peak plasma virus concentration, plasma virus in AJ11 became undetectable by our sensitive quantitative RT-PCR technique. Interestingly, this animal (AJ11) is Mamu-B*17 positive, an allele associated with the control of SIVmac239 (19). When compared to plasma viral concentrations in 13 other Mamu-B*17-positive macaques, the concentration of plasma virus was found not to differ in the acute phase, despite the high-dose challenge of SIVmac239 administered to these animals (Fig. 4B). Interestingly, comparison of the chronic-phase plasma virus concentration of AJ11 and the plasma virus concentration of the Mamu-A*01, B*17 double-positive long-term nonprogressors (shown in red in Fig. 5B) showed no difference. Animals 97009, 98019, AJ10, and 1941 were sacrificed due to SAIDS-related diseases at 26, 22, 26, and 10 weeks postinfection, respectively. We therefore conclude that low doses of SIVmac239 were pathogenic in these rhesus macaques regardless of dose or number of challenges received, and these findings fall within our expected range of life span with this virulent virus (19). Two animals remain asymptomatic to date (at 35 weeks postinfection), 96107 and AJ11, with the former having a set point viral load of >106 copies/ml, and we are still unable to detect plasma virus in the latter Mamu-B*17-positive animal.
FIG. 2.
Three groups of animals were repeatedly challenged with 30, 300, or 3,000 TCID50. Here we show the number of challenges administered to each animal to establish a SIVmac239 infection with respect to dose.
FIG. 3.
Longitudinal SIVmac239 plasma viral concentration and antibody responses for each low-dose-challenge group. The plasma viral concentration and antibodies were detected as outlined in Materials and Methods. Animals were euthanized due to SAIDS-related illness at the times indicated. The major histocompatibility complex alleles were determined for each animal by using methods described previously (12). OD450, optical density at 450 nm.
FIG. 4.
Plasma viral concentrations of low-dose-challenged animals compared to those of 23 control animals challenged with high doses of SIVmac239. (A) Viral loads for the 5 animals challenged with 30, 300, and 3,000 TCID50 doses of SIVmac239 (blue) and 10 control animals that received high doses of SIVmac239 (black) are shown. (B) Viral loads for AJ11 (blue) compared to those of 13 other Mamu-B*17-positive animals are shown. Included in this analysis are six long-term nonprogressor animals (red) that are phenotypically Mamu-A*01, B*17.
FIG. 5.
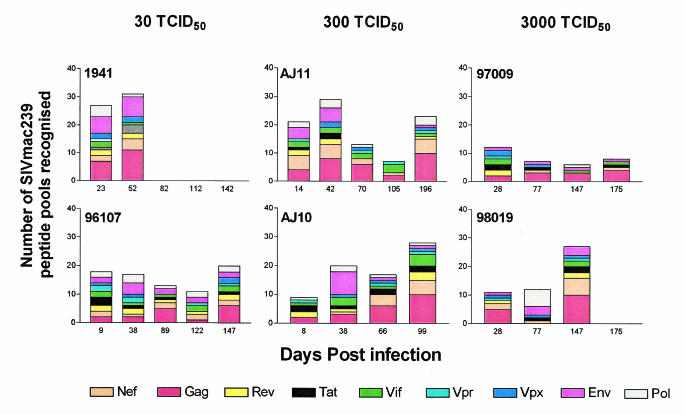
Breadth of T-cell responses over time (days postinfection) in each low-dose-challenge group. Here we show the number of pools and the SIVmac239 proteins recognized by the immune responses in each animal. Each animal was routinely monitored by using the whole-proteome ELISPOT assay, and the bar graphs represent the cumulative number of pools that were considered reactive by the criteria outlined in the text.
Humoral and cellular immune responses.
To investigate whether low-dose repeated challenge may result in antibody responses that differ from those seen in macaques exposed to a high-dose viral challenge, we monitored SIV-specific plasma antibody responses by using the commercially available HIV type 2-specific enzyme-linked immunosorbent assay kit from the Genetic Systems Corporation/Sanofi-Pasteur Diagnostics (Redmond, Wash.). The detection of antibody from the low-dose, repeatedly challenged animals produced the same kinetics as that seen in high-dose-challenge animals.
It is possible that infection with a low-dose viral inoculum might allow for the development of broad and vigorous SIV-specific cellular responses. In order to monitor the development and breadth of cellular responses before and after each virus challenge, we screened the animals regularly according to our animal protocols. Therefore, to assess SIV-specific T-cell responses, we used a gamma interferon ELISPOT assay (U-Cytech BV, Utrecht, The Netherlands) at regular intervals (Fig. 5) during the repeated intrarectal challenges using freshly isolated Ficoll-separated peripheral blood mononuclear cells (105/well). Peptide pools made up of 10 15-mers overlapping by 11 amino acids (final concentration, 5 μg/ml), which spanned the entire SIVmac239 proteome, were used to detect T-cell immune responses as described previously (11, 15-17). The peripheral blood mononuclear cells and peptides were coincubated for 16 to 18 h, and the spots were visualized directly by using an AID reader system (Cell Technologies, Inc., Columbia, Md.). Responses were considered positive when the frequency of gamma interferon-secreting T cells exceeded the mean spot-forming cell count of the negative controls plus twice the standard deviation. In all of the study animals, we detected T-cell responses across the SIVmac239 proteome from the earliest time point tested after successful intrarectal challenge until the time of sacrifice. At more than 27 weeks postinfection, the two animals that are still alive, AJ11 and 97106, had broad T-cell responses to 27 and 24% of the SIVmac239 pools tested, respectively (Fig. 4). Analysis of the average number of pools recognized by animals in the different groups shows that, at time points before 11 weeks, 11.5 (14%), 19.5 (23%), and 46.5 (56%) SIVmac239 pools per animal were recognized in the 3,000, 300, and 30 TCID50 groups, respectively. This would suggest that more immune responses are recognized in the lower-dose-challenge inoculum groups than in the 3,000-TCID50 dose of SIVmac239 early in infection. However, given the small number of animals and the inherent genetic variation in outbred rhesus macaques, this trend may not be significant.
Our low-dose-challenge experiments clearly show that infection can be achieved by repeated exposure to low virus doses across mucosal surfaces. Infection with these low virus doses results in typical plasma virus concentrations and the normal development of humoral immune responses. Furthermore, broad, multiepitope-specific cellular immune responses develop in animals that are infected with repeated exposure to low doses of SIVmac239. Since these repeated low-dose exposures more closely resemble exposure to HIV, it will be interesting to determine whether vaccine-induced CTL in the mucosa can ameliorate the disease course after challenge with a low dose of this highly pathogenic SIVmac239 clone.
Acknowledgments
We thank Bill Rehrauer and Tim Jacoby for major histocompatibility complex typing. We also thank Eva Rakasz for assistance with the antibody titers and for helpful discussions.
This work is supported by NIH grants AI46366, AI49120, RR15371, and RR00167. David Watkins is an Elizabeth Glaser Scientist.
REFERENCES
- 1.Allen, T. M., P. Jing, B. Calore, H. Horton, D. H. O'Connor, T. Hanke, M. Piekarczyk, R. Ruddersdorf, B. R. Mothe, C. Emerson, N. Wilson, J. D. Lifson, I. M. Belyakov, J. A. Berzofsky, C. Wang, D. B. Allison, D. C. Montefiori, R. C. Desrosiers, S. Wolinsky, K. J. Kunstman, J. D. Altman, A. Sette, A. J. McMichael, and D. I. Watkins. 2002. Effects of cytotoxic T lymphocytes (CTL) directed against a single simian immunodeficiency virus (SIV) Gag CTL epitope on the course of SIVmac239 infection. J. Virol. 76:10507-10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch, D. H., T. M. Fu, D. C. Montefiori, M. G. Lewis, J. W. Shiver, and N. L. Letvin. 2001. Vaccine-elicited immune responses prevent clinical AIDS in SHIV(89.6P)-infected rhesus monkeys. Immunol. Lett. 79:57-61. [DOI] [PubMed] [Google Scholar]
- 5.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty, H., P. K. Sen, R. W. Helms, P. L. Vernazza, S. A. Fiscus, J. J. Eron, B. K. Patterson, R. W. Coombs, J. N. Krieger, and M. S. Cohen. 2001. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 15:621-627. [DOI] [PubMed] [Google Scholar]
- 7.Crotty, S., C. J. Miller, B. L. Lohman, M. R. Neagu, L. Compton, D. Lu, F. X. Lu, L. Fritts, J. D. Lifson, and R. Andino. 2001. Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J. Virol. 75:7435-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 9.Garnett, G. P., and J. A. Rottingen. 2001. Measuring the risk of HIV transmission. AIDS 15:641-643. [DOI] [PubMed] [Google Scholar]
- 10.Gray, R. H., M. J. Wawer, R. Brookmeyer, N. K. Sewankambo, D. Serwadda, F. Wabwire-Mangen, T. Lutalo, X. Li, T. vanCott, and T. C. Quinn. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357:1149-1153. [DOI] [PubMed] [Google Scholar]
- 11.Horton, H., T. Vogel, D. O'Connor, L. Picker, and D. I. Watkins. 2002. Analysis of the immune response and viral evolution during the acute phase of SIV infection. Vaccine 20:1927-1932. [DOI] [PubMed] [Google Scholar]
- 12.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 13.McMichael, A. J., M. Callan, V. Appay, T. Hanke, G. Ogg, and S. Rowland-Jones. 2000. The dynamics of the cellular immune response to HIV infection: implications for vaccination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mock, P. A., N. Shaffer, C. Bhadrakom, W. Siriwasin, T. Chotpitayasunondh, S. Chearskul, N. L. Young, A. Roongpisuthipong, P. Chinayon, M. L. Kalish, B. Parekh, T. D. Mastro, et al. 1999. Maternal viral load and timing of mother-to-child HIV transmission, Bangkok, Thailand. AIDS 13:407-414. [DOI] [PubMed] [Google Scholar]
- 15.Mothe, B. R., H. Horton, D. K. Carter, T. M. Allen, M. E. Liebl, P. Skinner, T. U. Vogel, S. Fuenger, K. Vielhuber, W. Rehrauer, N. Wilson, G. Franchini, J. D. Altman, A. Haase, L. J. Picker, D. B. Allison, and D. I. Watkins. 2002. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mothe, B. R., J. Sidney, J. L. Dzuris, M. E. Liebl, S. Fuenger, D. I. Watkins, and A. Sette. 2002. Characterization of the peptide-binding specificity of Mamu-B*17 and identification of Mamu-B*17-restricted epitopes derived from simian immunodeficiency virus proteins. J. Immunol. 169:210-219. [DOI] [PubMed] [Google Scholar]
- 17.Mothe, B. R., J. Weinfurter, C. Wang, W. Rehrauer, N. Wilson, T. M. Allen, D. B. Allison, and D. I. Watkins. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77:2736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nixon, D. F., S. M. Donahoe, W. M. Kakimoto, R. V. Samuel, K. J. Metzner, A. Gettie, T. Hanke, P. A. Marx, and R. I. Connor. 2000. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and protection against challenge in rhesus macaques immunized with a live attenuated simian immunodeficiency virus vaccine. Virology 266:203-210. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor, D., B. R. Mothe, J. T. Weinfurter, S. Fuenger, W. M. Rehrauer, J. Peicheng, R. R. Rudersdorf, M. E. Loebl, K. Krebs, J. Vasquez, E. Dodds, J. Loffredo, S. Martin, A. B. McDermott, T. M. Allen, C. Wang, G. G. Doxiadis, D. C. Montefiori, A. Hughes, D. R. Burton, D. B. Allison, S. M. Wolinsky, R. Bontrop, L. J. Picker, and D. I. Watkins. 2003. Major histocompatibility class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 77:9029-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 21.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 22.Rowland-Jones, S. L., S. Pinheiro, R. Kaul, P. Hansasuta, G. Gillespie, T. Dong, F. A. Plummer, J. B. Bwayo, S. Fidler, J. Weber, A. McMichael, and V. Appay. 2001. How important is the “quality” of the cytotoxic T lymphocyte (CTL) response in protection against HIV infection? Immunol. Lett. 79:15-20. [DOI] [PubMed] [Google Scholar]
- 23.Shaffer, N., A. Roongpisuthipong, W. Siriwasin, T. Chotpitayasunondh, S. Chearskul, N. L. Young, B. Parekh, P. A. Mock, C. Bhadrakom, P. Chinayon, M. L. Kalish, S. K. Phillips, T. C. Granade, S. Subbarao, B. G. Weniger, T. D. Mastro, et al. 1999. Maternal virus load and perinatal human immunodeficiency virus type 1 subtype E transmission, Thailand. J. Infect. Dis. 179:590-599. [DOI] [PubMed] [Google Scholar]
- 24.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 25.Sutthent, R., S. Foongladda, S. Chearskul, N. Wanaprapa, S. Likanonskul, U. Kositanont, S. Riengrojpitak, S. Sahapong, and C. Wasi. 1997. Maternal and viral factors in vertical transmission of HIV-1 subtype E. Southeast Asian J. Trop. Med. Public Health 28:689-698. [PubMed] [Google Scholar]
- 26.Tachet, A., E. Dulioust, D. Salmon, M. De Almeida, S. Rivalland, L. Finkielsztejn, I. Heard, P. Jouannet, D. Sicard, and C. Rouzioux. 1999. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS 13:823-831. [DOI] [PubMed] [Google Scholar]
- 27.Thea, D. M., R. W. Steketee, V. Pliner, K. Bornschlegel, T. Brown, S. Orloff, P. B. Matheson, E. J. Abrams, M. Bamji, G. Lambert, E. A. Schoenbaum, P. A. Thomas, M. Heagarty, M. L. Kalish, et al. 1997. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. AIDS 11:437-444. [DOI] [PubMed] [Google Scholar]
- 28.Tranchat, C., P. Van de Perre, A. Simonon-Sorel, E. Karita, M. Benchaib, P. Lepage, C. Desgranges, V. Boyer, and C. Trepo. 1999. Maternal humoral factors associated with perinatal human immunodeficiency virus type-1 transmission in a cohort from Kigali, Rwanda, 1988-1994. J. Infect. 39:213-220. [DOI] [PubMed] [Google Scholar]
- 29.UNAIDS/W. H. O. 2002. AIDS epidemic update, December 2002. UNAIDS.
- 30.Vernazza, P. L., J. J. Eron, S. A. Fiscus, and M. S. Cohen. 1999. Sexual transmission of HIV: infectiousness and prevention. AIDS 13:155-166. [DOI] [PubMed] [Google Scholar]
- 31.Vogel, T. U., B. E. Beer, J. zur Megede, H. G. Ihlenfeldt, G. Jung, S. Holzammer, D. I. Watkins, J. D. Altman, R. Kurth, and S. Norley. 2002. Induction of anti-simian immunodeficiency virus cellular and humoral immune responses in rhesus macaques by peptide immunogens: correlation of CTL activity and reduction of cell-associated but not plasma virus load following challenge. J. Gen. Virol. 83:81-91. [DOI] [PubMed] [Google Scholar]