Abstract
At the uterine-placental interface, fetal cytotrophoblasts invade the decidua, breach maternal blood vessels, and form heterotypic contacts with uterine microvascular endothelial cells. In early gestation, differentiating- invading cytotrophoblasts produce high levels of matrix metalloproteinase 9 (MMP-9), which degrades the extracellular matrix and increases the invasion depth. By midgestation, when invasion is complete, MMP levels are reduced. Cytotrophoblasts also produce human interleukin-10 (hIL-10), a pleiotropic cytokine that modulates immune responses, helping to protect the fetal hemiallograft from rejection. Human cytomegalovirus (CMV) is often detected at the uterine-placental interface. CMV infection impairs cytotrophoblast differentiation and invasion, altering the expression of the cell adhesion and immune molecules. Here we report that infection with a clinical CMV strain, VR1814, but not a laboratory strain, AD169, downregulates MMP activity in uterine microvascular endothelial cells and differentiating-invading cytotrophoblasts. Infected cytotrophoblasts expressed CMV IL-10 (cmvIL-10) mRNA and secreted the viral cytokine, which upregulated hIL-10. Functional analyses showed that cmvIL-10 treatment impaired migration in endothelial cell wounding assays and cytotrophoblast invasion of Matrigel in vitro. Comparable changes occurred in cells that were exposed to recombinant hIL-10 or cmvIL-10. Our results show that cmvIL-10 decreases MMP activity and dysregulates the cell-cell and/or cell-matrix interactions of infected cytotrophoblasts and endothelial cells. Reduced MMP activity early in placental development could impair cytotrophoblast remodeling of the uterine vasculature and eventually restrict fetal growth in affected pregnancies.
Human cytomegalovirus (CMV) infection is asymptomatic in healthy individuals but causes serious morbidity and permanent sequelae in infants infected before birth (3, 40). Prenatal infections occur in 2% of births, and the risk of permanent sequelae, including neuronal defects and hearing loss, increases with a primary maternal infection. Early in gestation, CMV can infect the uterus, replicating in the vascular endothelium, the glandular epithelium, and decidual cells (42). CMV also replicates in placental cytotrophoblasts and dysregulates their functioning prior to their reaching the fetus (17, 21, 22, 34, 51). Innate cellular and adaptive immune responses protect the placenta from CMV infection in seropositive women with healthy, uncomplicated pregnancies (42). Decidual granular leukocytes include macrophages, dendritic cells, and natural killer cells that populate the pregnant uterus (15, 24-26, 55). In the decidua, these innate immune cells colocalize in islands where CMV-infected cells are present (42).
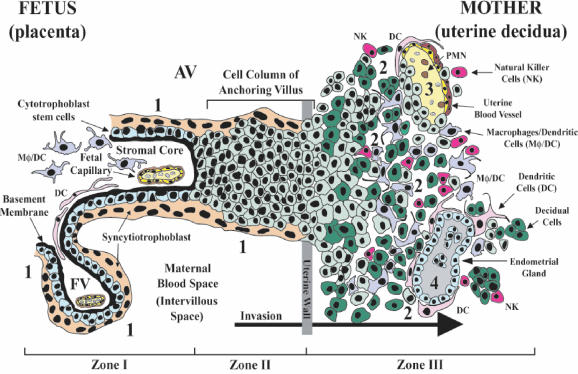
The placental-uterine interface provides nourishment and protects the fetus from immune rejection and local infections. The placenta is pivotal in CMV transmission to the fetus, as is suggested by the unusual anatomy of the maternal-fetal interface (Fig. 1) (10, 13). Cytotrophoblasts differentiate into the specialized trophoblast population of floating and anchoring chorionic villi, which have different properties and functions. Cytotrophoblasts in floating villi (Fig. 1, site 4) fuse into multinucleated syncytiotrophoblasts that cover the villus surface. These cells are in direct contact with maternal blood and exchange gas, nutrients, and waste with the maternal blood supply. Cytotrophoblasts in anchoring villi (Fig. 1, site 3) remain as single cells that aggregate into columns and invade the uterine wall up to the first third of the myometrium. Interstitial cytotrophoblasts invade the decidua and breach uterine spiral arterioles in a process with many similarities to tumor invasion, except that the extent and timing of invasion are carefully regulated (Fig. 1, site 2). Invasive cytotrophoblasts intercalate among innate immune cells in the decidua and remodel the uterine vasculature, replacing the endothelial cell lining and some of the smooth muscle cell wall (Fig. 1, sites 1 and 2). The result is a hybrid vasculature composed of fetal cytotrophoblasts and maternal endothelial cells that ultimately supplies vast quantities of blood to floating villi.
FIG. 1.
Anatomy of the maternal-fetal interface, where the fetus-derived placenta attaches to the mother's uterus. The basic structural unit of the placenta is the chorionic villus, composed of a stromal core with blood vessels, surrounded by a basement membrane, and overlaid by cytotrophoblast stem cells. As part of their differentiation pathway, stem cells detach from the basement membrane and adopt one of two lineage fates. They fuse to form the syncytiotrophoblast, which covers floating villi, or they join a column of extravillus cytotrophoblasts that invade the uterine stroma. The syncytiotrophoblast mediates nutrient and gas exchange across the maternal-fetal interface. The anchoring villi (AV) establish physical connections between the mother and fetus through the attachment of cytotrophoblast columns. The floating villi (FV), bathed by maternal blood, contain the fetal capillaries (zone I). Cytotrophoblasts in the AV attach the placenta to the uterine wall (zone II). Cytotrophoblasts then invade the decidua up to the first third of the myometrium (zone III), anchoring the placenta to the uterus and gaining access to the maternal circulation. Sites proposed as routes of CMV infection in utero are numbered 1 to 4.
During placental development, cytotrophoblasts initiate unusual, highly regulated molecular differentiation programs (10, 11, 19, 38). For example, differentiating cytotrophoblasts in columns begin to express novel adhesion molecules that are required for invasion and the attachment of the placenta to the uterine wall. Endovascular cytotrophoblasts transform their adhesion receptor phenotype to resemble the endothelial cells they replace (Fig. 1, site 1) (60). Like endothelial cells during angiogenesis, cytotrophoblasts express vasculogenic factors and receptors, including VE-(endothelial) cadherin, platelet endothelial adhesion molecule 1, and vascular endothelial adhesion molecule 1 as well as integrins α1β1 and αVβ3 (13).
Invasive cytotrophoblasts degrade the basement membrane and the extracellular matrix of the uterine stroma, a process that is precisely regulated during placentation. The cells upregulate matrix metalloproteinase 9 (MMP-9), a collagenase and urokinase-type plasminogen activator (30, 53), and tissue inhibitor of metalloproteinases 3, a regulator of proteolytic activity and invasion depth (2). Molecules that function in maternal immune tolerance, such as the nonclassical major histocompatibility complex class 1b molecule HLA-G (28, 35) and interleukin-10 (IL-10) (46, 47), are also produced.
MMPs are a family of degradative enzymes that remodel the extracellular matrix during many processes that include cell migration, vascularization, and invasion (7, 59). MMPs are highly regulated during translation and posttranslationally by activation and secretion (57, 58). Invasive cytotrophoblasts secrete relatively large amounts of MMP-9 during early gestation, when invasion peaks; later, when invasion is complete, MMP-9 levels decrease (30). Trophoblast invasion is also regulated by factors controlling MMP activation. The inactive proenzyme is activated by the cleavage and removal of an inhibitory domain. Activated MMP-9 is absolutely required for invasion, whereas pro-MMP-9 is associated with noninvasive cells (18, 30) and certain pregnancy complications (31). Several cytokines and growth factors regulate MMP expression and activity. For example, IL-1β is an autocrine stimulator of MMP-9 secretion and cytotrophoblast invasion of Matrigel in vitro (29). In contrast, IL-10 downregulates these processes and impairs cytotrophoblast invasion (46, 47). Cytokine expression and metalloproteinase activity are also regulated in endothelial cells (9, 39, 48).
Endothelial cells are targets of infection by CMV and may disseminate the virus in immune system-compromised patients (16, 20, 41, 49). The remodeling of uterine spiral arterioles suggests that CMV can spread from the maternal vasculature to endovascular cytotrophoblasts in the uterus (17). Uterine microvascular endothelial cells (UtMVEC) infected with VR1814, an endothelial cell-tropic CMV strain (45), transmit the infection to cocultured differentiating-invading cytotrophoblasts (33), suggesting a role for the uterine vasculature in virus transmission. Recently, we found that the decidua functions as a reservoir for CMV during early gestation and that virus replication in endovascular cytotrophoblasts correlates with infected endothelial cells and virus transmission at the maternal-fetal interface (42). CMV-infected cytotrophoblasts downregulate the key differentiation molecules HLA-G, which may play a role in immune tolerance, and α1β1 integrin, which is required for invasion (17). The presence of intimate contacts between endothelial cells and cytotrophoblasts in the hybrid vasculature suggests that similar changes in cell-cell and cell-matrix interactions could occur.
These observations, together with the capacity of CMV to modulate host immune responses (reviewed in reference 36), suggested to us that a virally encoded IL-10 homologue might, like the cellular molecule, impair the invasion of differentiating cytotrophoblasts. Thus, we examined the effect of CMV infection on MMP activity in cells that form heterotypic interactions in utero, namely UtMVEC and differentiating-invading cytotrophoblasts. We found that the expression of CMV IL-10 (cmvIL-10) and the upregulation of human IL-10 (hIL-10) reduced MMP activity and impaired endothelial cell migration and cytotrophoblast invasion in vitro. Diminished degradation of the extracellular matrix could contribute to the shallow invasion of the uterus and restriction of fetal growth observed in cases of CMV transmission in utero.
MATERIALS AND METHODS
Cell cultures, virus strains, and infection.
Approval for this project was obtained from the Institutional Review Board of the University of California, San Francisco. Highly purified cytotrophoblasts were isolated from 10- to 16-week placentas as previously described (30). Cells were plated in serum-free medium on wells coated with 30 μl of Matrigel (30). A small fraction of the cytotrophoblast preparation was evaluated for CMV infection in utero by PCR for CMV DNA (42) and for immediate-early protein expression by immunohistochemistry (17). Human foreskin fibroblasts were grown in Dulbecco's modified Eagle's medium (Life Technologies) supplemented with 10% Nu-serum (Becton Dickinson), 200 mM l-glutamine (Sigma), and penicillin-streptomycin (Sigma). Human umbilical vein endothelial cells (HUVEC) and UtMVEC (Clonetics, Biowhittaker, Inc.) were maintained in EBM-2 medium supplemented with EGM-2 and EGM-2-MV Singlequots (Clonetics). Endothelial cells that were found by immunohistochemistry with a rabbit antiserum to express the von Willebrand factor complex were used between passages 4 and 8. Human fibroblasts were infected (1 to 5 PFU/cell) with the laboratory CMV strain AD169. Fibroblasts and endothelial cells were infected with the clinical strain VR1814 (45) in Dulbecco's modified Eagle's medium containing 1% Nu-serum, and cells were then cultured for 4 days (cytotrophoblasts and fibroblasts) or 7 days (endothelial cells). The CMV infection efficiency in cytotrophoblasts was monitored by immunofluorescence assays for immediate-early protein expression at 24 h (37). Approximately 30 to 40% of cells were infected.
Antibodies and purified proteins.
The following commercial reagents were purchased: activated MMP-2, activated MMP-9, and pro-MMP-2 (Oncogene Research Products); polyclonal goat anti-cmvIL-10 (R&D Research); murine antibodies anti-MMP-2 (clone 42-5D11) and anti-MMP-9 (clone 7-11C) (Oncogene Research Products); rabbit antiserum against the von Willebrand factor complex (Novocastra Laboratories Ltd.); anti-species antibodies conjugated to horseradish peroxidase (Jackson Immunoresearch Laboratories); and recombinant proteins hIL-10 and cmvIL-10 expressed from the strain Towne sequence (27, 54) (R&D Research).
Sample preparation.
Conditioned medium (CM) and cells were collected separately. For the preparation of cell lysates, samples were solubilized in buffer containing 150 mM NaCl, 50 mM Tris HCl (pH 7.6), 2 mM EDTA, 1% NP-40, and 1 mM phenylmethylsulfonyl fluoride. CM and cell lysates were collected every other day, centrifuged (12,000 × g, 20 min), and stored at −80°C before testing. CM was filtered through 0.2-μm-pore-size filters (Millipore). For zymography, nonreducing gel loading buffer was added to CM (3×) and cell lysates (1×). For immunoblot analysis, loading buffer containing sodium dodecyl sulfate (SDS) (3×) was added.
Substrate gel zymography.
Proteins were analyzed for gelatinolytic activity by gelatin zymography (47). Briefly, proteins (20 μl of CM and 15 μg of total cell lysate) solubilized in nonreducing sample buffer were separated in an SDS-10% polyacrylamide gel containing 1 mg of swine gelatin (Sigma)/ml. After electrophoresis, the gels were washed in 2.5% Triton X-100 for 30 min to remove the SDS. Metalloproteinases were then activated by overnight incubation at 37°C in buffer containing 50 mM Tris-HCl, pH 7.8, and 5 mM CaCl2. For the visualization of proteinase activity, the gels were stained with 1% Coomassie brilliant blue R-250 for 2 h and were destained in 10% acetic acid and 30% methanol until the cleared bands, evidence of gelatinase activity, were visible. The gels were then photographed. Molecular masses were calculated by using a recombinant protein size marker (Full Range Rainbow; Amersham).
Immunoblot analyses.
Solubilized samples (20 μl of CM and 15 μg of total cell lysate) were subjected to SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Nonspecific reactivity was blocked overnight by incubating the membranes in phosphate-buffered saline containing 0.05% Tween 20 and 5% nonfat skim milk before the application of the primary antibody. Anti-species antibodies conjugated to horseradish peroxidase were used to detect antibody binding. Activated MMP-2 and MMP-9 recombinant proteins were used as standards to identify specific metalloproteinase bands. Proteins were detected by using an enhanced chemiluminescence system (ECL; Amersham Corp.).
Immunoprecipitation.
An antibody against cmvIL-10 was added to CM (2.5 ml) from VR1814-infected HUVEC and mock-infected cells (control), and binding was allowed to occur overnight at 4°C. Immune complexes, which were precipitated by the addition of protein G-Sepharose (Amersham), were resuspended in SDS sample buffer, electrophoresed, transferred to nitrocellulose, and subjected to immunoblot analyses as described above. cmvIL-10 was detected by using ECL Advance (Amersham Corp.).
Reverse transcription (RT)-PCR.
Total RNAs were isolated from CMV-infected human foreskin fibroblasts and HUVEC and from mock-infected control cells by use of an Absolutely RNA RT-PCR Miniprep kit (Stratagene) according to the manufacturer's instructions. The first-strand cDNAs were synthesized from total RNAs by use of a Reverse Transcription kit (Applied Biosystems). Control reactions were carried out in parallel. Reactions were incubated at 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min. Primers were selected with Primer 3 software to amplify a 237-bp region of the cmvIL-10 gene (forward, 5′-TCGGTGATGGTCTCTTCCTC-3′; reverse, 5′-CGTCGCAATAAACCGTACCT-3′). Reactions were done in a PTC-200 thermocycler (MJ Research, San Francisco, Calif.). cDNA (5 μl) was added to a master mix containing 1× RedTaq buffer (Sigma), a 0.2 mM concentration of each deoxynucleoside triphosphate (Sigma), a 0.2 μM concentration of each primer, and 1 Unit of RedTaq polymerase (Sigma). Cycling conditions were 2 min of denaturation at 95°C followed by 40 cycles of 95°C for 45 s, 54°C for 45 s, and 72°C for 1 min, with a final extension at 72°C for 2 min. Products were analyzed by 2% agarose gel electrophoresis.
hIL-10 protein assays.
hIL-10 in CM from infected and control cells was measured by using a commercial enzyme-linked immunosorbent assay (ELISA) (Chemicon International Inc.). In brief, sample and biotinylated anti-hIL-10 was added to microtiter plates coated with anti-hIL-10 antibody for 3 h at room temperature. After the addition of alkaline phosphatase, followed by color-generating solution, the plates were read at 490 nm. The amount of hIL-10 was determined by extrapolation from a standard curve.
Quantification of MMP activity.
MMP-2 and MMP-9 activity in CM and cell lysates from endothelial cells and cytotrophoblasts was measured by using a highly sensitive Biotrak assay system according to the manufacturer's instructions (Amersham Pharmacia Biotech). Briefly, 100 μl of sample was added to microplates coated with antibodies specific for MMP-2 or MMP-9 before overnight incubation at 4°C. After a washing step, immobilized MMP was activated by incubation with p-aminophenylmercuric acetate for 1.5 h at 37°C. After the addition of buffer containing modified urokinase (detection enzyme) and the peptide substrate S-2444, color development was recorded at 405 nm at various times up to 20 h. The activity was expressed in units defined as 1,000× (ΔA/h2). The level of endogenous activated MMP was detected without p-aminophenylmercuric acetate treatment. The statistical significance of the data was analyzed by Student's one-tailed t test. P values of <0.05 were considered significant.
Invasion and cell migration assays.
Assays to measure fibroblast and endothelial cell migration (14) and cytotrophoblast invasion (30) were performed as previously described. In cell migration assays, human fibroblast and HUVEC monolayers were scratched (“wounded”) with a sterile pipette and then infected with VR1814, cultured with medium alone (control), or cultured with medium containing cmvIL-10 or human IL-10 (100 ng/ml). Alternatively, HUVEC were infected with VR1814 and cultured for 4 days before the monolayer was wounded. The cells were incubated approximately 24 h until the controls reached confluence (“wound healing”). For the assessment of migration, 10 fields (×40) were examined under an Olympus LH50A microscope and photographed with an Olympus SC35 Type 12 camera. For the quantification of invasion, cytotrophoblasts were plated on Matrigel-coated Transwell polycarbonate filters (5-μm pores), and the cells were cultured in medium alone or treated with 100 ng of recombinant cmvIL-10 or hIL-10/ml. Cells were incubated for 48 h, fixed in 3% formaldehyde, and stained with a rat monoclonal antibody against cytokeratin (7D3) (12). Filters were mounted on a slide with the underside facing up so that cytokeratin-positive cells migrating through the filter pores could be counted.
RESULTS
Reduced MMP-2 activity in CMV-infected human fibroblasts.
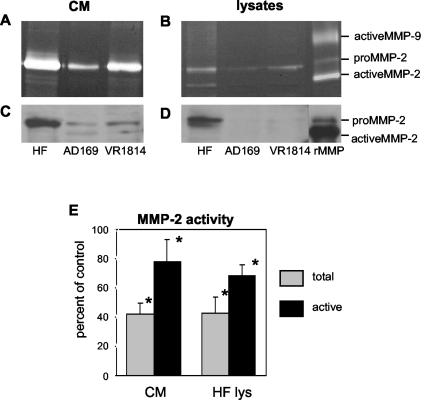
To test the hypothesis that CMV infection could affect MMP synthesis, we began by analyzing AD169- and VR1814-infected human fibroblasts for gelatinase activity by zymography (Fig. 2A and B). In control (uninfected) fibroblasts, the most abundant activity in CM and cell lysates was found in a 72-kDa band corresponding to pro-MMP-2. A smaller active fraction at 66 kDa corresponded to activated MMP-2. MMP-2 activity decreased after CMV infection of fibroblasts with AD169 (a laboratory strain) and less so with VR1814 (a clinical strain). No activated MMP-9 was found in CM or cell lysates. Immunoblots with an MMP-2-specific antibody showed decreased MMP activity in CMV-infected fibroblasts, corresponding to the reduced protein levels in CM and cell lysates (Fig. 2C and D). Because zymography, the most useful screening method for evaluating MMP activity, is only semiquantitative, we used a more sensitive assay, Biotrak, that measures the total amount and activated fraction of MMP (Fig. 2E). In CM, VR1814 infection reduced the total MMP-2 to 42% ± 7% (mean ± standard deviation [SD]) of the control and active MMP-2 to 78% ± 15%. In cell lysates, the total MMP-2 was reduced to 42.6% ± 11% of the control and active MMP-2 was reduced to 68% ± 7%. The finding that MMP-2 activity was significantly reduced in infected fibroblasts suggested that more invasive cell types might also be affected.
FIG. 2.
Downregulation of MMP-2 in CMV-infected human fibroblasts. CM and lysates from AD169- and VR1814-infected fibroblasts and uninfected fibroblasts were analyzed 4 days after infection by gelatin zymography (A and B) and immunoblotting (C and D). CMV reduced MMP-2 expression (both the 72-kDa proenzyme and the 66-kDa activated form) in CM (A and C) and cell lysates (B and D). MMP-2 activity was quantified in VR1814-infected fibroblast CM and cell lysates as a percentage of the control (E). Values are the means of four experiments and were highly reproducible. Asterisks indicate a significant difference between control and infected cells (P < 0.05; Fisher exact test). Error bars show SDs.
Reduced MMP-2 and MMP-9 activity in VR1814-infected endothelial cells.
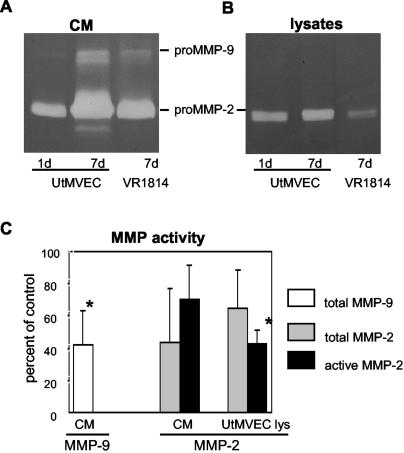
To test our hypothesis that MMP activity might be altered in endothelial cells, we analyzed UtMVEC infected with VR1814 (1 to 5 PFU/cell) by zymography (Fig. 3A). Control (uninfected) UtMVEC contained a major 72-kDa band with gelatinase activity and a trace 92-kDa band in CM at day 1 (Fig. 3A). The activities of both bands had increased dramatically by day 7 (Fig. 3A) and at each time point during this interval (data not shown). MMPs were identified as pro-MMP-2 (72 kDa) and pro-MMP-9 (92 kDa) by using recombinant MMP proteins as standards (see Fig. 2B, far right lane). After infection with VR1814, the pro-MMP-2 and pro-MMP-9 bands decreased (Fig. 3A). In lysates, MMP-2 remained constant in control cells and was diminished by infection; in contrast, pro-MMP-9 activity was barely detected under any of the conditions analyzed (Fig. 3B). Quantification of the activity in CM of VR1814-infected UtMVEC showed that the total MMP-9 decreased to 41% ± 21% and the total MMP-2 decreased to 43% ± 33% of control values; active MMP-2 was reduced to 70% ± 21%. In cell lysates, the total MMP-2 was reduced to 64% ± 23% and active MMP-2 was reduced to 42% ± 8% (Fig. 3C). Comparable changes were found in VR1814-infected HUVEC (data not shown). These results indicated that MMP-9 and MMP-2 activities are decreased in CM and lysates of CMV-infected endothelial cells.
FIG. 3.
Downregulation of MMP-2 and MMP-9 activity in VR1814-infected UtMVEC. (A and B) CM and lysates were analyzed by gelatin zymography after 1 and 7 days (uninfected control cells) and 7 days after the infection of experimental cells. (C) MMP-9 and MMP-2 activity in CM and cell lysates was quantified as a percentage of the control. Values are the means of four experiments. Asterisks indicate a significant difference between control and infected cells (P < 0.05). Error bars show SDs.
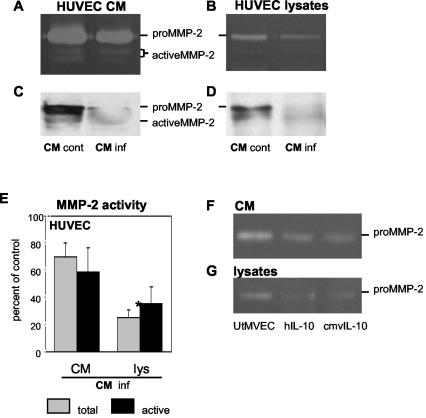
Reduced MMP-2 activity in HUVEC incubated with CM from infected UtMVEC.
Recent reports indicated that cmvIL-10 (27) binds the hIL-10 receptor 1 (IL-10R1) with an affinity similar to that of the natural ligand (23) and has a comparable immunosuppressive activity (54). An analysis of the cmvIL-10 genes from several strains showed a very high sequence conservation, suggesting that primers based on the Towne cmvIL-10 sequence might amplify cDNAs from VR1814-infected cells (27, 54).
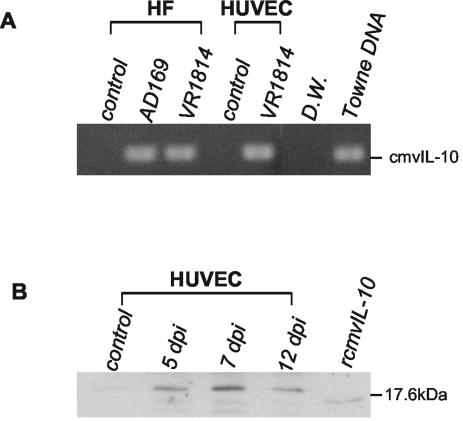
To better understand the mechanism of MMP dysregulation, we explored the possibility that cmvIL-10 might alter MMP activity in an autocrine fashion. First, we determined whether cmvIL-10 mRNA and protein could be detected in infected fibroblasts and HUVEC. Total RNAs extracted from AD169-infected fibroblasts and VR1814-infected fibroblasts and HUVEC were reverse transcribed, and PCR was performed with cmvIL-10-specific primers (Fig. 4A). A strong band amplified by RT-PCR primers for cmvIL-10 was detected with infected cells but not with uninfected controls. Immunoblotting indicated that a 20-kDa protein that immunoprecipitated with an antibody against cmvIL-10 was secreted into the CM of infected cells between 5 and 12 days after infection (Fig. 4B). The protein band detected for infected cells had a slightly larger molecular mass than the 17.6-kDa recombinant protein, which when expressed from a bacterial vector initiates at alanine 26 and terminates at lysine 126.
FIG. 4.
Expression of cmvIL-10 in VR1814-infected cells. (A) RT-PCR analysis of total RNA isolated 4 days after infection. HF, human fibroblasts. (B) Immunoblot analysis of cmvIL-10 protein complexes immunoprecipitated from infected cells 5, 7, and 12 days after infection (dpi).
We sought to determine whether cmvIL-10 secreted from VR1814-infected cells could alter MMP activity in uninfected cells. CM was collected from infected UtMVEC and uninfected control cells at 10 days, filtered to remove virions, and incubated with uninfected HUVEC. CM was replaced every other day for 6 days (Fig. 5). The MMP-2 activity in CM and cell lysates was reduced after exposure to CM from infected cells but not from control cells (Fig. 5A and B). Immunoblotting confirmed that the MMP-2 activity was reduced (Fig. 5C and D). An analysis of the CM from experimental cells showed that the total MMP-2 abundance was reduced to 70% ± 10% and active MMP-2 was reduced to 58% ± 18%. In the corresponding cell lysates, the total MMP was reduced to 25% ± 5% and active MMP was reduced to 35% ± 12% (Fig. 5E). Statistical analyses showed that the total MMP-2 activity was significantly reduced in HUVEC lysates by treatment with CM from infected cells.
FIG. 5.
CMV-infected endothelial cells secrete a soluble product that diminishes MMP-2 activity. Uninfected HUVEC were cultured for 6 days with CM from VR1814-infected UtMVEC (inf) or control cells (cont). CM and cell lysates were analyzed by gelatin zymography (A and B) and immunoblotting (C and D). MMP-2 activity was quantified in CM and lysates as a percentage of the control (E). Values are the means of two experiments. Asterisk indicates a significant difference between control and infected cells (P < 0.05). Error bars show SDs. UtMVEC that were infected or treated with purified recombinant cmvIL-10 or hIL-10 downregulated MMP-2 activity in CM (F) and cell lysates (G).
We next examined the effect of purified recombinant hIL-10 and cmvIL-10 on the MMP-2 activity in UtMVEC (Fig. 5F and G). After hIL-10 or cmvIL-10 treatment, CM and cell lysates contained less MMP activity, suggesting that cmvIL-10 inhibits endothelial cell MMP production in the absence of infection, and the effects were comparable to those for treatment with hIL-10. Together, these results indicate that VR1814-infected UtMVEC express cmvIL-10 and that the secreted proteins can reduce MMP activity.
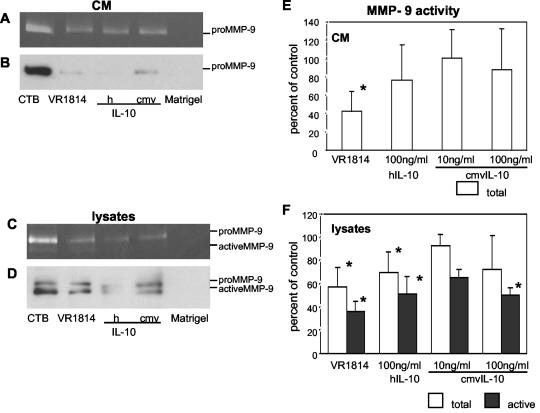
Reduced MMP-9 activity in VR1814-infected cytotrophoblasts.
Next, we determined whether VR1814 infection alters MMP activity in differentiating-invading cytotrophoblasts. Purified cells plated on Matrigel were infected for 4 days, after which CM and cell lysates (including Matrigel) were analyzed (Fig. 6). Zymography showed that both infected CM and cell lysates contained less MMP-9 activity than did control cells (Fig. 6A and C). Immunoblotting showed that the MMP-9 abundance was considerably reduced in CM but not in cell lysates (Fig. 6B and D).
FIG. 6.
Reduced MMP-9 activity in differentiating-invading cytotrophoblasts infected with VR1814 or treated with cmvIL-10. Cytotrophoblasts were untreated (CTB), infected, treated with CM, or treated with recombinant hIL-10 (h) or cmvIL-10 (cmv). Matrigel-coated wells without cells served as a control (Matrigel). CM and cell lysates were then analyzed for MMP-9 activity by gelatin zymography (A and C) and immunoblotting (B and D). CMV infection, hIL-10 treatment, and cmvIL-10 (100 ng/ml) treatment all decreased MMP-9 (both the 92-kDa proenzyme and the 86-kDa activated form) in CM (A and B) and cell lysates (C and D). Cytotrophoblasts were infected or treated with hIL-10 or cmvIL-10 for 4 days, and the CM (E) and lysates (F) were analyzed for MMP-9 activity, which was quantified as a percentage of the control. Values are the means of five experiments. Asterisks indicate a significant difference between control and infected or IL-10-treated cells (P < 0.05). Error bars show SDs.
The levels of MMP-9 activity in differentiating cytotrophoblasts infected with VR1814 or treated with hIL-10 or cmvIL-10 (100 ng/ml) were significantly decreased. After infection, the total MMP-9 levels declined to 42% ± 22% in CM and 57% ± 16% in cell lysates (Fig. 6E and F). The active form of MMP-9 declined to 36% ± 9% in cell lysates (Fig. 6F) and was under the limit of detection in CM (Fig. 6E). Treatment with hIL-10 alone decreased the total MMP-9 in CM to 76% ± 38%. In cell lysates, the total MMP-9 level was reduced to 69% ± 17%, and the active form was reduced to 51% ± 15%, confirming the results of a previous study (47). Likewise, treatment with cmvIL-10 (100 ng/ml) reduced the total MMP to 88% ± 7% in CM and 72% ± 29% in cell lysates. Active MMP-9 in CM was below the detection limit after hIL-10 or cmvIL-10 treatment (Fig. 6E). The downregulation of MMP activity was dose dependent: a 10-fold-lower dose of cmvIL-10 (1 ng/ml) had no effect (data not shown).
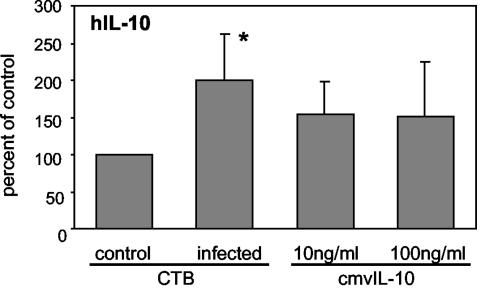
Early in the differentiation process, cytotrophoblasts produce hIL-10, which is downregulated by 12 h, when MMP-9 activity and invasion increase (46). Since cmvIL-10 binds IL-10R1, we asked whether cmvIL-10 might upregulate hIL-10 expression. Cytotrophoblasts were infected with VR1814 or treated with cmvIL-10 (10 or 100 ng/ml). CM was collected at 24 h, and hIL-10 was measured by ELISA. Significantly more hIL-10 was detected in CM from infected cytotrophoblasts than in CM from control (uninfected) cells (Fig. 7). cmvIL-10-treated cytotrophoblasts tended to increase intracellular levels of hIL-10 compared to controls, although the variability prevented the data from being statistically significant. In contrast, hIL-10 in VR1814-infected HUVEC was under the detection limit (data not shown). Together, the results of these experiments indicate that cells infected with VR1814 can upregulate cmvIL-10 and hIL-10 and have a synergistic effect on the suppression of MMP-9 activity of differentiating cytotrophoblasts by reducing the intracellular levels and secretion of the proteinase.
FIG. 7.
Upregulation of hIL-10 expression in differentiating-invading cytotrophoblasts after VR1814 infection and cmvIL-10 treatment. CM from VR1814- and mock-infected control or cmvIL-10-treated cytotrophoblasts was collected on day 1 and cultured for 24 to 36 h. hIL-10 was then quantified by ELISA. Results are expressed as percentages of the control. CM from untreated mock-infected cells served as a control. Values are the means of three experiments. Asterisks indicate a significant difference between control and infected or treated cells (P < 0.05). Error bars show SDs.
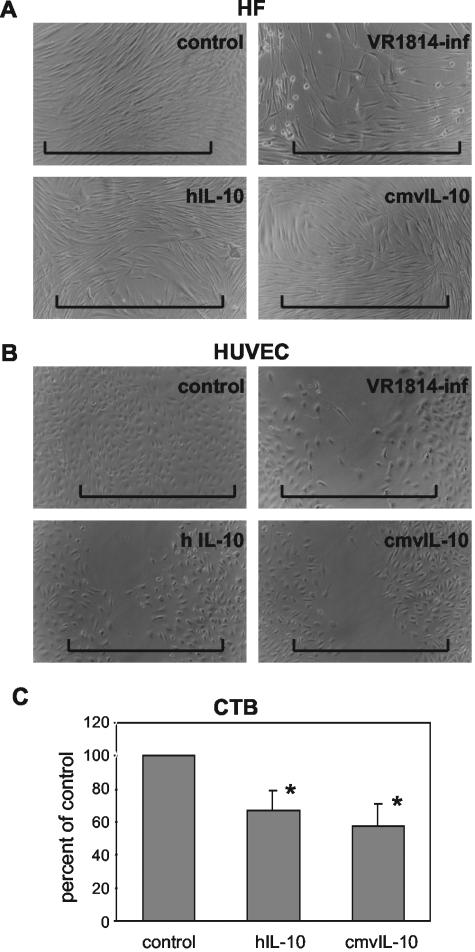
Impaired endothelial cell migration and cytotrophoblast invasiveness in vitro.
hIL-10 is an autocrine inhibitor of cytotrophoblast MMP-9 production and invasion of Matrigel in vitro (47) and could be one pathway by which CMV impairs cytotrophoblast functions (17). Having shown that VR1814-infected cells secrete cmvIL-10 and downregulate MMP activity, we next examined the effect of impaired proteinase activity on cell function in cell wound healing assays and cytotrophoblast invasion of Matrigel in vitro. Cells were infected or treated with cmvIL-10 or hIL-10, and functional assays were performed. In wound healing assays, subconfluent fibroblasts and endothelial cells were scratched at 4 days and incubated until migrating cells closed the wound (24 h for control cells) (Fig. 8). VR1814 infection slowed fibroblast migration in cell wound healing assays compared to control cells (Fig. 8A, top panels). In contrast, hIL-10 and cmvIL-10 treatment had no inhibitory effect on fibroblast migration (Fig. 8A). In HUVEC, we found that VR1814 infection and IL-10 treatment each impaired wound closure (Fig. 8B). When the cells were infected and then scratched, migration was impaired to a higher extent than vice versa. To assess the effect of IL-10 treatment on first-trimester cytotrophoblasts, we quantified the frequency with which cytotrophoblasts passed through narrow pores in a Matrigel-coated filter (Fig. 8C). The treatment of cytotrophoblasts with cmvIL-10 alone impaired invasion to a level comparable to that of hIL-10-treated cells. Fewer cells traversed the filter pores after treatment with cmvIL-10 (58% ± 13%) or hIL-10 (67% ± 12%) than did control untreated cells. Together, these results indicate that hIL-10 and cmvIL-10 impair endothelial cell migration in wound closure assays and also cytotrophoblast invasiveness, as was previously observed with CMV-infected cells in vitro (17).
FIG. 8.
Impaired cell motility in functional assays of endothelial cell wound healing and cytotrophoblast invasion of Matrigel in vitro. Human fibroblasts (A) and HUVEC (B) were infected with VR1814 and treated with hIL-10 or cmvIL-10 (100 ng/ml). The horizontal lines indicate the original widths of the wounded cell sheets. (C) Differentiating-invading cytotrophoblasts from first-trimester placentas were plated on Matrigel-coated filters and then were treated with cmvIL-10 or hIL-10, fixed at 48 h, and stained with a cytokeratin-specific antibody. Cells that reached the filter underside were counted. Values (percentages of the control) are expressed as the means ± SDs of three experiments. Asterisks indicate a significant difference between control and IL-10-treated cells (P < 0.05).
DISCUSSION
We have shown that CMV infection downregulates MMP activity indirectly through the expression of cmvIL-10 and the upregulation of hIL-10. Together, viral and human cytokines reduce proteinase activity and impair cytotrophoblast invasion and endothelial cell migration in vitro. The fact that cytotrophoblasts exposed to cmvIL-10 upregulated hIL-10 production suggests that the cytokines inhibit MMP synthesis via an autoregulatory mechanism that could contribute to impaired cell function. In infected cells, cmvIL-10 mRNA was expressed and the protein was secreted into the culture medium. In floating villi, cytotrophoblast progenitors produce hIL-10, which is downregulated as the cells differentiate in vitro, one mechanism that permits proteinase expression (46, 47). MMP-9 activity is also regulated posttranslationally by proteolytic cleavage (47). Given the importance of protease activity for extracellular matrix degradation and cytotrophoblast invasion of the uterus, downregulated MMP-9 expression at the protein and mRNA levels accompanies reduced invasive potential in the pregnancy complication preeclampsia, a syndrome characterized by maternal vascular damage, as evidenced by elevated blood pressure (new onset) and proteinuria (31).
During pseudovasculogenesis, differentiating cytotrophoblasts adopt a vascular adhesion phenotype, an unusual transformation process required for successful endovascular invasion and normal placentation (11, 60). We reported that UtMVEC transmit CMV to invasive cytotrophoblasts in vitro (33), suggesting that infection of the uterine vasculature and endovascular cytotrophoblasts in early gestation placentas could undermine vessel remodeling (17, 42). In the present study, we found that the invasion of IL-10-expressing cytotrophoblasts and migration of endothelial cells, but not fibroblasts, was impaired by cmvIL-10, suggesting possible paracrine effects in certain cell types. These findings suggest that the cmvIL-10 secreted from infected cells might also alter the invasiveness of uninfected cells nearby, one possible explanation for why cytotrophoblasts aggregated into cell columns show uniformly impaired invasion of Matrigel after CMV infection in vitro (17).
Like several other intracellular pathogens that infect macrophages, CMV exploits the IL-10 signaling pathway, expressing an IL-10 homologue and upregulating the cell's production of the cytokine (27, 44). Although cmvIL-10 shares only 27% sequence identity with hIL-10, the proteins have essentially identical affinities for the IL-10R1 and similarly reorganize the cell surface receptor complex (23). Both cytotrophoblasts and endothelial cells express IL-10R1, suggesting a possible autocrine and paracrine regulation by IL-10 (4, 47). hIL-10 in CM from cytotrophoblasts can suppress allogeneic lymphocyte reactivity (46), an important link between immune protection of the fetus and cytotrophoblast invasion of the uterus. Likewise, cmvIL-10 can inhibit the proliferation of mitogen-stimulated peripheral blood mononuclear cells and the production of proinflammatory cytokines at a level comparable to that of recombinant hIL-10 (54). cmvIL-10 alters the function of monocyte-derived dendritic cells, inducing hIL-10 production and inhibiting maturation and inflammatory cytokine production (W. Chang, N. Baumgarth, and P. Barry, personal communications). Likewise, murine CMV, which lacks a homologue, induces cellular IL-10 expression in macrophages, selectively reducing major histocompatibility complex class II expression and inflammatory cytokine production (36, 43). IL-10R1 protein expression in HUVEC is markedly upregulated in response to proinflammatory stimuli (e.g., gamma interferon or tumor necrosis factor alpha), implying that endothelial cells could become more responsive to IL-10 during an inflammatory episode (4). De novo synthesis of the receptor could be one explanation for the rapid upregulation of hIL-10 expression after cmvIL-10 treatment.
Clinical CMV strains contain genes that were lost from genomes of laboratory strains (5, 8) and confer a growth advantage in endothelial cells and macrophages (1, 32, 50, 52). The lower level of proteinase activity in cells infected with VR1814 than in cells infected with a laboratory strain or treated with cmvIL-10 suggests that impaired migration is a consequence of synergistic effects of diverse viral genes. Although cmvIL-10 encoded by UL111.5A is expressed as a γ gene product late in the viral life cycle (6), our results suggest that CMV infection may affect cytotrophoblast function earlier via other pathways. For example, VR1814 infection substantially increased tissue inhibitor of metalloproteinases 3 expression in cytotrophoblasts, further reducing the proteinase activity and the cells' invasiveness, whereas neither hIL-10 (47) nor cmvIL-10 altered the expression of the inhibitor (T. Tabata, S. McDonagh, H.-T. Chang, and L. Pereira, unpublished data). Interestingly, strain Toledo mediates the migration of vascular smooth muscle cells by the expression of US28, a viral chemokine receptor (56). Although we did not examine smooth muscle cells, this effect was not observed in VR1814-infected HUVEC. Altered migration is likely a consequence of several factors, including the viral strain, the time after infection of the assays, and the abundance of cell surface receptors that are responsive to ligand signaling. It is notable that AD169, a laboratory strain of CMV without endothelial cell tropism, downregulated the adhesion receptor, α1β1 integrin, which is required for invasion, and the immune molecule HLA-G in differentiating cytotrophoblasts (17) but had no effect on proteinase activity and IL-10-mediated dysregulated function in this study. Our results suggest that clinical strains trigger a constellation of events that impair cytotrophoblast function, directly by viral gene expression, indirectly by upregulating cellular genes, or both. Current efforts focus on identifying specific pathways whereby CMV undermines cytotrophoblast functions and placental development in utero.
Acknowledgments
We are grateful to members of the Pereira and Fisher laboratories for thoughtful discussions, Mirhan Kapidzic and Eduardo Caceres for technical assistance, and Mary McKenney for editing the manuscript.
This work was supported by Public Health Service grants AI46657, AI53782 (L.P. and S.F.), EY13683 (L.P.), and HD30367 (S.F.) from the National Institutes of Health and by grants from the March of Dimes Birth Defects Foundation and the University of California Academic Senate (L.P. and S.F.).
REFERENCES
- 1.Baldanti, F., M. G. Revello, E. Percivalle, N. Labo, and G. Gerna. 2003. Genomes of the endothelial cell-tropic variant and the parental Toledo strain of human cytomegalovirus are highly divergent. J. Med. Virol. 69:76-81. [DOI] [PubMed] [Google Scholar]
- 2.Bass, K. E., H. Li, S. P. Hawkes, E. Howard, E. Bullen, T. K. Vu, M. McMaster, M. Janatpour, and S. J. Fisher. 1997. Tissue inhibitor of metalloproteinase-3 expression is upregulated during human cytotrophoblast invasion in vitro. Dev. Genet. 21:61-67. [DOI] [PubMed] [Google Scholar]
- 3.Britt, W. J. 1999. Congenital cytomegalovirus infection, p. 269-281. In P. J. Hitchcock, H. T. MacKay, and J. N. Wasserheit (ed.), Sexually transmitted diseases and adverse outcomes of pregnancy. ASM Press, Washington, D.C.
- 4.Cattaruzza, M., W. Slodowski, M. Stojakovic, R. Krzesz, and M. Hecker. 2003. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J. Biol. Chem. 278:37874-37880. [DOI] [PubMed] [Google Scholar]
- 5.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers, J., A. Angulo, D. Amaratunga, H. Guo, Y. Jiang, J. S. Wan, A. Bittner, K. Frueh, M. R. Jackson, P. A. Peterson, M. G. Erlander, and P. Ghazal. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 73:5757-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, C., and Z. Werb. 2001. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 11:S37-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison, T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-170. [DOI] [PubMed] [Google Scholar]
- 9.Chi, L., Y. Li, L. Stehno-Bittel, J. Gao, D. C. Morrison, D. J. Stechschulte, and K. N. Dileepan. 2001. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J. Interferon Cytokine Res. 21:231-240. [DOI] [PubMed] [Google Scholar]
- 10.Cross, J. C., Z. Werb, and S. J. Fisher. 1994. Implantation and the placenta: key pieces of the development puzzle. Science 266:1508-1518. [DOI] [PubMed] [Google Scholar]
- 11.Damsky, C. H., and S. J. Fisher. 1998. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr. Opin. Cell Biol. 10:660-666. [DOI] [PubMed] [Google Scholar]
- 12.Damsky, C. H., M. L. Fitzgerald, and S. J. Fisher. 1992. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J. Clin. Investig. 89:210-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damsky, C. H., C. Librach, K. H. Lim, M. L. Fitzgerald, M. T. McMaster, M. Janatpour, Y. Zhou, S. K. Logan, and S. J. Fisher. 1994. Integrin switching regulates normal trophoblast invasion. Development 120:3657-3666. [DOI] [PubMed] [Google Scholar]
- 14.Denker, S. P., and D. L. Barber. 2002. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J. Cell Biol. 159:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake, P. M., M. D. Gunn, I. F. Charo, C. L. Tsou, Y. Zhou, L. Huang, and S. J. Fisher. 2001. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1 alpha. J. Exp. Med. 193:1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fish, K. N., C. Soderberg-Naucler, L. K. Mills, S. Stenglein, and J. A. Nelson. 1998. Human cytomegalovirus persistently infects aortic endothelial cells. J. Virol. 72:5661-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher, S., O. Genbacev, E. Maidji, and L. Pereira. 2000. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74:6808-6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher, S. J., T. Y. Cui, L. Zhang, L. Hartman, K. Grahl, G. Y. Zhang, J. Tarpey, and C. H. Damsky. 1989. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J. Cell Biol. 109:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher, S. J., and C. H. Damsky. 1993. Human cytotrophoblast invasion. Semin. Cell Biol. 4:183-188. [DOI] [PubMed] [Google Scholar]
- 20.Grefte, A., N. Blom, M. van der Giessen, W. van Son, and T. H. The. 1993. Ultrastructural analysis of circulating cytomegalic cells in patients with active cytomegalovirus infection: evidence for virus production and endothelial origin. J. Infect. Dis. 168:1110-1118. [DOI] [PubMed] [Google Scholar]
- 21.Halwachs-Baumann, G., M. Wilders-Truschnig, G. Desoye, T. Hahn, L. Kiesel, K. Klingel, P. Rieger, G. Jahn, and C. Sinzger. 1998. Human trophoblast cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Virol. 72:7598-7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmings, D. G., R. Kilani, C. Nykiforuk, J. Preiksaitis, and L. J. Guilbert. 1998. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J. Virol. 72:4970-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, B. C., N. J. Logsdon, K. Josephson, J. Cook, P. A. Barry, and M. R. Walter. 2002. Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL-10R1. Proc. Natl. Acad. Sci. USA 99:9404-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kammerer, U., A. O. Eggert, M. Kapp, A. D. McLellan, T. B. Geijtenbeek, J. Dietl, Y. Van Kooyk, and E. Kampgen. 2003. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 162:887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kammerer, U., K. Marzusch, S. Krober, P. Ruck, R. Handgretinger, and J. Dietl. 1999. A subset of CD56+ large granular lymphocytes in first-trimester human decidua are proliferating cells. Fertil. Steril. 71:74-79. [DOI] [PubMed] [Google Scholar]
- 26.Kammerer, U., M. Schoppet, A. D. McLellan, M. Kapp, H. I. Huppertz, E. Kampgen, and J. Dietl. 2000. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am. J. Pathol. 157:159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovats, S., E. K. Main, C. Librach, M. Stubblebine, S. J. Fisher, and R. DeMars. 1990. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248:220-223. [DOI] [PubMed] [Google Scholar]
- 29.Librach, C. L., S. L. Feigenbaum, K. E. Bass, T. Y. Cui, N. Verastas, Y. Sadovsky, J. P. Quigley, D. L. French, and S. J. Fisher. 1994. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J. Biol. Chem. 269:17125-17131. [PubMed] [Google Scholar]
- 30.Librach, C. L., Z. Werb, M. L. Fitzgerald, K. Chiu, N. M. Corwin, R. A. Esteves, D. Grobelny, R. Galardy, C. H. Damsky, and S. J. Fisher. 1991. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell Biol. 113:437-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim, K. H., Y. Zhou, M. Janatpour, M. McMaster, K. Bass, S. H. Chun, and S. J. Fisher. 1997. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 151:1809-1818. [PMC free article] [PubMed] [Google Scholar]
- 32.MacCormac, L. P., and J. E. Grundy. 1999. Two clinical isolates and the Toledo strain of cytomegalovirus contain endothelial cell tropic variants that are not present in the AD169, Towne, or Davis strains. J. Med. Virol. 57:298-307. [DOI] [PubMed] [Google Scholar]
- 33.Maidji, E., E. Percivalle, G. Gerna, S. Fisher, and L. Pereira. 2002. Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts. Virology 304:53-69. [DOI] [PubMed] [Google Scholar]
- 34.Markel, G., D. Wolf, J. Hanna, R. Gazit, D. Goldman-Wohl, Y. Lavy, S. Yagel, and O. Mandelboim. 2002. Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J. Clin. Investig. 110:943-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMaster, M. T., C. L. Librach, Y. Zhou, K. H. Lim, M. J. Janatpour, R. DeMars, S. Kovats, C. Damsky, and S. J. Fisher. 1995. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J. Immunol. 154:3771-3778. [PubMed] [Google Scholar]
- 36.Mocarski, E. S. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10:332-339. [DOI] [PubMed] [Google Scholar]
- 37.Navarro, D., P. Paz, S. Tugizov, K. Topp, J. La Vail, and L. Pereira. 1993. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology 197:143-158. [DOI] [PubMed] [Google Scholar]
- 38.Norwitz, E. R., D. J. Schust, and S. J. Fisher. 2001. Implantation and the survival of early pregnancy. N. Engl. J. Med. 345:1400-1408. [DOI] [PubMed] [Google Scholar]
- 39.Nystedt, S., V. Ramakrishnan, and J. Sundelin. 1996. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J. Biol. Chem. 271:14910-14915. [DOI] [PubMed] [Google Scholar]
- 40.Pass, B. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2, 4th ed. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 41.Percivalle, E., M. G. Revello, L. Vago, F. Morini, and G. Gerna. 1993. Circulating endothelial giant cells permissive for human cytomegalovirus (HCMV) are detected in disseminated HCMV infections with organ involvement. J. Clin. Investig. 92:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira, L., E. Maidji, S. McDonagh, O. Genbacev, and S. Fisher 2003. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol 77:13301-13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redpath, S., A. Angulo, N. R. Gascoigne, and P. Ghazal. 1999. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162:6701-6707. [PubMed] [Google Scholar]
- 44.Redpath, S., P. Ghazal, and N. R. Gascoigne. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9:86-92. [DOI] [PubMed] [Google Scholar]
- 45.Revello, M. G., F. Baldanti, E. Percivalle, A. Sarasini, L. De-Giuli, E. Genini, D. Lilleri, N. Labo, and G. Gerna. 2001. In vitro selection of human cytomegalovirus variants unable to transfer virus and virus products from infected cells to polymorphonuclear leukocytes and to grow in endothelial cells. J. Gen. Virol. 82:1429-1438. [DOI] [PubMed] [Google Scholar]
- 46.Roth, I., D. B. Corry, R. M. Locksley, J. S. Abrams, M. J. Litton, and S. J. Fisher. 1996. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 184:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roth, I., and S. J. Fisher. 1999. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev. Biol. 205:194-204. [DOI] [PubMed] [Google Scholar]
- 48.Silvestre, J. S., Z. Mallat, R. Tamarat, M. Duriez, A. Tedgui, and B. I. Levy. 2001. Regulation of matrix metalloproteinase activity in ischemic tissue by interleukin-10: role in ischemia-induced angiogenesis. Circ. Res. 89:259-264. [DOI] [PubMed] [Google Scholar]
- 49.Sinzger, C., A. Grefte, B. Plachter, A. S. Gouw, T. H. The, and G. Jahn. 1995. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 76:741-750. [DOI] [PubMed] [Google Scholar]
- 50.Sinzger, C., J. Knapp, B. Plachter, K. Schmidt, and G. Jahn. 1997. Quantification of replication of clinical cytomegalovirus isolates in cultured endothelial cells and fibroblasts by a focus expansion assay. J. Virol. Methods 63:103-112. [DOI] [PubMed] [Google Scholar]
- 51.Sinzger, C., H. Müntefering, T. Löning, H. Stöss, B. Plachter, and G. Jahn. 1993. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch. A 423:249-256. [DOI] [PubMed] [Google Scholar]
- 52.Sinzger, C., K. Schmidt, J. Knapp, M. Kahl, R. Beck, J. Waldman, H. Hebart, H. Einsele, and G. Jahn. 1999. Modification of human cytomegalovirus tropism through propagation in vitro is associated with changes in the viral genome. J. Gen. Virol. 80:2867-2877. [DOI] [PubMed] [Google Scholar]
- 53.Solberg, H., J. Rinkenberger, K. Dano, Z. Werb, and L. R. Lund. 2003. A functional overlap of plasminogen and MMPs regulates vascularization during placental development. Development 130:4439-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spencer, J. V., K. M. Lockridge, P. A. Barry, G. Lin, M. Tsang, M. E. Penfold, and T. J. Schall. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Starkey, P. M., I. L. Sargent, and C. W. Redman. 1988. Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology 65:129-134. [PMC free article] [PubMed] [Google Scholar]
- 56.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 57.Takeshita, S., J. R. Gage, T. Kishimoto, D. L. Vredevoe, and O. Martinez-Maza. 1996. Differential regulation of IL-6 gene transcription and expression by IL-4 and IL-10 in human monocytic cell lines. J. Immunol. 156:2591-2598. [PubMed] [Google Scholar]
- 58.Taraboletti, G., S. D'Ascenzo, P. Borsotti, R. Giavazzi, A. Pavan, and V. Dolo. 2002. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 160:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Werb, Z., C. M. Alexander, and R. R. Adler. 1992. Expression and function of matrix metalloproteinases in development. Matrix 1(Suppl.):337-343. [PubMed] [Google Scholar]
- 60.Zhou, Y., S. J. Fisher, M. Janatpour, O. Genbacev, E. Dejana, M. Wheelock, and C. H. Damsky. 1997. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Investig. 99:2139-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]