Abstract
Arenaviruses include Lassa fever virus (LFV) and the South American hemorrhagic fever viruses. These viruses cause severe human disease, and they pose a threat as agents of bioterrorism. Arenaviruses are enveloped viruses with a bisegmented negative-strand RNA genome whose proteomic capability is limited to four polypeptides: nucleoprotein (NP); surface glycoprotein (GP), which is proteolytically processed into GP1 and GP2; polymerase (L); and a small (11-kDa) RING finger protein (Z). Our investigators have previously shown that Z has a strong inhibitory activity on RNA synthesis mediated by the polymerase of the prototypic arenavirus, lymphocytic choriomeningitis virus (LCMV). In this report we show that cells transduced with a replication-deficient recombinant adenovirus expressing Z (rAd-Z) are resistant to LCMV and LFV infection. Virus cell entry mediated by LCMV or LFV GP was not affected in rAd-Z-transduced cells, but both virus transcription and replication were strongly and specifically inhibited, which resulted in a dramatic reduction in production of infectious virus. These findings open new avenues for developing antiviral strategies to combat the highly pathogenic human arenaviruses, including LFV.
Viral hemorrhagic fevers (HF) represent serious public health problems, causing devastating and often lethal disease. Several HF are caused by arenaviruses, among which Lassa fever virus (LFV) is the most important (8). Increased air travel has contributed to transport of LFV from its endemic niche in sub-Sahara Africa to other geographic areas, including Europe and the United States (20). Because of its severe morbidity and high mortality, together with the lack of immunization or effective treatment and the potential transmissibility from human to human, LFV and other HF arenaviruses are included in category A of potential bioterrorism microbial weapons (7). Therefore, the development of effective antiviral drugs to combat arenavirus infections is of high priority for many countries.
Arenaviruses are enveloped viruses with a bisegmented negative-strand (NS) RNA genome (8). The two genomic RNA segments are designated L and S and have approximate sizes of 7.2 and 3.4 kb, respectively. Each RNA segment directs the synthesis of two proteins in opposite orientations, separated by an intergenic region. The S RNA directs synthesis of the nucleoprotein (NP) and the two virion glycoproteins, GP1 and GP2, that are derived by posttranslational cleavage of a precursor polypeptide, GPC. Oligomeric structures of GP1 and GP2 make up the spikes on the virion envelope and mediate virus interaction with the host cell surface receptor (10). The L RNA segment codes for the virus RNA-dependent RNA polymerase (L) and a small (ca. 11-kDa) RING finger protein (Z) that interacts with several host cell proteins (5, 9) and has been implicated in several aspects of arenavirus biology (8).
Our investigators have developed a reverse genetic system for the prototypic arenavirus, lymphocytic choriomeningitis virus (LCMV) (23). Using this system we identified NP and L as the minimal trans-acting viral factors required for virus replication and transcription. Z was not required for intracellular transcription and replication of an LCMV minigenome (MG), but rather Z exhibited a dose-dependent inhibitory effect on both transcription and replication of LCMV MG (13). Here we show that cells transduced with a recombinant, replication-deficient, adenovirus expressing Z (rAd-Z) become resistant to LCMV and LFV. Z-mediated resistance was not due to a blockade of virus entry, but rather to a strong inhibitory effect of Z on the virus polymerase activity. These findings open novel avenues to combat infections with highly pathogenic human arenaviruses.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were maintained in 199 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 1.2 g of sodium bicarbonate/liter. Infections of Vero cells with LCMV strain Armstrong were done using a plaque-isolated clonal virus population (13). LCMV was titrated by plaque assay on Vero cells as described previously (1). LFV infections were done using strain Josiah. Virus inactivation in LFV-infected samples was done either by treatment with 2% paraformaldehyde (PFA) for 48 h (immunofluorescence [IF] assays) or by gamma irradiation (2 × 106 rad) using a cobalt 60 source (cell lysates for Western blot analysis). Measles virus (MV) infections and titrations were done using the Edmonston strain as described elsewhere (31).
Generation of rAd.
Replication-deficient rAd were generated using AdEasy technology (Quantum Biotechnologies). The LCMV Z and LFV Zmyc open reading frames flanked by a cytomegalovirus promoter and the polyadenylation signal of simian virus 40 were excised from pRK-Z and pRK-LFVZmyc plasmids, respectively, with KpnI and SpeI and cloned into the KpnI and XbaI sites of pShuttle. Expression of LFV Zmyc and LCMV Z was analyzed by Western blotting. AdEasy1 plasmid (100 ng) was combined with PmeI-linearized recombinant pShuttle (2 μg) and electroporated into Escherichia coli BJ5183 cells. Kanamycin-resistant colonies were selected and analyzed by restriction digestion. Plasmid DNA (5 μg) from correct clones was linearized with PacI and transfected into 293 cells for the generation of rAd. Whole populations of rAd were examined by Western blotting for their ability to express LCMV Z or LFV Zmyc. Clonal populations of each rAd were isolated by plaque purification, expanded, and tested for expression of Z by Western blotting.
Production of VSV pseudotypes.
Cells (293T) in six-well plates (80% confluent) were transfected with 2 μg of plasmid DNA expressing the glycoprotein of interest by using Lipofectamine. Thirty-two hours after transfection, cells were infected with a recombinant vesicular stomatitis virus (VSV), VSVΔG*-G, at a multiplicity of infection (MOI) of 3 PFU/cell for 1 h at 37°C (28). After a 1-h adsorption period, the inoculum was removed, cells were extensively washed with Dulbecco's modified Eagle's medium, and fresh culture medium was added. Twenty hours later culture supernatant was collected, clarified by low-speed centrifugation, and stored at −70°C. Pseudotyped viruses were titrated by infecting BHK-21 cells grown on 96-well plates as described previously (28).
Western blot assay.
Cells were directly harvested in sodium dodecyl sulfate (SDS) lysis buffer, and supernatants (75 μl) were mixed with 25 μl of 4× SDS lysis buffer. Samples were subjected to SDS-polyacrylamide gel electrophoresis followed by blotting on polyvinylidene difluoride Immobilon-P membrane (Millipore). Expression of LCMV Z was detected using a rabbit serum to an N-terminal peptide of the strain Armstrong Z (GQGKSREEKGTNSTNRAEI), whereas expression of c-myc-tagged LFV Z protein was detected with a mouse anti-c-myc antibody. Detection of LFV GPC and GP2 polypeptides was done using a rabbit serum to an LFV GP2 peptide provided by Olivier Lenz, Institut für Virologie, Philipps-University, Marburg, Germany. Antigen-antibody complexes were detected by incubation with an anti-rabbit peroxidase antibody (Roche), and proteins were visualized by enhanced chemiluminescence (Roche).
IF analysis.
Vero cells (2 × 105) seeded on glass coverslips in M24 wells were transduced with the indicated rAd and infected with LCMV, MV, or VSV pseudotypes. At the indicated time postinfection, cells were fixed with methanol-acetone (1:1) for 5 min at room temperature and processed for IF (28) using the following primary antibodies: guinea pig serum to LCMV, human serum from a subacute sclerosing panencephalitis patient (MV), and a rabbit polyclonal against VSV G cytoplasmic tail (VSVΔG/VSVG) or a mouse anti-LCMV GP2 (VSVΔG/LCMVGP). Cells infected with LFV were fixed in 2% PFA for 48 h, with one change of PFA prior to leaving containment. Subsequently, they were stained with a primate serum to LFV. Antigen-antibody complexes were detected using appropriate secondary antibodies conjugated to fluorescein isothiocyanate or rhodamine.
Cell death assay.
Cells were incubated with 10 μM Hoechst stain for 3 h at 37°C and photographed using a UV filter. Apoptotic cells were counted and recorded. Subsequently, cells were washed with phosphate-buffered saline (PBS), fixed for 30 min at room temperature with 4% PFA, washed with PBS, and permeabilized with 0.5% Triton X-100. Cells were then blocked in PBS-10% normal goat serum and analyzed by IF as described above using guinea pig and rabbit polyclonal sera to LCMV and Z, respectively.
Analysis of RNA expression.
Total cellular RNA was isolated using TRI reagent (Molecular Research Center) and analyzed by Northern blot hybridization using described procedures (13). LCMV NP and MV N [32P]DNA probes were generated according to Ambion's Decaprime-II protocol.
RESULTS
Cells transduced with rAd-Z are resistant to LCMV infection.
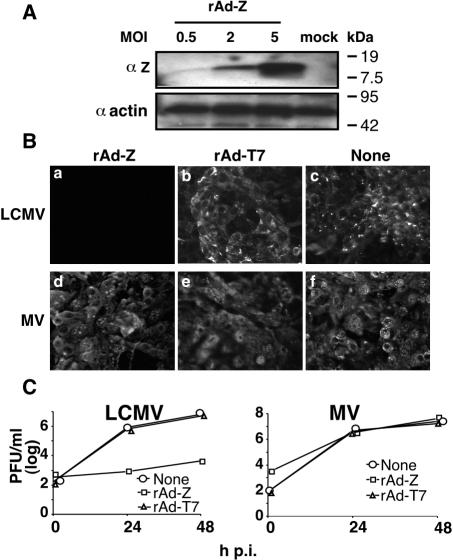
Using an LCMV MG rescue assay, our investigators showed a dose-dependent inhibitory effect of Z on RNA synthesis mediated by the LCMV polymerase (13). We therefore examined whether Z could also confer resistance to LCMV infection. For this we constructed a replication-deficient rAd-Z. Expression levels of rAd-Z-supplied Z depended on the MOI used to transduce cells (Fig. 1A). Using a rAd expressing green fluorescent protein (rAd-GFP), we determined that an MOI of 2 allowed for efficient transduction of Vero cells (>99% of the cells within the monolayer scored as GFP positive [data not shown]). Cells transduced with rAdZ (MOI of 2) expressed Z to levels similar to those documented in LCMV-infected cells (12), and they became specifically resistant to LCMV infection (Fig. 1B and C). In contrast, cells transduced with a control rAd expressing T7 RNA polymerase (rAd-T7) remained fully susceptible to LCMV infection (Fig. 1B and C).
FIG. 1.
Vero cells transduced with rAd-Z are specifically resistant to LCMV infection. (A) Expression levels of rAd-supplied Z protein depended on the MOI of rAd-Z. Cells were transduced with rAd-Z at various MOIs. Twenty hours later, cell lysates were prepared and analyzed by Western blotting using antibodies to Z or actin. (B and C) Vero cells were transduced with either rAd-Z or rAd-T7, both at an MOI of 2, and subsequently infected with either LCMV or MV (both at an MOI of 2). LCMV antigens were not detected in rAd-Z-transduced cells (B). At 48 h postinfection (p.i.), cells were fixed and analyzed by IF using antibodies to LCMV and MV. Production of infectious LCMV particles was dramatically and specifically reduced in rAd-Z-transduced cells (C). At the indicated time p.i., tissue culture supernatants were collected and titers of LCMV and MV were determined by plaque assay.
Z protects cells against infection with LFV.
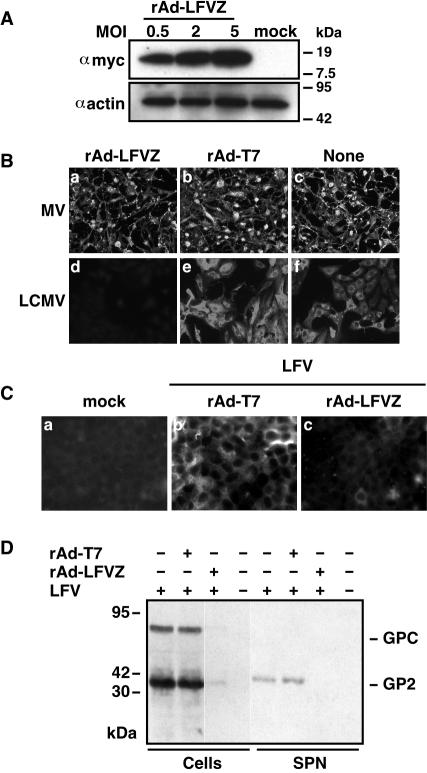
Z inhibitory activity on LCMV MG expression is influenced by the genetic distance between the arenavirus source of Z and LCMV (12). We therefore considered that LCMV Z might not provide efficient protection against LFV infection. To avoid this potential problem, we constructed a rAd expressing LFV Z (rAd-LFVZ). Since no antibodies were available to us for the detection of LFV Z, we used a c-myc-tagged version of LFV Z for the generation of rAd-LFVZ. Our investigators previously documented that c-myc tagging of the C terminus of LFV Z did not interfere with its inhibitory activity on LCMV MG expression (12).
Cells transduced with rAd-LFVZ expressed LFV Z at levels comparable to those observed for LCMV Z in cells transduced with rAd-Z (compare Fig. 1A and 2A). As with rAd-Z, transduction of cells with rAd-LFVZ resulted in specific resistance to LCMV infection (Fig. 2B). More importantly, cells transduced with rAd-LFVZ, but not those transduced with rAd-T7, were resistant to LFV infection as determined by IF (Fig. 2C) and Western blot analysis of cell extracts and corresponding tissue culture supernatant samples (Fig. 2D).
FIG. 2.
Vero cells expressing a Myc-tagged LFV Z (LFVZmyc) are specifically resistant to LCMV and LFV infection. (A) Expression levels of rAd-supplied LFVZ depended on the MOI of rAd-LFVZ. Cells were transduced with rAd-LFVZ at the indicated MOI. Twenty hours later, cell lysates were prepared and analyzed by Western blotting using antibodies to Myc and actin. (B and C) Vero cells were transduced with either rAd-LFVZ or rAd-T7, both at an MOI of 2, and subsequently infected with either LCMV (MOI of 2), MV (MOI of 2), or LFV (MOI of 2). At 48 h postinfection (p.i), cells were fixed and analyzed by IF with antibodies to LCMV, MV, or LFV. LCMV (B) and LFV (C) antigens were not detected in rAd-LFVZ-transduced cells. (D) Expression of LFV GP2 was dramatically reduced in both whole-cell extracts and tissue culture supernatants of rAd-LFV-transduced cells. Vero cells were transduced with either rAd-T7 or rAd-LFVZ (MOI of 2) and were subsequently infected with LFV (MOI of 2). Seven days after LFV infection, supernatants and whole-cell lysates were analyzed by Western blotting by using an antibody to LFV GP2.
Mechanism of Z-mediated resistance to arenavirus infection.
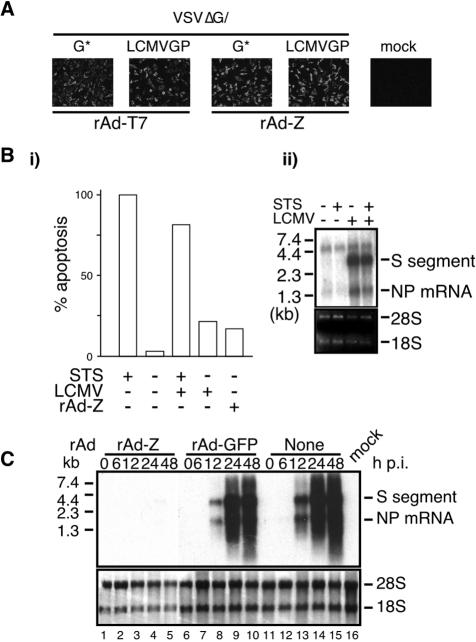
We next investigated possible mechanisms responsible for Z-mediated resistance to LCMV and LFV infection. Resistance to superinfection due to receptor downregulation has been documented for retroviruses (29) as well as other viruses. However, cells transduced with either rAd-Z or rAd-T7 were equally susceptible to infection by VSV pseudotyped particles decorated with the LCMV GP (Fig. 3A) or LFV GP (data not shown).
FIG. 3.
(A) Cell entry mediated by LCMV or LFV GP is not affected in rAdZ-transduced cells. Vero cells were transduced with either rAd-T7 or rAdZ (both at an MOI of 2) and subsequently infected with VSVΔG pseudotyped with either VSV G or LCMV GP. Twelve hours later, cells were fixed and analyzed by IF using an antibody to the nucleoprotein of VSV. (B) Low levels of apoptosis induced by rAd-supplied Z do not affect LCMV replication. (i) Levels of apoptosis (as a percentage) were normalized with respect to those seen in STS (50 μM)-treated cells. (ii) STS (50 μM)-treated and untreated Vero cells were infected with LCMV (MOI of 1). At 24 h postinfection (p.i.), total cellular RNA was isolated and analyzed by Northern blot hybridization using an LCMV NP probe. (C) LCMV RNA synthesis, both transcription and replication, is inhibited in cells transduced with rAdZ. Cells were transduced with either rAd-Z or rAd-GFP or were nontransduced, and subsequently cells were infected with LCMV. At the indicated time p.i., total cell RNA was isolated and analyzed by Northern blot hybridization using a [32P]NP double-stranded DNA probe that hybridizes to both NP mRNA (transcription) and S RNA (replication).
Arenavirus Z has been proposed to induce changes in cell physiology that promote apoptosis (6). Viruses can benefit from either promoting or inhibiting programmed cell death (PCD), including apoptosis (19). Hence, Z-induced PCD could contribute to rAd-Z-mediated resistance to arenavirus infection. To investigate this issue, we analyzed the effect of PCD on LCMV multiplication. For this, we used treatment of Vero cells with staurosporine (STS), a well-established method to induce apoptosis in these cells (30). Treatment with STS (50 μM) induced levels of cell death significantly higher than those seen in rAd-Z-transduced Vero cells (Fig. 3B, panel i), but LCMV multiplication was not affected in STS-treated Vero cells (Fig. 3B, panel ii).
We finally examined whether LCMV RNA synthesis was affected in rAd-Z-transduced cells. For this we determined levels of both NP mRNA and S RNA as surrogate markers of LCMV transcription and replication, respectively. Both viral transcription and RNA replication were inhibited in cells transduced with rAd-Z, whereas cells transduced with rAd-T7 exhibited levels of NP mRNA and S RNA similar to those seen in nontransduced cells (Fig. 3C).
DISCUSSION
The L segment of the arenavirus genome directs the synthesis of a small RING finger protein called Z that is present in virions and lacks an obvious counterpart in other NS RNA viruses (8). The role of Z in the arenavirus life cycle is poorly understood. Early studies implicated Z in both mRNA synthesis and genome replication (16). Nevertheless, Z was not required for the expression of an LCMV MG, but rather Z exhibited a dose-dependent inhibitory effect on both transcription and replication of the LCMV MG (13). Similar findings also have been now documented for Tacaribe virus Z protein (25). Z has been shown to interact with the eukaryotic initiation factor 4E, which resulted in a reduced affinity of eukaryotic initiation factor 4E for its substrate, the 5′-m7G cap structure (22). Acting as a translational repressor, Z could affect expression of proteins required for viral RNA synthesis. This attractive model is difficult to reconcile with the observation that overexpression of Z did not affect cap-dependent expression of LCMV NP and L proteins (13). We cannot, however, rule out that Z could inhibit translation of a cellular factor required for RNA synthesis mediated by the arenavirus polymerase. Our investigators have obtained evidence that Z is the arenavirus functional counterpart of the matrix (M) protein found in other NS RNA viruses (24). M proteins of NS RNA viruses play an essential role in virus assembly and budding (17). M proteins of several NS RNA viruses are known to inhibit RNA synthesis by the virus polymerase (11, 27). This inhibitory activity has been attributed to the M-mediated condensation of RNPs prior to virus egress (15). The finding that Z causes a linear dose-dependent decrease in RNA synthesis by the virus polymerase (13) argues against inhibition by RNP condensation, a process expected to require a threshold level of available Z protein. Moreover, transduction of cells with rAd-Z shortly after infection with LCMV (MOI of 0.1) did not affect virus production from cells infected during the first cycle of virus infection but effectively prevented subsequent rounds of cell infection. This finding suggests that Z-mediated inhibition of virus multiplication likely involves interference with a very early step of viral RNA synthesis rather than a blockade of the synthetic activity of RNP at later times during infection. The M protein of rabies virus has been shown to inhibit virus transcription but to stimulate virus replication (15). This finding suggests that M proteins of NS viruses might play a role in the regulation of the activities of the viral polymerases. The interdependency of transcription and replication in arenavirus-infected cells complicates the assessment of a Z-mediated inhibition of these processes. In contrast, in the LCMV MG system the viral trans-acting factors are plasmid supplied; hence, MG transcription is not required for its replication. Z exhibited a similar strong inhibitory activity on both transcription and replication of an LCMV MG by the virus polymerase. Therefore, contrary to the M protein of rabies virus, the inhibitory activity of arenavirus Z does not appear to discriminate between the transcriptase and replicase activities of arenavirus polymerase. However, it cannot be ruled out that some of the Z effects on viral RNA synthesis in LCMV-infected cells are not recreated in our MG system.
Virus interference due to virus-induced receptor downregulation has been implicated in resistance to superinfection in several viral systems, including the lentivirus human immunodeficiency virus type 1 (29). Superinfection exclusion has been demonstrated with several arenaviruses, and its degree appears to correlate with the genetic relationship of the viruses (14, 33). The molecular bases for arenavirus superinfection exclusion remain to be determined, but receptor downregulation is currently a favored mechanism. Therefore, receptor downregulation in rAd-Z-transduced cells might contribute to their resistance to infection. However, rAd-Z-transduced cells were fully susceptible to infection by VSV pseudotyped particles decorated with LCMV or LFV GP. The same cells, however, were resistant to infection with rLCMV/VSVG (data not shown). This recombinant LCMV expresses the glycoprotein of vesicular stomatitis virus (VSVG) instead of its own GP, and thereby cell entry is mediated by the VSV G. These findings argue against Z-mediated resistance being related to receptor downregulation and suggest a possible role of Z in arenavirus superinfection exclusion.
Our data do not allow us to discern whether Z is inhibiting a very early step of RNA synthesis by the virus polymerase or inhibiting virus disassembly. The latter mechanism underlies the documented coat protein-mediated resistance seen in plant viruses (3), where transgenically expressed coat protein disrupts virus disassembly, which results in plant protection against the infecting virus. Our findings would be more consistent with recently documented evidence indicating that Tacaribe virus Z protein interaction with the virus L polymerase might be responsible for the inhibitory effect of Z on RNA synthesis mediated by the virus polymerase (21).
The Bunyamwera virus nonstructural protein NSs and the NS1 protein of respiratory syncytial virus have been also shown to inhibit viral RNA synthesis in a minireplicon system (2, 32). Likewise, single viral nucleocapsid proteins have been reported to interfere with the replication of Borna disease virus (18). In addition, cells expressing the human foamy retrovirus accessory Bet protein are resistant to productive human foamy retrovirus superinfection (4). These findings raise the question of why viruses would encode a protein with an activity that would seem to be counterproductive for viral replication. Negative regulatory proteins could contribute to modulate virus replication and gene expression, which under certain circumstances would be advantageous for the virus (26). Z-mediated superinfection exclusion might influence arenavirus evolution and contribute to the phenomenon of population partitioning in the field, which allows the maintenance of independent evolutionary lineage of the same strain within a small geographic range (8). By having a negative regulatory effect on RNA synthesis, Z might also contribute to the noncytopathic properties characteristic of many arenaviruses.
Whether cell-type-specific factors can contribute to this inhibitory activity of Z is unknown, but we obtained similar results with BHK-21 and Vero cells. Likewise, it needs to be determined whether Z can exert its inhibitory effect on cells persistently infected with arenavirus.
A detailed understanding of the mechanisms whereby arenavirus Z exerts its inhibitory activity could open new avenues for the development of antiviral strategies to target highly pathogenic human arenaviruses, such as LFV.
Acknowledgments
We thank Oliver Lenz, Institut für Virologie, Philipps-University, for providing the anti-LFV-GP2 antiserum; Mike Garbutt, Special Pathogens Program, National Microbiology Laboratory Health Canada, for assistance with the biocontainment work; and Mar Perez and Michael Oldstone for helpful discussions.
This work was supported by National Institutes of Health grant RO1 AI47140 to J.C.D.L.T.
REFERENCES
- 1.Ahmed, R., R. S. Simon, M. Matloubian, S. R. Kolhekar, P. J. Southern, and D. M. Freedman. 1988. Genetic analysis of in vivo-selected viral variants causing chronic infection: importance of mutation in the L RNA segment of lymphocytic choriomeningitis virus. J. Virol. 62:3301-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atreya, P. L., M. E. Peeples, and P. L. Collins. 1998. The NS1 protein of human respiratory syncytial virus is a potent inhibitor of minigenome transcription and RNA replication. J. Virol. 72:1452-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beachy, R. N. 1999. Coat-protein-mediated resistance to tobacco mosaic virus: discovery mechanisms and exploitation. Philos. Trans. R. Soc. Lond. B 354:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock, M., M. Heinkelein, D. Lindemann, and A. Rethwilm. 1998. Cells expressing the human foamy virus (HFV) accessory Bet protein are resistant to productive HFV superinfection. Virology 250:194-204. [DOI] [PubMed] [Google Scholar]
- 5.Borden, K. L., E. J. Campbell Dwyer, and M. S. Salvato. 1998. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J. Virol. 72:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borden, K. L., E. J. Campbell Dwyer, and M. S. Salvato. 1997. The promyelocytic leukemia protein PML has a pro-apoptotic activity mediated through its RING domain. FEBS Lett. 418:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borio, L., T. Inglesby, C. J. Peters, A. L. Schmaljohn, J. M. Hughes, P. B. Jahrling, T. Ksiazek, K. M. Johnson, A. Meyerhoff, T. O'Toole, M. S. Ascher, J. Bartlett, J. G. Breman, E. M. Eitzen, Jr., M. Hamburg, J. Hauer, D. A. Henderson, R. T. Johnson, G. Kwik, M. Layton, S. Lillibridge, G. J. Nabel, M. T. Osterholm, T. M. Perl, P. Russell, and K. Tonat. 2002. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 287:2391-2405. [DOI] [PubMed] [Google Scholar]
- 8.Buchmaier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviridae: the virus and their replication, p. 1635-1668. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 9.Campbell Dwyer, E. J., H. Lai, R. C. MacDonald, M. S. Salvato, and K. L. Borden. 2000. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. J. Virol. 74:3293-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 11.Carroll, A. R., and R. R. Wagner. 1979. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J. Virol. 29:134-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornu, T. I., and J. C. de la Torre. 2002. Characterization of the arenavirus RING finger Z protein regions required for Z-mediated inhibition of viral RNA synthesis. J. Virol. 76:6678-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornu, T. I., and J. C. de la Torre. 2001. RING finger Z protein of lymphocytic choriomeningitis virus (LCMV) inhibits transcription and RNA replication of an LCMV S-segment minigenome. J. Virol. 75:9415-9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damonte, E. B., S. E. Mersich, and C. E. Coto. 1983. Response of cells persistently infected with arenaviruses to superinfection with homotypic and heterotypic viruses. Virology 129:474-478. [DOI] [PubMed] [Google Scholar]
- 15.Finke, S., R. Mueller-Waldeck, and K. K. Conzelmann. 2003. Rabies virus matrix protein regulates the balance of virus transcription and replication. J. Gen. Virol. 84:1613-1621. [DOI] [PubMed] [Google Scholar]
- 16.Garcin, D., S. Rochat, and D. Kolakofsky. 1993. The Tacaribe arenavirus small zinc finger protein is required for both mRNA synthesis and genome replication. J. Virol. 67:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garoff, H., R. Hewson, and D. J. E. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geib, T., C. Sauder, S. Venturelli, C. Hassler, P. Staeheli, and M. Schwemmle. 2003. Selective virus resistance conferred by expression of Borna disease virus nucleocapsid components. J. Virol. 77:4283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hay, S., and G. Kannourakis. 2002. A time to kill: viral manipulation of the cell death program. J. Gen. Virol. 83:1547-1564. [DOI] [PubMed] [Google Scholar]
- 20.Isaacson, M. 2001. Viral hemorrhagic fever hazards for travelers in Africa. Clin. Infect. Dis. 33:1707-1712. [DOI] [PubMed] [Google Scholar]
- 21.Jacamo, R., N. Lopez, M. Wilda, and M. T. Franze-Fernandez. 2003. Tacaribe virus Z protein interacts with the L polymerase protein to inhibit viral RNA synthesis. J. Virol. 77:10383-10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kentsis, A., E. C. Dwyer, J. M. Perez, M. Sharma, A. Chen, Z. Q. Pan, and K. L. Borden. 2001. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. J. Mol. Biol. 312:609-623. [DOI] [PubMed] [Google Scholar]
- 23.Lee, K. J., I. S. Novella, M. N. Teng, M. B. Oldstone, and J. C. de La Torre. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 74:3470-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, K. J., M. Perez, D. D. Pinschewer, and J. C. de la Torre. 2002. Identification of the lymphocytic choriomeningitis virus (LCMV) proteins required to rescue LCMV RNA analogs into LCMV-like particles. J. Virol. 76:6393-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez, N., R. Jacamo, and M. T. Franze-Fernandez. 2001. Transcription and RNA replication of Tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J. Virol. 75:12241-12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meiering, C. D., and M. L. Linial. 2002. Reactivation of a complex retrovirus is controlled by a molecular switch and is inhibited by a viral protein. Proc. Natl. Acad. Sci. USA 99:15130-15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez, D. R., and R. O. Donis. 1998. The matrix 1 protein of influenza A virus inhibits the transcriptase activity of a model influenza reporter genome in vivo. Virology 249:52-61. [DOI] [PubMed] [Google Scholar]
- 28.Perez, M., M. Watanabe, M. A. Whitt, and J. C. de la Torre. 2001. N-terminal domain of Borna disease virus G (p56) protein is sufficient for virus receptor recognition and cell entry. J. Virol. 75:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potash, M. J., and D. J. Volsky. 1998. Viral interference in HIV-1 infected cells. Rev. Med. Virol. 8:203-211. [DOI] [PubMed] [Google Scholar]
- 30.Pugachev, K. V., and T. K. Frey. 1998. Rubella virus induces apoptosis in culture cells. Virology 250:359-370. [DOI] [PubMed] [Google Scholar]
- 31.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber, F., E. F. Dunn, A. Bridgen, and R. M. Elliott. 2001. The Bunyamwera virus nonstructural protein NSs inhibits viral RNA synthesis in a minireplicon system. Virology 281:67-74. [DOI] [PubMed] [Google Scholar]
- 33.Welsh, R. M., and C. J. Pfau. 1972. Determinants of lymphocytic choriomeningitis interference. J. Gen. Virol. 14:177-187. [DOI] [PubMed] [Google Scholar]