Abstract
Dengue virus (DEN) causes dengue fever and dengue hemorrhagic fever/dengue shock syndrome, which are major public health problems worldwide. The immune factors that control DEN infection or contribute to severe disease are neither well understood nor easy to examine in humans. In this study, we used wild-type and congenic mice lacking various components of the immune system to study the immune mechanisms in the response to DEN infection. Our results demonstrate that alpha/beta interferon (IFN-α/β) and IFN-γ receptors have critical, nonoverlapping functions in resolving primary DEN infection. Furthermore, we show that IFN-α/β receptor-mediated action limits initial DEN replication in extraneural sites and controls subsequent viral spread into the central nervous system (CNS). In contrast, IFN-γ receptor-mediated responses seem to act at later stages of DEN disease by restricting viral replication in the periphery and eliminating virus from the CNS. Mice deficient in B, CD4+ T, or CD8+ T cells had no increased susceptibility to DEN; however, RAG mice (deficient in both B and T cells) were partially susceptible to DEN infection. In summary, (i) IFN-α/β is critical for early immune responses to DEN infection, (ii) IFN-γ-mediated immune responses are crucial for both early and late clearance of DEN infection in mice, and (iii) the IFN system plays a more important role than T- and B-cell-dependent immunity in resistance to primary DEN infection in mice.
Dengue virus (DEN) is a member of the Flavivirus genus in the Flaviviridae family of single-stranded, positive-polarity, enveloped RNA viruses. DEN causes dengue fever (DF) and dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), the most common mosquito-borne viral illnesses in humans (3, 5). An estimated 50 million new cases of DF and over 250,000 cases of DHF/DSS occur per year in the subtropical and tropical regions of the world (3). Typically, individuals with primary infection by any one of the four distinct DEN serotypes develop DF, an acute febrile illness with arthralgia, myalgia, and headache (13). In some cases, individuals with primary infection or secondary infection by a different serotype may develop the severe, life-threatening form of DF, called DHF/DSS, with increased vascular permeability, thrombocytopenia, focal or generalized hemorrhages, and shock in cases of DSS (15). A small subset of DHF/DSS patients also exhibit severe central nervous system (CNS) symptoms, such as reduced consciousness, convulsions, and encephalitis (4, 44, 50). Currently, no specific treatment for or vaccines against DEN exist, despite an increase in the geographic distribution of the DEN-transmitting Aedes aegypti and Aedes albopictus mosquitoes, the cocirculation of different DEN serotypes, and the increased frequency of DEN epidemics (14, 45). Thus, dengue is an emerging disease and a major public health concern.
At present, the mechanisms of DEN-induced disease and immunity are poorly defined, and the protective versus the pathogenic nature of the immune response to DEN infection is as yet unclear. Clinical, epidemiological, and laboratory studies suggest that the protective components of the immune system against DEN infection include interferons (IFNs) (7, 8, 31), antibodies (Abs) (10, 12, 29, 47), and T cells (30, 38), whereas the immunopathogenic mechanisms may involve subneutralizing concentrations of DEN-specific Abs (26), Abs cross-reactive to host antigens (19, 33, 34), and DEN serotype-cross-reactive Abs and T cells (15, 20, 46). IFNs may be involved in DEN pathogenesis as well, since pretreatment of U937 cells, a human monocytic cell line, leads to an increase in both the expression of Fc-γ receptors and DEN infection (27). These findings are based primarily on in vitro data, due to the lack of an adequate animal model for DEN infection and disease. Since in vitro findings may not reflect in vivo activity, animal model-based studies that identify the precise immune mechanisms against DEN infection in vivo are required.
As a first step to understanding the role of the immune system in DEN infection in vivo, we recently characterized a model for primary DEN infection in A/J mice by using a non-mouse-adapted strain of DEN serotype 2 (DEN2) (49). A subset (50%) of A/J mice that were intravenously inoculated with a high dose (108 PFU) of DEN2 developed paralysis, with infectious virus in both the brain and the spinal cord. We have now started to identify immune components that are required for protection following primary DEN infection in vivo by using a variety of loss-of-function mice that are deficient in immune cells and molecules. Previous work from our laboratory demonstrated that DEN infection of human cells in vitro was inhibited by pretreatment of cells with alpha/beta IFN (IFN-α/β) and IFN-γ (7, 8), and other investigators have shown that peripheral blood mononuclear cells from naïve humans produced both IFN-α/β and IFN-γ upon exposure to DEN-infected cells in culture (31). More recent studies with dendritic cells, which may be the initial cellular targets for DEN infection in vivo (41, 53, 55), have revealed that human peripheral blood mononuclear cell-derived dendritic cells produce several cytokines, including IFN-α, after infection by DEN (17, 32). Together, these results suggest a role for the two IFN systems in limiting DEN infection in vivo. Indeed, Johnson and Roehrig (21) have demonstrated that mice lacking receptors for both IFN-α/β and IFN-γ succumb to DEN infection, whereas all wild-type mice survive. Therefore, in this study, we have further defined the role of IFN-α/β and IFN-γ receptor-dependent immunity in controlling primary DEN infection in vivo by using mice of the 129/Sv/Ev strain that are deficient in the IFN-α/β receptor, the IFN-γ receptor, or both.
Additionally, immunologic analysis of the A/J mouse model revealed early activation of B cells in the spleen at day 3 postinfection (p.i.) and the presence of DEN-specific immunoglobulin M (IgM) at day 3 p.i. and IgG at day 7 p.i (49). The kinetics of isotype production in the A/J mouse model parallels that in human cases, and human Abs against DEN presumably provide protection from reinfection (20, 28). In a mouse model for DEN vaccine testing, immunization with DEN vaccine strains resulted in the production of neutralizing Ab titers and protection from lethal DEN challenge (21). In the same study (21), the adoptive transfer of anti-DEN Abs failed to protect mice from subsequent virus infection; however, other passive transfer experiments (16, 24, 25, 52) have shown that the administration of anti-DEN Abs can protect mice from lethal DEN challenge, indicating that DEN-specific Abs alone may prevent or control infection. Therefore, in this study, we have examined the role of B cells in protection against primary DEN infection in vivo by using μMT mice, which lack B cells, and RAG1−/− or RAG2−/− mice, which are devoid of both T and B lymphocytes.
Here, using two different DEN serotypes and two mouse strains, we have confirmed that the combined activities of IFN-α/β and IFN-γ receptors are essential for protection against primary DEN infection. Additionally, we demonstrate that the IFN-α/β receptor pathway limits early DEN viral load and that the IFN-γ receptor pathway may be more important than the IFN-α/β receptor pathway in resistance to DEN-induced disease. Furthermore, we show that B, CD4+, or CD8+ cells alone are not necessary for resolving primary DEN infection in mice; however, B and T cells in combination offer partial protection against DEN-induced disease. These results demonstrate that IFN-α/β and IFN-γ receptor-dependent immunity is more important than B- and T-cell-dependent adaptive immunity in controlling primary DEN infection in mice.
MATERIALS AND METHODS
Mice.
Mice were either obtained through scientific collaboration or purchased from the Jackson Laboratory (Bar Harbor, Maine) or Taconic (Germantown, N.Y.), and they were housed and bred under specific pathogen-free conditions at the University of California, Berkeley (UC Berkeley). All experiments were approved and conducted in accordance with the guidelines of the Office of Laboratory Animal Care at UC Berkeley. Mice were used at 5 to 6 weeks of age, and all experiments were performed in the biosafety level 2 animal facility at UC Berkeley. 129/Sv/Ev (WT129) mice and mice with null mutations in the IFN-α/β receptor (A129 mice), the IFN-γ receptor (G129 mice), or both the IFN-α/β and the IFN-γ receptors (AG129 mice) were obtained from M. Aguet (Swiss Institute for Experimental Cancer Research, Epalinges, Switzerland). C57BL/6J (B6) mice deficient in CD4 (CD4−/− mice), the CD8 α chain (CD8−/− mice), and the IgM heavy chain (μMT mice) were obtained from M. Diamond (Washington University School of Medicine, St. Louis, Mo.). B6 mice (Jackson 000664), RAG1-deficient B6 (RAGB6) mice (Jackson 002216), and RAG2-deficient 129/Sv/Ev (RAG129) mice (Taconic RAG2-M) were purchased.
Virus and mouse infection.
DEN2 (PL046 strain, non-mouse adapted, Taiwanese isolate) and DEN1 (Mochizuki strain, mouse adapted, Japanese isolate) were obtained from H.-Y. Lei (National Cheng Kung University, Taiwan) and R. Tesh (University of Texas Medical Branch, Galveston, Tex.), respectively. Both viral stocks were passaged in the Aedes albopictus C6/36 cell line for amplification, since mosquito cells are natural hosts and are highly susceptible to DEN infection, yielding high viral titers. Culture supernatants containing virus were harvested, frozen, and stored at −80°C, as previously described (6, 8). Viral titers were determined by a standard plaque assay using baby hamster kidney clone 21 (BHK-21) cells (6, 8). Although the C6/36 cell passage history of both viral strains prior to our acquisition of them was unknown, DEN2 stocks from our passage 2 and DEN 1 stocks from our passages 4 and 5 were used in all experiments in this study. For injection into mice, frozen stocks containing 5 × 106 to 5 × 107 PFU of DEN2/ml and 1 × 103 to 5 × 105 PFU of DEN1/ml were thawed, ultracentrifuged (43,000 × g for 2.5 h), and resuspended in endotoxin-free, ice-cold phosphate buffered saline (PBS). Our initial experiments had shown that A/J and AG129 mice were more susceptible to DEN infection via an intravenous route than by an intraperitoneal method of inoculation. Therefore, all mice in this study were intravenously injected with 100 to 250 μl of the inoculum via the tail vein. The exact viral doses and numbers of mice per group are indicated in the figures and the figure legends.
Quantitation of virus in infected mice.
Mice were euthanized via isofluorane inhalation, and blood was immediately collected by cardiac puncture to isolate serum. Tissues were harvested, weighed, and homogenized by using zirconia-silica beads (1.0-mm diameter) with a Mini-Beadbeater-8 (BioSpec Products, Bartlesville, Okla.) and microcentrifuged to pellet debris as previously described (49). Viral burden in the supernatants of tissue homogenates and serum samples was assessed by both direct and indirect plaque assays, as previously described (49). Direct plaque assays were performed using BHK cells, and results were measured as PFU per gram (tissue weight). In indirect plaque assays, C6/36 cells were inoculated, and standard plaque assays with BHK cells were performed with the resulting supernatants; thus, indirect plaque assay results were scored for the presence or absence of virus and were not quantitative. For analysis of virus in tissues of infected mice at early time points after infection, mice were cardiac perfused with ice-cold PBS (30 to 50 ml) prior to tissue dissection. At late time points p.i., a majority of mice with paralysis were not flushed with PBS before tissues were harvested, since no difference in viral titers was observed between mice with and without PBS perfusion.
Hematocrit measurement.
After cardiac puncture, blood was collected into an EDTA-coated Microtainer tube (Becton Dickinson Vacutainer Systems, Franklin Lakes, N.J.), drawn into a microhematocrit capillary tube (Fisher Scientific, Pittsburgh, Pa.), and spun in a microhematocrit centrifuge, as previously described (49). After centrifugation, the hematocrit was read on a microhematocrit capillary tube reader (Sherwood Medical, St. Louis, Mo.).
Statistical analyses.
The major clinical phenotype in susceptible mice with DEN infection was paralysis. As per the UC Berkeley guidelines, mice were sacrificed as soon as they developed paralysis. Paralysis was scored as death, since mice with severe paralysis sometimes died while preparations for harvesting tissue samples were in progress, indicating that paralysis does in fact lead to death. Kaplan-Meier analysis was performed to generate survival curves with Prism software (version 3.0cx; GraphPad Software, Inc., San Diego, Calif.). Statistical analyses of hematocrit and direct plaque assay results were performed with Microsoft Excel X (Microsoft Corporation, Seattle, Wash.), and values are shown as means ± standard deviations.
RESULTS
AG129 mice lacking both IFN-α/β and IFN-γ receptors are completely susceptible to DEN2-induced disease.
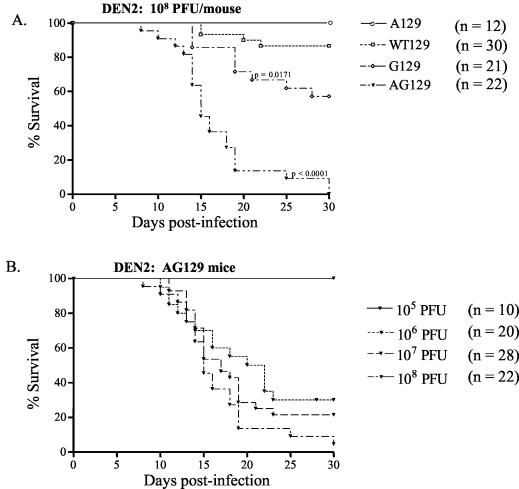
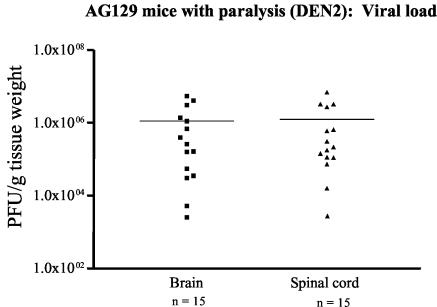
To evaluate the role of the IFN system during DEN infection in vivo, we compared the abilities of wild-type 129/Sv/Ev mice and congenic strains bearing null mutations in the IFN-α/β receptor and/or IFN-γ receptor genes to control primary DEN2 infection. At a high dose of a non-mouse-adapted strain of DEN2 (108 PFU), AG129 mice that were deficient in both IFN receptors began to develop limb paralysis more rapidly than WT129, A129, or G129 mice starting at day 8 p.i., and none survived past day 30 p.i. (Fig. 1A). A dose-response study revealed that 0, 70, 79, and 100% of AG129 mice manifested paralysis and died after infection with 105, 106, 107, and 108 PFU of DEN2, respectively (Fig. 1B), demonstrating the dose-dependent nature of DEN-induced disease. DEN2-infected AG129 mice with paralysis carried high viral loads in the brain and spinal cord, as determined by plaque assay (Fig. 2). In another assay for measuring DEN-induced illness, the hematocrit was measured, since hemoconcentration is observed in human DHF/DSS. The paralytic mice had significantly elevated hematocrits compared to those of mock-infected mice (the mean hematocrit ± the standard deviation was 54.7 ± 2.4 in DEN2-infected mice with paralysis [n = 16 mice] versus 46.5 ± 1.4 in mock-infected mice [n = 7 mice]; P < 0.0001). In contrast, 87% of WT129 mice, 100% of A129 mice, and 57% of G129 mice survived the DEN2 infection without exhibiting any clinical signs, such as fur ruffling, weight loss, hunchback posture, or paralysis (Fig. 1A). Log rank test results demonstrated significant differences in mortality rates between WT129 and AG129 mice (P < 0.0001) and between WT129 and G129 mice (P = 0.0171). Thus, the AG129 mice, which developed paralysis, elevated hematocrits, and infectious DEN in the central nervous system, were extremely vulnerable to infection, suggesting an essential role for the combined actions of IFN-α/β and IFN-γ receptors in controlling primary DEN2 infection in mice. Additionally, a significant number of G129, but not A129, mice succumbed to infection, suggesting a more important role for IFN-γ than for IFN-α/β in resistance against DEN-induced disease.
FIG. 1.
(A) Susceptibility of wild-type and IFN receptor-deficient mice to primary DEN2 infection. 129/Sv/Ev wild-type (WT129), IFN-α/β receptor−/− (A129), IFN-γ receptor−/− (G129), and IFN-α/β receptor−/− × IFN-γ receptor−/− (AG129) mice were inoculated via the tail vein with 108 PFU of DEN2 (PL046 strain). Mice were euthanized as soon as they developed paralysis, as per the guidelines of the Office of Laboratory Animal Care at UC Berkeley. Kaplan-Meier analysis was performed, and the log rank test yielded P values of <0.0001 for WT129 versus AG129 and 0.0171 for WT129 versus G129. The survival curves shown represent combined data from three to five separate experiments (n = number of mice per group). (B) Dose dependence of AG129 mice for DEN2 infection. Mice were intravenously injected with 105, 106, 107, or 108 PFU of DEN2 (PL046 strain), and their times of survival (see above) were recorded until day 30 p.i. Results from two to three different experiments were pooled (n = total number of mice per group) and plotted as Kaplan-Meier survival curves. All experiments shown were terminated at day 30 p.i.
FIG. 2.
Scatter plots of the titers of infectious DEN in the brains and spinal cords of paralytic AG129 mice. AG129 mice were infected with 108 PFU of DEN2 (PL046 strain), and the viral loads in the brain and spinal cord homogenates of 15 AG129 mice with paralysis were titrated by use of a direct plaque assay on BHK cells. Values are expressed as PFU per gram (tissue weight). Each symbol represents an individual mouse (▪, brain; ▴, spinal cord), the line indicates the mean, and n is the total number of mice. Paralytic mice from two independent experiments are represented, and the limit of detection of the assay is 10 PFU per gram (tissue weight).
A129 mice lacking IFN-α/β receptors are unable to control early viral burden.
To begin to understand how each IFN receptor pathway controls DEN infection, viral levels in various tissues of WT129, A129, G129, and AG129 mice were measured at early time points after infection with 108 PFU of DEN2. At both day 3 and day 7 p.i., infectious virus was detected by direct plaque assays in tissues from AG129 mice but not in tissues from WT129, A129, and G129 mice (Fig. 3 and data not shown). At day 3 p.i., AG129 mice contained virus in the spleen, lymph nodes, brain, and spinal cord, with higher viral titers in the spleen and lymph nodes than in the CNS. By day 7 p.i., AG129 mice no longer had detectable virus in the spleen and lymph nodes, whereas they harbored increased viral loads in the brain and spinal cord. These results reveal that DEN2 may replicate in both extraneural and neural sites early, at day 3 p.i., with higher levels in extraneural tissues than in neural tissues; however, by day 7 p.i, viral replication was detectable only in the CNS.
FIG. 3.
Levels of infectious DEN2 in tissues of AG129 mice at day 3 and day 7 p.i. AG129 mice were inoculated with 108 PFU of DEN2 (PL046 strain), and viral titers in the spleen, lymph nodes, brain, and spinal cord at days 3 and 7 after infection were quantitated by a direct plaque assay of BHK cells. Results are expressed as PFU per gram (tissue weight) and are shown as the averages of results from two to three experiments ± standard deviations (n = 7 mice for day 3 and n = 9 mice for day 7). The limit of sensitivity of the plaque assay is 10 PFU per gram (tissue weight).
Indirect plaque assays, in which virus was amplified in C6/36 cells before plaquing, detected infectious virus in the CNSs of A129 mice as well as AG129 mice at both day 3 and day 7 p.i. and in additional nonneural tissues at day 3 p.i. (Table 1). Specifically, at day 3 p.i., the sera, livers, spleens, lymph nodes, brains, and spinal cords of A129 and AG129 mice, but not those of WT129 and G129 mice, contained infectious virus. By day 7 p.i., DEN2 was detected only in the brains and spinal cords of these mice (Table 1). Thus, in both A129 and AG129 mice, the initial distribution of DEN2 included nonneural and neural tissues, followed by a more limited pattern of replication in the CNS. Moreover, the widespread distribution of infectious virus in the A129 mice, but not in the WT129 and G129 mice, at an early time point after infection indicates that the IFN-α/β receptor pathway plays a role in controlling initial viral replication and/or spread during primary DEN2 infection. In the absence of IFN-α/β receptor-mediated responses, the IFN-γ receptor pathway also contributes to limiting the early DEN load, since AG129 mice had higher levels of DEN than A129 mice at both day 3 and day 7 p.i.
TABLE 1.
Detection of infectious DEN2 in day 3 and day 7 p.i. tissues by indirect plaque assaysa
| Mouse strain | Assay results forb:
|
|||||
|---|---|---|---|---|---|---|
| Serum | Liver | Spleen | Lymph nodes | Brain | Spinal cord | |
| Day 3 p.i. | ||||||
| WT129 | − | − | − | − | − | − |
| A129 | + | + | + | + | +/− | +/− |
| G129 | − | − | − | − | − | − |
| AG129 | + | + | + | + | + | + |
| Day 7 p.i. | ||||||
| WT129 | − | − | − | − | − | − |
| A129 | − | − | − | − | +/− | +/− |
| G129 | − | − | − | − | − | − |
| AG129 | − | − | − | − | + | + |
Mice were intravenously inoculated with 108 PFU of DEN2 (PL046 strain). At day 3 p.i., tissues were harvested and processed for the indirect plaque assay. Each group consisted of three to five mice per time point. This experiment was performed three times, and similar results were obtained in all experiments.
+, virus was detectable; −, virus was undetectable; +/−, virus was detected in only some mice or some experiments.
AG129 mice with primary DEN1 infection confirm the essential role of IFN-α/β and IFN-γ receptors in defense against DEN.
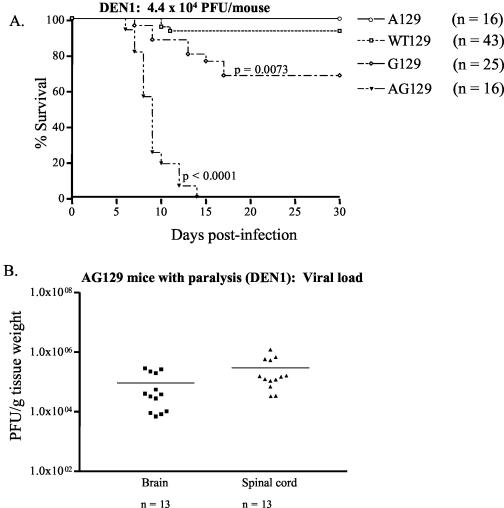
We next verified the critical role of the IFN system in resolving primary DEN infection in mice by using a different DEN serotype. After infection with 4.4 × 104 PFU of a mouse-adapted strain of DEN1, 100% of AG129 mice developed paralysis within 7 to 14 days p.i. (Fig. 4A) (P < 0.0001) and carried high viral loads in the brain and spinal cord at the time of death (Fig. 4B). Similar to the results obtained with DEN2, 93% of WT129 mice, 100% of A129 mice, and 70% of G129 mice survived the DEN1 infection, and the difference in mortality rates between the WT129 and G129 mice was significant (P = 0.0162). These results show that all four strains of mice infected with DEN1 exhibited susceptibilities to infection similar to those of mice that were infected with DEN2. Moreover, log rank test results demonstrated a significant difference (P < 0.0001) between survival curves for DEN1-infected AG129 mice (median survival day, day 9) and DEN2-infected AG129 mice (median survival day, day 15), indicating that the mouse-adapted DEN1 strain used in this study, even at a lower dose, was more virulent than the non-mouse-adapted DEN2 strain. Collectively, these data confirm that the IFN-α/β and IFN-γ receptors in combination are essential for resolving primary DEN infection in mice. In addition, the increased susceptibilities of G129 mice to both DEN1 and DEN2 infection compared to those of the WT129 and A129 mice implicates a more important role for the IFN-γ receptor pathway than for IFN-α/β receptor action in providing protection against DEN-induced disease.
FIG. 4.
Susceptibility of mice deficient in IFN receptors to infection with DEN1. Groups of wild-type (WT129), IFN-α/β receptor−/− (A129), IFN-γ receptor−/− (G129), and IFN-α/β receptor−/− × IFN-γ receptor−/− (AG129) 129/Sv/Ev mice were infected intravenously with 4.4 × 104 PFU of DEN1 (Mochizuki strain). Mice were monitored daily for the development of paralysis until day 30 p.i. and were euthanized as soon as they exhibited paralysis. (A) Kaplan-Meier survival curves. Data from three to five separate experiments were pooled. A log rank test revealed significant differences between WT129 and AG129 mice (P < 0.0001) and between WT129 and G129 mice (P = 0.0073). (B) Levels of infectious virus in AG129 mice with paralysis. Brains and spinal cords were harvested from mice that were euthanized due to the onset of paralysis. Samples were homogenized and subjected to a direct plaque assay. Each symbol represents a mouse, and results for two different infections are shown. ▪, brain; ▴, spinal cord, —, mean; n, total number of mice.
μMT mice lacking B lymphocytes clear primary DEN infection.
Since Abs and B lymphocytes may protect against DEN infection in humans (20), we next examined the role of B cells in limiting primary DEN infection by using B-cell-deficient μMT mice in the C57BL/6J background. After infection with 4.4 × 104 PFU of DEN1, 79% of μMT mice and 78% of WTB6 animals survived (Table 2), indicating a normal ability of μMT mice to resist DEN1-induced disease. At day 3 p.i., infectious virus was undetectable in both extraneural and neural tissues of μMT mice, as determined by an indirect plaque assay (data not shown), demonstrating that μMT mice can mediate early DEN clearance. Likewise, 75% of CD4+ T-cell-deficient (CD4−/−) mice and 86% of CD8+ T-cell-deficient (CD8−/−) mice survived DEN1 infection (Table 2), and virus was not recovered from tissues of infected mice at day 3 p.i. (data not shown), revealing little defect in the ability of these mutant mouse strains to clear primary DEN1 infection. Similarly, μMT, CD4−/−, and CD8−/− mice did not exhibit increased susceptibility to DEN2 infection at 108 PFU (Table 2), indicating that B cells, CD4+ T cells, and CD8+ T cells by themselves do not appear to play a significant role in limiting primary DEN infection in mice.
TABLE 2.
Survival data for wild-type and immunodeficient C57BL/6J mice after primary DEN infectiona
| Mouse strain | No. of mice | No. of survivors | % Survival |
|---|---|---|---|
| DEN1 | |||
| WTB6 | 46 | 38 | 83 |
| CD4−/− | 12 | 9 | 75 |
| CD8−/− | 36 | 31 | 86 |
| μmMT | 28 | 22 | 79 |
| RAGB6 | 33 | 16 | 48b |
| DEN2 | |||
| WTB6 | 16 | 16 | 100 |
| CD4−/− | 6 | 6 | 100 |
| CD8−/− | 8 | 8 | 100 |
| μMT | 9 | 9 | 100 |
| RAGB6 | 16 | 11 | 69c |
Mice were intravenously inoculated with 4.4 × 104 PFU of DEN1 (Mochizuki) or 108 PFU of DEN2 (PL046). Mice were observed daily for the development of paralysis, and mice that exhibited paralysis were euthanized and scored as dead. Experiments were terminated on day 30 p.i., and data from two to five separate experiments were pooled.
P = 0.0039 for RAGB6 mice versus WTB6 mice.
P = 0.0895 for RAGB6 mice versus WTB6 mice.
RAGB6 and RAG129 mice, which lack both T and B lymphocytes, have increased susceptibilities to primary infection with a mouse-adapted DEN strain.
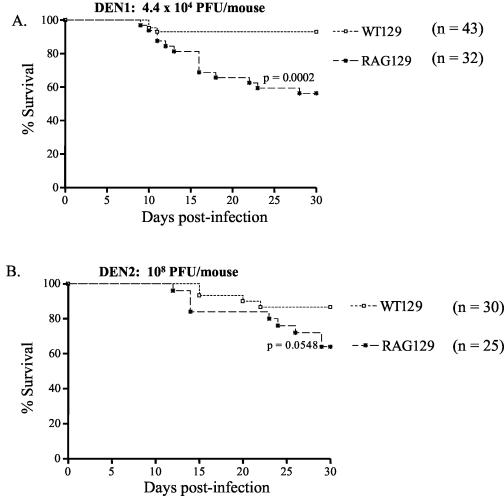
After examining the contribution of each individual component of adaptive immunity, we next defined the role of the entire adaptive immune response in controlling primary DEN infection by using RAG knockout mice, which are deficient in both T and B cells. A significant percentage of RAG mice in the 129/Sv/Ev genetic background (RAG129 mice) succumbed to infection with 4.4 × 104 PFU of DEN1 compared to the percentage of WT129 control mice that did (mortality rate of 44% for RAG129 mice versus 7% for WT129 mice; P < 0.0002) (Fig. 5A). However, the percentage of RAG129 mice that developed paralysis when infected with 108 PFU of the non-mouse-adapted DEN2 strain was not significant compared to the percentage of the WT129 mice that did (mortality rate of 28% for RAG129 mice versus 13% for WT129 mice; P = 0.0548) (Fig. 5B). These results indicate that some RAG129 mice are unable to resolve primary infection with a mouse-adapted DEN1 strain, despite their nearly normal ability to contain the challenge with a non-mouse-adapted DEN2 strain. Indirect plaque assays did not detect infectious DEN1 or DEN2 in tissues of RAG129 mice at day 3 p.i. (data not shown), implying that RAG129 mice, like WT129 and G129 mice, are able to limit early viral titers.
FIG. 5.
Susceptibility of wild-type and T- and B-cell-deficient (RAG2−/−) mice in the 129/Sv/Ev background to primary DEN infection. Wild-type (WT129) and RAG2−/− (RAG129) mice were intravenously inoculated with 4.4 × 104 PFU of DEN1 (Mochizuki strain) (A) or 108 PFU of DEN2 (PL046 strain) (B). Mice were observed for paralysis on a daily basis until day 30 p.i. and were euthanized immediately after the onset of paralysis (see above). Data were combined from four or five independent experiments (n = total number of mice per group) and graphed as Kaplan-Meier survival curves. The log rank test revealed a significant difference between WT129 and RAG129 mice after DEN1 infection (P = 0.0002) (A), whereas no significant difference was observed between WT129 and RAG129 mice after DEN2 infection (P = 0.0548) (B).
To confirm these findings, infection experiments were repeated with T- and B-cell-deficient RAGB6 mice of the C57BL/6J strain (Table 2). Similar to results obtained with RAG129 mice, the mortality rate for RAGB6 mice after infection with 4.4 × 104 PFU of DEN1 was significantly higher than that for WTB6 mice (52% mortality for RAGB6 mice versus 17% mortality for WTB6 mice; P < 0.0039) (Table 2). Again, as observed in experiments with RAG129 mice, at 108 PFU of the non-mouse-adapted DEN2 strain, the mortality percentage for RAGB6 mice was not significant compared to that for WTB6 control mice (31% mortality for RAGB6 mice versus 0% mortality for WTB6 mice; P = 0.0895) (Table 2). Additionally, infectious DEN1 and DEN2 were not detected in either extraneural or neural tissues of RAGB6 mice at day 3 p.i., suggesting that the viral load was below the limit of our indirect plaque assay and/or present in tissues that were not examined. Regardless, the survival data demonstrate that both RAGB6 and RAG129 mice have decreased resistances to disease induced by a mouse-adapted DEN strain but not to disease induced by a non-mouse-adapted DEN strain. Therefore, the T- and B-cell-dependent adaptive immunity as a whole provides some protection from DEN-induced disease but is not absolutely required for controlling primary DEN infection in mice.
DISCUSSION
In this report, we identified immune mechanisms that protect against primary DEN infection in mice by comparing infection in wild-type mice to that in congenic mice that are deficient in various components of the immune system. Specifically, the IFN-α/β and IFN-γ receptor-dependent immune responses together are essential for resistance to DEN-induced disease in mice. Furthermore, IFN-α/β receptor-mediated action is crucial for limiting initial DEN replication in extraneural sites and for controlling subsequent viral spread into the CNS, but it is not required for protection from disease. In contrast, the IFN-γ receptor pathway provides partial resistance against DEN-induced disease and appears to be less important in controlling the early viral load. However, in the absence of a functional IFN-α/β receptor pathway, IFN-γ receptor responses can mediate early viral clearance as well, as evidenced by a higher viral load in mice lacking both receptors. Thus, we have demonstrated for the first time significant contributions by each of the two IFN receptor pathways in controlling primary DEN infection. In comparison, B-, CD4+ T-, or CD8+ T-cell-mediated functions by themselves are not required to limit DEN infection, although the combined activities of T and B cells appear to contribute to the control of infection by a mouse-adapted DEN strain but not to the control of infection by a non-mouse-adapted DEN strain.
Infection in AG129 mice.
AG129 mice lacking both IFN-α/β and IFN-γ receptors were extremely susceptible to primary DEN infection. They developed rapid-onset paralysis and had elevated hematocrits, as observed in a recent study with DEN2-infected A/J mice (49) and with the HepG2-SCID mouse chimera model for DEN infection (1). In our studies involving both AG129 and A/J mice, the hematocrit data alone represent another marker for DEN-induced illness, not necessarily for plasma leakage, since an elevated hematocrit may be a result of dehydration in the paralytic mice with a limited ability to move. Thus, additional studies are necessary to determine the presence of plasma leakage in DEN-infected mice. As expected, AG129 mice with primary DEN infection carried high viral burdens in the brain and spinal cord by 7 to 30 days p.i. At day 3 p.i., infectious virus was detected in both neural and nonneural tissues, including the serum, liver, spleen, lymph nodes, and CNS. These results concur with a published report in which AG129 mice that were infected intraperitoneally with a mouse-adapted DEN2 strain (New Guinea C) carried high viral loads in their sera and spleens in the early days after infection, followed by increasingly higher viral titers in the brain in later days (21). Both studies demonstrate an absolute requirement for the combined activities of IFN-α/β and IFN-γ receptors in the control of primary DEN infection, similar to reports of increased susceptibility of AG129 mice to other RNA viruses. For example, studies with the arenavirus lymphocytic choriomeningitis virus (54) and a calicivirus, murine norovirus 1 (23), demonstrated that AG129 mice had a more severe disease phenotype than A129 or G129 mice.
Infection in A129 mice and G129 mice.
In general, IFN-α/β, produced within hours by virus-infected and uninfected cells, limits early viral replication and spread by activating the transcription of IFN-inducible genes, such as protein kinase R and 2′-5′ oligoadenylate synthetase (OAS). In our study, IFN-α/β receptor-deficient A129 mice did not present clinical symptoms when infected with DEN, although virologic analysis revealed that A129 mice harbored infectious virus in multiple tissues, including the serum, the liver, the spleen, the lymph nodes, and the CNS, at day 3 p.i. This phenotype indicates a role for the IFN-α/β receptor pathway in regulating early viral replication and spread. Additional experiments have revealed that some of the A129 mice develop paralysis after DEN1 infection, indicating that A129 and WT129 mice have a similar susceptibility to primary DEN1 infection.
In contrast to IFN-α/β, IFN-γ is secreted later in the course of infection by NK and T cells, modulates the immune response, and mediates antiviral activities primarily through the induction of nitric oxide synthase in macrophages (40). Unlike IFN-α/β receptor-deficient A129 mice, G129 mice lacking the IFN-γ receptor had undetectable amounts of virus in tissues at both day 3 and day 7 p.i., and a significant proportion developed paralysis, thereby suggesting a role for the IFN-γ receptor pathway in resistance to disease. This decreased survival of G129 mice is not consistent with a previously published report in which IFN-γ receptor-deficient BALB/c mice were found to be no more susceptible than wild-type controls to DEN infection (21). Genotypic differences in the DEN2 strains used (New Guinea C versus PL046) as well as the difference in routes of infection (intraperitoneal versus intravenous) may have affected the outcome of infection in mice lacking IFN-γ receptors. Additionally, differences in the genetic backgrounds of the 129/Sv/Ev and the BALB/c strains may account for this discrepancy. In particular, the levels of expression of IFN-responsive gene products, including OAS, that are important in flavivirus infection may differ among inbred mouse strains. For example, a mutation in the OAS1b gene has recently been discovered to be associated with West Nile virus susceptibility in the mouse model (37, 42).
The distinct disease phenotypes of DEN-infected A129 and G129 mice imply that the IFN-α/β and IFN-γ receptor pathways act synergistically in response to DEN infection. In the absence of IFN-α/β receptor-mediated activities, the IFN-γ receptor pathway limits early viral burden, since higher levels of infectious virus were present in AG129 mouse tissues than in A129 mouse tissues at both day 3 and day 7 p.i. Similarly, the higher mortality rate for AG129 mice than for G129 mice implies that the IFN-α/β receptor pathway can prevent DEN-induced disease under conditions in which IFN-γ receptor-mediated responses are lacking.
Taken together, our results demonstrate both synergistic and nonredundant activities of the two IFN receptor pathways. Previous work has shown that resistance to murine infection by the picornavirus Theiler's murine encephalomyelitis virus also involves nonredundant functions of the two IFN receptor pathways (11). In the immune defense against Murray Valley encephalitis virus, a flavivirus that is related to DEN, IFN-α/β receptor-mediated responses play a more dominant role than IFN-γ-mediated mechanisms (36). Similarly, IFN-α/β receptor-mediated activity, but not IFN-γ receptor-mediated activity, limits disease resulting from other RNA viruses, including the rhabdovirus vesicular stomatitis virus and the togavirus Semliki Forest virus (39). Thus, in contrast to these other RNA virus infections, resistance to primary DEN infection in mice involves complementary roles for the two IFN receptor pathways.
Infection in μMT, CD4−/−, and CD8−/− mice.
Our results with μMT mice lacking B cells indicate that an Ab response to DEN is not required to resolve primary infection in mice. These results contrast with a recent finding that Abs are critical for defense against the flavivirus West Nile virus in mice (9), highlighting the control of closely related flavivirus infections by different immune mechanisms. Our previous results for A/J mice revealed early B-cell activation and production of DEN-specific antibodies at day 3 p.i (49). However, this finding may simply indicate general immune activation without direct involvement in the resolution of the infection. Although several reports have shown the production of neutralizing Abs in response to DEN infection in humans, a crucial role of these Abs in the control of primary DEN infection in people has yet to be proven. Similar to humans, DEN-infected mice produce neutralizing antibodies by day 10 p.i. (our unpublished observations), but these antibodies may protect against reinfection and may not necessarily prevent early viral dissemination in primary infection.
The CD4−/− and CD8−/− mice were resistant to DEN-induced disease and had a normal ability to limit early viral infection, suggesting that these cells are not required for resolving primary DEN infection. This finding agrees with previous work using the Mochizuki strain of DEN1 to infect T-cell-deficient nude (nu/nu) mice, in which no significant difference was observed in the mortality rates between nu/nu mice and nu/+ mice (18). Our results with CD4−/− or CD8−/− mice are not surprising given the existence of redundant and compensatory mechanisms in vivo. Although DEN-specific CD8+ CTL clones have previously been generated from murine splenocytes and tested for antigen cross-reactivity (51), CD8+ CTLs do not appear to be essential in our mouse model of primary DEN infection. In CD8−/− mice, virus may be eliminated by CD4+ or NK cell-dependent functions in the absence of a vigorous CD8+ cytotoxic T-lymphocyte (CTL) response. The phenotype of CD4−/− mice is more surprising, since (i) our present results demonstrate that IFN-γ is required for resolving DEN infection and (ii) we have previously shown that CD4+ T cells produce IFN-γ at day 14 p.i. in A/J mice (49). However, CD4−/− mice are likely to be compensated by other IFN-γ-producing cells, including NK (2), NKT (2), γδ T (48), and CD8+ T (43) cells.
Infection in RAG129 and RAGB6 mice.
DEN1 and DEN2 infection of both RAG129 and RAGB6 mice resulted in increased mortality compared to that for wild-type mice, yet this increased mortality was statistically significant only with the mouse-adapted DEN1 infection. Published studies using human cell-engrafted scid mice (35, 56) have also shown that innate immunity was sufficient to clear DEN infection. In our studies, the mouse-adapted DEN1 Mochizuki strain was more lethal than the non-mouse-adapted DEN2 PL046 strain, requiring 4.4 × 104 PFU of DEN1 and 108 PFU of DEN2 to induce disease in 100% of AG129 mice and some RAG129 mice. In comparison, 79% of AG129 mice infected with 107 PFU of DEN2 develop paralysis, compared to 0% of RAG129 mice (data not shown). Therefore, depending on the viral dose and virulence, T and B cells together may play an active role in fighting DEN infection but may not be absolutely required for the elimination of virus. Since the survival curve for DEN-infected RAG mice is similar to that for G129 mice, the T- and B-cell-dependent immune responses may contribute to DEN clearance via IFN-γ.
In summary, IFN-α/β and IFN-γ receptors have nonoverlapping yet critical functions in resolving primary infection with DEN. In the presence of a functional IFN system, CD4+, CD8+, or B cells alone appear to be unnecessary for controlling primary DEN infection in mice. However, the collective activities of T and B cells together are important but not absolutely required for protection against DEN infection. The finding of a lack of a severe defect in the ability of RAG mice to resist DEN infection is similar to a recent finding that AG129 mice, but not RAG mice, succumb to acute murine norovirus 1 infection (23), confirming that the IFN system plays a more dominant role than T- and B-cell-dependent immunity in controlling certain viral infections. Since T cells are one of the major sources of IFN-γ production during viral infection in vivo (22), the partial susceptibility of RAG mice to DEN-induced disease may be due to an insufficient level of IFN-γ produced solely by NK cells. The decreased IFN-γ level may allow the virus to enter the CNS and replicate there with greater efficiency, leading to paralysis. Further experiments are now necessary to determine the precise mechanisms by which the IFN system mediates the antiviral response in mice with primary DEN infection.
Acknowledgments
We thank Huan-Yao Lei for providing DEN2 (PL046 strain), Robert Tesh for providing DEN1 (Mochizuki strain), and Michael Diamond and Joel Ernst for helpful discussions. We are grateful to Marija Helt for critical reading of the manuscript.
This work was supported by the NIH (grant NRSA 1F32 AI51070-01 [S.S.]), the Ellison Medical Foundation (grant EMF 1D-1A-0031 [E.H.]), and the UC Berkeley Committee on Research (E.H.).
REFERENCES
- 1.An, J., J. Kimura-Kuroda, Y. Hirabayashi, and K. Yasui. 1999. Development of a novel mouse model for dengue virus infection. Virology 263:70-77. [DOI] [PubMed] [Google Scholar]
- 2.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 3.Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1126. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 4.Cam, B. V., L. Fonsmark, N. B. Hue, N. T. Phuong, A. Poulsen, and E. D. Heegaard. 2001. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 65:848-851. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44:649-688. [DOI] [PubMed] [Google Scholar]
- 6.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, M. S., and E. Harris. 2001. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 289:297-311. [DOI] [PubMed] [Google Scholar]
- 8.Diamond, M. S., T. G. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falgout, B., M. Bray, J. J. Schlesinger, and C.-J. Lai. 1990. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J. Virol. 64:4356-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiette, L., C. Aubert, U. Muller, S. Huang, M. Aguet, M. Brahic, and J. F. Bureau. 1995. Theiler's virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J. Exp. Med. 181:2069-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry, M. K., E. A. Henchal, J. M. McCown, W. E. Brandt, and J. M. Dalrymple. 1982. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 31:548-555. [DOI] [PubMed] [Google Scholar]
- 13.George, R., and L. C. S. Lum. 1997. Clinical spectrum of dengue infection, p. 89-113. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 14.Gibbons, R. V., and D. W. Vaughn. 2002. Dengue: an escalating problem. BMJ 324:1563-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 16.Henchal, E. A., L. S. Henchal, and J. J. Schlesinger. 1988. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J. Gen. Virol. 69:2101-2107. [DOI] [PubMed] [Google Scholar]
- 17.Ho, L. J., J. J. Wang, M. F. Shaio, C. L. Kao, D. M. Chang, S. W. Han, and J. H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 166:1499-1506. [DOI] [PubMed] [Google Scholar]
- 18.Hotta, H., I. Murakami, K. Miyasaki, Y. Takeda, H. Shirane, and S. Hotta. 1981. Inoculation of dengue virus into nude mice. J. Gen. Virol. 52:71-76. [DOI] [PubMed] [Google Scholar]
- 19.Huang, Y. H., B. I. Chang, H. Y. Lei, H. S. Liu, C. C. Liu, H. L. Wu, and T. M. Yeh. 1997. Antibodies against dengue virus E protein peptide bind to human plasminogen and inhibit plasmin activity. Clin. Exp. Immunol. 110:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innis, B. L. 1997. Antibody responses to dengue virus infection, p. 221-243. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 21.Johnson, A. J., and J. T. Roehrig. 1999. New mouse model for dengue virus vaccine testing. J. Virol. 73:783-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kambayashi, T., E. Assarsson, A. E. Lukacher, H. G. Ljunggren, and P. E. Jensen. 2003. Memory CD8+ T cells provide an early source of IFN-gamma. J. Immunol. 170:2399-2408. [DOI] [PubMed] [Google Scholar]
- 23.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman, B. M., P. L. Summers, D. R. Dubois, W. H. Cohen, M. K. Gentry, R. L. Timchak, D. S. Burke, and K. H. Eckels. 1989. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 41:576-580. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman, B. M., P. L. Summers, D. R. Dubois, and K. H. Eckels. 1987. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 36:427-434. [DOI] [PubMed] [Google Scholar]
- 26.Kliks, S. C., S. Nimmanitya, A. Nisalak, and D. S. Burke. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 38:411-419. [DOI] [PubMed] [Google Scholar]
- 27.Kontny, U., I. Kurane, and F. A. Ennis. 1988. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J. Virol. 62:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koraka, P., C. Suharti, T. E. Setiati, A. T. Mairuhu, E. Van Gorp, C. E. Hack, M. Juffrie, J. Sutaryo, G. M. Van Der Meer, J. Groen, and A. D. Osterhaus. 2001. Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J. Clin. Microbiol. 39:4332-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurane, I., D. Hebblewaite, and F. A. Ennis. 1986. Characterization with monoclonal antibodies of human lymphocytes active in natural killing and antibody-dependent cell-mediated cytotoxicity of dengue virus-infected cells. Immunology 58:429-436. [PMC free article] [PubMed] [Google Scholar]
- 30.Kurane, I., B. L. Innis, A. Nisalak, C. Hoke, S. Nimmannitya, A. Meager, and F. A. Ennis. 1989. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J. Clin. Investig. 83:506-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurane, I., A. Meager, and F. A. Ennis. 1986. Induction of interferon alpha and gamma from human lymphocytes by dengue virus-infected cells. J. Gen. Virol. 67:1653-1661. [DOI] [PubMed] [Google Scholar]
- 32.Libraty, D. H., S. Pichyangkul, C. Ajariyakhajorn, T. P. Endy, and F. A. Ennis. 2001. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J. Virol. 75:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, C. F., H. Y. Lei, C. C. Liu, H. S. Liu, T. M. Yeh, S. T. Wang, T. I. Yang, F. C. Sheu, C. F. Kuo, and Y. S. Lin. 2001. Generation of IgM anti-platelet autoantibody in dengue patients. J. Med. Virol. 63:143-149. [PubMed] [Google Scholar]
- 34.Lin, C. F., H. Y. Lei, A. L. Shiau, C. C. Liu, H. S. Liu, T. M. Yeh, S. H. Chen, and Y. S. Lin. 2003. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J. Med. Virol. 69:82-90. [DOI] [PubMed] [Google Scholar]
- 35.Lin, Y. L., C. L. Liao, L. K. Chen, C. T. Yeh, C. I. Liu, S. H. Ma, Y. Y. Huang, Y. L. Huang, C. L. Kao, and C. C. King. 1998. Study of dengue virus infection in SCID mice engrafted with human K562 cells. J. Virol. 72:9729-9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobigs, M., A. Mullbacher, Y. Wang, M. Pavy, and E. Lee. 2003. Role of type I and type II interferon responses in recovery from infection with an encephalitic flavivirus. J. Gen. Virol. 84:567-572. [DOI] [PubMed] [Google Scholar]
- 37.Mashimo, T., M. Lucas, D. Simon-Chazottes, M. P. Frenkiel, X. Montagutelli, P. E. Ceccaldi, V. Deubel, J. L. Guenet, and P. Despres. 2002. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl. Acad. Sci. USA 99:11311-11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathew, A., I. Kurane, A. L. Rothman, L. L. Zeng, M. A. Brinton, and F. A. Ennis. 1996. Dominant recognition by human CD8+ cytotoxic T lymphocytes of dengue virus nonstructural proteins NS3 and NS1.2a. J. Clin. Invest. 98:1684-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 40.Nathan, C. F., and J. B. Hibbs, Jr. 1991. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 3:65-70. [DOI] [PubMed] [Google Scholar]
- 41.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perelygin, A. A., S. V. Scherbik, I. B. Zhulin, B. M. Stockman, Y. Li, and M. A. Brinton. 2002. Positional cloning of the murine flavivirus resistance gene. Proc. Natl. Acad. Sci. USA 99:9322-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pien, G. C., K. B. Nguyen, L. Malmgaard, A. R. Satoskar, and C. A. Biron. 2002. A unique mechanism for innate cytokine promotion of T cell responses to viral infections. J. Immunol. 169:5827-5837. [DOI] [PubMed] [Google Scholar]
- 44.Ramos, C., G. Sanchez, R. H. Pando, J. Baquera, D. Hernandez, J. Mota, J. Ramos, A. Flores, and E. Llausas. 1998. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J. Neurovirol. 4:465-468. [DOI] [PubMed] [Google Scholar]
- 45.Rigau-Perez, J. G., G. G. Clark, D. J. Gubler, P. Reiter, E. J. Sanders, and A. V. Vorndam. 1998. Dengue and dengue haemorrhagic fever. Lancet 352:971-977. [DOI] [PubMed] [Google Scholar]
- 46.Rothman, A. L., and F. A. Ennis. 1999. Immunopathogenesis of dengue hemorrhagic fever. Virology 257:1-6. [DOI] [PubMed] [Google Scholar]
- 47.Russell, P. K., S. Udomsakdi, and S. B. Halstead. 1967. Antibody response in dengue and dengue hemorrhagic fever. Jpn. J. Med. Sci. Biol. 20:103-108. [PubMed] [Google Scholar]
- 48.Selin, L. K., P. A. Santolucito, A. K. Pinto, E. Szomolanyi-Tsuda, and R. M. Welsh. 2001. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J. Immunol. 166:6784-6794. [DOI] [PubMed] [Google Scholar]
- 49.Shresta, S., J. L. Kyle, P. R. Beatty, and E. Harris. 2004. Early activation of natural killer and B cells in response to dengue virus infection in A/J mice. Virology 319:262-273. [DOI] [PubMed]
- 50.Solomon, T., N. M. Dung, D. W. Vaughn, R. Kneen, L. T. Thao, B. Raengsakulrach, H. T. Loan, N. P. Day, J. Farrar, K. S. Myint, M. J. Warrell, W. S. James, A. Nisalak, and N. J. White. 2000. Neurological manifestations of dengue infection. Lancet 355:1053-1059. [DOI] [PubMed] [Google Scholar]
- 51.Spaulding, A. C., I. Kurane, F. A. Ennis, and A. L. Rothman. 1999. Analysis of murine CD8(+) T-cell clones specific for the Dengue virus NS3 protein: flavivirus cross-reactivity and influence of infecting serotype. J. Virol. 73:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan, C. H., E. H. Yap, M. Singh, V. Deubel, and Y. C. Chan. 1990. Passive protection studies in mice with monoclonal antibodies directed against the non-structural protein NS3 of dengue 1 virus. J. Gen. Virol. 71:745-749. [DOI] [PubMed] [Google Scholar]
- 53.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]
- 56.Wu, S. J., C. G. Hayes, D. R. Dubois, M. G. Windheuser, Y. H. Kang, D. M. Watts, and D. G. Sieckmann. 1995. Evaluation of the severe combined immunodeficient (SCID) mouse as an animal model for dengue viral infection. Am. J. Trop. Med. Hyg. 52:468-476. [DOI] [PubMed] [Google Scholar]