Abstract
The high prevalence of preexisting immunity to adenovirus serotype 5 (Ad5) in human populations will likely limit the immunogenicity and clinical utility of recombinant Ad5 vector-based vaccines for human immunodeficiency virus type 1 and other pathogens. Ad5-specific neutralizing antibodies (NAbs) are thought to contribute substantially to anti-Ad5 immunity, but the potential importance of Ad5-specific T lymphocytes in this setting has not been fully characterized. Here we assess the relative contributions of Ad5-specific humoral and cellular immune responses in blunting the immunogenicity of a rAd5-Env vaccine in mice. Adoptive transfer of Ad5-specific NAbs resulted in a dramatic abrogation of Env-specific immune responses following immunization with rAd5-Env. Interestingly, adoptive transfer of Ad5-specific CD8+ T lymphocytes also resulted in a significant and durable suppression of rAd5-Env immunogenicity. These data demonstrate that NAbs and CD8+ T lymphocytes both contribute to immunity to Ad5. Novel adenovirus vectors that are currently being developed to circumvent the problem of preexisting anti-Ad5 immunity should therefore be designed to evade both humoral and cellular Ad5-specific immune responses.
Replication-defective recombinant adenovirus serotype 5 (rAd5) vector-based vaccines elicit potent and protective immune responses in a variety of animal models (4, 19, 22, 23). Clinical trials of rAd5 vaccines for human immunodeficiency virus type 1 (HIV-1) and other pathogens are therefore currently in progress (14). A major limitation of this approach, however, is that the majority of the human population has preexisting anti-Ad5 immunity that may substantially reduce the immunogenicity and clinical utility of rAd5 vaccines. In fact, anti-Ad5 immunity has already been demonstrated to suppress the immunogenicity of rAd5 vaccines in studies in mice (3, 31), rhesus monkeys (4), and humans in early phase I clinical trials.
It is generally accepted that potent Ad5-specific neutralizing antibodies (NAbs) contribute substantially to the suppressive effects of anti-Ad5 immunity (7, 8, 12, 25, 27, 32). Far less is known about the biological importance of Ad5-specific T-lymphocyte responses. Ad5-specific CD4+ and CD8+ T lymphocytes have been found in both humans (9, 17, 18, 20) and mice (11, 13, 21, 29, 30). However, the importance of Ad5-specific T lymphocytes in suppressing the immunogenicity of rAd5 vaccines has not been fully characterized.
In this study, we investigate the relative contributions of Ad5-specific humoral and cellular immune responses in suppressing the immunogenicity of a rAd5-Env vaccine in mice. By adoptive-transfer studies, we demonstrate that NAbs and CD8+ T lymphocytes both contribute to immunity to Ad5. These data suggest that novel Ad vector-based vaccines should overcome both humoral and cellular immune responses to circumvent preexisting anti-Ad5 immunity.
MATERIALS AND METHODS
Mice and immunizations.
BALB/c mice, 6 to 8 weeks old, were purchased from Charles River Laboratories (Wilmington, Mass.). For rAd5 immunizations, mice were injected intramuscularly with various doses of E1-deleted, replication-incompetent rAd5-HIV-1 Env IIIB gp140ΔCFI (3) in 100 μl of sterile phosphate-buffered saline (PBS). To induce anti-Ad5 immunity, mice were preimmunized intramuscularly, either once or twice (4 weeks apart), with 1010 virus particles (vp) of rAd5-Empty, containing no insert, in 100 μl of sterile PBS.
Adoptive transfers.
To study the inhibitory effects of Ad5-specific humoral and cellular immunity, 5 × 107 splenocytes or 500 μl of serum pooled from donor mice with or without anti-Ad5 immunity were adoptively transferred to naive recipient mice by tail vein injection. On the day following adoptive transfer, recipient mice were immunized with 108 vp of rAd5-Env. Purified immunoglobulin G (IgG) was prepared from serum with a protein A antibody purification kit (Sigma, St. Louis, Mo.) and dialyzed into endotoxin-free Dulbecco's PBS (Life Technologies, Gaithersberg, Md.) before being used in adoptive-transfer studies. Serum and purified IgG from mice with anti-Ad5 immunity exhibited comparable Ad5-specific NAb titers. Purified CD8+ T lymphocytes were prepared by negative selection with a murine CD8+ T-cell isolation kit (Miltenyi Biotec, Auburn, Calif.) and were >95% pure as assessed by flow cytometry. Splenocytes and fractionated cell populations were resuspended at a density of 108 cells/ml in endotoxin-free Dulbecco's PBS before being used in adoptive transfer studies.
Tetramer staining.
Tetrameric H-2Dd complexes folded around the HIV-1 IIIB V3 loop optimal P18 epitope peptide (P18-I10; RGPGRAFVTI) (24) were prepared and used to stain P18-specific CD8+ T lymphocytes essentially as described previously (1, 2). Mouse blood was collected in RPMI 1640 containing 40 U of heparin per ml. Following lysis of the red blood cells (RBCs), 0.1 μg of phycoerythrin-labeled Dd/P18 tetramer in conjunction with allophycocyanin-labeled anti-CD8α monoclonal antibody (MAb) (Ly-2; Caltag, San Francisco, Calif.) was used to stain P18-specific CD8+ T lymphocytes. The cells were washed in PBS containing 2% fetal bovine serum and fixed in 0.5 ml of PBS containing 1.5% paraformaldehyde. Samples were analyzed by two-color flow cytometry on a FACSCalibur instrument (Becton Dickinson Pharmingen, Mountain View, Calif.). Gated CD8+ T lymphocytes were examined for staining with the Dd/P18 tetramer. CD8+ T lymphocytes from naive mice were used as negative controls and exhibited <0.1% tetramer staining.
Env-specific ELISPOT assays.
Enzyme-linked immunospot (ELISPOT) assays were used to assess gamma interferon (IFN-γ) production by splenocytes from vaccinated mice in response to the P18 epitope peptide or a pool of 47 overlapping 15-amino-acid peptides derived from HIV-1 Env IIIB gp120 (Centralised Facility for AIDS Reagents, Potters Bar, United Kingdom). ELISPOT assays were performed 4 weeks following immunization. Multiscreen plates with 96 wells (Millipore, Bedford, Mass.) coated overnight with 100 μl of 10-μg/ml rat anti-mouse IFN-γ (Pharmingen, San Diego, Calif.) in PBS per well were washed with endotoxin-free Dulbecco's PBS containing 0.25% Tween 20 and blocked for 2 h at 37°C with PBS containing 5% fetal bovine serum. The plates were washed three times with Dulbecco's PBS containing 0.25% Tween 20, rinsed with RPMI 1640 containing 10% FBS, and incubated in triplicate with 5 × 105 splenocytes per well in a 100-μl reaction volume containing 2 μg of peptide per ml. For studies utilizing the Env peptide pool, each peptide in the pool was present at 2 μg/ml. Following an 18-h incubation, the plates were washed nine times with Dulbecco's PBS containing 0.25% Tween 20 and once with distilled water. The plates were then incubated for 2 h with 75 μl of 5-μg/ml biotinylated rat anti-mouse IFN-γ per well, washed six times with Coulter Wash (Coulter Corp., Miami, Fla.), and incubated for 2 h with a 1:500 dilution of streptavidin-AP (Southern Biotechnology Associates, Birmingham, Ala.). Following five washes with Coulter Wash and one wash with PBS, the plates were developed with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-β-d-galactoside (NBT/BCIP) chromogen (Pierce, Rockford, Ill.), the reaction was stopped by washing with tap water, and the plates were air dried and read using an ELISPOT reader (Hitech Instruments, Edgement, Pa.).
Env-specific ELISA.
Serum anti-gp120 antibody titers from immunized mice were measured by a direct enzyme-linked immunosorbent assay (ELISA) essentially as described previously (2). ELISAs were performed 0, 2, and 4 weeks following immunization. Plates (96 wells) coated overnight with 100 μl of 1-μg/ml recombinant HIV-1 Env IIIB gp120 (Intracel, Cambridge, Mass.) in PBS per well were blocked for 2 h with PBS containing 2% bovine serum albumin and 0.05% Tween 20. Sera were then added in serial dilutions and incubated for 1 h. The plates were washed three times with PBS containing 0.05% Tween 20 and incubated for 1 h with a 1:2,000 dilution of a peroxidase-conjugated affinity-purified rabbit anti-mouse secondary antibody (Jackson Laboratory, Bar Harbor, Maine). The plates were then washed three times and developed with tetramethylbenzidine (KPL, Gaithersburg, Md.), the reaction was stopped with 1% HCl, and the plates were analyzed at 450 nm with a Dynatech MR5000 ELISA plate reader.
Ad-specific ELISPOT assays.
Ad5-specific cellular immune responses were assessed by IFN-γ ELISPOT assays using BALB/c mouse splenocytes incubated with Ad5-infected syngeneic NIH 3T3 stimulator cells essentially as described previously (26). 3T3 cells were plated at a density of 106 cells per well in a six-well plate and infected with E1-deleted rAd5-Empty at a multiplicity of infection of 2 × 104 for 3 days. ELISPOT assays using splenocytes from immunized mice were then performed as described above, with 5 × 105 splenocytes per well and 1 × 105 Ad5-infected 3T3 stimulator cells per well instead of peptide antigens. For negative controls, splenocytes were incubated with uninfected 3T3 cells or medium alone.
Ad-specific NAb assay.
Ad5-specific NAb responses were assessed by luciferase-based virus neutralization assays essentially as described previously (26). A549 human lung carcinoma cells were plated at a density of 104 cells per well in 96-well plates and infected with E1-deleted rAd5-luciferase reporter constructs at a multiplicity of infection of 500 with two-fold serial dilutions of serum in 200-μl reaction volumes. Following a 24-h incubation, luciferase activity in the cells was measured using the Steady-Glo luciferase reagent system (Promega, Madison, Wis.). The 90% neutralization titers were defined as the maximum serum dilution that neutralized 90% of luciferase activity.
Statistical analyses.
Data are presented as means and standard errors. Statistical analyses were performed with GraphPad Prism version 2.01 (GraphPad Software, Inc., 1996). Comparisons of mean immune responses among groups of mice were performed by analyses of variance with Bonferroni adjustments to account for multiple comparisons. In all cases, P values of less than 0.05 were considered significant.
RESULTS
Ad5-specific humoral and cellular immune responses.
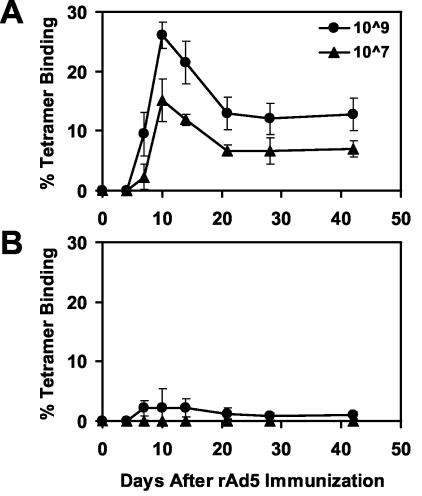
Our laboratory and others have previously demonstrated that anti-Ad5 immunity markedly suppresses immune responses elicited by E1-deleted, replication-incompetent rAd5 vector-based vaccines in mice (3, 31). In this study, we sought to define the primary immune effector mechanisms responsible for these suppressive effects. Groups of BALB/c mice (four mice per group) were preimmunized with one injection of 1010 vp of rAd5-Empty to induce anti-Ad5 immunity. After 4 weeks, these mice were immunized with different doses of the rAd5 vaccine expressing HIV-1 Env IIIB gp140ΔCFI (3). Vaccine-elicited CD8+ T-cell responses specific for the immunodominant H-2Dd-restricted P18 epitope (24) were assessed following immunization by tetramer binding to CD8+ T lymphocytes (1, 2). As shown in Fig. 1, mice with anti-Ad5 immunity exhibited a dramatic 90% reduction of tetramer+ CD8+ T-lymphocyte responses elicited by 109 vp of rAd5-Env and a complete abrogation of responses elicited by 107 vp of rAd5-Env compared with mice without anti-Ad5 immunity.
FIG. 1.
Anti-Ad5 immunity suppresses immune responses elicited by rAd5-Env. Groups of mice (four mice per group) in either the absence (A) or presence (B) of anti-Ad5 immunity were immunized with 109 or 107 vp of rAd5-Env vaccine. Vaccine-elicited cellular immune responses were assessed by Dd/P18 tetramer binding to CD8+ T lymphocytes. Anti-Ad5 immunity was induced by preimmunizing mice with 1010 vp of rAd5-Empty 4 weeks prior to rAd5-Env immunization.
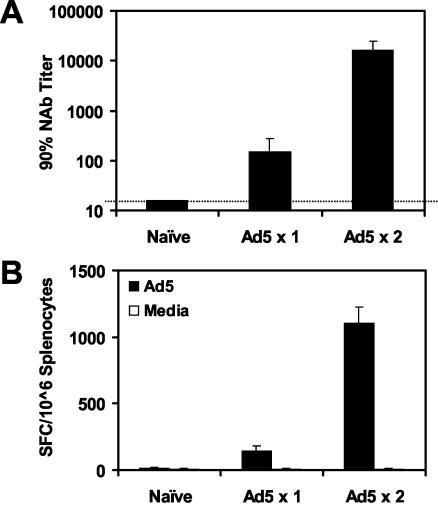
We next assessed the magnitude of Ad5-specific NAb and T-lymphocyte responses in mice with anti-Ad5 immunity. Ad5-specific NAb titers were measured by luciferase-based virus neutralization assays, and Ad5-specific T-lymphocyte responses were measured by ELISPOT assays using Ad5-infected syngeneic stimulator cells (26). As shown in Fig. 2, administration of one injection of 1010 vp of rAd5-Empty elicited clearly detectable mean Ad5-specific NAb titers of 148 and Ad5-specific ELISPOT responses of 142 spot-forming cells (SFC)/106 splenocytes at week 4. Administration of two injections of rAd5-Empty at weeks 0 and 4 elicited potent mean Ad5-specific NAb titers of 16,384 and Ad5-specific ELISPOT responses of 1,105 SFC/106 splenocytes by week 8. Thus, both humoral and cellular immune responses to the Ad5 vector were readily detected in mice with anti-Ad5 immunity. The titers of Ad5-specific NAbs induced by one and two injections of rAd5-Empty were comparable to the range of Ad5-specific NAb titers typically found in humans (reference 26 and unpublished data), suggesting that these levels of anti-vector immunity are biologically relevant.
FIG. 2.
Ad5-specific humoral and cellular immune responses. Naive mice or mice preimmunized with one or two injections of 1010 vp of rAd5-Empty were assessed for Ad5-specific NAb titers (A) and Ad5-specific IFN-γ ELISPOT responses (B). ELISPOT assays were performed using splenocytes incubated with Ad5-infected or uninfected syngeneic 3T3 stimulator cells.
Adoptive transfers using mice with low levels of anti-Ad5 immunity.
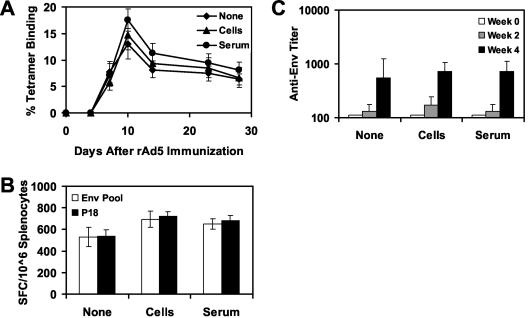
We next sought to determine whether cellular or humoral immune responses were responsible for the suppressive effects of anti-Ad5 immunity on the immunogenicity of rAd5-Env. We therefore performed a series of adoptive-transfer studies using splenocytes or serum from donor mice with or without anti-Ad5 immunity. For these studies, 5 × 107 freshly isolated splenocytes or 500 μl of serum from donor mice was adoptively transferred to naive recipient mice by tail vein injection. The recipient mice were then immunized with 108 vp of rAd5-Env, and vaccine-elicited cellular and humoral immune responses to Env were assessed by Dd/P18 tetramer binding, Env pooled peptide and P18 epitope peptide-specific ELISPOT assays, and Env-specific ELISAs. As shown in Fig. 3, adoptive transfer of splenocytes or serum from donor mice without anti-Ad5 immunity did not nonspecifically suppress the magnitude or kinetics of immune responses elicited by rAd5-Env.
FIG. 3.
Control adoptive transfer of splenocytes or serum from naive mice. A total of 5 × 107 splenocytes or 500 μl of serum from naive donor mice was adoptively transferred to groups of recipient mice (four mice per group) prior to immunization of the recipient mice with 108 vp of rAd5-Env. Vaccine-elicited immune responses were assessed by Dd/P18 tetramer binding to CD8+ T lymphocytes (A), Env peptide pool and P18 epitope peptide-specific IFN-γ ELISPOT assays (B), and Env-specific ELISAs (C).
We next performed adoptive-transfer studies using donor mice with low levels of anti-Ad5 immunity. Donor mice received one injection of 1010 vp of rAd5-Empty and were sacrificed after 4 weeks. Splenocytes or serum from these mice were adoptively transferred to naive recipient mice (four mice per group) prior to immunization with 108 vp of rAd5-Env. As shown in Fig. 4A, adoptive transfer of splenocytes resulted in a 31% trend toward reduction of peak tetramer CD8+ T-lymphocyte responses on day 10 following immunization. By days 21 to 28 following immunization, however, there was only a marginal reduction of memory tetramer+ CD8+ T-lymphocyte responses in these mice. Adoptive transfer of serum resulted in 74 and 49% reductions of peak and memory tetramer+ CD8+ T-lymphocyte responses, respectively. Adoptive transfer of serum also resulted in a trend toward lower ELISPOT responses (Fig. 4B) and Env-specific antibody titers (Fig. 4C). As expected, mice with active anti-Ad5 immunity developed no detectable Env-specific cellular or humoral immune responses following rAd5-Env immunization.
FIG. 4.
Adoptive transfer of splenocytes or serum from mice with low levels of anti-Ad5 immunity. A total of 5 × 107 splenocytes or 500 μl of serum from donor mice preimmunized with one injection of 1010 vp of rAd5-Empty was adoptively transferred to groups of recipient mice (four mice per group) prior to immunization of the recipient mice with 108 vp of rAd5-Env. A group of mice with active anti-Ad5 immunity was included as a control. Vaccine-elicited immune responses were assessed by Dd/P18 tetramer binding to CD8+ T lymphocytes (A), Env peptide pool and P18 epitope peptide-specific IFN-γ ELISPOT assays (B), and Env-specific ELISAs (C).
Adoptive transfers using mice with high levels of anti-Ad5 immunity.
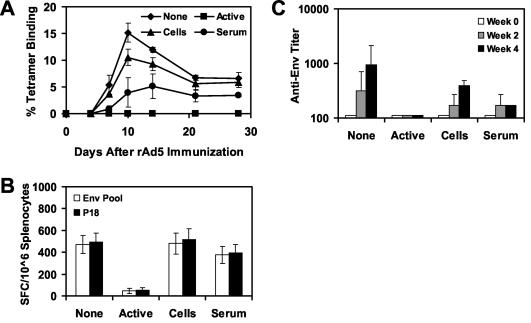
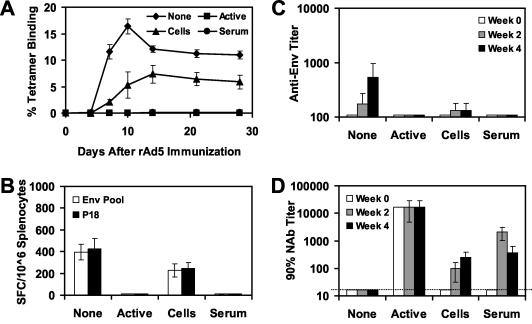
To investigate further the suppressive effects of Ad5-specific cellular and humoral immune responses on the immunogenicity of rAd5-Env, we performed similar adoptive-transfer studies using donor mice with high levels of anti-Ad5 immunity. Donor mice received two injections of 1010 vp of rAd5-Empty at week 0 and 4 and were sacrificed at week 8. These mice developed potent Ad5-specific NAb and T-lymphocyte responses as shown in Fig. 2. Splenocytes or serum from these mice was adoptively transferred to naive recipient mice (four mice per group) prior to immunization of the recipient mice with 108 vp of rAd5-Env. As shown in Fig. 5A, adoptive transfer of splenocytes from these donor mice resulted in a 68% reduction of peak tetramer+ CD8+ T-lymphocyte responses on day 10 following immunization and a durable 47% reduction of memory tetramer+ CD8+ T-lymphocyte responses by days 21 to 28 following immunization (P < 0.01 when comparing responses between groups on day 10 or 28 following immunization, using analyses of variance with Bonferroni adjustments to account for multiple comparisons). As shown in Fig. 5B and C, adoptive transfer of splenocytes from these donor mice similarly resulted in significant reductions of ELISPOT and ELISA responses (P < 0.01). Adoptive transfer of serum from these donor mice resulted in a >95% abrogation of vaccine-elicited tetramer+ CD8+ T-lymphocyte, ELISPOT, and ELISA responses (P < 0.001), presumably as a result of primary neutralization of the vaccine vector.
FIG. 5.
Adoptive transfer of splenocytes or serum from mice with high levels of anti-Ad5 immunity. A total of 5 × 107 splenocytes or 500 μl of serum from donor mice preimmunized with two injections of 1010 vp of rAd5-Empty was adoptively transferred to groups of recipient mice (four mice per group) prior to immunization of the recipient mice with 108 vp of rAd5-Env. A group of mice with active anti-Ad5 immunity was included as a control. Vaccine-elicited immune responses were assessed by Dd/P18 tetramer binding to CD8+ T lymphocytes (A), Env peptide pool and P18 epitope peptide-specific IFN-γ ELISPOT assays (B), and Env-specific ELISAs (C). (D) Ad5-specific NAb titers of these mice were assessed before and after the adoptive transfer.
We next assessed the evolution of Ad5-specific NAbs in these mice following adoptive transfer of splenocytes or serum. As shown in Fig. 5D, mice that did not receive any adoptive transfer failed to generate detectable Ad5-specific NAbs (titers of <16), suggesting that the rAd5-Env vaccine at a dose of 108 vp was insufficient to elicit high-titer Ad5-specific NAbs. As expected, adoptive transfer of serum resulted in early, high-titer NAbs that waned over time. Interestingly, adoptive transfer of splenocytes from mice with anti-Ad5 immunity resulted in the emergence of Ad5-specific NAbs that increased in titer from week 2 to week 4. These data suggest that adoptively transferred B lymphocytes or CD4+ T lymphocytes contributed to the generation of Ad5-specific NAbs following rAd5-Env immunization.
Ad5-specific NAbs and CD8+ T lymphocytes both contribute to Ad5 immunity.
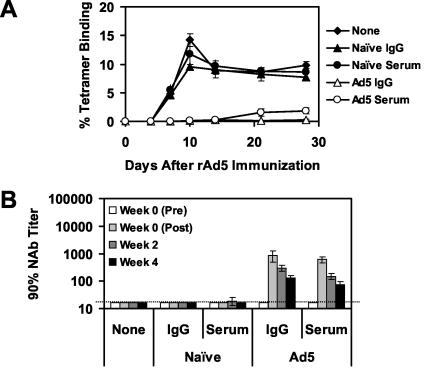
To define more precisely the immunologic mechanisms by which serum and splenocytes from mice with anti-Ad5 immunity suppressed the immunogenicity of rAd5-Env, we performed adoptive-transfer studies using purified IgG and CD8+ T lymphocytes. IgG was purified from serum with protein A columns. Purified IgG and serum exhibited comparable Ad5-specific NAb titers (data not shown). As shown in Fig. 6A, adoptive transfer of purified IgG from mice with anti-Ad5 immunity was as effective as serum in suppressing tetramer+ CD8+ T-lymphocyte responses elicited by the rAd5-Env vaccine. Purified IgG and serum were also comparable in their ability to suppress vaccine-elicited ELISPOT and ELISA responses (data not shown). Ad5-specific NAb titers in mice that received purified IgG or serum showed similar early peaks and clearance kinetics (Fig. 6B).
FIG. 6.
Adoptive transfer of purified IgG from mice with high levels of anti-Ad5 immunity. Serum or purified IgG from naive donor mice or donor mice preimmunized with two injections of 1010 vp of rAd5-Empty were adoptively transferred to groups of recipient mice (four mice per group) prior to immunization of the recipient mice with 108 vp of rAd5-Env. (A) Vaccine-elicited immune responses were assessed by Dd/P18 tetramer binding to CD8+ T lymphocytes. (B) Ad5-specific NAb titers of these mice were assessed before and after the adoptive transfer.
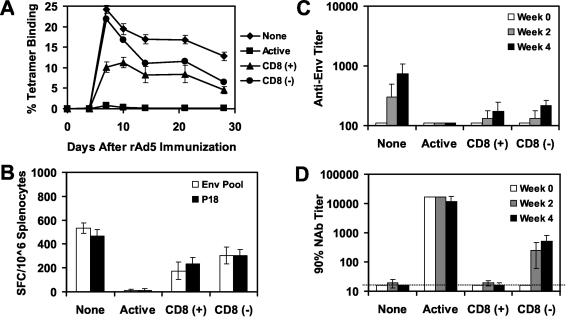
The suppression of rAd5-Env immunogenicity that we observed following adoptive transfer of splenocytes (Fig. 5) may have reflected either Ad5-specific CD8+ T lymphocytes or the higher levels of Ad5-specific NAbs that developed following immunization in these mice. To differentiate between these possibilities, we performed a similar adoptive-transfer study using purified CD8+ T lymphocytes from mice with high levels of anti-Ad5 immunity. CD8+ T lymphocytes were purified from splenocytes by negative selection using antibody-coated magnetic beads. As shown in Fig. 7A, adoptive transfer of purified CD8+ T lymphocytes resulted in 58 and 50% reductions of peak and memory tetramer+ CD8+ T-lymphocyte responses, respectively, elicited by the rAd5-Env vaccine (P < 0.01). As shown in Fig. 7B and C, these mice also exhibited significant reductions of vaccine-elicited ELISPOT and ELISA responses (P < 0.01). The lack of detectable Ad5-specific NAb responses in these mice following immunization [Fig. 7D, CD8 (+)] confirmed that this effect was mediated solely by CD8+ T lymphocytes.
FIG. 7.
Adoptive transfer of purified CD8+ T lymphocytes from mice with high levels of anti-Ad5 immunity. Purified CD8+ T lymphocytes [CD8 (+)] or splenocytes depleted of CD8+ T lymphocytes [CD8 (−)] from donor mice preimmunized with two injections of 1010 vp of rAd5-Empty were adoptively transferred to groups of recipient mice (four mice per group) prior to immunization of the recipient mice with 108 vp of rAd5-Env. A group of mice with active anti-Ad5 immunity was included as a control. Vaccine-elicited immune responses were assessed by Dd/P18 tetramer binding to CD8+ T lymphocytes (A), Env peptide pool and P18 epitope peptide-specific IFN-γ ELISPOT assays (B), and Env-specific ELISAs (C). (D) Ad5-specific NAb titers of these mice were assessed before and after the adoptive transfer. This study was repeated three times.
Interestingly, adoptive transfer of splenocytes depleted of CD8+ T lymphocytes failed to blunt the early kinetics of the vaccine-elicited tetramer+ CD8+ T-lymphocyte responses but did result in a partial suppression of these responses by days 14 to 28. This late suppression corresponded to the emergence of Ad5-specific NAbs in these animals [Fig. 7D, CD8 (−)]. These data suggest that adoptively transferred B lymphocytes or CD4+ T lymphocytes have the ability to mediate a degree of late suppression of vaccine-elicited immune responses, presumably through the generation of Ad5-specific NAbs.
DISCUSSION
Recombinant Ad5 vector-based vaccines for HIV-1 and other pathogens have shown considerable promise in preclinical studies and are currently being advanced into clinical trials (4, 19, 22, 23). The high prevalence of preexisting anti-Ad5 immunity in human populations, however, may substantially limit the immunogenicity and clinical utility of these vaccines. A detailed understanding of the immunologic effector mechanisms by which anti-Ad5 immunity blunts the immunogenicity of rAd5 vaccines is therefore critical in the development of strategies to overcome this problem. In this study, we modeled the suppressive effects of anti-Ad5 immunity on the immunogenicity of a rAd5 vaccine by performing adoptive-transfer studies with mice. We showed that Ad5-specific NAbs and CD8+ T lymphocytes both contribute to immunity to the Ad5 vector and that the degree of suppression of vaccine-elicited immune responses was dependent on the titer of Ad5-specific NAbs and the frequency of Ad5-specific T lymphocytes.
The dramatic abrogation of vaccine-elicited immune responses following adoptive transfer of Ad5-specific NAbs demonstrates that humoral immunity plays a central role in anti-Ad5 immunity. These results are consistent with previous reports showing that antibodies against viral capsid proteins exhibit potent neutralizing activity (7, 8, 25, 27). We also observed that Ad5-specific CD8+ T lymphocytes contribute substantially to anti-Ad5 immunity, although to a lesser extent than Ad5-specific NAbs. These data suggest that NAbs may represent the primary immunologic effector mechanism of anti-Ad5 immunity and that CD8+ T lymphocytes probably play an important but secondary role. We suspect that Ad5-specific CD8+ T lymphocytes will prove most relevant in individuals with high levels of anti-Ad5 immunity, although the frequency of Ad5-specific CD8+ T lymphocytes in humans has not been clearly established.
Containment of viral infections often involves both humoral and cellular virus-specific immune responses. NAbs function by neutralizing free virus and blocking infection of cells, whereas CD8+ T lymphocytes typically function by eliminating virally infected cells and suppressing viral replication. For a replication-defective vaccine vector that does not undergo multiple rounds of reinfection in vivo, however, the role of vector-specific CD8+ T lymphocytes in reducing transgene expression and vaccine-elicited immune responses is less well defined. We hypothesize that Ad5-specific CD8+ T lymphocytes function by rapidly eliminating virally infected antigen-presenting cells prior to optimal immune priming.
Previous studies have suggested that CD8+ T lymphocytes can contribute to the eventual clearance of virally infected hepatocytes in a murine model of Ad5-based cystic fibrosis gene therapy, suggesting that cellular immune responses may present an obstacle to sustained transgene expression (29, 30). Our results extend these observations and demonstrate that Ad5-specific CD8+ T lymphocytes also suppress immune responses elicited by rAd5 vaccines that do not require long-term transgene expression. In contrast to our results, however, a recent study reported that adoptive transfer of CD8+ T lymphocytes from mice with anti-Ad5 immunity did not in fact suppress the immunogenicity of a rAd5-Gag vaccine (6). These apparent differences may be explained by the lower level of anti-Ad5 immunity induced by 108 vp of Ad5 in this previous study. Interestingly, even this low frequency of Ad5-specific CD8+ T lymphocytes was sufficient to reduce the immunogenicity of a heterologous chimpanzee rAd68-Gag vaccine (6). These data suggest that the functional importance of cross-reactive T-lymphocyte responses between serologically distinct Ads may be substantially greater than has previously been appreciated. Other investigators have similarly observed that anti-Ad5 immunity was able to suppress chimeric Ad vectors that were designed to evade Ad5-specific NAb responses (8, 32). These effects may in part reflect cross-reactive Ad5-specific T-lymphocyte responses, although this possibility was not directly studied.
Our results have substantial implications for the development of second-generation Ad vaccine vectors that aim to circumvent the problem of preexisting anti-Ad5 immunity. Novel Ad vectors are currently being developed both from rare human serotypes (12, 15, 16, 26) and from species other than humans (5, 6, 10, 28). The experiments presented here suggest that novel vectors will probably need to evade both Ad5-specific NAbs and T-lymphocyte responses to overcome anti-Ad5 immunity. Further studies are therefore warranted to determine the primary Ad5-specific NAb and T-lymphocyte epitopes in both humans and animal models and the extent of their cross-reactivity with Ads of other serotypes.
Acknowledgments
We thank Raphael Dolin, Michael Seaman, Sampa Santra, Kristi Martin, Gary Nabel, Bimal Chakrabarti, and Ling Xu for generous advice, assistance, and reagents. The HIV-1 IIIB Env overlapping peptides were obtained from the United Kingdom Centralised Facility for AIDS Reagents (EU contract QLK2-CT-1999-01215).
We acknowledge support from NIH grant AI-51223 (D.H.B.), a Harvard Medical School CFAR Pilot Project Award (D.H.B.), the Harvard Medical School PASTEUR program (S.M.S.), and CFAR P30 AI-28691 (N.L.L.). D.H.B. is a recipient of a Doris Duke Clinical Scientist Development Award.
REFERENCES
- 1.Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168:562-568. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting HIV-1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farina, S. F., G. P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, H. C. Ertl, and J. M. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416-1422. [DOI] [PubMed] [Google Scholar]
- 7.Gahery-Segard, H., F. Farace, D. Godfrin, J. Gaston, R. Lengagne, T. Tursz, P. Boulanger, and J. G. Guillet. 1998. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 72:2388-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gall, J. G., R. G. Crystal, and E. Falck-Pedersen. 1998. Construction and characterization of hexon-chimeric adenoviruses: specification of adenovirus serotype. J. Virol. 72:10260-10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heemskerk, B., L. A. Veltrop-Duits, T. van Vreeswijk, M. M. ten Dam, S. Heidt, R. E. Toes, M. J. van Tol, and M. W. Schilham. 2003. Extensive cross-reactivity of CD4+ adenovirus-specific T cells: implications for immunotherapy and gene therapy. J. Virol. 77:6562-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann, C., P. Loser, G. Cichon, W. Arnold, G. W. Both, and M. Strauss. 1999. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 73:6930-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jooss, K., H. C. Ertl, and J. M. Wilson. 1998. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J. Virol. 72:2945-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kass-Eisler, A., L. Leinwand, J. Gall, B. Bloom, and E. Falck-Pedersen. 1996. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 3:154-162. [PubMed] [Google Scholar]
- 13.Kast, W. M., R. Offringa, P. J. Peters, A. C. Voordouw, R. H. Meloen, A. J. van der Eb, and C. J. Melief. 1989. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell 59:603-614. [DOI] [PubMed] [Google Scholar]
- 14.Letvin, N. L., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 15.Mack, C. A., W. R. Song, H. Carpenter, T. J. Wickham, I. Kovesdi, B. G. Harvey, C. J. Magovern, O. W. Isom, T. Rosengart, E. Falck-Pedersen, N. R. Hackett, R. G. Crystal, and A. Mastrangeli. 1997. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8:99-109. [DOI] [PubMed] [Google Scholar]
- 16.Mastrangeli, A., B. G. Harvey, J. Yao, G. Wolff, I. Kovesdi, R. G. Crystal, and E. Falck-Pedersen. 1996. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum. Gene Ther. 7:79-87. [DOI] [PubMed] [Google Scholar]
- 17.Molinier-Frenkel, V., H. Gahery-Segard, M. Mehtali, C. Le Boulaire, S. Ribault, P. Boulanger, T. Tursz, J. G. Guillet, and F. Farace. 2000. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J. Virol. 74:7678-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olive, M., L. Eisenlohr, N. Flomenberg, S. Hsu, and P. Flomenberg. 2002. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum. Gene Ther. 13:1167-1178. [DOI] [PubMed] [Google Scholar]
- 19.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 20.Smith, C. A., L. S. Woodruff, C. Rooney, and G. R. Kitchingman. 1998. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum. Gene Ther. 9:1419-1427. [DOI] [PubMed] [Google Scholar]
- 21.Sparer, T. E., S. G. Wynn, D. J. Clark, J. M. Kaplan, L. M. Cardoza, S. C. Wadsworth, A. E. Smith, and L. R. Gooding. 1997. Generation of cytotoxic T lymphocytes against immunorecessive epitopes after multiple immunizations with adenovirus vectors is dependent on haplotype. J. Virol. 71:2277-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 30:605-609. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, L. Xu, Z. Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi, H., Y. Nakagawa, C. D. Pendleton, R. A. Houghten, K. Yokomuro, R. N. Germain, and J. A. Berzofsky. 1992. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science 255:333-336. [DOI] [PubMed] [Google Scholar]
- 25.Toogood, C. I., J. Crompton, and R. T. Hay. 1992. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J. Gen. Virol. 73:1429-1435. [DOI] [PubMed] [Google Scholar]
- 26.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Ceechini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell interaction aand bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlfart, C. 1988. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 62:2321-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang, Z., G. Gao, A. Reyes-Sandoval, C. J. Cohen, Y. Li, J. M. Bergelson, J. M. Wilson, and H. C. Ertl. 2002. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 76:2667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433-442. [DOI] [PubMed] [Google Scholar]
- 30.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, Z. Y., L. S. Wyatt, W. P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youil, R., T. J. Toner, Q. Su, M. Chen, A. Tang, A. J. Bett, and D. Casimiro. 2002. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 13:311-320. [DOI] [PubMed] [Google Scholar]