Abstract
In this study, reverse transcriptase (RT)- and integrase (IN)-defective human immunodeficiency virus type 1 (HIV-1) was transcomplemented with Vpr-RT-IN fusion proteins to delineate pol sequences important for HIV-1 replication. Our results reveal that a 194-bp sequence encompassing the 3′end of the IN gene and containing the central DNA flap is necessary and sufficient for efficient HIV-1 single-cycle replication in dividing and nondividing cells. Furthermore, we show that the central DNA flap enhances HIV-1 single-round replication by five- to sevenfold, primarily by facilitating nuclear import of proviral DNA. In agreement with previous reports, our data support a functional role of the central DNA flap during the early stages of HIV-1 infection.
The complexity of human immunodeficiency virus type 1 (HIV-1) replication is attributed in large part to the intricate interplay that takes place between cis-acting sequences present on viral nucleic acids and viral or host cell proteins that function in trans (22). The HIV-1 pol gene encodes three enzymatic proteins, including protease, reverse transcriptase (RT), and integrase (IN), which play critical roles during specific stages of the virus infection cycle. Soon after virus entry, RT catalyzes the conversion of the viral RNA genome into double-stranded proviral DNA, while IN mediates proviral DNA integration into the host cell genome (for a review, see references 10, 15, and 21). Even though extensive in vitro biochemical and mechanistic studies have greatly contributed to a better understanding of the primary function and mode of action of RT and IN enzymes, studies performed in the context of HIV-1 infectious proviral clones have revealed that some mutations and/or internal deletions in RT or IN can significantly alter steps other than reverse transcription or integration, such as virus assembly or release, or even inactivate virus infectivity (1, 2, 4, 14, 20, 35). These pleiotropic phenotypes resulting from mutagenic analysis suggest that RT and IN may play other roles which are independent of their enzymatic activities. Furthermore, introduction of mutations in RT and/or IN sequences may simultaneously affect a cis-acting element(s) present within the pol gene sequence that is required for efficient virus replication.
In contrast to oncoretroviruses, HIV-1 and other lentiviruses have the capacity to infect nondividing cell populations, such as macrophages, mucosal dendritic cells, and nondividing T cells, since they do not depend on host cell mitosis to mediate the nuclear translocation of their preintegration complex (PIC) (5, 12, 27, 28, 31, 40). At the molecular level, HIV-1 PIC nuclear transport was shown to proceed through intact nuclear pore complexes by an active and energy-dependent mechanism that involves the karyophilic properties of several PIC-associated viral proteins, including Matrix (MAp17gag), IN, and Vpr (3, 6, 12, 19, 20, 23, 24, 32, 39). In addition, a cis-acting element designated the central DNA flap located in the 3′ region of the pol gene sequence was also shown to contribute to the nuclear import of HIV-1 proviral DNA in both dividing and nondividing cells (45). The central DNA flap is a region of triple-stranded DNA created by two discrete half-genomic fragments with a central strand displacement event controlled in cis by a central polypurine tract (cPPT) and a central termination sequence (CTS) during HIV-1 reverse transcription (7, 8). HIV-1 viruses carrying an inactivated cPPT or CTS were reported to exhibit a considerable impairment of viral replication in different dividing and nondividing target cells (7, 8), presumably because of a defect at the level of the nuclear import of the PIC (45). However, these results have been put into question recently by two other studies (13, 29), which provided evidence indicating that the central DNA flap did not play a major role in either PIC nuclear import or HIV-1 replication in a variety of cell lines. Interestingly, in contrast to these studies that used replication-competent viruses, numerous other studies have reported that the central DNA flap conferred a transduction advantage of approximately 2- to 10-fold on HIV-1-derived lentiviral vectors, thus suggesting that the central DNA flap facilitated an early step(s) in lentiviral infection (11, 17, 30, 33, 34, 37, 46). The exact impact of the central DNA flap on the early steps of HIV-1 infection is still an open question that remains to be clarified.
Requirement of pol gene sequence for efficient HIV-1 single-cycle infection.
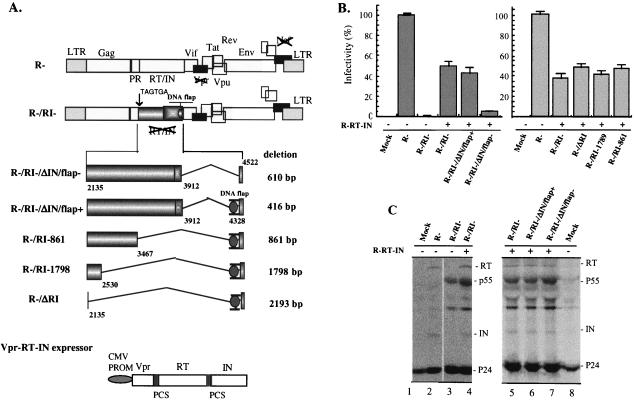
In order to investigate the impact of the HIV-1 pol gene sequence on virus replication, we generated a previously described HIV-1 RT and IN transcomplemented replication system (16, 41). An RT- and IN-defective HIV-1 provirus (R−/RI−) with an intact pol gene sequence was constructed by replacing the first two amino acids of RT with two premature stop codons (TGA TAG) in a Vpr- and Nef-defective HxBc2-derived HIV-1 provirus (R−) by using a two-step PCR-based method (44). To transcomplement the RT and IN defect of HIV-1 virus, a Vpr-RT-IN fusion protein expression plasmid (CMV-R-RT-IN) was also made by inserting a PCR-amplified HIV-1 RT and IN gene cDNA in frame with Vpr into the SVCMV-Vpr plasmid (44) (Fig. 1A). It has been shown that HIV-1 RT and IN enzymatic defects can be restored in trans through Vpr-mediated virion incorporation of Vpr-RT-IN fusion protein, leading to production of viral particles that can undergo a single-round replication (16, 41). To test the infectivity of the transcomplemented RT- and IN-defective virus, 293T cells were transfected with R−/RI− provirus or cotransfected with CMV-R-RT-IN plasmid by using the calcium phosphate DNA precipitation method (42). In parallel, the wild-type (wt) provirus (R−) was used as a positive control. At 48-h posttransfection, virus stocks were generated from supernatants and quantitated by p24 measurements by using an HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) kit (AIDS Vaccine Program of the Frederick Cancer Research and Development Center) as described previously (43). Then the infectivity of each virus was examined by infecting HeLa-CD4-β-galactosidase (HeLa-CD4-[β-Gal]) cells with equal amounts (15 ng of p24gag antigen/well) of virus and evaluated by MAGI assay 48-h postinfection (p.i.), as described previously (26). Consistent with a previous report (41), RT- and IN-defective R−/RI− viruses were found to be infectious by MAGI assay only when they were transcomplemented with RT and IN during viral production, reaching infectivity levels corresponding to 40 to 50% of the wt level (R− virus) (Fig. 1B, left panel). To test the efficiency of Vpr-mediated RT and IN transincorporation, transfected 293T cells were radiolabeled and the resulting viral particles were analyzed for viral protein content by immunoprecipitation by using anti-HIV-1 serum (42). Results reveal that, as expected, the R−/RI− viral particles did not contain any RT or IN proteins (Fig. 1C, lane 3), whereas the transcomplemented R−/RI− virus incorporated RT and IN proteins at levels comparable to those for the wt R− virus (Fig. 1C, compare lane 4 to lane 2). Interestingly, it was noted that significant amounts of unprocessed Pr55gag accumulated in R−/RI− viral particles (Fig. 1C, compare lanes 3 and 4 to lane 2). This maturation defect is likely to result from an impairment of protease activation, given that the Gag-Pol polyprotein precursor was truncated by early termination of RT and IN and is believed to be responsible for the observed 50 to 60% reduction of virus infectivity (Fig. 1B).
FIG. 1.
Effect of the HIV-1 IN and RT gene sequences on the infectivity of RT/IN-transcomplemented virus. (A) Schematic structure of HIV-1 proviruses carrying a mutation and/or deletions in pol and of the plasmid encoding the Vpr-RT-IN fusion protein. Provirus R−/RI− was constructed by replacing the first two amino acids of RT with two premature stop codons (TGA TAG) in HxBruR−(R−) provirus. In R−/RI−/ΔIN/flap− provirus, a 610-bp fragment of the IN gene sequence (including cPPT/CTS) was deleted. The R−/RI−/ΔIN/flap+, R−/RI-861, R−/RI-1798, and R−/ΔRI proviruses harbor different deletions within the RT and/or IN gene sequences but contain the 194-bp sequence in the 3′-end region of IN, which harbors the cPPT/CTS cis-acting elements. PR, protease. (B) The infectivity of trans-complemented virus produced in 293T cells was evaluated by MAGI assay. Equal amounts (15 ng of p24gag antigen) of the different viruses were used to infect HeLa-CD4-β-Gal cells, and the number of infected cells was monitored by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining. The infectivity (% infectivity) of each virus stock was calculated as the ratio of the number of β-Gal-positive cells relative to the number of β-Gal-positive cells obtained with the wt virus (R−). The number of β-Gal-positive cells detected with the R− virus ranged between 330 to 410 and was set at 100%. The results are representative of three independent experiments. (C) To evaluate Vpr-mediated transincorporation of RT and IN in viral particles, radiolabeled viruses were isolated from cell supernatants, lysed, immunoprecipitated with anti-HIV antibodies, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% acrylamide). LTR, long terminal repeat.
We next analyzed the requirement of RT and IN gene sequences for virus replication by using this Vpr-RT-IN transcomplementation system. A series of proviruses with deletions of the RT and/or IN gene derived from the R−/RI− provirus were constructed as indicated in Fig. 1A. In the R−/RI−/ΔIN/flap− provirus, a 610-bp sequence encompassing a large part of the IN gene and including the cPPT and CTS cis-acting sequences (from nucleotide 3,912 to 4,522; +1 corresponds to the transcription initiation site of the BRU strain), which were previously shown to play an important role in HIV-1 replication, were deleted (7, 8, 11). In the R−/RI−/ΔIN/flap+ provirus, a smaller deletion of 494 bp was introduced, thus leaving intact a 194-bp sequence, including cPPT/CTS elements at the 3′ end of the IN gene. To further test the impact of the RT gene sequence on virus replication, different regions of the RT gene sequence were further deleted, based on the R−/RI−/ΔIN/flap+ provirus and designated R−/RI-861, R−/RI-1798, and R−/ΔRI (Fig. 1A). In the R−/ΔRI provirus, all the RT and IN gene sequences except the 194 bp containing the cPPT/CTS sequences were deleted (a deletion of 2,193 bp). The infectivity of each transcomplemented virus with a deletion of the RT and/or IN gene was analyzed by MAGI assay. Deletion of the 3′ region of IN encompassing the cPPT/CTS elements resulted in a substantial five- to sevenfold decrease of viral infectivity compared to that of the transcomplemented R−RI− virus (Fig. 1B, left panel). This sharp decrease in viral infectivity was not due to variation in the levels of RT and/or IN transincorporated into viral particles, since they were found to be similar in both transcomplemented viruses (Fig. 1C, compare lanes 7 and 5). In contrast, maintenance of a 194-bp sequence in the 3′ region of the IN gene sequence, which includes the cPPT/CTS elements (R−/RI−/ΔIN/flap+), restored infectivity to a level similar to that of the transcomplemented R−/RI− virus (Fig. 1B, left panel). Interestingly, deletion of RT gene sequences (R−/RI-861, R−/RI-1798, and R−/ΔRI) had no impact on viral infectivity as long as the 194-bp sequence in the 3′ end region of IN was intact (Fig. 1B, right panel). Furthermore, maintenance of this 194-bp fragment was found to confer a six- to sevenfold infectivity advantage to single-cycle replicating virus in dividing and aphidicolin growth-arrested C8166 T cells (data not shown). Overall, these results indicate that the 3′ region of the IN gene sequence harbors cis-acting determinants(s) that substantially enhance the replication of HIV-1 toward dividing and nondividing CD4+ T cells in the context of a single-cycle infection system, while other RT and IN gene sequences are clearly dispensable.
The cPPT contributes to efficient HIV-1 single-cycle replication.
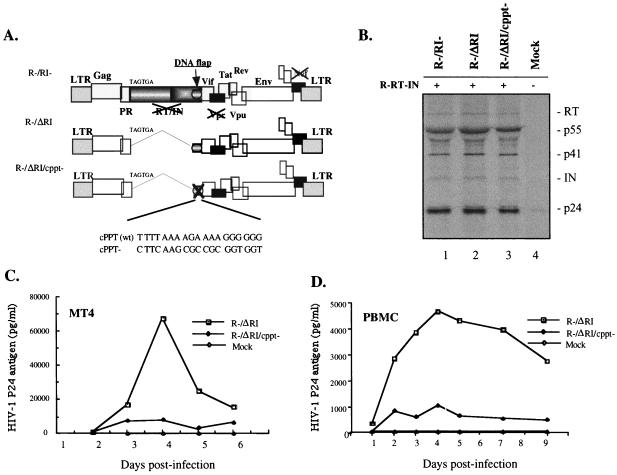
To further confirm that cis-acting element(s) in the 3′ region of the IN gene sequence contribute to efficient single-cycle replication, we introduced a 10-bp substitution in the cPPT element in the R−/ΔRI provirus and generated a cPPT-defective mutant designated R−/ΔRI/cPPT− (Fig. 2A). These specific mutations in the cPPT element have previously been reported to prevent the formation of the central DNA flap during reverse transcription (13, 29, 45). Prior to testing virus infectivity, the levels of transincorporated RT and IN in both the cPPT mutant and the control virus were examined by radiolabeling and immunoprecipitation as described in Fig. 1. Similar levels of virion-associated RT, IN, p24gag, and p55gag were detected in transcomplemented R−/RI−, R−/ΔRI, and R−/ΔRI/cPPT− virus preparations (Fig. 2B). To compare the replication potential of transcomplemented R−/ΔRI/cPPT− and R−/ΔRI viruses, CD4+ MT4 cells and phytohemagglutinin-stimulated human peripheral blood mononuclear cells (h-PBMCs) were infected with equal amounts of each virus stock for 8 h, and at different time intervals, virion-associated p24gag antigen levels in the supernatant were measured by anti-p24 ELISA. Disruption of the cPPT was found to decrease by five- to sevenfold viral replication in both MT4 T cells and activated h-PBMCs compared to that in the trans-complemented R−/ΔRI control virus (Fig. 2C and D). Hence, we conclude that the central DNA flap is the necessary determinant in the 3′ region of the IN gene sequence that contributes to efficient single-cycle virus replication.
FIG. 2.
The central DNA flap contributes to efficient HIV-1 single-cycle replication. (A) Schematic structure of HIV-1 provirus with the RT/IN gene deleted (R−/ΔRI) and the cPPT− mutant (R−/ΔRI/cPPT−). The cPPT element was inactivated by introduction of ten nucleotide substitution mutations, as indicated. (B) To evaluate RT and IN transincorporation, [35S]-methionine-radiolabeled viruses were collected from transfected 293T cells, lysed, and analyzed by immunoprecipitation by using anti-HIV antibodies. To test the replication potential of each virus stock, CD4+ MT4 T cells (C) or PHA-stimulated human PBMCs (D) were infected with equal amounts of R−/ΔRI or R−/ΔRI/cPPT− viruses. At different time intervals after infection, viral production was monitored in the supernatants by measurement of HIV-1 p24gag antigen by using a p24 ELISA assay. The results are representative of two independent experiments.
Effect of the central DNA flap on late-reverse-transcribed DNA products, viral cDNA nuclear import, and proviral DNA integration in h-PBMCs.
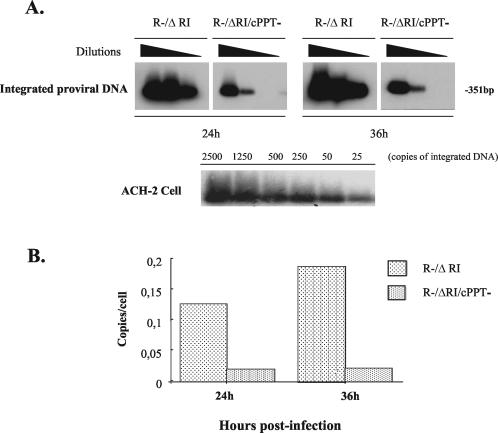
To investigate the mechanism(s) underlying the action of the central DNA flap during single-cycle replication, we first analyzed the efficiency of proviral DNA integration in h-PBMCs infected with cPPT+ or cPPT− transcomplemented viruses by using a previously described, sensitive, two-step Alu-PCR technique (9). Results reveal that levels of integrated proviral DNA detected in the R−/ΔRI/cPPT− sample were five- to sevenfold lower than those detected in the R−/ΔRI sample at both 24- and 36-h p.i. (Fig. 3A and B). Interestingly, this significant decrease in proviral DNA integration correlated well with the five- to sevenfold replication defect observed with the cPPT-defective virus, indicating that the central DNA flap contributes to efficient viral single-cycle replication by acting on an early stage(s) of viral replication at and/or prior to viral integration. To further investigate at which early step(s) of the infection cycle the central DNA flap acts, we analyzed the total amounts of viral cDNA present at different early time points by PCR following infection of h-PBMCs with equal amounts of R−/ΔRI or R−/ΔRI/cPPT− viruses. Briefly, an equal number (2 × 106 cells) of h-PBMCs were lysed in lysis buffer (20 mM Tris-HCl [pH 8.0], 100 mM KCl, 0.05% NP-40, 0.05% Tween 20) and treated with proteinase K (100 μg/ml) prior to phenol-chloroform DNA purification. Then fivefold-serially diluted DNA samples were subjected to PCR analysis with specific primers (5′-U3, 5′-GGATGGTGCTTCAAGCTAGTACC-3′, and 3′-Gag, 5′-ACTGACGCTCTCGCACCCATCTCTCTC-3′) and further analyzed by Southern blotting by using specific PCR DIG-Labeling probes (Roche Diagnostics, Laval, Quebec, Canada). As shown in Fig. 4A, at 6-h p.i., similar amounts of total viral cDNA were detected in R−/ΔRI- and R−/ΔRI/cPPT−-infected cells (4.9 copies/cell versus 4.6 viral copies/cell), suggesting that both transcomplemented viruses entered cells with similar efficiencies and underwent uncoating and reverse transcription at comparable rates. In contrast, between 12- and 24-h p.i., total amounts of late cPPT− reverse-transcribed products decreased at a rate that was clearly different from that of viral cPPT+ cDNA products. At 12-h and 24-h p.i, levels of viral cPPT− cDNA were reduced by approximately 45 and 40% compared to the levels of cPPT+ cDNA, which stayed quite stable during the same time interval (between approximately 87 and 88% of their levels at 6 h) (Fig. 4A). At later time points (between 24 and 48 h), both cPPT+ and cPPT− viral cDNAs decreased at similar rates, most probably as a result of the dilution of unintegrated viral cDNA that occurs upon cell division. This difference in the rate of viral cDNA decrease detected between R−/ΔRI and R−/ΔRI/cPPT− infection was not due to intrinsic variation between samples, since similar levels of a control cellular DNA (human β-2-adrenergic receptor [β2-AR] gene) were detected by PCR in each sample (Fig. 4B, left panel). These results suggest that the central DNA flap does not interfere with the rate of the reverse transcription step per se but appears to influence the rate of accumulation of total viral cDNA product.
FIG. 3.
Effect of the central DNA flap on HIV-1 proviral DNA integration in h-PBMC. (A) h-PBMCs were infected with R−/ΔRI or R−/ΔRI/cPPT− virus (125 ng of p24/106 cells). At 24- and 36-h p.i., the cells were lysed, and serially diluted cell lysates were analyzed by two-step Alu-PCR and Southern blotting for specific detection of integrated proviral DNA from infected PBMCs (upper panel) or from ACH-2 cells as the quantitative control (lower panel). (B) Quantitative analysis of integrated proviral DNA in single-cycle infection. The bands in panel A were quantified by laser densitometry, and the number of integrated proviral DNA copies per cell was determined by using the PCR-generated standard curve derived from ACH-2 cells.
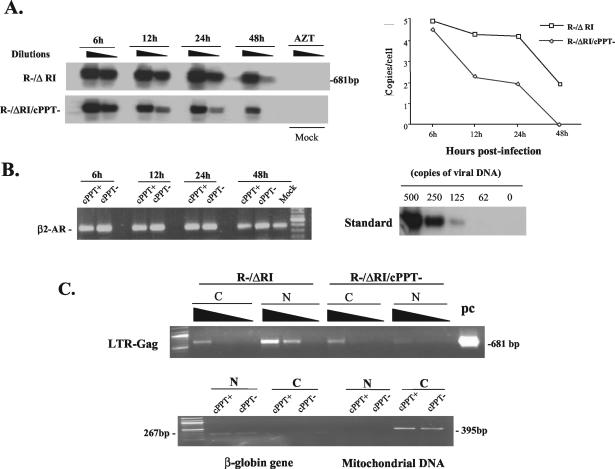
FIG. 4.
Effect of the central DNA flap on HIV-1 cDNA nuclear import. (A) h-PBMCs were infected with transcomplemented R−/ΔRI (cPPT+) and R−/ΔRI/cPPT− (cPPT−) viruses (125 ng of p24/106 cells) for 2 h. As the negative control, 3′-azido-3′-deoxythymidine (AZT; 10 μM)-pretreated PBMCs were infected with the same amounts of transcomplemented R−/ΔRI virus. At each indicated time point, serial dilutions of extracted total DNA were analyzed for late-reverse-transcription products by PCR by using long terminal repeat (LTR)-Gag primers and Southern blotting. HIV-1 late-reverse-transcription products detected in the left panel were quantified by laser densitometry. The diagram at the right shows the number of HIV-1 cDNA copies per cell as determined by using the PCR-generated standard curve (B, right panel). These results are representative of those obtained in two independent experiments. Serially diluted R-/ΔRI plasmid DNA was used as a standard for DNA copy quantification (right panel). To evaluate cellular DNA levels in each sample, the cellular β2-AR gene was amplified by PCR and visualized by ethidium bromide staining (left panel). (C) At 24-h p.i., 2 × 106 infected h-PBMCs were fractionated into cytoplasmic and nuclear fractions as described previously (36). The amounts of viral DNA in the cytoplasmic and nuclear fractions were evaluated by PCR by using HIV-1 LTR-Gag primers and were visualized by ethidium bromide staining. The R−/ΔRI plasmid DNA was used as a PCR-positive control (pc) (upper panel). In parallel, the purity and DNA content of each subcellular fraction were evaluated by PCR detection of the human globin gene and mitochondrial DNA and were visualized by ethidium bromide staining (lower panel). N, nuclear fraction; C, cytoplasmic fraction.
In parallel, we analyzed viral cDNA nuclear import by subcellular fractionation and subsequent detection of viral cDNA associated with nuclear or cytoplasmic fractions as previously described (36). Human PBMCs were infected with equivalent amounts of transcomplemented R−/ΔRI/cPPT− or R−/ΔRI viruses, and cytoplasmic and nuclear fractions were isolated from the same number of cells at 24-h p.i. All fractions were then analyzed by PCR as described in the legend to Fig. 4A. The presence of total viral DNA was visualized by ethidium bromide staining, and the staining intensity of each amplified DNA product was quantified by using a ChemiImager 5500 system with AlphaEaseFC software (Alpha Innotech Corporation). The results shown in Fig. 4C (upper panel) reveal that at 24-h p.i. total amounts of viral cDNA in the R−/ΔRI-infected sample (including cytoplasmic and nuclear fractions) were approximately threefold higher than those detected in the R−/ΔRI/cPPT− infected sample, thus confirming the data obtained from Fig. 4A. Interestingly, while approximately 75% of total viral cDNA was detected in the nuclear fraction of R−/ΔRI-infected cells, only 30% of total viral cDNA was found in the nuclei of R−/ΔRI/cPPT−-infected cells (Fig. 4C, upper panel). Moreover, the absolute levels of nuclear-associated viral cDNA were approximately sevenfold higher with the wt virus than with the cPPT-defective virus. The integrity of the fractionation procedure was validated by detection of mitochondrial DNA and β-globin DNA, as described previously (18, 36, 38). Results showed that mitochondrial and β-globin DNAs were found solely in the cytoplasm and the nucleus, respectively (Fig. 4C, lower panel). In addition, levels of each of these control cellular DNAs were similar in both R−/ΔRI and R−/ΔRI/cPPT− subcellular fractions, confirming that equivalent amounts of nuclear and cyctoplasmic fractions were analyzed.
Several recent studies have investigated the role of the central DNA flap in HIV-1 replication and nuclear import and have reached conflicting conclusions (11, 13, 17, 29, 30, 33, 34, 37, 45, 46). In this study, we reexamined this question by using RT and IN transcomplemented HIV-1 viral particles capable of a single round of replication. Our results reveal that the central DNA flap was not essential to HIV-1 replication but conferred a five- to sevenfold infectivity advantage to single-cycle replicating viruses in a variety of cellular systems, including MAGI cells, MT4, dividing and nondividing C8166 T-cell lines, and h-PBMCs (Fig. 1and 2 and data not shown). These results are consistent with findings reported by several previous studies that the central DNA flap conferred a transduction advantage of 2- to 10-fold on vesicular stomatitis virus-G-pseudotyped HIV-1 vectors (11, 13, 17, 29, 30, 33, 34, 37, 45, 46). At this point, it is still unclear why this substantial defect in single-round infectivity caused by disruption of the DNA flap does not translate into a detectable difference when the replication kinetic is monitored by using replication-competent virus, as shown recently (13, 29). Clearly, more studies in this area are required to understand this discrepancy.
In an attempt to understand the mechanism(s) underlying the effect of the central DNA flap during HIV-1 single-cycle replication, we analyzed by PCR the amount of integrated proviral DNA in the presence or absence of the central DNA flap. Our results clearly show that disruption of the DNA flap results in a five- to sevenfold decrease in proviral DNA integration (Fig. 4), suggesting that the central DNA flap contributes to efficient single-cycle viral replication by acting on an early stage(s) of the HIV-1 infection cycle at and/or prior to viral integration. We further analyzed total late-reverse-transcribed DNA products over time during cPPT+ or cPPT− single-cycle viral infection and determined the distribution of total viral cDNA in the nucleus and the cytoplasm. Our results reveal that the presence of the central DNA flap does not significantly influence the amount of viral transcripts produced at early time points (6 h) but contributes primarily to an accumulation of viral cDNA in the nucleus (Fig. 4). These results are consistent with findings made by several previous studies using either replication-competent viruses or single-round HIV-1 vector transduction systems that the central cDNA flap enhances the establishment of HIV-1 infection by facilitating the nuclear import of proviral DNA (17, 30, 45).
In addition to the effect on viral cDNA nuclear import, our data also suggest that the presence of the central DNA flap might have a stabilizing and/or a protective effect on viral cDNA (Fig. 4). Indeed, it is possible that the central DNA flap might contribute to a correct conformation of viral cDNA and/or be implicated in the recruitment of host cell proteins to form a functional PIC capable of effective proviral DNA nuclear import. Absence of an intact central DNA flap might lead to immature PIC where viral cDNA is less stable or subject to rapid degradation. In this regard, it has recently been reported that the central DNA flap region of viral cDNA was resistant to DNase I digestion when viral PIC complexes were isolated from the cytoplasm of infected cells at 10 h, whereas it was sensitive to degradation when complexes were isolated at 8.5 h after infection (25). Alternatively, it may also be possible that the presence of the central DNA flap positively modulates the stability of viral cDNA in the nucleus. Interestingly, a similar difference in the rate of decline of wt and cPPT-defective total viral cDNA was observed by Limon et al. (29) in infected PBMCs, although the effect was not as pronounced as in our study. Altogether, these observations point toward a possible role of the central DNA flap in the formation and maturation of HIV-1 PICs; such a role is likely to influence viral cDNA stability and nuclear import.
Acknowledgments
We thank Nicole Rougeau, Johanne Mercier, and Serge Senechal for technical support. We thank Sylvie Beaulieu, Luchino Y. Cohen, and Ghislaine Duisit for fruitful discussions. We are also grateful to M. Emerman for the HeLa-CD4-β-Gal cells, which were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
Xiaojian Yao is a recipient of a Médecine-Relève 2000-Messenger Foundation Award from the Faculté de Médecine, Université de Montréal. Éric A. Cohen is the recipient of the Canada Research Chair in Human Retrovirology. This work was supported by grants from the Canadian Foundation for AIDS Research (CANFAR) (X.Y), the Canadian Institute of Health Research (CIHR), and the Fonds de la Recherche en Santé du Québec (FRSQ) (E.A.C.).
REFERENCES
- 1.Ansari-Lari, M. A., L. A. Donehower, and R. A. Gibbs. 1995. Analysis of human immunodeficiency virus type 1 integrase mutants. Virology 211:332-335. [DOI] [PubMed] [Google Scholar]
- 2.Ansari-Lari, M. A., and R. A. Gibbs. 1996. Expression of human immunodeficiency virus type 1 reverse transcriptase in trans during virion release and after infection. J. Virol. 70:3870-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouyac-Bertoia, M., J. D. Dvorin, R. A. Fouchier, Y. Jenkins, B. E. Meyer, L. I. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. [DOI] [PubMed] [Google Scholar]
- 4.Bukovsky, A., and H. Gottlinger. 1996. Lack of integrase can markedly affect human immunodeficiency virus type 1 particle production in the presence of an active viral protease. J. Virol. 70:6820-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukrinsky, M., and O. K. Haffar. 1998. HIV-1 nuclear import: matrix protein is back on center stage, this time together with Vpr. Mol. Med. 4:138-143. [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charneau, P., M. Alizon, and F. Clavel. 1992. A second origin of plus-strand synthesis is required for optimal human immunodeficiency virus replication. J. Virol. 66:2814-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription: a termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 9.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffin, J. M. 1990. Retroviridae and their replication. In B. N. Fields and D. M. Knipe (ed.), Fields virology, 2nd ed. Raven Press, New York, N.Y. 1437-1500.
- 11.Dardalhon, V., B. Herpers, N. Noraz, F. Pflumio, D. Guetard, C. Leveau, A. Dubart-Kupperschmitt, P. Charneau, and N. Taylor. 2001. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 8:190-198. [DOI] [PubMed] [Google Scholar]
- 12.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 13.Dvorin, J. D., P. Bell, G. G. Maul, M. Yamashita, M. Emerman, and M. H. Malim. 2002. Reassessment of the roles of integrase and the central DNA flap in human immunodeficiency virus type 1 nuclear import. J. Virol. 76:12087-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman, A., G. Englund, J. M. Orenstein, M. A. Martin, and R. Craigie. 1995. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 69:2729-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farnet, C. M., and W. A. Haseltine. 1996. HIV cDNA integration: molecular biology and inhibitor development. AIDS 10:S3-S11. [PubMed] [Google Scholar]
- 16.Fletcher, T. M., III, M. A. Soares, S. McPhearson, H. Hui, M. Wiskerchen, M. A. Muesing, G. M. Shaw, A. D. Leavitt, J. D. Boeke, and B. H. Hahn. 1997. Complementation of integrase function in HIV-1 virions. EMBO J. 16:5123-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Follenzi, A., L. E. Ailles, S. Bakovic, M. Geuna, and L. Naldini. 2000. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat. Genet. 25:217-222. [DOI] [PubMed] [Google Scholar]
- 18.Forget, J., X. J. Yao, J. Mercier, and E. A. Cohen. 1998. Human immunodeficiency virus type 1 Vpr protein transactivation function: mechanism and identification of domains involved. J. Mol. Biol. 284:915-923. [DOI] [PubMed] [Google Scholar]
- 19.Fouchier, R. A., B. E. Meyer, J. H. Simon, U. Fischer, A. V. Albright, F. Gonzalez-Scarano, and M. H. Malim. 1998. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J. Virol. 72:6004-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallay, P., T. Hope, D. Chin, and D. Trono. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. USA 94:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goff, S. P. 1992. Genetics of retroviral integration. Annu. Rev. Genet. 26:527-544. [DOI] [PubMed] [Google Scholar]
- 22.Greene, W. C., and B. M. Peterlin. 2002. Charting HIV's remarkable voyage through the cell: basic science as a passport to future therapy. Nat. Med. 8:673-680. [DOI] [PubMed] [Google Scholar]
- 23.Haffar, O. K., S. Popov, L. Dubrovsky, I. Agostini, H. Tang, T. Pushkarsky, S. G. Nadler, and M. Bukrinsky. 2000. Two nuclear localization signals in the HIV-1 matrix protein regulate nuclear import of the HIV-1 pre-integration complex. J. Mol. Biol. 299:359-368. [DOI] [PubMed] [Google Scholar]
- 24.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khiytani, D. K., and N. J. Dimmock. 2002. Characterization of a human immunodeficiency virus type 1 pre-integration complex in which the majority of the cDNA is resistant to DNase I digestion. J. Gen. Virol. 83:2523-2532. [DOI] [PubMed] [Google Scholar]
- 26.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Rouzic, E., A. Mousnier, C. Rustum, F. Stutz, E. Hallberg, C. Dargemont, and S. Benichou. 2002. Docking of HIV-1 Vpr to the nuclear envelope is mediated by the interaction with the nucleoporin hCG1. J. Biol. Chem. 277:45091-45098. [DOI] [PubMed] [Google Scholar]
- 28.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limon, A., N. Nakajima, R. Lu, H. Z. Ghory, and A. Engelman. 2002. Wild-type levels of nuclear localization and human immunodeficiency virus type 1 replication in the absence of the central DNA flap. J. Virol. 76:12078-12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maele, B. V., J. D. Rijck, E. D. Claercq, and Z. Debyser. 2003. Impact of the central polypurine tract on the kinetics of human immunodeficiency virus type 1 vector transduction. J. Virol. 77:4685-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maertens, G., P. Cherepanov, W. Pluymers, K. Busschots, E. De Clercq, Z. Debyser, and Y. Engelborghs. 2003. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J. Biol. Chem. 278:33528-33539. [DOI] [PubMed] [Google Scholar]
- 32.Nie, Z., D. Bergeron, R. A. Subbramanian, X. J. Yao, F. Checroune, N. Rougeau, and E. A. Cohen. 1998. The putative alpha helix 2 of human immunodeficiency virus type 1 Vpr contains a determinant which is responsible for the nuclear translocation of proviral DNA in growth-arrested cells. J. Virol. 72:4104-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, F., and M. A. Kay. 2001. Modified HIV-1 based lentiviral vectors have an effect on viral transduction efficiency and gene expression in vitro and in vivo. Mol. Ther. 4:164-173. [DOI] [PubMed] [Google Scholar]
- 34.Parolin, C., B. Taddeo, G. Palu, and J. Sodroski. 1996. Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology 222:415-422. [DOI] [PubMed] [Google Scholar]
- 35.Petit, C., O. Schwartz, and F. Mammano. 2000. The karyophilic properties of human immunodeficiency virus type 1 integrase are not required for nuclear import of proviral DNA. J. Virol. 74:7119-7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon, J. H. M., and M. H. Malim. 1996. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J. Virol. 70:5297-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sirven, A., F. Pflumio, V. Zennou, M. Titeux, W. Vainchenker, L. Coulombel, A. Dubart-Kupperschmitt, and P. Charneau. 2000. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood 96:4103-4110. [PubMed] [Google Scholar]
- 38.Vandegraaff, N., R. Kumar, H. Hocking, T. R. Burke, Jr., J. Mills, D. Rhodes, C. J. Burrell, and P. Li. 2001. Specific inhibition of human immunodeficiency virus type 1 (HIV-1) integration in cell culture: putative inhibitors of HIV-1 integrase. Antimicrob. Agents Chemother. 45:2510-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg, J. B., T. J. Matthews, B. R. Cullen, and M. H. Malim. 1991. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J. Exp. Med. 174:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, X., H. Liu, H. Xiao, J. A. Conway, E. Hunter, and J. C. Kappes. 1997. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. EMBO J. 16:5113-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao, X. J., G. Kobinger, S. Dandache, N. Rougeau, and E. Cohen. 1999. HIV-1 Vpr-chloramphenicol acetyltransferase fusion proteins: sequence requirement for virion incorporation and analysis of antiviral effect. Gene Ther. 6:1590-1599. [DOI] [PubMed] [Google Scholar]
- 43.Yao, X. J., A. J. Mouland, R. A. Subbramanian, J. Forget, N. Rougeau, D. Bergeron, and E. A. Cohen. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao, X. J., R. A. Subbramanian, N. Rougeau, F. Boisvert, D. Bergeron, and E. A. Cohen. 1995. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J. Virol. 69:7032-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]
- 46.Zennou, V., C. Serguera, C. Sarkis, P. Colin, E. Perret, J. Mallet, and P. Charneau. 2001. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat. Biotechnol. 19:446-450. [DOI] [PubMed] [Google Scholar]