Abstract
3′-deoxy-3′-[18F]fluoro-L-thymidine (FLT) and 2′-deoxy-2′-[18F]fluoro-D-glucose (FDG) are used to visualize proliferative and metabolic activity of tumors. In this study we aimed at evaluating the prognostic value of FLT and FDG uptake measured by positron emission tomography (PET) in patients with metastatic non-small cell lung cancer (NSCLC) prior to systemic therapy with erlotinib. FLT and FDG maximum standardized uptake (SUVmax) values per patient were analyzed in 40 chemotherapy naive patients with advanced NSCLC (stage IV) before treatment with erlotinib. Prior therapy median SUVmax was 6.6 for FDG and 3.0 for FLT, respectively. In univariate analysis, patients with an FDG SUVmax <6.6 had a significantly better overall survival (16.3 months [95% confidence interval [CI] 7.1–25.4 months]) compared to patients with an FDG SUVmax ≥6.6 (3.1 months [95% CI 0.6–5.5 months]) (p<0.001, log rank). Similarly, low FLT uptake (SUVmax <3.0) was associated with significantly longer survival (10.3 months (0–23.3 months, 95% CI) compared to high FLT uptake (3.4 months (0–8.1 months, 95% CI) (p = 0.027). The independent prognostic value of baseline FDG uptake was demonstrated in multivariate analysis (p = 0.05, Cox regression). These data suggest that baseline SUVmax values for both FDG and FLT PET might be further developed as markers for prognostic stratification of patients in advanced NSCLC treated with tyrosine kinase inhibitors (TKI) directed against the epidermal growth factor receptor (EGFR).
Trial Registration
Clinicaltrials.gov, Identifier: NCT00568841
Introduction
Prognostic factors may help to understand the biological heterogeneity of malignant disease and, ultimately, to develop individualized therapeutic strategies for distinct subgroups. In advanced NSCLC, several pretherapeutic prognostic factors have been identified, among these disease stage and performance state [1], [2], [3]. Increasingly, genetic alterations are identified with prognostic as well as predictive potential concerning the use of molecularly targeted drugs. Activating mutations in the epidermal growth factor receptor (EGFR) for instance indicate a better prognosis independent from therapy as well as a favorable outcome with EGFR tyrosine kinase inhibitor (TKI) therapy [4], [5], [6], [7], [8], [9]. However, molecular analyses are not always feasible due to limitations regarding tissue availability and quality [10]. These problems might be circumvented by noninvasive methods.
Molecular imaging tools gain in importance for assessment of tumor biology with and without therapy. 2′-deoxy-2′-[18F]fluorodeoxyglucose (FDG) is by far the most commonly used PET tracer, visualizing glucose metabolism. In early stage NSCLC, reports were ambiguous concerning the prognostic value of preoperative FDG uptake, whereas there was no prognostic value in advanced NSCLC treated with standard chemotherapy [1], [11], [12], [13]. In two recent trials, FDG was superior to 3′-Deoxy-3′-[18F]fluorothymidine (FLT) in early predicting response and nonprogression in NSCLC patients treated with erlotinib [14], [15]. The use of FDG as a tool for early response prediction was also confirmed in patients with advanced NSCLC undergoing chemoradiotherapy [16]. In esophageal cancer, FDG baseline activity is predictive for response [17]. In BRAF-mutated advanced melanoma treated with vemurafenib, there was a trend for longer profession-free survival (PFS) in patients with low metabolic disease assessed by FDG-PET [18].
FLT is a noninvasive marker of proliferation and has been shown to correlate with Ki-67 expression in NSCLC [19], [20], [21], [22]. Proliferative activity has been discussed to have a negative impact on survival [23], [24], [25], although the definitive relationship remains unclear [26]. In NSCLC, the ability of FLT as a PET tracer to early visualize G1-cell cycle arrest and induction of apoptosis was demonstrated in xenotransplanted cell lines sensitive to erlotinib, and early reduction of FLT uptake predicted response in patients treated with gefitinib and erlotinib [27], [28], [29]. In patients with aggressive B-cell lymphomas treated with the R-CHOP regimen high baseline FLT uptake is a negative predictor for response [30]. In patients with NPM-ALK-positive lymphomas treated with targeted therapy, FLT-PET was superior to FDG-PET for very early response prediction [31].
Based on results of a monocentric clinical trial, we analyzed if already the initial proliferative (FLT) or metabolic (FDG) activity of NSCLC tumors assessed by PET is associated with overall survival irrespective of clinical trial protocol adherence, follow-up treatments or very early progression and how EGFR mutational status and Ki-67 immunohistochemistry as well as clinical parameters contribute to these findings.
Patients and Methods
Patients
Between September 2007 and September 2009, patients with cytologically or histologically confirmed metastatic NSCLC (International Union Against Cancer [UICC] stage IV) and without prior systemic treatment had undergone one FDG-PET and one FLT-PET prior to systemic therapy within the screening program of the ERLOPET trial (NCT00568841), which was approved by the institutional review board, the local ethics committee and the respective federal and state authorities, including the German Authority for Radiation Safety. 34 of the 40 patients presented here could be analyzed in the ERLOPET trial. The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1. All patients gave written informed consent. As part of the screening process, the patients had to be at least 18 years old, with an Eastern Cooperative Oncology Group (ECOG) performance state ≤2, neither decompensated liver nor heart failure, a serum creatinine level <1.7 mg/dL, and normal blood glucose levels (<120 mg/dl). Patients with brain metastases requiring further local treatment were not excluded. For this analysis all patients that underwent baseline PET were evaluated irrespective of later trial exclusion e.g. due to stop of medication.
Treatment
All patients were intended to start with erlotinib 150 mg/d for at least six weeks or until disease progression. In case of progression, a platinum-based combination therapy was the recommended treatment option. After progress to platinum-based chemotherapy patients were treated either with chemotherapy (pemetrexed, docetaxel, gemcitabine or vinorelbine) or targeted therapy (sorafenib plus everolimus within a clinical trial (NCT00933777) or afatinib within a compassionate use program). The mean number of treatment regimens was 2 (range, 0 to 5), with the following regimens used: carboplatin/paclitaxel +/−bevacizumab, cisplatin/vinorelbine +/− cetuximab, pemetrexed, pemetrexed maintenance, docetaxel, gemcitabine, oral vinorelbine, sorafenib/everolimus (one patient in forth-line setting,) and afatinib. Early palliative care was performed as described [32]. Radiation therapy was performed whenever indicated: 9 patients (23%) were pretreated with radiation therapy (5 patients receiving either whole-brain radiation or stereotactic intervention due to brain metastases, 2 patients with local treatment of symptomatic bone metastases, 2 patients with mediastinal/lung radiation due to local complications), 7 patients (18%) received radiation therapy while being treated with erlotinib (2 whole-brain radiation, 5 bone metastases), and 5 patients were irradiated after stop of erlotinib treatment and change to an alternative systemic therapy (1 whole-brain radiation, 2 local irradiation, 2 bone metastases). One patient died before start of therapy. 13 patients [32.5%] had brain metastases at baseline. In general, radiation therapy was performed in 21 (52.5%) patients, whereof 9 patients (22.5%) had to start radiation therapy due to local complications (brain, bones) before administration of systemic therapy. Some of the details are also shown in table 1 .
Table 1. Patient characteristics.
| Characteristics | Number (%) | |
| All patients | 40 (100) | |
| Gender | female | 21 (53) |
| male | 19 (47) | |
| Histology | Adeno/BAC | 34 (85) |
| others | 6 (15) | |
| ECOG | 0 | 17 (42.5) |
| 1 | 17 (42.5) | |
| 2 | 6 (15) | |
| EGFR mutation detected | yes | 5 (12.5) |
| no | 35 (87.5) | |
| Brain metastases | yes | 13 (32.5) |
| no | 27 (67.5) | |
| Local radiation | yes | 21 (52.5) |
| no | 19 (47.5) |
Response evaluation
Response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.0 [33]. The first computed tomography (CT) scan was performed after six weeks of treatment. Follow-up CT scans were performed every 12 weeks or in case of clinically suspected progression. A 16-slice multidetector CT scanner (Brilliance 16, Philips Medical Systems, Eindhoven, the Netherlands) was used.
Clinical parameters
The following parameters were assessed prior to therapy start to evaluate their impact on overall survival: age (dichotomized), ECOG (0–2), EGFR mutational status, histology (adeno/bronchiolo alveolar carcinoma histology vs non-adeno/BAC), gender.
PET-Imaging
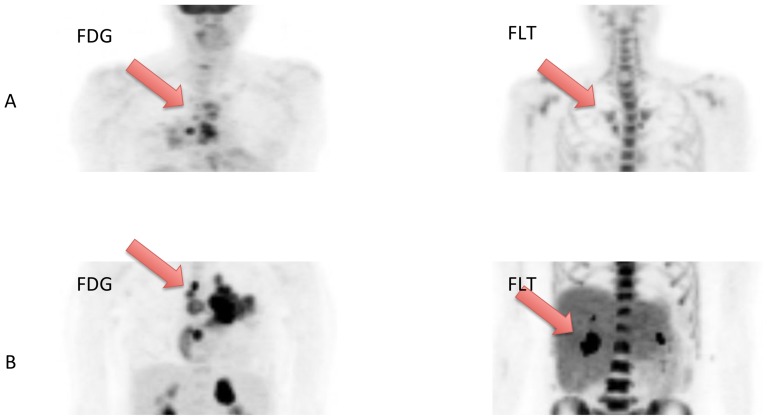
FLT-PET and FDG-PET were performed before treatment administration. Both tracers were synthesized as described before [34]. The images were obtained using an ECAT EXACT 47 (Siemens, Erlangen, Germany). Patients had to be fasting for at least 6 hours. 60 minutes after injection of 300 MBq FLT or 370 MBq FDG, the PET acquisition started. The attenuation-corrected scan trajectory covered 90 cm (6 bed positions: 5 min emission, 3 min transmission). All scans were corrected for decay, dead time, scatter and randoms, and reconstructed by ordered subset expectation maximization. The same protocol for acquisition and the same software for reconstruction were used. The maximum standardized uptake value (SUVmax) normalized to body weight was assessed using the voxel with the maximum uptake on reconstructed PET images without additional rebinning, resampling, or smoothing. Up to five lesions with the highest SUVmax uptakes were coregistered. The highest SUVmax for the respective tracer, not necessarily the same lesions, were taken into analysis ( figure 1 ).
Figure 1. Example of two patients with low and high baseline uptake of FDG and FLT.
The patient shown in figure A with low uptake is a 66-year old female patient who had an overall survival of 21.3 months, whereas the patient in B with a high uptake is a 56-year old female patient with an overall survival of only 1.5 months. In both cases, the respective most active lesion was chosen for assessment.
Molecular analysis
Tumor material from the initial diagnosis of NSCLC was analysed for EGFR mutational status. If there was still tumor material left after the mutational analyses, Ki-67 immunohistochemistry staining was performed.
EGFR mutational status was assessed as recently reported, using PCR and dideoxy sequencing, pyrosequencing and massively parallel sequencing analyses dependent on tissue quality and amount of tumor cells . Ki-67 immunohistochemistry staining was performed using standard techniques.
Statistical analysis
The trial was powered for its primary objective [14]. Time-to-event analyses were exploratorily assessed. Overall survival (OS) and PFS were defined as the time from start of treatment until the respective event and analysed using Kaplan-Meier estimates and log rank tests for univariate analysis. For continuous parameters, the median was chosen to divide the cohort in order to build homogenous subgroups of patients. Parameters showing statistical significance (p≤0.05) in univariate analysis were included in a Cox regression for multivariate analysis. FDG- and FLT-SUVmax values and age were dichotomized by their median to achieve homogenous subgroups for Kaplan-Meier estimates. For correlation analysis, Pearson's correlations were used. For response analysis, receiver-operator-characteristics (ROC) curves were created. Students T test was performed were applicable.
Results
Patients
40 patients received both FLT-PET and FDG-PET prior to treatment start. At data cut-off (May 18th, 2011), 4 patients (10%) were still alive, with a median follow-up of 25.6 months (range, 23.4–34.0). 3 of these patients are women with adenocarcinoma, one with a detected EGFR mutation. The fourth is a male patient with squamous-cell carcinoma. 34 patients had adenocarcinoma/BAC histology (85%). The mean age was 62.5 years (range, 38–78 years). 31 patients (77.5%) had tumor tissue available (formalin-fixed paraffin-embedded biopsies, stained cytospins) for EGFR mutational analysis. Thereafter, 18 tissue samples remained for Ki-67 staining. Five patients (12.5%) had sensitizing EGFR mutations (4 deletions in exon 19, one L858R). Patient characteristics are shown in table 1 . Six patients (15%) had an ECOG 2 performance state, 17 patients (42.5%) ECOG 1. One patient died immediately (3 days) after PET scans due to deterioration of an underlying pneumonia. For this patient, time from the PET scans until death was calculated for time-to-event analyses.
5 patients (12.5%) responded to erlotinib, and additional 7 patients (17.5%) had a stable disease lasting for at least 18 weeks. 28 patients (70%) had either documented progressive-disease (PD) in the first CT scan after six weeks of treatment or clinical progression before, including rapid deaths.
Clinical parameters
Of the clinical parameters tested, only the division of patients by the median of age (62.5 years) led to significantly different groups regarding median OS (mOS), favoring older patients (14.9 months [95% CI, 3.0–26.7 months] vs 3.4 months [0–6.8 months, 95% CI], p = 0.030). Presence of an activating EGFR mutation status had no significant impact on survival in our cohort (EGFR mut: mOS of 21.3 months (9.9–32.8 months; EGFR wt: 4.8 months (2.3–7.3); log-rank (p = 0.087)). Similarly, OS differences did not reach significance in log rank for gender (mOS in women 10.3 months (2.3–18.2) compared to 4.8 months (2.6–7.0) in men; p = 0.214, log rank), histology (mOS in non-adeno/BAC 0.9 months (0–4.2) compared with 5.4 months in adenocarcinoma (0–12.4); p = 0.467, log rank) and performance state (ECOG 0 = 14.9 (7.0–22.8); ECOG 1 = 1.4 (2.6–8.1) ECOG 3 = 0.3 (0.1–1.2); p = 0.141, log rank).
In contrast, EGFR mutational status (p = 0.008, log rank), age (p = 0.009, log rank, in favor of the older patients), and ECOG performance status (p = 0.004, log rank) were significantly associated with a longer progression free survival.
PET analysis
The majority of patients (n = 36, 90%) underwent PET scans on consecutive days. In one patient, FDG-PET scan was conducted two days prior to FLT-PET scan. In another patient, FDG-PET was performed four days before FLT-PET, while in two patients, FLT-PET was performed three days prior to FDG-PET. Table 2 shows the individual patient characteristics and PET results.
Table 2. Individual patient characteristics and PET results.
| Pat-ID | Gender | Age | Histology | SUVmax (FDG) | Tissue (SUVmax FDG) | SUVmax (FLT) | Tissue (SUVmax FLT) | Differernt lesions |
| 01-01 | f | 53 | Adeno | 7,9 | lung | 2,6 | lymph node | yes |
| 01-02 | m | 69 | Adeno | 7,5 | adrenal gland | 3,3 | adrenal gland | no |
| 01-03 | m | 58 | Adeno | 7,8 | lung | 3,3 | adrenal gland | yes |
| 01-04 | f | 69 | Adeno | 13,0 | lung | 2,5 | lung | no |
| 01-05 | m | 67 | SCC | 11,9 | lung | 3,3 | lung | no |
| 01-06 | m | 69 | SCC | 8,3 | lung | 2,9 | lung | no |
| 01-07 | f | 69 | SCC | 5,7 | lung | 3,9 | lymph node | yes |
| 01-08 | m | 64 | Adeno | 4,0 | pleura | 2,5 | lung | yes |
| 01-09 | m | 55 | Adeno | 9,3 | pleura | 5,5 | bone | yes |
| 01-10 | m | 38 | SCC | 5,5 | lymph node | 4,0 | thoracic wall | yes |
| 01-11 | m | 51 | Adeno | 3,4 | bone | 1,3 | bone | no |
| 01-12 | f | 53 | Adeno | 1,6 | adrenal gland | 1,3 | adrenal gland | no |
| 01-13 | m | 55 | Adeno | 6,6 | thoracic wall | 4,6 | pleura | yes |
| 01-14 | m | 60 | Adeno | 4,5 | lung | 2,5 | lung | no |
| 01-15 | m | 53 | Adeno | 7,0 | lymph node | 3,4 | lymph node | no |
| 01-16 | f | 45 | Large cell | 6,6 | bone | 1,9 | bone | no |
| 01-17 | f | 72 | Adeno | 5,7 | lung | 5,5 | lung | no |
| 01-18 | f | 78 | BAC | 2,0 | lung | 1,8 | lung | no |
| 01-19 | m | 67 | BAC | 3,9 | lung | 1,6 | lung | no |
| 01-20 | m | 60 | BAC | 4,0 | bone | 1,5 | bone | no |
| 01-21 | f | 57 | Adeno | 11,0 | adrenal gland | 5,5 | lung | yes |
| 01-22 | f | 61 | Adeno | 5,8 | thoracic wall | 5,0 | bone | yes |
| 01-23 | f | 55 | Adeno | 2,8 | lung | 2,0 | lymph node | yes |
| 01-24 | m | 67 | Adeno | 6,3 | lung | 2,3 | lung | no |
| 01-25 | f | 61 | Adeno | 1,9 | pleura | 1,4 | pleura | no |
| 01-26 | m | 63 | Adeno | 8,0 | lung | 5,0 | lung | no |
| 01-27 | f | 66 | Adeno | 5,1 | lymph node | 3,0 | lymph node | no |
| 01-28 | f | 71 | Adeno | 7,2 | lymph node | 2,7 | lymph node | no |
| 01-29 | f | 57 | SCC | 3,9 | lymph node | 4,1 | lymph node | yes |
| 01-30 | f | 73 | Adeno | 2,5 | lymph node | 1,5 | lymph node | no |
| 01-31 | f | 68 | Adeno | 3,6 | lung | 1,3 | lung | no |
| 01-32 | f | 48 | Adeno | 9,1 | lung | 5,0 | lung | no |
| 01-33 | m | 71 | SCC | 3,0 | lymph node | 3,0 | lymph node | no |
| 01-34 | m | 75 | Adeno | 13,0 | lung | 3,0 | lung | no |
| 01-35 | m | 77 | Adeno | 8,5 | bone | 3,0 | lymph node | yes |
| 01-36 | f | 72 | Adeno | 13,3 | lymph node | 5,3 | lymph node | no |
| 01-37 | f | 56 | Adeno | 9,7 | lung | 2,8 | lung | no |
| 01-38 | f | 58 | Adeno | 12,3 | bone | 2,2 | bone | no |
| 01-39 | f | 78 | Adeno | 7,1 | lung | 3,0 | lung | no |
| 01-40 | m | 62 | BAC | 7,4 | lung | 4,8 | lung | yes |
By analysing up to 5 lesions per patient we assessed the lesions with the highest SUVmax values in both FDG and FLT. In 13 patients (32.5%) the lesions differed between FDG and FLT (see table 2 ).
A total of 157 lesions (mean, 3.9 lesions per patient) detected with FDG-PET were analyzed. Of these 157 lesions, 134 (85.4%) showed activity in FLT-PET, too (mean, 3.4 lesions per patient). The 23 discrepant lesions, which did not show activity in FLT-PET, were located within the following tissues: bone (n = 8, 34.8%), lymph nodes (n = 6, 26.1%), liver (n = 3, 13.0%), thoracic wall (n = 3, 13.0%), pleura (n = 2) and adrenal gland (n = 1).
Association of baseline SUVmax and overall survival (OS)
All patients underwent both FDG- and FLT-PET 0–9 days prior to start of therapy.
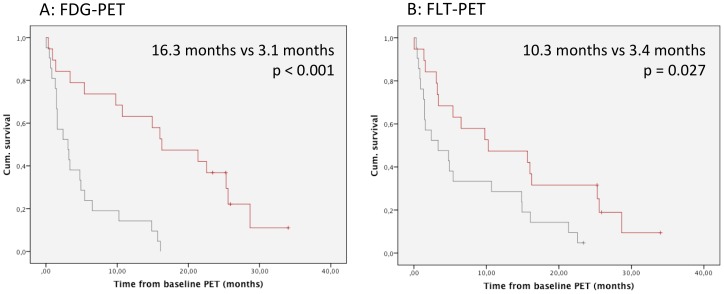
No significant differences in mean SUVmax of FLT and FDG could be noted between patients with or without adeno/BAC histology (p = 0.921 for FDG and p = 0.873 for FLT, T-test). The SUVmax values of the most active tumor manifestation for FDG had a mean of 6.7 (5.7–7.7) and a median of 6.6. Taking this value as a cut-off, two groups were built. Patients with low SUVmax (SUV<6.6) demonstrate a significantly longer survival (Hazard ratio [HR] 4.3, [95% CI 1.9–9.6]; p<0.001) of 16.3 months (7.1–25.4, n = 19) when compared to patients with high SUVmax (3.1 months, 0.6–5.5, n = 21)( figure 2A ).
Figure 2. Kaplan Meier curves showing overall survival depending on SUVmax values.
A) Overall survival of patients with high (>6.7; grey) or low (<6.7; red) baseline SUVmax in FDG-PET. (B) Overall survival of patients with high (>3; grey) or low (<3; red) baseline SUVmax in FLT-PET.
For FLT, SUVmax had a mean value of 3.1 (2.7–3.6), confirming the reported ratio of FDG/FLT [20], [35]. The median value of SUVmax for FLT was 3.0. Patients with an SUVmax <3.0 (n = 19) had a mOS of 10.3 months (0–23.3), and patients with an SUVmax ≥3.0 (n = 21) had a mOS of 3.4 months (0–8.1)(HR 2.2 [95% CI 1.1–4.4]; p = 0.027) ( figure 2B ).
The SUVmax values of FDG and FLT were significantly correlated (Pearson correlation coefficient 0.468, p = 0.002). Baseline FDG-PET was shown to be an independent prognostic factor in a multivariate cox regression model including FLT, FDG and age as categorial (p = 0.05) or continous (p = 0.001) variables. Even when adding EGFR mutation into the model, baseline FDG SUVmax remained an independent prognostic factor (p = 0.002) Also in the group of patients without detected EGFR mutation low SUVmax (<6.6) in FDG-PET was associated with a significantly better overall survival (10.7 months mOS (0.7–20.8 months) vs 3.1 (0.8–5.4 months)(p = 0.002). No such association was observed for FLT-PET in this group (p = 0.077).
We also investigated if one of the parameters (FDG-/FLT-uptake) acts as a prognostic marker in the absence of the other. For FDG SUVmax in Cox regression with age, a p-value<0.001 was reached (age, p = 0.016). For FLT SUVmax, both FLT and age reached significance (p = 0.017 and p = 0.018).
Association of baseline SUVmax and PFS and response
The mean SUVmax values of EGFR-mutated tumors for both FDG and FLT were significantly lower than in tumors not harboring a mutation (p = 0.033 for FDG and p = 0.027 for FLT, T-test). Consequently, we observed an association between SUVmax in baseline FDG- and FLT-PET and response to erlotinib treatment (AUC of 0.79 (FDG) and 0.78 (FLT) (p = 0.035 for FDG, p = 0.043 for FLT). This higher probability of response in patients with low FDG and FLT uptake did not transfer into a prolonged PFS.
Ki-67 staining and correlation with PET and EGFR mutational status
Ki-67 was stained in 18 samples to independently assess the proliferative status of these tumors. Overall, no significant correlation between Ki-67 staining and SUVmax of FDG and FLT was found, but patients with low FLT SUVmax values tended to have low Ki-67 activity. Nevertheless, this was not significant (p = 0.168). For FDG, virtually no difference of Ki-67 percentage could be determined in patients with high or low baseline SUVmax (p = 0.936, T-test).
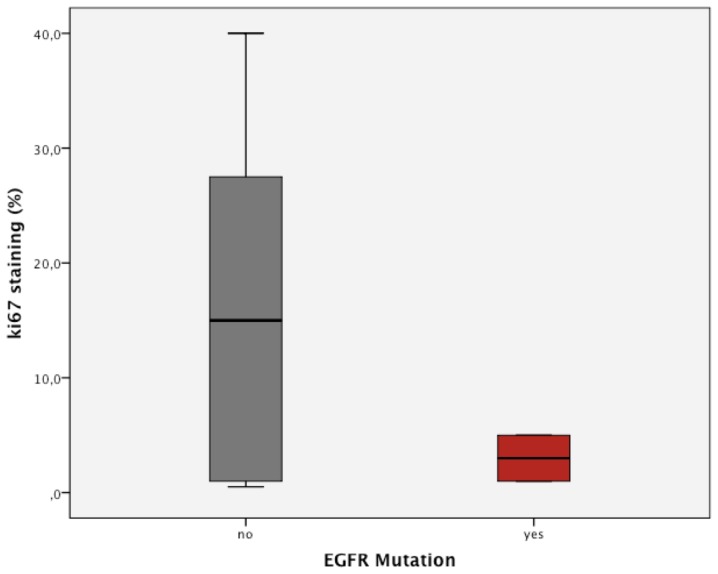
Patients with EGFR mutations showed a significantly lower percentage of Ki-67 positive cells compared to wildtype patients (p = 0.01, T-test) ( figure 3 ). Further, the three responding patients had a significantly lower Ki-67 percentage than patients not responding (p = 0.002, T-test).
Figure 3. Correlation of the EGFR mutational status with Ki-67 staining in %.
The median positive staining percentage was 10%. There was no significant difference between patients with a percentage <10% and patients with a percentage ≥10% regarding OS (p = 0.225, log rank), but PFS differed significantly (6.0 [3.8–8.2] vs 1.6 [1.4–1.8] months, p = 0.030, log rank; for the low Ki-67 group).
Discussion
The aim of this hypothesis generating analysis was to evaluate and compare FDG and FLT baseline activity in the most active tumor manifestations regarding their impact on the prognosis of patients with newly diagnosed advanced NSCLC before start with erlotinib treatment. The baseline uptakes of both tracers are shown to be prognostic in univariate analysis, with FDG being an independent prognostic factor in multivariate analysis as the major finding. These results show that the initial metabolic (FDG) and proliferative (FLT) activity is associated with survival of the patients. Similarly, low metabolic and proliferative activity was associated with a higher probability of response in this group of patients.
Sensitizing EGFR mutations in NSCLC are the strongest predictors of PFS, response and OS under gefitinib and erlotinib therapy [9]. As the five patients with EGFR mutations in our analysis had significantly lower baseline uptakes of both FDG and FLT, it is tempting to speculate that EGFR mutated tumors have lower proliferative activity and that this might contribute to the better prognosis of these patients even if not treated with erlotinib or gefitinib. Similarly, Ki-67 staining demonstrated significantly lower Ki-67 positive cells in EGFR mutated tumors compared to EGFR wildtype tumors as has been reported previously [36]. Nevertheless, even after excluding the five patients with EGFR mutations from the analysis, the baseline FDG uptake remained a strong and independent prognostic factor.
Our results are in line with recent results showing the prognostic value of FDG-PET concerning OS in patients with NSCLC treated with standard chemotherapy and radiotherapy [37]. In contrast, the prognostic significance of FDG-PET was limited for patients with advanced ovarian cancer and in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy and radical surgery [38], [39] One thus might speculate, that the pretherapeutic tumor glucose metabolism per se is not a strong and independent predictor of overall survival, independent from tumor type and therapeutic modality.
FLT was introduced in cancer imaging as a proliferation-specific marker [40]. In our analysis, FLT was strongly prognostic in univariate analysis, but not in multivariate analysis. Thus, compared to FDG-PET, FLT does not add more specific information regarding prognosis. Whether this is due to the lack of tumor specificity of FLT as recently reported, remains an open question [38], [41], [42]. However, also the prognostic value of the tissue-based proliferation marker Ki-67 has not been established unequivocally so far for advanced NSCLC, most studies available focus on resectable tumors [25], [43], [44]. Surprisingly, in our dataset, there was no significant association between FLT uptake in the hottest lesion and Ki-67 staining in the tumor tissue obtained for diagnosis. This might, however, be due to the fact that in our series in many patients biopsy was not obtained from the lesion with the maximum SUVmax, clearly a limitation of this analysis. In addition, this observation underlines once more again the limited informative value of tissue-based biomarkers requiring invasive biopsy and thus being restricted to one tumor site. A recent meta-analysis describes the difficulties in interpreting findings from clinical trials [22]. Taken together, although FLT-PET seems to be a good tool for response prediction in some tumor entities, its prognostic value remains unclear.
In our analyses we selected up to 5 lesions with the highest activity in FDG- or FLT_PET for evaluation of the prognostic value in concordance with recent studies and recommendations for response prediction [45]. Clearly, this procedure does not allow any conclusion of the total tumor burden, which might be considered a further limitation of this study. On the other hand it is also conceivable to assume that the most active lesion in PET is the prognostically limiting lesion. Finally, we chose this procedure because of its easy-to-access character in clinical settings with the advantages of real-time detection and low inter-observer variability. This consideration also underlied the use of SUVmax and not other PET parameters like SUVpeak, SUVmean or SUVdispersion. We have recently shown that there are no significant differencies concerning the predictive potential of SUVmax and SUVpeak early after initiation of EGFR-therapy [46].
In summary, we show that the identification of the lesion with the highest metabolic activity in FDT-PET has significant prognostic relevance before initiation of erlotinib therapy independent of the EGFR-mutational status. Thus, pretherapeutic metabolic activity might be established in further studies as a risk-stratification tool for clinical trials in advanced NSCLC.
Supporting Information
CONSORT checklist of the trial. Please note that this trial was not randomized.
(DOC)
Trial protocol of the underlying trial of this study.
(PDF)
Funding Statement
This work was supported by the German Cancer Aid as part of the Interdisciplinary Oncology Centers of Excellence program to the Center for Integrated Oncology Köln Bonn and by the Federal German Ministry of Science and Education (BMBF) as part of the National Genome Research Network program (NGFNplus, grants 01GS08100 and 01GS08101) to JW and RT. MS was partly supported by the German Federal Ministry of Research and Education (BMBF grant 01KN0706). Erlotinib was supplied by Roche. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE 2nd, et al. (2008) Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26: 1459–1464. [DOI] [PubMed] [Google Scholar]
- 2. Paesmans M, Sculier JP, Libert P, Bureau G, Dabouis G, et al. (1995) Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 13: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 3. Takigawa N, Segawa Y, Okahara M, Maeda Y, Takata I, et al. (1996) Prognostic factors for patients with advanced non-small cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation. Lung cancer 15: 67–77. [DOI] [PubMed] [Google Scholar]
- 4. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, et al. (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353: 123–132. [DOI] [PubMed] [Google Scholar]
- 5. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139. [DOI] [PubMed] [Google Scholar]
- 6. Pao W, Miller V, Zakowski M, Doherty J, Politi K, et al. (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101: 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 8. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, et al. (2009) Screening for epidermal growth factor receptor mutations in lung cancer. The New England journal of medicine 361: 958–967. [DOI] [PubMed] [Google Scholar]
- 9. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, et al. (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29: 2866–2874. [DOI] [PubMed] [Google Scholar]
- 10. Querings S, Altmuller J, Ansen S, Zander T, Seidel D, et al. (2011) Benchmarking of mutation diagnostics in clinical lung cancer specimens. PloS one 6: e19601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, et al. (2005) [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23: 1136–1143. [DOI] [PubMed] [Google Scholar]
- 12. Vesselle H, Freeman JD, Wiens L, Stern J, Nguyen HQ, et al. (2007) Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clinical cancer research : an official journal of the American Association for Cancer Research 13: 3255–3263. [DOI] [PubMed] [Google Scholar]
- 13. Agarwal M, Brahmanday G, Bajaj SK, Ravikrishnan KP, Wong CY (2010) Revisiting the prognostic value of preoperative (18)F-fluoro-2-deoxyglucose ((18)F-FDG) positron emission tomography (PET) in early-stage (I & II) non-small cell lung cancers (NSCLC). European journal of nuclear medicine and molecular imaging 37: 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zander T, Scheffler M, Nogova L, Kobe C, Engel-Riedel W, et al. (2011) Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [(18)F]fluorodeoxyglucose and [(18)F]fluorothymidine positron emission tomography. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29: 1701–1708. [DOI] [PubMed] [Google Scholar]
- 15. Mileshkin L, Hicks RJ, Hughes BG, Mitchell PL, Charu V, et al. (2011) Changes in 18F-fluorodeoxyglucose and 18F-fluorodeoxythymidine positron emission tomography imaging in patients with non-small cell lung cancer treated with erlotinib. Clinical cancer research : an official journal of the American Association for Cancer Research 17: 3304–3315. [DOI] [PubMed] [Google Scholar]
- 16. Huang W, Zhou T, Ma L, Sun H, Gong H, et al. (2011) Standard uptake value and metabolic tumor volume of (1)(8)F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. European journal of nuclear medicine and molecular imaging 38: 1628–1635. [DOI] [PubMed] [Google Scholar]
- 17. Hatt M, Visvikis D, Pradier O, Cheze-le Rest C (2011) Baseline (1)(8)F-FDG PET image-derived parameters for therapy response prediction in oesophageal cancer. European journal of nuclear medicine and molecular imaging 38: 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McArthur GA, Puzanov I, Amaravadi R, Ribas A, Chapman P, et al. (2012) Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30: 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, et al. (2003) Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 44: 1426–1431. [PubMed] [Google Scholar]
- 20. Yap CS, Czernin J, Fishbein MC, Cameron RB, Schiepers C, et al. (2006) Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. Chest 129: 393–401. [DOI] [PubMed] [Google Scholar]
- 21. Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, et al. (2002) In vivo validation of 3′deoxy-3′-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 8: 3315–3323. [PubMed] [Google Scholar]
- 22. Chalkidou A, Landau DB, Odell EW, Cornelius VR, O'Doherty MJ, et al. (2012) Correlation between Ki-67 immunohistochemistry and 18F-Fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. European journal of cancer [DOI] [PubMed] [Google Scholar]
- 23. Hommura F, Dosaka-Akita H, Mishina T, Nishi M, Kojima T, et al. (2000) Prognostic significance of p27KIP1 protein and ki-67 growth fraction in non-small cell lung cancers. Clinical cancer research : an official journal of the American Association for Cancer Research 6: 4073–4081. [PubMed] [Google Scholar]
- 24. Shiba M, Kohno H, Kakizawa K, Iizasa T, Otsuji M, et al. (2000) Ki-67 immunostaining and other prognostic factors including tobacco smoking in patients with resected nonsmall cell lung carcinoma. Cancer 89: 1457–1465. [DOI] [PubMed] [Google Scholar]
- 25. Martin B, Paesmans M, Mascaux C, Berghmans T, Lothaire P, et al. (2004) Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. British journal of cancer 91: 2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dosaka-Akita H, Hommura F, Mishina T, Ogura S, Shimizu M, et al. (2001) A risk-stratification model of non-small cell lung cancers using cyclin E, Ki-67, and ras p21: different roles of G1 cyclins in cell proliferation and prognosis. Cancer research 61: 2500–2504. [PubMed] [Google Scholar]
- 27. Ullrich RT, Zander T, Neumaier B, Koker M, Shimamura T, et al. (2008) Early detection of erlotinib treatment response in NSCLC by 3′-deoxy-3′-[F]-fluoro-L-thymidine ([F]FLT) positron emission tomography (PET). PloS one 3: e3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sohn HJ, Yang YJ, Ryu JS, Oh SJ, Im KC, et al. (2008) [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clinical cancer research : an official journal of the American Association for Cancer Research 14: 7423–7429. [DOI] [PubMed] [Google Scholar]
- 29. Scheffler M, Kobe C, Zander T, Nogova L, Kahraman D, et al. (2012) Monitoring reversible and irreversible EGFR inhibition with erlotinib and afatinib in a patient with EGFR-mutated non-small cell lung cancer (NSCLC) using sequential [18F]fluorothymidine (FLT-)PET. Lung cancer 77: 617–620. [DOI] [PubMed] [Google Scholar]
- 30. Herrmann K, Buck AK, Schuster T, Junger A, Wieder HA, et al. (2011) Predictive value of initial 18F-FLT uptake in patients with aggressive non-Hodgkin lymphoma receiving R-CHOP treatment. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 52: 690–696. [DOI] [PubMed] [Google Scholar]
- 31. Li Z, Graf N, Herrmann K, Junger A, Aichler M, et al. (2012) FLT-PET Is Superior to FDG-PET for Very Early Response Prediction in NPM-ALK-Positive Lymphoma Treated with Targeted Therapy. Cancer research 72: 5014–5024. [DOI] [PubMed] [Google Scholar]
- 32. Gaertner J, Wolf J, Hallek M, Glossmann JP, Voltz R (2011) Standardizing integration of palliative care into comprehensive cancer therapy–a disease specific approach. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 19: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 33. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 34. Hamacher K, Coenen HH, Stocklin G (1986) Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 27: 235–238. [PubMed] [Google Scholar]
- 35. van Westreenen HL, Cobben DC, Jager PL, van Dullemen HM, Wesseling J, et al. (2005) Comparison of 18F-FLT PET and 18F-FDG PET in esophageal cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 46: 400–404. [PubMed] [Google Scholar]
- 36. Lara-Guerra H, Chung CT, Schwock J, Pintilie M, Hwang DM, et al. (2012) Histopathological and immunohistochemical features associated with clinical response to neoadjuvant gefitinib therapy in early stage non-small cell lung cancer. Lung cancer 76: 235–241. [DOI] [PubMed] [Google Scholar]
- 37. Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, et al. (2012) Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. European journal of nuclear medicine and molecular imaging 39: 27–38. [DOI] [PubMed] [Google Scholar]
- 38. Bats AS, Hugonnet F, Huchon C, Bensaid C, Pierquet-Ghazzar N, et al. (2012) Prognostic significance of mediastinal 18F-FDG uptake in PET/CT in advanced ovarian cancer. European journal of nuclear medicine and molecular imaging 39: 474–480. [DOI] [PubMed] [Google Scholar]
- 39. Martoni AA, Di Fabio F, Pinto C, Castellucci P, Pini S, et al. (2011) Prospective study on the FDG-PET/CT predictive and prognostic values in patients treated with neoadjuvant chemoradiation therapy and radical surgery for locally advanced rectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO 22: 650–656. [DOI] [PubMed] [Google Scholar]
- 40. Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, et al. (1998) Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nature medicine 4: 1334–1336. [DOI] [PubMed] [Google Scholar]
- 41. Zhang CC, Yan Z, Li W, Kuszpit K, Painter CL, et al. (2012) [(18)F]FLT-PET imaging does not always “light up” proliferating tumor cells. Clinical cancer research : an official journal of the American Association for Cancer Research 18: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 42. Aarntzen EH, Srinivas M, De Wilt JH, Jacobs JF, Lesterhuis WJ, et al. (2011) Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3′-fluoro-3′-deoxy-thymidine ([18F]FLT) PET imaging. Proceedings of the National Academy of Sciences of the United States of America 108: 18396–18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang J, Ramnath N, Moysich KB, Asch HL, Swede H, et al. (2006) Prognostic significance of MCM2, Ki-67 and gelsolin in non-small cell lung cancer. BMC cancer 6: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsubochi H, Sato N, Hiyama M, Kaimori M, Endo S, et al. (2006) Combined analysis of cyclooxygenase-2 expression with p53 and Ki-67 in nonsmall cell lung cancer. The Annals of thoracic surgery 82: 1198–1204. [DOI] [PubMed] [Google Scholar]
- 45. Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 50 Suppl 1: 122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kahraman D, Scheffler M, Zander T, Nogova L, Lammertsma AA, et al. (2011) Quantitative analysis of response to treatment with erlotinib in advanced non-small cell lung cancer using 18F-FDG and 3′-deoxy-3′-18F-fluorothymidine PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 52: 1871–1877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT checklist of the trial. Please note that this trial was not randomized.
(DOC)
Trial protocol of the underlying trial of this study.
(PDF)