Abstract
Background
The association of hypertension and mortality is attenuated in elderly adults. Walking speed, as a measure of frailty, may identify which elders are most at risk for the adverse effects of hypertension. We hypothesized that elevated blood pressure (BP) would be associated with a greater risk of mortality in faster, but not slower, walking older adults.
Methods
Participants included 2,340 persons ≥65 years in the National Health and Nutrition Examination Survey, 1999–2000 and 2001–2002. Mortality data was linked to death certificates in the National Death Index. Walking speed was measured over a 20-foot walk and classified as faster (≥ 0.8 meters/second, n=1,307), slower (n=790), or incomplete (n=243). Potential confounders included age, sex, race, survey year, lifestyle and physiologic variables, health conditions, and antihypertensive medications.
Results
There were 589 deaths through December 31st, 2006. The association of BP and mortality varied by walking speed. Among faster walkers, those with elevated systolic BP (≥140 mmHg) had a greater adjusted risk of mortality compared to those without (Hazard Ratio (HR): 1.35, 95% confidence interval (CI): 1.03, 1.77). Among slower walkers, neither elevated systolic nor diastolic BP (≥90 mmHg) was associated with mortality. In participants who did not complete the walk test, elevated BP was strongly and independently associated with a lower risk of death: HR: 0.38, 95% CI: 0.23, 0.62 (systolic) and HR: 0.10, 95% CI: 0.01, 0.81 (diastolic).
Conclusions
Walking speed could be a simple measure to identify elderly adults who are most at risk for adverse outcomes related to high BP.
Introduction
Hypertension is a major public health problem in the United States that has prompted extensive efforts to increase awareness and treatment.1 The prevalence of hypertension is increasing, and rates of control remain suboptimal.2 The high prevalence of hypertension has been, at least in part, attributed to the increase in blood pressure (BP) with age and the rapid growth of the elderly population (≥ 65 years).3 Despite the impact of age on BP, the evidence on BP control in elderly adults is limited and treatment recommendations are the same in older and younger adults.4 Joint National Committee (JNC) guidelines suggest that “therapy should not be withheld on the basis of age”, and recommended BP goals do not differ by age.5
The data on optimal BP goals are especially limited in the oldest old.4 A recent expert consensus document noted that it is “unclear whether target systolic BP should be the same in patients 65 to 79 years of age as in patients ≥80 years of age”.4 There is substantial evidence from epidemiologic studies demonstrating an attenuated or inverted association between higher BP and mortality at older ages.6,7 Nonetheless, the Hypertension in the Very Elderly Trial (HYVET), demonstrated a beneficial effect of antihypertensive therapy in adults 80 years and older on stroke, cardiovascular events, heart failure and death, based on a goal BP of <150/80 mmHg.8 Notably, the participants in this trial were healthier than average, and had lower prevalence of comorbid conditions. In clinical practice, decisions regarding BP targets are particularly difficult in poor functioning older adults who frequently do not meet the inclusion criteria of randomized controlled trials. Thus, whether current targets are appropriate for this population remains unclear, and how to identify elderly persons who may benefit from lower BP goals is unknown.
We propose that age may be an inadequate measure of the factors that determine the importance of elevated BP. Other measures that better capture frailty and health status may better identify which elderly persons are most at risk for the adverse consequences of hypertension, and which may benefit from higher levels of BP. Walking speed is an excellent integrative measure of health; it incorporates function across multiple organ systems, and it is strongly associated with mortality and other adverse events9–11. In a previous study of older Latino adults, we demonstrated that systolic BP was associated mortality only in participants with fast self-reported walking speed.12 The present study extends this research, and examines whether the relationship between elevated BP and mortality varies by objectively-measured walking speed among a nationally-representative sample of elderly adults. This is timely and important question as the clinician strives to reconcile the evidence on the impact of hypertension in the growing elderly population.
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative survey of the civilian, noninstitutionalized U.S. population, conducted by the National Center for Health Statistics (NCHS) of the Center for Disease Control and Prevention.13 Standardized questionnaires were administered in the home, followed by a detailed physical exam at the mobile examination center. This study includes data from participants aged 65 and older in two waves of the survey (1999–2000 and 2001–2002); 2,438/2,855 (85.4%) completed both the interview and exam.
Measures
The NCHS has linked mortality data from NHANES to death certificate data in the National Death Index (NDI).13 Mortality data were available from the date of the survey participation through December 31st, 2006, based on a probabilistic match between NHANES and NDI death certificate records.
Three or four BP measurements were taken in sequence the mobile examination center on seated participants using a mercury sphygmomanometer. If only one BP reading was obtained, that reading was recorded. If there was more than one BP reading, the first reading was excluded, and the BP was recorded as the average of all subsequent readings. Persons were considered to have elevated BP at levels ≥ 140 mmHg (systolic) and 90 mmHg (diastolic), based on current guidelines.4,5
Prior research has demonstrated slow gait speed has the strongest prognostic ability of the traditional components used to assess frailty.14–16 A 20-foot long test area was set up in a corridor of the mobile examination center. Adhesive tape strips on the floor indicated the start and stop points, and the walk was timed using a hand-held stopwatch. The participant was asked to walk at their usual pace. Start and stop times were defined as when the participant’s first foot touched the floor across the start line and finish line. We classified slower walkers as <0.8 m/s, based on the prior literature recommending this cut point,10 and because it best discriminated between survival times of the fast and slow walkers. A total of 243 participants did not complete the timed walk. The comments on the incomplete walking tests are as follows: 21 safety exclusion, 22 participant refusal, 69 no time/came late/left early, 77 physical limitation, 13 ill/emergency, 36 other reasons, and 5 were missing an explanation.
Age, sex, race, education, smoking, alcohol use were determined through questionnaire. Height and weight were measured by standard protocol at the mobile examination center, and fasting total and high-density lipoprotein (HDL)-cholesterol, triglycerides, and cystatin C were measured in blood samples also taken during the exam. Estimated glomerular filtration rate was calculated based on the CKD-EPI group formula (76.7 * cystatin C−1.19).17 Self-rated health and whether the participant had anyone to ask for emotional support were assessed by questionnaire. Average daily physical activity, history of diabetes, coronary heart disease, stroke, heart failure, and antihypertensive medication use were also assessed by questionnaire.
Statistical Analysis
NHANES uses a complex, multistage, probability sampling design; we appropriately accounted for this in all analyses by specifying the sampling design parameters. All analyses were conducted using Stata svy commands (Stata Corporation, College Station, TX).
We first compared the baseline characteristics of the participants with faster and slower walking speed, and in those who did not complete the walking test. We calculated the weighted mean and proportions across the continuous and categorical characteristics, respectively, and tested for differences based on a Wald test. We next summarized the mortality rates by level of BP and walking speed, and calculated the rate difference in persons with and without elevated BP (≥140 mmHg systolic and ≥90 mmHg diastolic).
We examined the adjusted association of elevated systolic and diastolic BP with mortality in Cox proportional hazards models. We used the Schoenfeld residuals and Kaplan-Meier survival curves to test if the proportional hazards assumption was met. Age, sex, black race, and survey year were included as potential confounders in all models; other candidate confounders included education (less than high school, high school, more than high school), smoking status (never, former/current), self-rated health (excellent/very good/good, fair/poor), emotional support, physical activity (low, medium, high), body mass index (BMI), total and HDL-cholesterol, triglycerides, cystatin C, and history of diabetes, coronary heart disease, stroke, and heart failure. A covariate was included in the adjusted model if was associated with mortality and BP at a significance level of p<0.20.Of measures examined, education, smoking status, cholesterol, heart failure, coronary heart disease, and stroke met these criteria. We included multiplicative interaction terms between elevated systolic and diastolic BP and walking speed, and used a Wald test for interaction. Subsequently, models were stratified on walking speed. We repeated these analyses with systolic and diastolic BP classified as a linear variable.
We conducted several sensitivity analyses. First, we restricted deaths to those with underlying cause of death due to cardiovascular disease (ICD10 I00-78). Because falling BP levels can be an indicator of the end-of-life, we conducted an analysis excluding deaths in the 1st year of follow-up. As an additional sensitivity analysis we also explored the inclusion of antihypertensive medication use in the multivariable models, and also stratified based on medication status. We also stratified by age above and below 75 years. Finally, because not completing the 20-ft walk test may be an indicator of frailty, we grouped the slower walkers and those participants who did not complete the walk test (due to reasons other than logistical) together as a combined, lower functioning group.
Results
A total of 2,340 participants in NHANES waves 1999–2000 and 2001–2002 were aged 65 and older and had measured systolic BP; 2,282 had measured diastolic BP. The NHANES participants represent a total of 32 million noninstitutionalized U.S. adults. Of these participants, 56% had a walking speed of ≥0.8 m/s, 34% had a walking speed of <0.8 m/s and, and 10% did not complete the walk for various reasons (described in Methods). Faster walkers were younger, less often female and black, and were more likely to have a high school education compared with slower walkers. They were more likely to smoke and had better kidney function. In addition, persons with faster walking speed had less comorbidities compared with those with slower walking speed; they had a lower prevalence of diabetes, coronary heart disease, stroke, and heart failure. Persons with faster walking speed were less likely to be on antihypertensive medications, and had lower systolic BP and higher diastolic BP compared with slower walking participants. Persons who did not complete the timed walk had the highest systolic BP levels. Overall, elevated systolic BP (>140 mmHg) was prevalent in approximately 50% of all participants (1,164/2,340), whereas elevated diastolic BP (>90 mmHg) was only prevalent in 6% (133/2,282) of participants. The proportion of men and women who did not complete the walk test was highest in those 85 years and older (Table 2). In all age groups, women were slightly less likely to have a usual walking speed of ≥ 0.8 m/s compared with men. (Table 2)
Table 2.
Walking speed by age and sex in NHANES participants age 65 and older (1999–2002)
| Walking Speed | ||||
|---|---|---|---|---|
| Faster ≥ 0.8 m/s (n = 1,307) |
Slower <0.8 m/s (n = 790) |
Did not complete (n = 243) |
||
| Men | 65–69 years | 243 (77%) | 52 (16%) | 21 (7%) |
| 70–74 years | 204 (66%) | 77 (25%) | 28 (9%) | |
| 75–79 years | 131 (60%) | 67 (30%) | 22 (10%) | |
| 80–84 years | 103 (50%) | 91 (44%) | 12 (6%) | |
| 85+ years | 30 (35%) | 62 (53%) | 26 (22%) | |
| Women | 65–69 years | 206 (65%) | 80 (25%) | 31 (10%) |
| 70–74 years | 175 (61%) | 88 (31%) | 24 (8%) | |
| 75–79 years | 109 (54%) | 71 (35%) | 23 (11%) | |
| 80–84 years | 72 (33%) | 122 (56%) | 24 (11%) | |
| 85+ years | 34 (23%) | 80 (55%) | 32 (22%) |
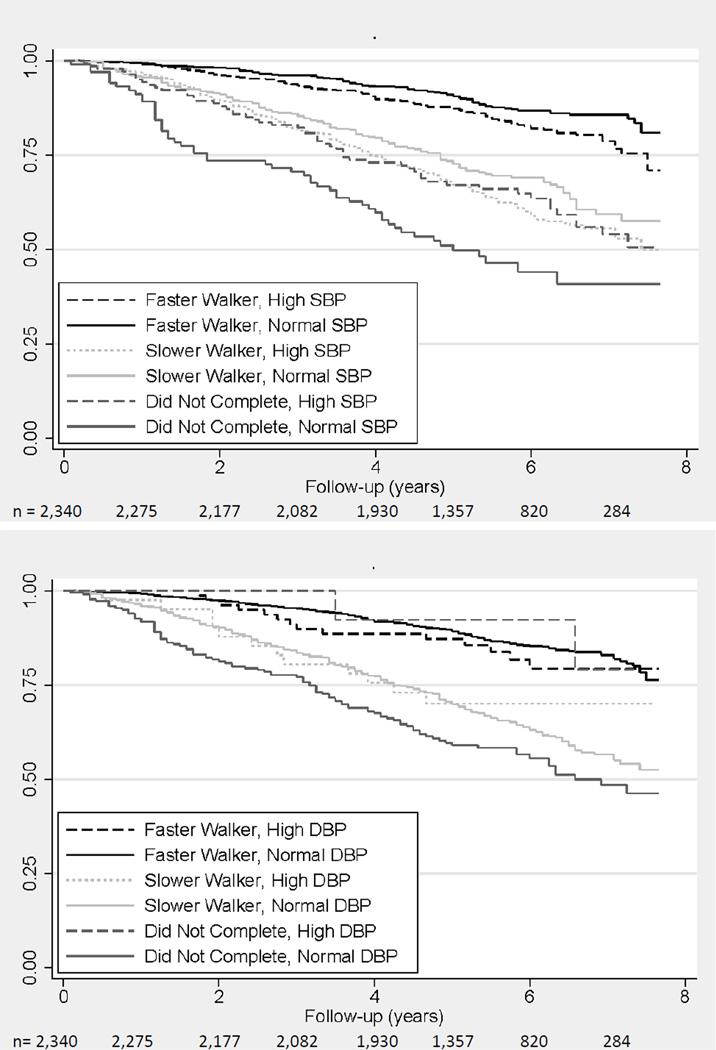
By December 31st, 2006, there were 589 deaths (representing 7 million U.S. adults); the average mortality rate in this population was 41.7 per 1,000 person-years. Faster-walking participants with elevated systolic BP (≥140 mmHg) had a higher mortality rate compared to those with systolic BP <140 mmHg; whereas slower walkers with elevated systolic BP did not appear to have increased mortality (Table 3 and Figure 1a). In contrast, among those participants who did not complete the walk test; participants with elevated systolic BP had a lower mortality rate (Table 3 and Figure 1a).
Table 3.
The association of elevated blood pressure and mortality, across strata of walking speed, in NHANES participants aged 65 and older (1999–2002)
| Walking Speed | ||||
|---|---|---|---|---|
| Faster ≥ 0.8 m/s (n = 1,307) |
Slower <0.8 m/s (n = 790) |
Did not complete (n = 243) |
||
| Systolic BP | ||||
| Mortality Rate (per 1,000 person-years) | ||||
| ≥ 140 mmHg | 28.1 | 72.1 | 62.4 | |
| < 140 mmHg* | 20.4 | 67.6 | 133.5 | |
| Rate Difference | 7.7 | 4.5 | −71.1 | |
| Unadjusted Hazard Ratio (95% CI)†,‡ | 1.39 (0.98, 1.96) | 1.06 (0.84, 1.34) | 0.47 (0.29, 0.73) | |
| Adjusted Hazard Ratio (95% CI)†,‡ | 1.35 (1.03, 1.77) | 1.12 (0.87, 1.45) | 0.38 (0.23, 0.62) | |
| Diastolic BP | ||||
| Mortality Rate (per 1,000 person-years) | ||||
| ≥ 90 mmHg | 24.0 | 42.5 | 8.4 | |
| < 90 mmHg* | 23.5 | 71.3 | 95.6 | |
| Rate Difference | 0.5 | −28.8 | −87.2 | |
| Unadjusted Hazard Ratio (95% CI)†,‡ | 1.01 (0.49, 2.07) | 0.60 (0.26, 1.40) | 0.09 (0.01, 0.62) | |
| Adjusted Hazard Ratio (95% CI)†,‡ | 0.94 (0.38, 2.28) | 0.75 (0.32, 1.75) | 0.10 (0.01, 0.81) | |
referent group
Adjusted for survey year, age, gender, black race, education, smoking status, cholesterol, coronary heart disease, heart failure, and stroke
p-values for Wald test for interaction of walking speed categories and elevated systolic BP: <0.001 (unadjusted), <0.001 (adjusted); diastolic BP: 0.07 (unadjusted), 0.21 (adjusted)
Figure 1.
Kaplan-Meier survival plots of persons with elevated 1) systolic > 140 mmHg (top) and b) diastolic >90 mmHg (bottom) blood pressure, stratified by walking speed, in NHANES participants aged 65 and older (1999–2002) followed until December 31st, 2006
The adjusted association between elevated systolic BP and mortality varied across walking speed (p-value for interaction < 0.001). Higher systolic BP was associated with a 35% elevated risk of mortality in the fast walkers, even after adjustment for potential confounders (p=0.03) (Table 3). In contrast, there was no association between elevated systolic BP and mortality in the slower walkers (p=0.37). There was an inverted relationship between elevated systolic BP and mortality in those participants who did not complete the walk test; higher systolic BP was associated with a lower risk of death, even after accounting for potential confounders (p<0.001).
Faster walkers with elevated diastolic BP did not have a higher mortality rate compared with those without elevated diastolic BP (Table 3 and Figure 1b). Slower walkers with elevated diastolic BP had a lower mortality rate, although the hazard ratio was not statistically significant. In participants who did not complete the walk test, those with elevated diastolic BP had a lower mortality rate compared to those with lower diastolic BP levels, and the adjusted association was strongly in the protective direction (p=0.03).
Similar patterns of association were present when we classified BP as a linear variable, although the association of higher systolic BP and mortality in the faster walkers no longer reached statistical significance after adjustment for potential confounders. (Table 4) In participants who did not complete the walk test, elevated systolic and diastolic BP were associated with a lower adjusted risk of mortality.
Table 4.
The association of linear blood pressure and mortality, stratified by walking speed, in NHANES participants aged 65 and older (1999–2002)
| Walking Speed | ||||||||
|---|---|---|---|---|---|---|---|---|
| Faster ≥0.8 m/s |
Slower < 0.8 m/s |
Did not complete | ||||||
| HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | p-value for interaction |
||
| Systolic BP (per 10 mmHg Higher) |
Unadjusted | 1.10 (1.01, 1.21) |
0.03 | 1.04 (0.99, 1.09) |
0.11 | 0.80 (0.73, 0.87) |
<0.001 | <s0.001 |
| Adjusted* | 1.10 (0.99, 1.22) |
0.07 | 1.06 (1.01, 1,11) |
0.02 | 0.75 (0.67, 0.84) |
<0.001 | <0.001 | |
| Diastolic BP (per 10 mmHg Higher) |
Unadjusted | 0.97 (0.86, 1.11) |
0.67 | 0.91 (0.82, 1.02) |
0.10 | 0.70 (0.58, 0.85) |
0.001 | 0.03 |
| Adjusted* | 1.05 (0.92, 1.20) |
0.44 | 0.95 (0.85, 1.06) |
0.32 | 0.77 (0.60, 0.98) |
0.04 | 0.04 | |
Adjusted for survey year, age, gender, black race, education, smoking status, cholesterol, coronary heart disease, heart failure, and stroke
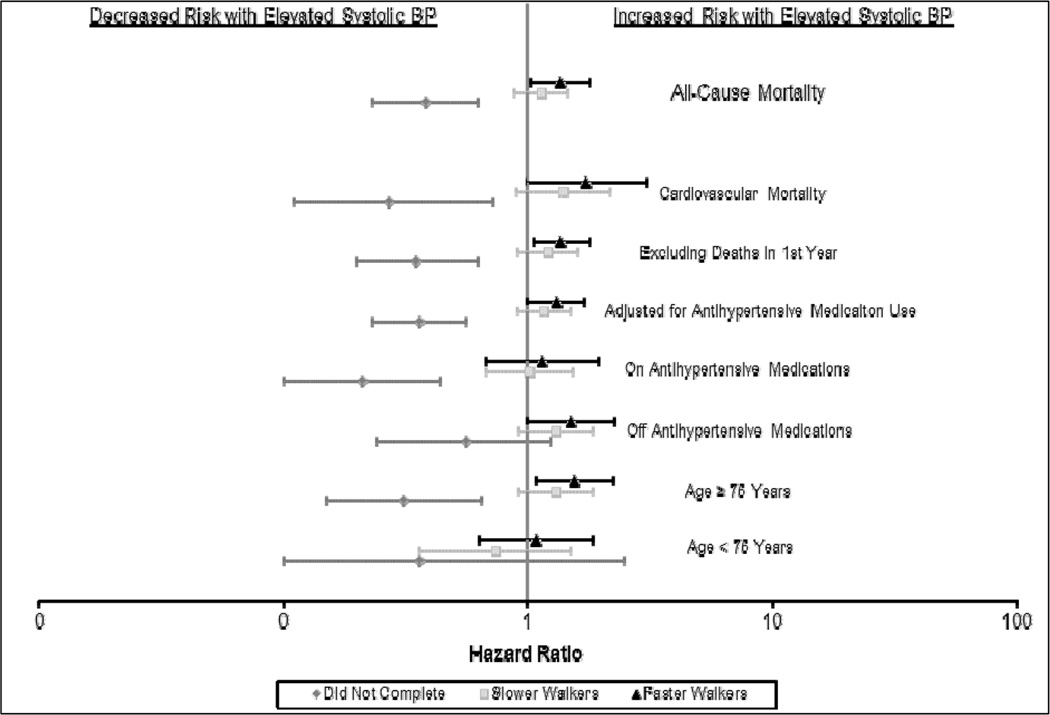
The estimates were robust against multiple sensitivity analyses, although there was limited power in several of these analyses. (Figure 2) Finally, as an alternative classification of frail participants, we grouped the slower walkers and those who did not complete the walk for non-logistical reasons, there was no association between higher systolic (HR: 1.00, 95% CI: 0.78, 1.28) or diastolic (HR: 0.57, 95% CI: 0.23, 1.40) BP and mortality in this lower functioning group.
Figure 2.
Sensitivity analyses of the association of elevated systolic BP (>140 mmHg) and mortality, stratified by walking speed, in NHANES participants aged 65 and older (1999–2002) followed until December 31st, 2006.
Discussion
In this nationally representative sample of elderly adults, we found that the association of BP with mortality varied by walking speed. Higher systolic BP was associated with an increased risk of mortality only among elderly adults who had a medium to fast walking pace. In contrast, among slower walking older adults, there was not an association between elevated systolic or diastolic BP and mortality. Strikingly, we found elevated systolic and diastolic BP was strongly and independently associated with a lower mortality risk in participants who did not complete the walk test. Our findings suggest that walking speed may be useful measure to identify older adults who are most at risk for the adverse effects of high blood pressure.
Our findings are consistent with prior studies that have found that the association of BP and mortality diminishes with age,7 because the prevalence of frailty increases with age.18 The present findings are also consistent with a recent study from our group.12 We previously examined the relationship between BP, self-reported walking speed, and mortality in a cohort of older Latino adults. In participants who reported fast walking speed, there was an association of higher systolic BP with mortality, whereas there was no association in slower walkers. Latino adults may be at an increased risk for functional impairment due to the high prevalence of obesity and diabetes in this population. The present study extends our findings to a nationally-representative study population, and uses an objective measure of walking speed.
The association of elevated systolic BP and mortality in the faster walking elders is also congruent with findings from randomized controlled trials that have demonstrated a benefit of treatment of antihypertensive therapy in study participants. Participants in trials are often healthier compared with the general population due to study inclusion criteria that may limit the types and severity of comorbid conditions. In the Systolic Hypertension in the Elderly Program (SHEP), investigators reported a beneficial effect of antihypertensive therapy on stroke, major cardiovascular events, and all-cause mortality.19 A large randomized controlled trial, the HYpertension in the Very Elderly Trial (HYVET), also demonstrated that persons aged 80 and older on antihypertensive therapy had a reduced risk of all-cause mortality, after two years of treatment.8
There has been substantial evidence published regarding the pathophysiologic consequences of hypertension in older adults.4 Less literature has focused on the potential harm of lower BP, and the majority has focused on orthostatic hypotension as a risk factor for falls.4,20 In older, frail adults, elevated BP may be necessary to maintain perfusion of the vital organs, especially the heart, which is perfused during diastole.21 This may explain the apparently protective association we observed between higher diastolic BP and lower mortality in participants who did not complete the walk test. Low diastolic pressure may also contribute to high pulse pressure, which is a strong risk factor for coronary events in elderly adults22. Older, frail adults may be at higher risk from aggressive therapeutic interventions, and our findings warrant further investigation of the effects of BP control in this population.
This finding of an inverted association between BP and mortality in those who did not complete the walk test was striking, although the wide range of reasons for not completing the walk precludes us from making any definitive conclusions regarding the mechanism for this inverted association in this population. There was a wide variety of comments regarding this missing data in the NHANES data, which included both logistical and safety reasons. Because only 243 participants had missing data, we did not have the statistical power to determine if the relation between BP and mortality differed within subgroups of these participants. Based on their baseline characteristics and mortality rate, the participants with missing walk test data appeared to have worse health status compared to those with complete data. Given the substantial difference in associations in those with and without walk test data, we suggest that future studies in older adults use methods to address this potential source of bias. Healthy participant bias is a well-described source of bias and may be of particular importance in older populations where frailty and disability are common23. This bias may help explain the apparently discordant findings between epidemiologic studies and randomized controlled trials of BP and antihypertensive medications in older adults.
This study also has limitations that should be considered. Both walking speed and BP often vary over time, and because NHANES only had a one-time measure of each, we were unable to account for changes in these factors over time. We assessed one domain of frailty, whereas the underlying process is complex and likely best assessed with a constellation of measures. In addition, many of the variables were self-reported, and there may be some misclassification of these measures. We classified walking speed into two levels in order to maximize statistical power within the strata, although the relationship between BP, walking speed, and mortality could likely be better described across multiple levels of walking speed in a larger study population.
In summary, this is the first study to examine the relationship between BP and walking speed, and mortality in a representative sample of U.S. adults age 65 and older. We found that systolic BP is associated with an increased risk of mortality in adults with medium to fast walking speed. The association of BP and mortality is less clear in slower walking adults, and future research should aim to characterize this relationship better in frail, older adults and the institutionalized population. Some researchers have recommended that walking speed be incorporated into regular geriatric assessment as a predictive tool to identify those at risk for future adverse events and identify functional impairment in one or more of the systems that contribute to the ability to walk11. Walking speed appears to be a simple measure to explain the differential association of high BP and mortality in older adults.
Supplementary Material
Table 1.
Characteristics of NHANES participants age 65 and older (1999–2002), stratified by walking speed
| Walking Speed | ||||
|---|---|---|---|---|
| Faster ≥0.8 m/s (n = 1,307) |
Slower < 0.8 m/s (n = 790) |
Did not complete (n = 243) |
||
| Characteristic | Mean (SD) or % | P-value | ||
| Age (years) | 72 ± 6 | 77 ± 6 | 77 ± 6 | <0.001 |
| Female | 52% | 67% | 61% | <0.001 |
| Black Race | 5% | 10% | 14% | 0.001 |
| Less than High School Education | 24% | 48% | 45% | <0.001 |
| Current/Prior Smoking | 56% | 50% | 47% | 0.02 |
| BMI (m/kg2) | 27 ± 5 | 28 ± 6 | 29 ± 6 | 0.01 |
| Cholesterol (mg/dL) | 212 ± 39 | 212 ± 45 | 206 ± 40 | 0.25 |
| HDL-Cholesterol (mg/dL) | 54 ± 16 | 54 ± 18 | 53 ± 17 | 0.56 |
| Estimated GFR (ml/min/1.73m2) | 77 ± 19 | 64 ± 20 | 64 ± 24 | <0.001 |
| History of Diabetes | 10% | 23% | 23% | <0.001 |
| History of CHD | 11% | 13% | 12% | 0.72 |
| History of Stroke | 5% | 12% | 23% | <0.001 |
| History of CHF | 4% | 13% | 15% | <0.001 |
| Antihypertensive Medication Use | 39% | 54% | 46% | <0.001 |
| Systolic BP (mmHg) | 139 ± 21 | 143 ± 23 | 147 ± 30 | 0.003 |
| Diastolic BP (mmHg) | 70 ± 13 | 66 ± 15 | 68 ± 14 | 0.001 |
Acknowledgements
Dr. Odden is supported by a the American Heart Association Western States Affiliate Clinical Research Program and the National Institute on Aging (K01AG039387)
References
- 1.Institute of Medicine Committee on Public Health Priorities to Reduce and Control Hypertension. A Population-Based Policy and Systems Change Approach to Prevent and Control Hypertension. The National Academies Press. 2010 [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011 Aug 30;124(9):1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997 Jul 1;96(1):308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 4.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011 May 31;123(21):2434–2506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6.Langer RD, Criqui MH, Barrett-Connor EL, Klauber MR, Ganiats TG. Blood pressure change and survival after age 75. Hypertension. 1993 Oct;22(4):551–559. doi: 10.1161/01.hyp.22.4.551. [DOI] [PubMed] [Google Scholar]
- 7.Satish S, Freeman DH, Jr, Ray L, Goodwin JS. The relationship between blood pressure and mortality in the oldest old. J Am Geriatr Soc. 2001 Apr;49(4):367–374. doi: 10.1046/j.1532-5415.2001.49078.x. [DOI] [PubMed] [Google Scholar]
- 8.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008 May 1;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 9.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011 Jan 5;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The Journal of Nutrition, Health & Aging. 2009 Dec;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 11.Studenski S. Bradypedia: is gait speed ready for clinical use? The Journal of Nutrition, Health & Aging. 2009 Dec;13(10):878–880. doi: 10.1007/s12603-009-0245-0. [DOI] [PubMed] [Google Scholar]
- 12.Odden MC, Covinsky KE, Neuhaus J, Mayeda ER, Peralta CA, Haan MN. The Association of Blood Pressure and Mortality Differs by Self-Reported Walking Speed in Older Latinos. J Gerontol A Biol Sci Med Sci. 2012 Mar 1; doi: 10.1093/gerona/glr245. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention. [Accessed August 25, 2008];2008 www.cdc.gov/nchs/nhanes.htm.
- 14.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006 Nov;54(11):1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 16.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008 Dec;56(12):2211. doi: 10.1111/j.1532-5415.2008.02008.x. 2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008 Mar;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991 Jun 26;265(24):3255–3264. [PubMed] [Google Scholar]
- 20.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007 Oct;120(10):841–847. doi: 10.1016/j.amjmed.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin JS. Embracing complexity: A consideration of hypertension in the very old. J Gerontol A Biol Sci Med Sci. 2003 Jul;58(7):653–658. doi: 10.1093/gerona/58.7.m653. [DOI] [PubMed] [Google Scholar]
- 22.Franklin SS, Lopez VA, Wong ND, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009 Jan 20;119(2):243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benfante R, Reed D, MacLean C, Kagan A. Response bias in the Honolulu Heart Program. Am J Epidemiol. 1989 Dec;130(6):1088–1100. doi: 10.1093/oxfordjournals.aje.a115436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.