Abstract
Rationale
The Iowa Gambling Task (IGT) can be used to quantify impulsive and risky choice behaviors in psychiatric patients, e.g. Bipolar Disorder (BD) sufferers. Although developing treatments for these behaviors is important, few predictive animal models exist. Inhibition of the dopamine transporter (DAT) can model profiles of altered motor activity and exploration seen in patients with BD. The effect of DAT inhibition on impulsive choices related to BD has received limited study however. We used a rodent IGT to elucidate the effects of similarly acting drugs on risky choice behavior.
Objectives
We hypothesized that 1) C57BL/6 mice could adopt the ‘safe’ choice options in the IGT and 2) DAT inhibition would alter risk preference.
Methods
Mice were trained in the IGT to a stable risk-preference and then administered the norepinephrine/DAT inhibitor amphetamine, or the more selective DAT inhibitors modafinil or GBR12909.
Results
Mice developed a preference for the ‘safe’ option, which was potentiated by amphetamine administration. GBR12909 or modafinil administration increased motor impulsivity, motivation significantly, and risk preference subtly.
Conclusions
The rodent IGT can measure different impulse-related behaviors and differentiate similarly acting BD-related drugs. The contrasting effects of amphetamine and modafinil in mice are similar to effects in rats and humans in corresponding IGT tasks, supporting the translational validity of the task. GBR12909 and modafinil elicited similar behaviors in the IGT, likely through a shared mechanism. Future studies using a within-session IGT are warranted to confirm the suitability of DAT inhibitors to model risk-preference in BD.
Keywords: Iowa Gambling Task, dopamine transporter, bipolar disorder, risk-taking, mice, modafinil, GBR12909, amphetamine, mania, impulsivity
INTRODUCTION
Mania is a cardinal feature of Bipolar Disorder (BD), a debilitating psychiatric disorder affecting approximately 1% of the US population for BD-I and BD-II combined (Merikangas et al. 2011). Impulsive behavior is a critical aspect of mania and one of the diagnostic criteria according to the DSM-IV. Self-report questionnaires identify higher levels of impulsivity or poor risk assessment in depressed, euthymic, and manic phases of BD (Swann et al. 2009; 2008), corroborated using laboratory-based assessments (Adida et al. 2011). Treatment options for patients with BD, especially those directed at symptoms such as impulsivity and poor risk assessment, are limited and require development.
To quantify such impulsive aspects of mania and develop treatments, laboratory tests with real world translational validity are now being utilized. Such tests include the Iowa Gambling Task (IGT) (Bechara et al. 1994), which measures decision-making for risk, choosing between ‘high yield/high risk options versus low risk/low yield options’. In short, subjects in the IGT have to deduce and select the best of four options that vary in both the size and probability of reward and losses (Bechara et al. 1997; 2005) (Table 1). As the session continues, healthy subjects readily select the safe options after initially trying all decks (Bechara et al. 1999), whereas patients with BD take longer to adopt this strategy irrespective of state (Adida et al. 2011; Clark et al. 2001). Thus, the IGT quantifies the uncontrolled risk-taking trait of patients with BD across the spectrum of the disorder (Adida et al. 2011; Kim et al. 2006). The IGT enables examination of the putative mechanisms underlying risk-taking behavior in BD mania by linking performance with self-report questionnaires (Adida et al. 2008; Yechiam et al. 2008). The IGT can differentiate between clinical populations whereby BD patients take longer to adopt a safe strategy, substance-abusers learn at different rates (van der Plas et al. 2008), and less clear impairments are observed in patients with schizophrenia (Sevy et al. 2007).
Table 1.
The gain-loss structure in the human and mouse Iowa Gambling Tasks
| Sequence | Human Iowa Gambling Task | Mouse Iowa Gambling Task | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| A (risky) | B (risky) | C (safe) | D (safe) | P1 (safe) | P2 (safe) | P3 (risky) | P4 (risky) | |
| 1 | 100 | 100 | 50 | 50 | 1 | 2 | 3 | 4 |
| 2 | 100 | 100 | 50 | 50 | 1 | 2 | −30 | 4 |
| 3 | −50 | 100 | 0 | 50 | 1 | 2 | 3 | −40 |
| 4 | 100 | 100 | 50 | 50 | 1 | −10 | −30 | 4 |
| 5 | −200 | 100 | 0 | 50 | 1 | 2 | 3 | −40 |
| 6 | 100 | 100 | 50 | 50 | 1 | 2 | −30 | −40 |
| 7 | −100 | 100 | 0 | 50 | 1 | 2 | −30 | −40 |
| 8 | 100 | 100 | 50 | 50 | 1 | −10 | 3 | 4 |
| 9 | −150 | −1150 | 0 | 50 | 1 | 2 | 3 | −40 |
| 10 | −250 | 100 | 0 | −200 | −5 | 2 | −30 | −40 |
|
| ||||||||
| Outcome +/− rate | −250 | −250 | +250 | +250 | 9+/5s | 16+/20s | 15+/150s | 16+/240s |
| 5+ | 9+ | 5+ | 9+ | 9+ | 8+ | 5+ | 4+ | |
| 5− | 1− | 5− | 1− | 1− | 2− | 5− | 6− | |
To better understand these discrepancies and neurobiological underpinnings of risk-taking/gambling behavior, researchers have developed animal versions of the IGT (Homberg et al. 2008; Rivalan et al. 2009; van den Bos et al. 2006; Zeeb et al. 2009), see (de Visser et al. 2011) for a review. These paradigms may facilitate development of therapeutics for risk-taking behavior and have already been used to study both dopaminergic (Zeeb et al. 2009) and serotonergic (Homberg et al. 2008; Zeeb et al. 2009) manipulations in rats. Based initially on the rat paradigm (Zeeb et al. 2009), we developed a mouse IGT to investigate the genetic underpinnings of performance (Young et al. 2011c). In short, analogous to the human IGT, the rodent version presents the animal with a choice between four distinct options with different probabilities and magnitudes of expected gains and losses (Table 1). Two options are ultimately advantageous whereas the other two ultimately have a disadvantageous outcome. Motor impulsivity as measured by premature responses (before choice stimuli appear) and risk-taking behavior as measured by percentage advantageous choices (propensity of an animal to choose advantageous/safe options or disadvantageous/risky options) can be measured simultaneously in the rodent IGT (de Visser et al. 2011). The rodent IGT therefore provides measurements of impulsive activity and impulsive choice respectively (Evenden 1999), the latter of which more closely resembles the choice/risk taking measured in the human IGT. Maximizing reward requires learning the gain/loss contingencies available and selecting safe options.
Dysregulation of dopaminergic homeostasis plays an important role in the mechanisms underlying mania in BD (Manji et al. 2003; Vawter et al. 2000). Specifically, genetic linkage studies have identified abnormalities in the human dopamine transporter (DAT) in BD (Greenwood et al. 2001; 2006; Pinsonneault et al. 2011). Such mutations lead to reduced cell surface expression of DAT in human cells (Horschitz et al. 2005), corroborated by lower striatal DAT levels in unmedicated BD patients (Anand et al. 2011). Consistent with reduced DAT functioning producing mania-like behavior, we observed that mice with reduced DAT functioning (via either genetic or pharmacological manipulation) exhibit complex exploratory profiles similar to those of acutely manic patients with BD in the cross-species translational behavioral pattern monitor (BPM) (Minassian et al. 2010; Perry et al. 2010; 2009; Young et al. 2010a; 2007). In our recently described rodent IGT, we observed that DAT knockdown (KD) mice exhibited increased risk preference, both within the first session and across sessions (Young et al. 2011c), providing further support for the DAT KD mouse as a model for mania.
Besides genetic models, pharmacological agents have also been used to model BD. Traditionally, the non-selective DAT inhibitor amphetamine is a widely used pharmacological model of BD mania (Arban et al. 2005; Berggren et al. 1978; Frey et al. 2006; Gould et al. 2001), despite having several limitations (Young et al. 2011a). The more selective DAT inhibitor GBR12909 has also been investigated as a model of mania. In contrast with amphetamine, GBR12909 mimicked the behavioral exploratory profile of patients with BD mania as assessed by the BPM (Perry et al. 2009; Young et al. 2010a).
The effects of amphetamine or GBR12909 have not yet been compared in the rodent IGT. Amphetamine reduced risk choice preference in the rodent IGT, leading to a more conservative choice strategy (Zeeb et al. 2009). Assessing the effects of amphetamine in mice may provide evidence of cross-species translational validity of the rodent IGT between rats and mice. Investigating the effects of GBR12909 in this paradigm will be useful to further determine the suitability of this treatment as a model of mania. Modafinil, a drug used to treat narcolepsy, also inhibits the DAT in rodents, monkeys, and humans (Madras et al. 2006; Volkow et al. 2009; Zolkowska et al. 2009) and can increase risk-taking in the human IGT (Zack and Poulos 2009).
These DAT inhibitors may help in delineating a) the effects of acute increases in extracellular dopamine levels on risk-taking behavior and b) the pharmacological predictive validity of the IGT across species. Hence we investigated the effects of d-amphetamine, GBR12909, and modafinil administration on mouse performance of the IGT. We hypothesized that: 1) C57BL/6 mice would readily learn to choose the less ‘risky’ options; 2) amphetamine would increase the choice of less punishing stimuli (make the mice risk aversive) consistent with effects on rats; and 3) GBR12909 and modafinil would increase risk preference in the IGT similar to patients with BD.
MATERIALS AND METHODS
Mice
Male C57BL/6N mice (Charles Rivers Laboratories) were trained in the IGT (n=21), and were approximately 3 months of age and weighed between 20–30 g at the start of the studies. All animals were group housed (maximum 4/cage), maintained in a temperature-controlled vivarium (21±1 °C) on a reversed day-night cycle (lights on at 8.00 PM, off at 8.00 AM), and were tested during the dark phase of the cycle. All mice had ad libitum access to water, were food-restricted, and maintained at 85% of their free-feeding weight during the periods of training and testing described below. Mice were brought to the testing area 45 min before testing between 2.00 PM and 5.00 PM. All procedures were approved by the UCSD Institutional Animal Care and Use Committee and the “Principles of laboratory animal care” were followed. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
Apparatus
Mice were trained and tested in sixteen 5-hole operant chambers (25×25×25 cm, Med Associates Inc., St. Albans, VT). Each chamber consisted of an array of five square holes (2.5×2.5×2.5 cm) arranged horizontally on a curved wall 2.5 cm above the grid floor. A food delivery magazine (Lafayette Instruments, Lafayette, IN) was on the opposite panel at floor level. The chamber had a house-light near the ceiling and was enclosed in a sound-attenuating box, which was ventilated by a fan that provided a low level of background noise. An infra-red camera installed in each chamber enabled the monitoring of performance during training and testing. The animals were trained to respond with a holepoke to an illuminated LED recessed into the holes. Infrared beams, mounted vertically and located 3 mm from the opening of the hole, were used to detect the responses. The food delivery magazine opposite to the middle hole contained a well in which liquid reinforcement utilized in the form of strawberry milkshake (Nesquik® plus non-fat milk, 30 μl) was delivered by a peristaltic pump (Lafayette Instruments, Lafayette, IN). An infrared beam mounted horizontally, 5 mm from the floor and recessed 6 mm into the magazine, was used to detect magazine entries. The control of stimuli and recording of responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc., St. Albans, VT) using custom programming.
Behavioral handling and training
Prior to training, mice were acclimated to the reinforcer. Training on days 1–3 lasted 10 min and reinforcement was dispensed every 15 s into the magazine well while the magazine was lit. Magazine entry extinguished the magazine light until the next reinforcement delivery. Magazine entries were counted. On day 4, to obtain reinforcement mice had to holepoke in 1 of the 4 lit holes opposite the magazine (central hole was never lit; Fig. 1). This session was repeated daily (Monday–Friday) until >70 responses were recorded within 30 min for 2 consecutive days. Upon attainment of criterion, mice were trained in this session only on Tuesday and Friday in order to maintain responding while training continued for other mice to avoid over-training on what is an intermediary stage (Young et al. 2009).
Fig. 1.
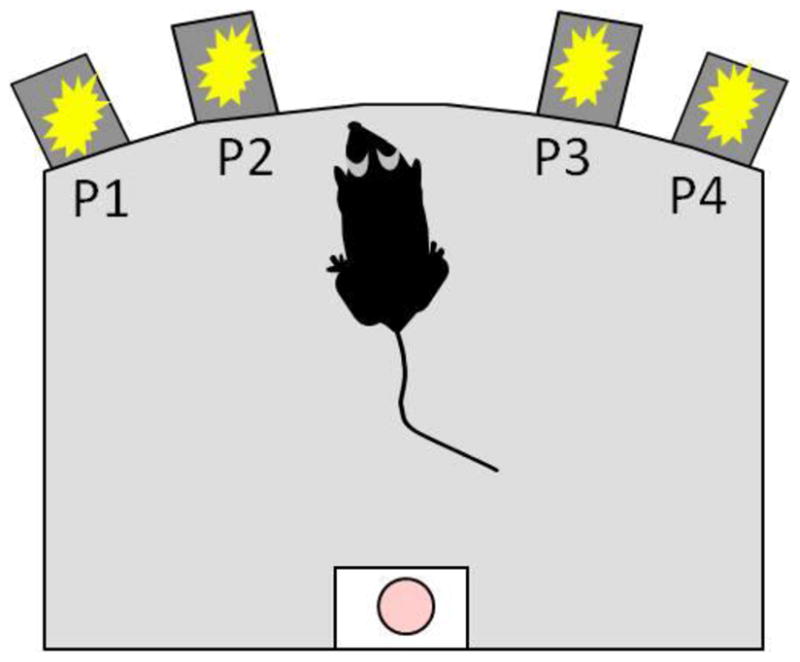
A visual schematic of the rodent IGT. Mice can holepoke among four illuminated holes (P1–P4) during the 10s stimulus duration to obtain food reward (strawberry milkshake, delivered opposite the holes) or punishment (cue light flashing and all apertures unresponsive for a predetermined period) depending on a predefined schedule (see table 1 for an example). Each session lasts for 100 trials or 30 min, whichever is completed first. Two options (P1, P2) deliver small rewards and low punishments, ultimately leading to an advantageous outcome, while the other two options (P3, P4) deliver high rewards and high punishments, ultimately leading to disadvantageous final outcome.
Rodent Iowa Gambling Task
For the IGT sessions we utilized the protocol described previously (Young et al. 2011c). The first 3 days, mice were presented with a forced choice version of the IGT to acquaint the mice with the different reinforcement and punishment schedules associated with each hole, where only 1 of the 4 holes was illuminated randomly and after holepoking, the appropriate reinforcement or punishment schedule followed. For both the IGT program as well as for its forced choice version, two different forms were used to counterbalance for possible hole preferences.
After 3 days of forced choice IGT, the mice were moved onto the full IGT lasting 30 min or 100 trials, whichever was completed first. The session began with the magazine being illuminated and each trial was initiated by holepoking then exiting the magazine. An intertrial interval (ITI) of 5 s preceded illumination of the cue array. If the mouse holepoked in any cue hole during this 5 s ITI, a premature response was recorded and did not count as a completed trial, stimuli were not presented, and the house light illuminated for a 5 s time-out period in which all holes were unresponsive (Table 2). The next trial began when the house light was extinguished and the mouse holepoked the magazine. If the mouse withheld from responding during the ITI period, holes 1, 2, 4, and 5 were illuminated. These lights remained lit until the mouse holepoked in one of these holes or until 10 s had passed. Failure to respond in any hole during the light stimuli was registered as an omission. Omissions did not trigger a time-out period, but rather resulted in the cue lights being extinguished and the magazine being illuminated so that another trial could be started. If the animal did holepoke in 1 of the 4 lit holes during the stimulus, a ‘correct’ response and the hole choice were recorded. All cue lights were then extinguished and the mouse rewarded or punished depending on the reward schedule (Table 1). For rewards, the magazine light was illuminated and delivered the appropriate level of reinforcer. Retrieving the reward initiated the next trial. If a punishment occurred however, no reward was given and a punishing time-out was triggered whereby the light stimulus of the chosen hole flashed at a frequency of 0.5 Hz for the duration of the time-out period, during which all apertures were unresponsive. After the time-out period, the flashing light was extinguished and the magazine light illuminated for a new trial to begin. Repeated holepoke responses were counted as response perseveratives but not punished. Repeated poking in the same hole when rewarded were counted as reward perseveratives and when punished as punishment perseveratives. Finally, the latency to collect rewards (mean reward latency) was also recorded. As to the different response options, data were collected as P1, P2, P3, and P4 responses corresponding to different reward schedules. Responses were measured as a percentage of the total trials completed. Where appropriate due to reduced sample size, data were grouped by advantageous (P1 and P2) and disadvantageous options (P3 and P4) and response options were measured as a percentage of the advantageous choices (P1+P2)/(P1+P2+P3+P4)*100 as described previously (Young et al. 2011c). Mice were trained continuously on the IGT until they exhibited stable performance after 20 sessions (no main effect of day when analyzed over 4 consecutive days). Because drug and genetic effects on IGT performance can be observed within a session (Young et al. 2011c; Zack and Poulos 2009), with individual differences in performance interacting with drug effects (Granon et al. 2000), within-session performance of good and poor performing animals were also analyzed (see statistics).
Table 2.
Summary of measures used to assess different behaviors in the IGT
| Measure & description | Outcome |
|---|---|
| Trials completed: Number of trials completed per session (maximum = 100) | Degree of session completion |
| Premature responses: Response in any cue hole during the 5s intertrial interval (ITI) preceding illumination of the cue array → 5s time-out period | Motoric impulsivity |
| Omissions (%): Failure to respond in any hole during the light stimulus duration of 10s | May reflect inattention or amotivation |
| ‘Correct’ response: Response in any of the 4 lit cue holes during the stimulus of 10s | A choice between the 4 cues is made |
| Responses (%): Response to P1, P2, P3, or P4 as a percentage of total holepokes | Preference for 1 cue compared to others |
| Advantageous choices (%): Advantageous response options (P1+P2)/total (P1+P2+P3+P4)*100 | Represents choice preference |
| Punishment perseverative responses: Repeated responses in the same hole when punished | Perseveration during punishment |
| Reward perseverative responses: Repeated responses in the same hole when rewarded | Perseveration after being rewarded |
| Mean reward latency: The latency to collect rewards in seconds | Reflects aspects of motivation |
Drug Testing Procedure
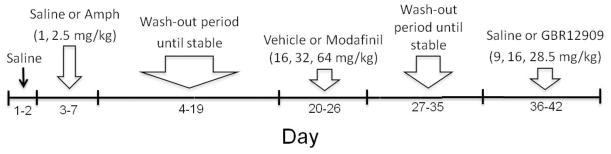
Each mouse received saline for the 2 training days prior to drug testing to habituate the animals to being injected. In the amphetamine study, mice were given their assigned amphetamine dose (1.0 and 2.5 mg/kg) (Helms et al. 2006; Perry et al. 2009; Wade et al. 2000) or saline on 3 drug testing days with saline days in between, 10 min prior to assessing performance in the IGT (Fig. 2). After amphetamine testing, a wash-out of 16 days followed until the mice were again stable. For the modafinil study, mice received 3 different doses of modafinil (16, 32, and 64 mg/kg) (Qu et al. 2008; Waters et al. 2005) or vehicle on 4 drug testing days, again with saline days in between. A wash-out of 9 days followed after modafinil testing. For the last challenge, the animals received 3 different doses of GBR12909 (9.0, 16.0, and 28.5 mg/kg) (van den Buuse and de Jong 1989; Young et al. 2010a) or saline on 4 drug testing days, with saline days in between. In each study, the range of doses were chosen based on cognitive effects described in the literature, as well as their effects on exploratory behavior we have described previously.
Fig. 2.
Timeline of drug testing procedure for amphetamine, modafinil, and GBR12909. Each mouse received saline for 2 training days prior to drug testing to habituate the animals to being injected. Before each drug study, mice were allocated to drug dose or vehicle in randomized order. Following a within-subject design, mice were administered their assigned drug dose or vehicle on 3 (amphetamine) or 4 (modafinil and GBR12909) drug testing days with saline administration training days in between testing.
Drugs
D-amphetamine sulfate, modafinil, and GBR12909 dihydrochloride were purchased from Sigma Aldrich (St Louis, MO USA). D-amphetamine sulfate was dissolved in saline, GBR12909 dihydrochloride was dissolved in saline after heating (45°C for 60 min), and modafinil was dissolved in vehicle (5% Tween and 1% Methylcellulose dissolved in saline). Drugs were injected intraperitoneally, with amphetamine at a volume of 5 ml/kg body weight, and modafinil and GBR12909 at a volume of 10 ml/kg due to poor solubility. Free-base drug weights were used in all drug calculations. Drug solutions were prepared just prior to testing.
Statistics
Stable performance was compared using a repeated measure analysis of variance (ANOVA) with days as a within-subject factor. Data obtained were subjected to a repeated measure ANOVA with dose and response option as within-subject factors. To assess within-session performance, sessions were divided into the first and second halves by trials completed (Grottick and Higgins 2002) and analyzed using a repeated measure ANOVA with session half, dose, and response option as within-subject factors. Because different within-session performances were observed across the group, mice were further separated into good and poor performers using a median split on their learning performance (% advantageous choice session-half 2 – % advantageous choice session-half 1) during saline treatment of each drug study. Subjects with less than 15 responses in a half were excluded from each within-session analysis. Where appropriate, planned comparison paired t-tests were conducted between groups. Pearson r correlation coefficients measured the relationship between risk-taking and motor impulsivity measures in each drug study. Tukey post hoc analyses of statistically significant main or interaction effects were performed where applicable. The level of probability for statistical significance was defined at 0.05. All statistics were performed using SPSS (19.0, Chicago, IL, USA).
RESULTS
Baseline performance in the IGT
After 20 days of training on the IGT, the C57BL/6 mice exhibited a stable and significant preference for a response option (F(3,171)=3.1, p<0.05; Fig. 3), choosing P2 more than P3 and P4 (p<0.05), and tended to choose P2 more than P1 (p<0.1). No significant effect of day was observed for response option or any other measure (F<1, ns).
Fig. 3.
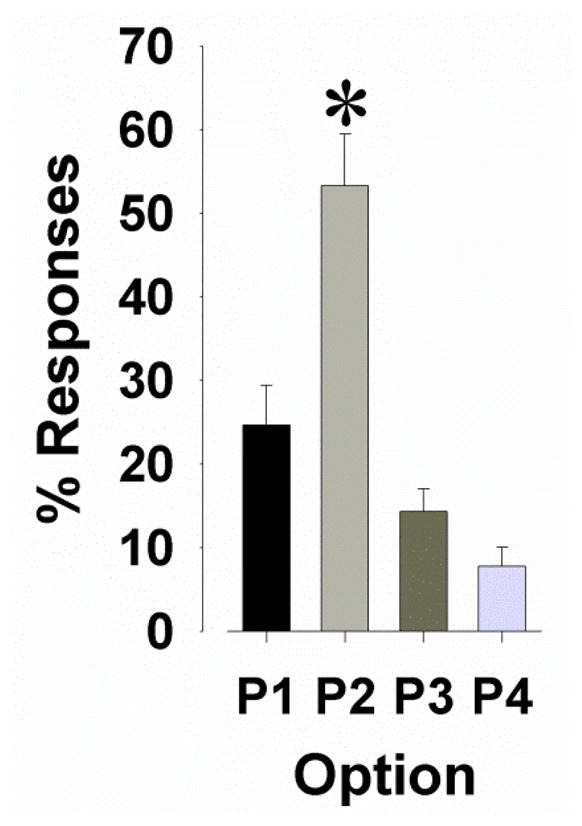
Baseline choice preference of C57BL/6 mice in the IGT after 20 days of training. Mice (n=21) consistently showed a preference for the safe option P2 over options P3 and P4 as indicated by a higher % of responses in P2. Data are shown as the mean + SEM, * denotes p<0.05 when compared with P3, and P4.
Experiment 1: The effects of amphetamine on IGT performance
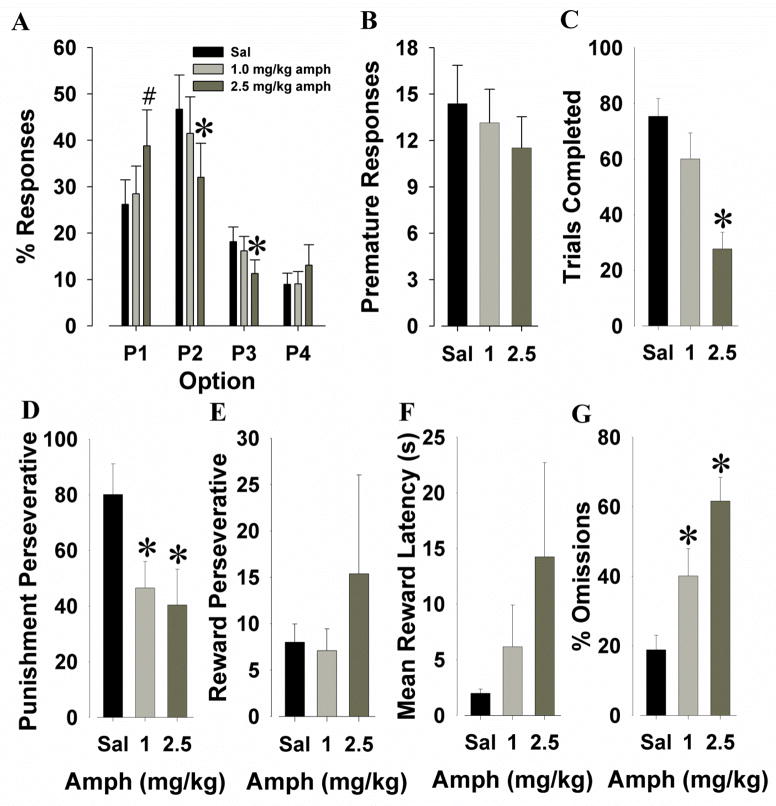
Amphetamine was administered to mice to determine whether this drug would affect their risk-taking behavior in the IGT. Significant main effects of response option were observed (F(3,60)=6.4, p<0.01), as was a dose by response-option interaction (F(6,120)=3.2, p<0.01; Fig. 4A). No main effect of dose was observed (F<1, ns). Post hoc analyses revealed that within P1 there was a trend toward 2.5 mg/kg amphetamine inducing more responses than saline (p=0.056). Within P2, amphetamine at 2.5 mg/kg resulted in fewer responses than saline (p<0.05), similarly for P3 (p<0.05). Although an increase in response to P4 was observed at the highest dose, this difference was not significant (p>0.1).
Fig. 4.
Effects of amphetamine on performance of C57BL/6 mice in the IGT. Amphetamine (1 and 2.5 mg/kg) administration changed choice preference at the highest dose to the lower punishing P1 option (A), did not affect premature responding (B), and significantly decreased trials completed at the highest dose (C). Both doses of amphetamine decreased punishment perseveratives (D), but had no significant effect on reward perseveratives (E). The time animals took to collect their reward was slowed by the highest dose of amphetamine although not significantly (F). At both doses, amphetamine increased the percentage of omitted trials (G). Data are shown as the mean + SEM, * denotes p<0.05 when compared with saline, # denotes p<0.1 when compared with saline.
Significant main effects of amphetamine were observed for trials completed (F(2,40)=18.5, p<0.001; Fig. 4C), punishment perseveratives (F(2,40)=4.6, p<0.05; Fig. 4D), and percentage omissions (F(2,34)=21.0, p<0.001; Fig. 4G). Post hoc analyses revealed that the highest dose of amphetamine reduced trials completed compared to both saline and 1 mg/kg amphetamine (p<0.01). A decrease in punishment perseveratives at both 1 and 2.5 mg/kg amphetamine compared to saline was also observed (p<0.05). Increased percentage omissions was observed at 1 mg/kg amphetamine compared to saline (p<0.05) and at 2.5 mg/kg compared to both saline and 1 mg/kg (p<0.01). No main effects of dose were observed for premature responses (F(2,40)=2.2, p>0.1; Fig. 4B), reward perseveratives (F<1, ns; Fig. 4E), or mean reward latencies (F(2,36)=1.3, p>0.1; Fig. 4F).
Within-session risk preference
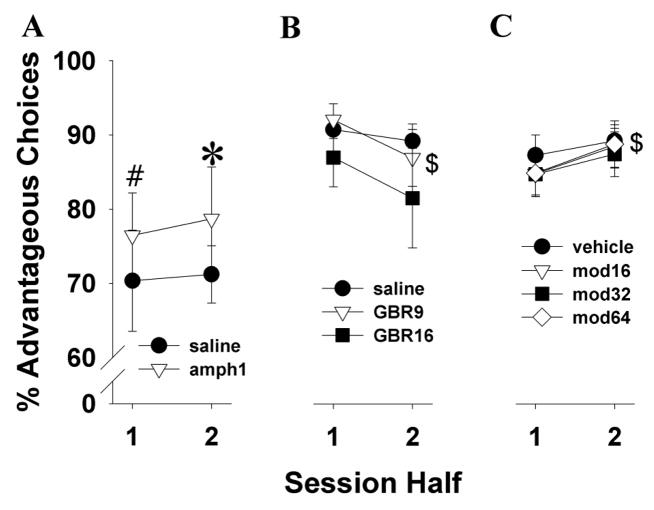
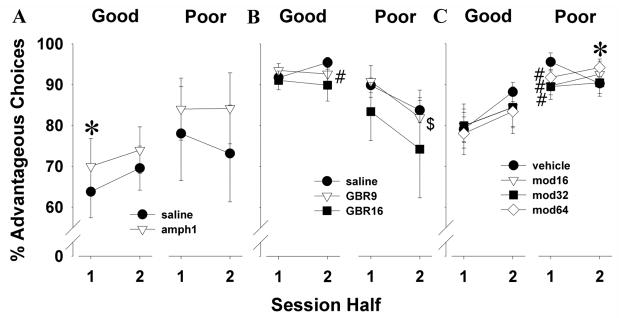
To determine whether the animals’ initial choice strategy was affected by drug, choice preference within the drug-challenged session was examined. Because the highest dose decreased trials completed, we excluded animals given amphetamine at 2.5 mg/kg in this analysis. When performance was split by session half, 1 mg/kg amphetamine significantly increased safe option preference of mice (F(1,12)=6.0, p<0.05; Fig. 5A). Planned comparisons revealed that 1 mg/kg amphetamine resulted in a trend to more safe choices compared to saline during the first half (t=−2.1, p<0.1) and significantly more safe choices compared to saline in the second half (t=−2.3, p<0.05). No main effect of session-half or interaction with amphetamine was observed (F<1, ns).
Fig. 5.
Effects of treatments on choice strategy within the testing sessions. Amphetamine at 1 mg/kg resulted in mice selecting significantly more safe options compared to saline when examined across the session (A). GBR12909 at 9 mg/kg increased risky choice preference of mice in the second half of the session compared to the first, but was not significantly different from saline (B). Modafinil at 64 mg/kg decreased risk choice preference in the second half of the session compared to the first half driven by their riskier preference in the first half of the session (C). Reductions in risk preference across the session induced by modafinil were predominantly observed in poor learners. Data are shown as the mean ± SEM, * denotes p<0.05 when compared with saline, # denotes p<0.1 when compared with saline, and $ denotes p<0.05 when compared with the first session-half.
Experiment 2: The effects of GBR12909 on IGT performance
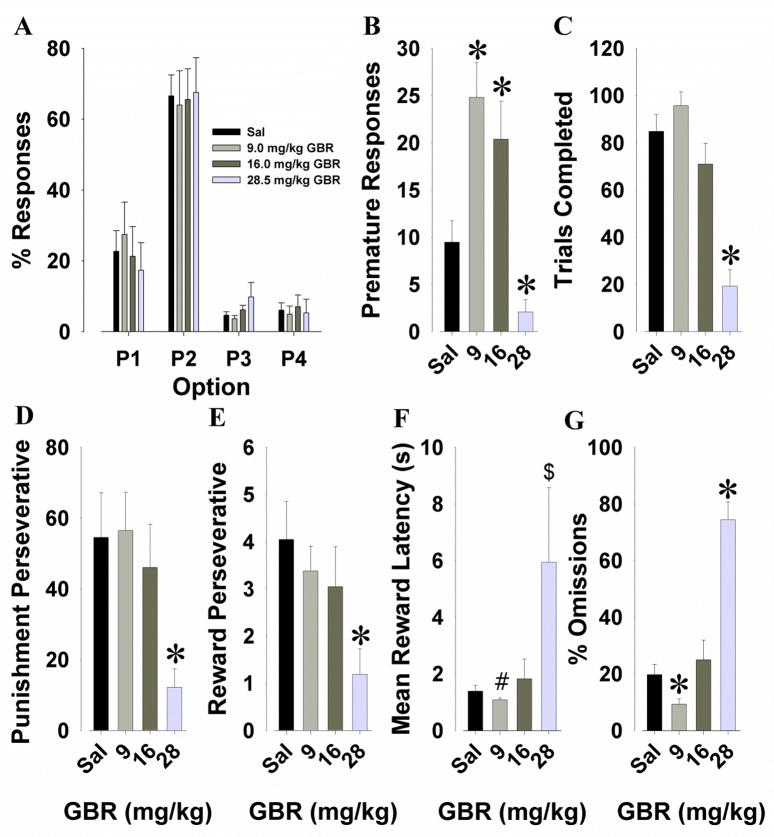
The selective DAT inhibitor GBR12909 was administered to mice to examine its effects on risk-taking behavior. A main effect of response option was observed (F(3,27)=22.5, p<0.001), with no interaction between response option and drug (F<1, ns; Fig. 6A).
Fig. 6.
Effects of GBR12909 on performance of C57BL/6 mice in the IGT. GBR12909 (9, 16, and 28.5 mg/kg) did not impair the animals’ choice preference (A). GBR12909 decreased premature responding at the highest dose, while increasing it at the lower doses (B). At the highest dose, GBR12909 decreased the total trials completed (C), as well as punishment (D) and reward perseveratives (E). GBR12909 at the highest dose tended to slow the latency to collect reward compared to 9 and 16 mg/kg while 9 mg/kg tended to speed latencies compared with saline (F). GBR12909 decreased the percentage of omitted trials at the lowest dose but increased it at the highest dose (G). Data are shown as the mean + SEM, * denotes p<0.05 when compared with saline, # denotes p<0.1 when compared with saline, and $ denotes p<0.1 when compared with 9 and 16 mg/kg GBR12909.
Significant main effects of GBR12909 were observed for premature responses (F(3,60)=11.9, p<0.001; Fig. 6B), trials completed (F(3,60)=33.1, p<0.001; Fig. 6C), punishment perseveratives (F(3,60)=7.9, p<0.001; Fig. 6D), reward perseveratives (F(3,60)=4.1, p<0.05; Fig. 6E), mean reward latencies (F(3,36)=3.3, p<0.05; Fig. 6F), and percentage omissions (F(3,60)=38.9, p<0.001; Fig. 6G). Post hoc analyses revealed that both lower doses (9 and 16 mg/kg) increased premature responding compared to saline, while 28.5 mg/kg resulted in fewer premature responses than saline (p<0.05). Decreased trials completed was observed at 28.5 mg/kg compared to saline and all other doses (p<0.001), with similar 28.5 mg/kg-induced reductions in punishment perseveratives (p<0.05) and reward perseveratives (p<0.05) compared to saline. Further analyses revealed that the lowest dose (9 mg/kg) decreased the percentage of omissions whereas the highest dose increased percentage omissions compared to saline (p<0.01). There was a trend toward 9 mg/kg inducing faster mean reward latencies than saline (p<0.1). In contrast, the highest dose resulted in a trend towards inducing slower reward latencies compared to 9 (p<0.1) and 16 mg/kg (p<0.1).
Within-session risk preference
Mice administered 28.5 mg/kg GBR12909 were discarded from this analysis because of their low level of responding. There was a trend towards a main session-half effect (F(1,14)=3.1, p<0.1; Fig. 5B). No main effect of GBR12909 (F(2,28)=2.1, ns) nor interaction with session-half (F<1, ns) was observed. Planned comparisons revealed that GBR12909 at 9 mg/kg induced significantly more risky choices during the second half compared to the first (t=2.5, p<0.05).
Experiment 3: The effects of modafinil on IGT performance
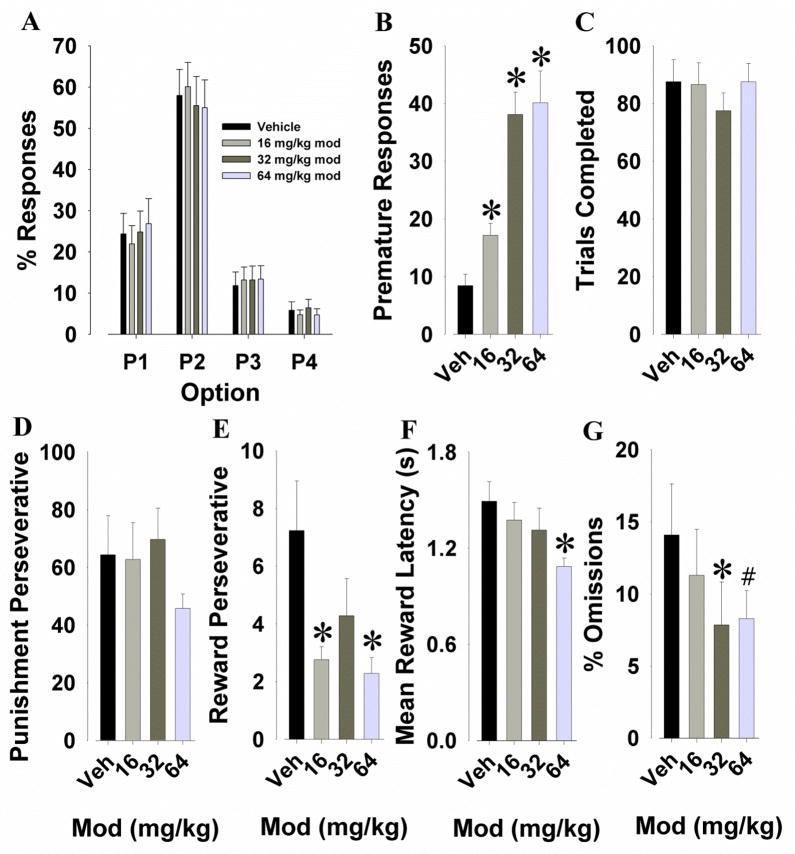
We tested the effects of modafinil on risk-taking behavior in the IGT to determine whether it would have effects similar to GBR12909. For response option, a main effect was observed (F(3,60)=21.1, p<0.001), with no dose by response-option interaction (F<1, ns; Fig. 7A).
Fig. 7.
Effects of modafinil on performance of C57BL/6 mice in the IGT. Modafinil (16, 32, and 64 mg/kg) did not affect the animals’ overall choice preference (A). Premature responding was significantly increased after administration of modafinil at all doses (B). Modafinil had no effect on the animals’ total trials completed (C), nor did it affect punishment perseveratives (D). Mice exhibited less reward perseveratives after treatment with modafinil at both the lowest as well as the highest doses (E), while the latency to collect rewards was significantly decreased by modafinil at the highest dose (F). Modafinil reduced the percentage of omitted trials at the highest doses (G). Data are shown as the mean + SEM, * denotes p<0.05 when compared with vehicle, # denotes p<0.1 when compared with vehicle.
Main effects of modafinil were observed for premature responses (F(3,60)=23.7, p<0.001; Fig. 7B), reward perseveratives (F(3,60)=4.1, p<0.05; Fig. 7E), and mean reward latencies (F(3,60)=3.5, p<0.05; Fig. 7F). Post hoc analyses revealed that all doses increased premature responses compared to vehicle (p<0.001), with 32 mg/kg and 64 mg/kg increasing premature responses more than 16 mg/kg modafinil (p<0.005). Analyses revealed that modafinil induced a decrease in reward perseveratives at 16 mg/kg (p<0.05) and 64 mg/kg (p<0.01) compared to vehicle. A significant decrease in mean reward latency was observed following modafinil administration at 64 mg/kg compared to vehicle (p<0.005), 16 mg/kg (p<0.05), and 32 mg/kg (p<0.1). No main effects of drug were observed for trials completed (F(3,60)=1.1, p>0.1; Fig. 7C), or punishment perseveratives (F(3,60)=1.9, p>0.1; Fig. 7D). Modafinil tended to reduce percentage omissions (F(3,60)=2.5, p<0.1; Fig. 7G), with post hoc analyses revealing that modafinil at 32 mg/kg reduced omissions (p<0.05), while 64 mg/kg tended to reduce omissions (p<0.1) compared to vehicle.
Within-session risk preference
A main effect of session-half (F(1,17)=4.9, p<0.05; Fig. 5C) was observed. No main effect of modafinil or interaction with session-half was observed however (F<1, ns). Post hoc analysis revealed that mice treated with the highest dose of modafinil exhibited more risky choices in the first half of the session compared to the second session-half where they selected more from the safe cues (t=−2.4, p<0.05).
‘Poor learners’ vs. ‘good learners’
In a recent human placebo-controlled IGT study it was observed that modafinil increases risky choice in ‘low-impulsive’ gamblers, but decreases risky choice in ‘high-impulsive’ gamblers (Zack and Poulos 2009). We separated our subjects’ into poor and good learners using a median split on the learning performance of the vehicle-administered mice of the particular drug study (% advantageous choice session-half 2 – % advantageous choice session-half 1).
Amphetamine - Good/poor learners
We observed a main effect of amphetamine (F(1,11)=5.8, p<0.05; Fig. 8A) and a session-half by poor/good learners interaction (F(1,11)=5.1, p<0.05). The good learners (n=7) chose significantly more safe options during the second vs. the first half (70 vs. 64%) (saline, t=−3.1, p<0.05). During the first half, amphetamine at 1 mg/kg increased safe choices compared to saline (70.0 vs. 64%) in the good learners (t=−2.9, p<0.05). No effects of amphetamine were observed in the poor learners (n=6).
Fig. 8.
Effects of DAT inhibitor treatments on choice strategy of good and poor IGT learners. Amphetamine significantly increased safe choice preference of the good learners in the first half of trials compared to saline (A). GBR12909 at 9 mg/kg increased risk choice preference of the good learners in the second half compared to saline and of the poor learners in the second half compared to the first half, but not compared to saline (B). All three doses of modafinil increased risk choice preference of the poor learners in the first half compared to saline, while the highest dose increased safe choice preference in the latter half (C). Data are shown as the mean ± SEM, * denotes p<0.05 when compared with saline, # denotes p<0.1 when compared with saline, and $ denotes p<0.05 when compared with the first session-half.
GBR12909 - Good/poor learners
We observed a trend effect of half (F(1,13)=3.3, p<0.1; Fig. 8B) and half by poor/good learners interaction (F(1,13)=4.4, p<0.1). Poor learners (n=8) chose fewer safe options during the second vs. the first half (84 vs. 90%) (saline, t=2.6, p<0.05). GBR12909 at 9 mg/kg led to fewer safe choices during the second vs. the first half in the poor learners (82 vs. 91%) (t=2.9, p<0.05). The good learners (n=7) chose significantly more safe options during the second vs. the first half (96 vs. 92%) (saline, t=−4.1, p<0.05). During the second half, GBR12909 at 9 mg/kg decreased safe choice preference compared to saline in the good learners (93 vs. 96%) (t=2.2, p<0.1).
Modafinil - Good/poor learners
A main effect of half (F(1,16)=6.1, p<0.05; Fig. 8C) and half by poor/good learners interaction (F(1,16)=5.2, p<0.05) and a trend effect of modafinil by half by poor/good learners interaction (F(3,48)=2.4, p<0.1) was observed. Further analyses revealed that the poor learners (n=9) chose significantly fewer safe options during the second vs. the first half (90 vs. 96%) (saline, t=3.6, p<0.05). All three doses of modafinil induced a trend towards fewer safe choices compared to saline (90, 90, and 92 from low to high dose vs. 96% for saline) during the first half of the session in the poor learners (p<0.1). During the second half however, modafinil at 64 mg/kg led to more safe choices compared to saline (94 vs. 90%) in the poor learners (t=−2.8, p<0.05). The good learners (n=9) chose significantly more safe options during the second vs. the first half (88 vs. 79%) (saline, t=−3.6, p<0.05). Modafinil did not affect the choice performance of good learners.
Correlation of impulsivity measures: Risk preference and premature responding
Analyses revealed that there were individual differences between the animals’ performance on different parameters. No correlation (r=0.07, p>0.1) was observed between the individual differences on % advantageous choices (risk-taking behavior) and ‘premature responses’ (motoric impulsivity). At effective doses of modafinil (r=0.26, p>0.1) and GBR12909 (r=0.19, p>0.1), still no correlations were observed. A correlation between % advantageous choices and premature responses was observed for mice during amphetamine administration (r=0.47, p<0.05), but this did not pass the Bonferroni correction (p>0.0125).
DISCUSSION
With repeated training, C57BL/6 mice learned to choose the less risky options in the IGT resulting in frequent small rewards and little punishment. Importantly, we observed no correlation between risk choice preference and premature responding, suggesting that these two variables measure two different forms of impulsive behavior, impulsive choice and motoric impulsivity respectively (Evenden 1999). Moreover, we have demonstrated that performance in the IGT can be differentially influenced by drugs with similar dopaminergic mechanisms. Administration of the mixed NET/DAT inhibitor amphetamine resulted in a risk-averse preference with no effect on premature responding. In contrast, the more selective DAT inhibitors GBR12909 and modafinil modestly increased risk preference in the IGT, but only when within-session learning rates were controlled for. Unlike amphetamine, these more selective DAT inhibitors also increased measures of motivation and motor impulsivity.
Amphetamine altered the risk preference of mice in a manner that was consistent with its effect in rats (Zeeb et al. 2009), supporting the cross-species translational validity of the rodent IGT. The shift to the more likely rewarded and less risk-prone option P1 indicates a more conservative punishment-averse choice strategy. Zeeb et al. (2009) suggest that amphetamine could make animals hypersensitive to punishments, leading to this increased preference of the small reward/punishment option. This interpretation is supported by amphetamine-induced anxiogenic effects in rodents (Lapin 1993; Onaivi and Martin 1989). Moreover, Rivalan et al. (2009) observed that punishment-averse strategies in the IGT correlated with risk-averse (high anxiety) behaviors in other rat paradigms such as the light/dark emergence and elevated plus-maze test. In approach/avoidance conflict situations, which engender a tradeoff between risk-taking and anxiety, the anxiogenic effects of amphetamine may result in more avoidance behavior. The effects of amphetamine may depend on dose, strain, the presence of a conditioned stimulus during a delayed reward (Cardinal et al. 2000), or the specific cost-benefit decisions the animal is faced with (Floresco et al. 2008). The use of signaled punishing time-outs in the rodent IGT may thus have promoted an amphetamine-induced conservative strategy. In the present studies, we have also examined within-session task performance in an attempt to track the subject’s ongoing decision-making behavior as is performed in human IGT testing and as we have presented previously (Young et al. 2011c). These analyses demonstrated that amphetamine increased the preference for safe options across the whole session. Because the highest dose – tested for comparability with exploratory studies (Perry et al. 2009; Young et al. 2010a) – also reduced trials completed, decreasing perseveration and increasing omissions, future studies could examine the effects of lower doses to determine whether there is a dose-dependent effect on risk preference. The current data underscore the limited aspects of BD that can be modeled by amphetamine (Silverstone et al. 1998; Young et al. 2011a), since patients with BD exhibit increased risk preference in the IGT (Adida et al. 22008; Yechiam et al. 2008), opposite to what was observed here in mice.
In contrast with amphetamine administration to rats in the IGT or 5-choice serial reaction time task (5CSRTT) (Harrison et al. 1997; Pattij et al. 2007; Zeeb et al. 2009), amphetamine administration did not increase premature responding in mice in the IGT. This result may reflect species differences because previous studies in mice have reported an increased although less robust effect of amphetamine on premature responding than in rats (Loos et al. 2010; Yan et al. 2011). Amphetamine has 5- to 9-fold less potency at the DAT than the norepinephrine transporter (NET) in mice and humans (Han and Gu 2006), but with greater selectivity to the DAT over NET in rats (Rothman and Baumann 2003). Given that selective NET inhibition reduces premature responding (Navarra et al. 2008; Paine et al. 2007; Robinson et al. 2008), while selective DAT inhibition increases premature responding (Loos et al. 2010), combined NET and DAT inhibition may obfuscate the effect of DAT inhibition alone, supporting this rat/mouse species difference of amphetamine effects.
Selective DAT inhibition with GBR12909 (9 mg/kg) increased risk preference in mice, albeit only in one session-half. Treatment with the highest dose, 28.5 mg/kg, decreased the number of trials and increased omissions consistent with amphetamine at 2.5 mg/kg (reflecting stereotypy; (Kelley and Lang 1989)), and supporting similar effects on exploratory behavior in the mouse BPM at these doses (Perry et al. 2009; Young et al. 2010b). Despite the greater selectivity for the DAT, at a high dose GBR12909 is also likely to inhibit the NET (Heikkila and Manzino 1984), thus producing amphetamine-like effects such as no increase in premature responding. At low to moderate doses however, GBR12909 induced more premature responses, indicative of motor impulsivity consistent with 5CSRTT studies (Loos et al. 2010). GBR12909 at 9 mg/kg also decreased the amount of omitted trials and the time to obtain reward, supporting drug-induced increases in motivation (Robbins 2002) as seen previously in mice in a progressive ratio breakpoint study (PRBS) (Young and Geyer 2010). This increased motivation could be interpreted as hedonic-like behavior (Flaisher-Grinberg et al. 2009; Gould and Einat 2007), which would support the use of selective DAT inhibition to model mania since patients with BD mania also exhibit hedonic behavior (Cassidy et al. 2002). Increased risk preference in the IGT induced by GBR12909, would further support its use as a model of BD (Clark et al. 2001). The subtlety of the effect observed here limits the possible utility of this model however, given the striking difference between patients with BD and healthy subjects (Adida et al. 2011; Clark et al. 2001).
Consistent with GBR12909, modafinil induced a subtle increase in risk preference when analyzed across session halves. Effects of modafinil on other IGT measures were also consistent with the effects of GBR12909 at low to moderate doses, supporting the interpretation of a similar underlying DAT inhibitory mechanism of these two drugs (Madras et al. 2006; Volkow et al. 2009). In support of DAT inhibition-mediated effects of modafinil, this drug produces the same behavioral effects as GBR12909 in rat activity (Zolkowska et al. 2009), the mouse BPM (Young et al. 2010a; 2011b), and in a mouse PRBS (Young and Geyer 2010). In the human IGT, ‘low-impulsive’ gamblers treated with modafinil chose more risky options compared to placebo, whereas the opposite was observed in ‘high-impulsive’ gamblers within one IGT session (Zack and Poulos 2009). When we separated our subjects in a similar manner, we observed that modafinil increased safe choice preference in the poor within-session learners, similar to the human findings. In the good within-session learners, modafinil did not increase safe choice preference. Hence, these data provide some limited support for the pharmacological predictive validity of the rodent IGT for testing in humans.
We recently reported that DAT KD mice chose riskier options than their WT littermates during initial learning of the IGT (session 1) (Young et al. 2011c). Moreover, DAT KD mice exhibited increased risk preference throughout acquisition of the IGT. Furthermore, DAT KD mice exhibited the same decreased reward latencies and omissions and increased premature responding (Young et al. 2011c) as was observed after modafinil and low-dose GBR12909 administration to C57BL/6 mice. These similar increases in hedonic-like behavior and motor impulsivity support the use of selective pharmacological DAT inhibition in an attempt to model BD mania (Perry et al. 2009; Young et al. 2010a). The consistent risk preference of DAT KD mice during training contrasts with the subtlety of effects observed with acute DAT inhibition once the task was acquired. Differences may therefore exist between affecting performances during within-session learning vs. once trained (de Visser et al. 2011; Zeeb and Winstanley 2011), an important aspect for cross-species translational research because the human IGT assesses within-session learning of risk preference.
Modafinil- and GBR12909-induced increases in risk preference were only detected by analysis of changes in performance across the session. Specifically, modafinil induced more risky choices in the first half of the session, while GBR12909 increased the risk preference of the mice during the second half. This disparity in timing of effects may relate to the pharmacokinetics of the two drugs. GBR12909 did not affect exploration in mice for the first 10 min of a BPM session, while modafinil affected several aspects of exploration (Young et al. 2010a; 2011b). Alternatively, these drugs’ ability to change choice preference within a session may have been influenced by repeated training in the rodent IGT given that a single session is used in the human version. The GBR12909 study was conducted last in these mice after re-baselining performance (Fig. 2), which may have reduced the sensitivity of the mice to DAT inhibition on this behavior. Although performance was stable prior to each drug study, the animals gradually increased advantageous choices, from approx. 70% in study 1 to 90% in study 3). Thus, repeated testing in this rodent IGT may not provide the best means with which to assess the effects of drug manipulation on risk choice. Future studies will examine the effects of these drugs within an extended single training session to determine whether they affect risk learning behavior within a session. Risk preference learning in rats has been measured in a single session in the rodent IGT without prolonged training (de Visser et al. 2011; Rivalan et al. 2009). Experimental effects can differ between these within- and between-session risk learning paradigms. Lesions of the orbitofrontal cortex (OFC) before acquisition in a between-session risk learning rodent IGT led to delayed development of safe choice preference, but with no effect on decision making if lesioned after repeated training (Zeeb and Winstanley 2011). In a within-session rodent IGT however, lesions of the OFC decreased the amount of good decision-making rats (Rivalan et al. 2011), highlighting the difference between learned and within-session learning risk behaviors. Future studies in mice will track the ongoing decision process - like the human IGT - in one session in order to develop a robust cross-species translational task and animal models of BD. Besides DAT inhibition, testing the effects of dopamine agonists on IGT performance may further elucidate the role of the dopaminergic system in risk-taking.
In summary, the present data demonstrate that C57BL/6 mice can be trained to perform the IGT. Moreover, this task can discriminate between two different aspects of impulsive behavior, impulsive choice and motor impulsivity. These two behaviors are differentially influenced by very similar drugs that modulate norepinephrine and dopamine levels and are used to model BD. Both modafinil and GBR12909 induced similar increases in motor impulsivity, motivation, and a modest increase in risk-preference, consistent with, but less striking than, those observed in patients with BD mania. Repeated training in the IGT may impede drug-induced risk-taking effects however. The rodent IGT is a novel tool for investigating the genetic and neurobiological basis of risk-taking behavior. With further refinement, it could be used to screen other animal models of neuropsychiatric disorders and test novel treatments.
Acknowledgments
We thank Dr. Berend Olivier and Mahalah Buell for their support. These studies were supported by NIH grants R01-MH071916, and R21-MH091571, as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. The experiments comply with all US federal and Californian state requirements for animal care and were approved by the American Association for Accreditation of Laboratory Animal Care.
Footnotes
The authors report no conflict of interest.
References
- Adida M, Clark L, Pomietto P, Kaladjian A, Besnier N, Azorin JM, Jeanningros R, Goodwin GM. Lack of insight may predict impaired decision making in manic patients. Bipolar Disord. 2008;10:829–37. doi: 10.1111/j.1399-5618.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–65. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Anand A, Barkay G, Dzemidzic M, Albrecht D, Karne H, Zheng QH, Hutchins GD, Normandin MD, Yoder KK. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar Disord. 2011;13:406–13. doi: 10.1111/j.1399-5618.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Arban R, Maraia G, Brackenborough K, Winyard L, Wilson A, Gerrard P, Large C. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav Brain Res. 2005;158:123–32. doi: 10.1016/j.bbr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9:159–62. doi: 10.1016/j.tics.2005.02.002. discussion 162–4. [DOI] [PubMed] [Google Scholar]
- Berggren U, Tallstedt L, Ahlenius S, Engel J. The effect of lithium on amphetamine-induced locomotor stimulation. Psychopharmacology (Berl) 1978;59:41–5. doi: 10.1007/BF00428028. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–75. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Ahearn EP, Carroll BJ. Symptom profile consistency in recurrent manic episodes. Compr Psychiatry. 2002;43:179–81. doi: 10.1053/comp.2002.32357. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry. 2001;158:1605–11. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- de Visser L, Homberg JR, Mitsogiannis M, Zeeb FD, Rivalan M, Fitoussi A, Galhardo V, van den Bos R, Winstanley CA, Dellu-Hagedorn F. Rodent versions of the iowa gambling task: opportunities and challenges for the understanding of decision-making. Front Neurosci. 2011;5:109. doi: 10.3389/fnins.2011.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Overgaard S, Einat H. Attenuation of high sweet solution preference by mood stabilizers: a possible mouse model for the increased reward-seeking domain of mania. J Neurosci Methods. 2009;177:44–50. doi: 10.1016/j.jneumeth.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–89. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Frey BN, Valvassori SS, Reus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci. 2006;31:326–32. [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci Biobehav Rev. 2007;31:825–31. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium’s reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. Journal of Neuroscience. 2000;20:1208–15. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, Kelsoe JR. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. Am J Med Genet. 2001;105:145–51. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1161>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Molecular Psychiatry. 2006;11:125–133. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Assessing a vigilance decrement in aged rats: effects of pre-feeding, task manipulation, and psychostimulants. Psychopharmacology. 2002;164:33–41. doi: 10.1007/s00213-002-1174-3. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 1997;133:329–42. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L. Behavioral properties of GBR 12909, GBR 13069 and GBR 13098: specific inhibitors of dopamine uptake. Eur J Pharmacol. 1984;103:241–8. doi: 10.1016/0014-2999(84)90483-7. [DOI] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology (Berl) 2006;188:144–51. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Homberg JR, van den Bos R, den Heijer E, Suer R, Cuppen E. Serotonin transporter dosage modulates long-term decision-making in rat and human. Neuropharmacology. 2008;55:80–4. doi: 10.1016/j.neuropharm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Lau T, Rietschel M, Schloss P. A dopamine transporter mutation associated with bipolar affective disorder causes inhibition of transporter cell surface expression. Mol Psychiatry. 2005;10:1104–9. doi: 10.1038/sj.mp.4001730. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Lang CG. Effects of GBR 12909, a selective dopamine uptake inhibitor, on motor activity and operant behavior in the rat. European Journal of Pharmacology. 1989;167:385–95. doi: 10.1016/0014-2999(89)90447-0. [DOI] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Eckert ED, Faris PL, Hartman BK. Pathological gambling and mood disorders: clinical associations and treatment implications. J Affect Disord. 2006;92:109–16. doi: 10.1016/j.jad.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Anxiogenic effect of phenylethylamine and amphetamine in the elevated plus-maze in mice and its attenuation by ethanol. Pharmacology, Biochemistry and Behavior. 1993;44:241–3. doi: 10.1016/0091-3057(93)90305-d. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer AN, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–24. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–9. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Payne JL, Singh J, Lopes BP, Viegas JS, Zarate CA. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–46. [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord. 2010;120:200–6. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Martin BR. Neuropharmacological and physiological validation of a computer-controlled two-compartment black and white box for the assessment of anxiety. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1989;13:963–76. doi: 10.1016/0278-5846(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry. 2007;62:687–93. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 2007;191:587–98. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA. Quantifying overactivity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res. 2010;178:84–91. doi: 10.1016/j.psychres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–80. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, Bertolino A, Malhotra AK, Gu HH, Sadee W. Dopamine Transporter Gene Variant Affecting Expression in Human Brain is Associated with Bipolar Disorder. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–9. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalan M, Ahmed SH, Dellu-Hagedorn F. Risk-Prone Individuals Prefer the Wrong Options on a Rat Version of the Iowa Gambling Task. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Rivalan M, Coutureau E, Fitoussi A, Dellu-Hagedorn F. Inter-individual decision-making differences in the effects of cingulate, orbitofrontal, and prelimbic cortex lesions in a rat gambling task. Front Behav Neurosci. 2011;5:22. doi: 10.3389/fnbeh.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–37. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, Bechara A. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007;92:74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone PH, Pukhovsky A, Rotzinger S. Lithium does not attenuate the effects of D-amphetamine in healthy volunteers. Psychiatry Res. 1998;79:219–26. doi: 10.1016/s0165-1781(98)00037-7. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disord. 2009;11:280–8. doi: 10.1111/j.1399-5618.2009.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Steinberg JL, Lijffijt M, Moeller FG. Impulsivity: differential relationship to depression and mania in bipolar disorder. J Affect Disord. 2008;106:241–8. doi: 10.1016/j.jad.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, Lasthuis W, den Heijer E, van der Harst J, Spruijt B. Toward a rodent model of the Iowa gambling task. Behav Res Methods. 2006;38:470–8. doi: 10.3758/bf03192801. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, de Jong W. Differential effects of dopaminergic drugs on open-field behavior of spontaneously hypertensive rats and normotensive Wistar-Kyoto rats. J Pharmacol Exp Ther. 1989;248:1189–96. [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: A comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2008:1–14. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Freed WJ, Kleinman JE. Neuropathology of bipolar disorder. Biol Psychiatry. 2000;48:486–504. doi: 10.1016/s0006-3223(00)00978-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Waters KA, Burnham KE, O’Connor D, Dawson GR, Dias R. Assessment of modafinil on attentional processes in a five-choice serial reaction time test in the rat. J Psychopharmacol. 2005;19:149–58. doi: 10.1177/0269881105048995. [DOI] [PubMed] [Google Scholar]
- Yan TC, Dudley JA, Weir RK, Grabowska EM, Pena-Oliver Y, Ripley TL, Hunt SP, Stephens DN, Stanford SC. Performance deficits of NK1 receptor knockout mice in the 5-choice serial reaction-time task: effects of d-amphetamine, stress and time of day. PLoS One. 2011;6:e17586. doi: 10.1371/journal.pone.0017586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Hayden EP, Bodkins M, O’Donnell BF, Hetrick WP. Decision making in bipolar disorder: a cognitive modeling approach. Psychiatry Res. 2008;161:142–52. doi: 10.1016/j.psychres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of modafinil--increased motivation via the dopamine transporter inhibition and D1 receptors? Biol Psychiatry. 2010;67:784–7. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010a;208:443–54. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol Biochem Behav. 2010b;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA. Predictive animal models of mania, hits, misses, and future directions. Br J Pharmacol. 2011a doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kooistra K, Geyer MA. Dopamine receptor mediation of the exploratory/hyperactivity effects of modafinil. Neuropsychopharmacology. 2011b;36:1385–96. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev. 2007;31:882–96. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J Psychopharmacol. 2011c;25:934–43. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23:660–71. doi: 10.1177/0269881108091072. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–43. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J Neurosci. 2011;31:2197–204. doi: 10.1523/JNEUROSCI.5597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–46. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]