Abstract
Alcoholism and HIV-1 infection each affect components of selective attention and cognitive control that may contribute to deficits in emotion processing based on closely interacting fronto-parietal attention and frontal-subcortical emotion systems. Here, we investigated whether patients with alcoholism, HIV-1 infection, or both diseases have greater difficulty than healthy controls in resolving conflict from emotional words with different valences. Accordingly, patients with alcoholism (ALC, n = 20), HIV-1 infection (HIV, n = 20), ALC + HIV comorbidity (n = 22), and controls (CTL, n = 16) performed an emotional Stroop Match-to-Sample task, which assessed the contribution of emotion (happy, angry) to cognitive control (Stroop conflict processing). ALC + HIV showed greater Stroop effects than HIV, ALC, or CTL for negative (ANGRY) but not for positive (HAPPY) words, and also when the cue color did not match the Stroop stimulus color; the comorbid group performed similarly to the others when cue and word colors matched. Furthermore, emotionally salient face cues prolonged color-matching responses in all groups. HIV alone, compared with the other three groups, showed disproportionately slowed color-matching time when trials featured angry faces. The enhanced Stroop effects prominent in ALC + HIV suggest difficulty in exercising attentional top-down control on processes that consume attentional capacity, especially when cognitive effort is required to ignore negative emotions.
Keywords: Emotional Stroop, Selective attention, Top-down and bottom-up processes, Faces, Fronto-parietal attention system, Frontal-subcortical emotion system
INTRODUCTION
Chronic alcoholism has a detrimental impact on brain systems involved in cognitive control and emotional regulation (Makris et al., 2008). Such deficits can compromise impulse control and may promote risky behavior, actions likely to lead to HIV-1 infection (Leigh & Stall, 1993). Impaired executive control over attentional and emotion systems occurs in alcoholism (Stormark, Laberg, Nordby, & Hugdahl, 2000) and HIV-1 infection (Hinkin, Castellon, Hardy, Granholm, & Siegle, 1999). The difficulty that alcoholics and HIV-1 infected individuals experience with attention and cognitive control may also influence their response to emotionally relevant stimuli and may contribute to experiencing their illness as uncontrollable and promote feelings of depression (Bauer & Shanley, 2006; Clark, Oscar-Berman, Shagrin, & Pencina, 2007; Hutton, Lyketsos, Zenilman, Thompson, & Erbelding, 2004). Despite high prevalence rates of alcoholism–HIV-infection comorbidity estimated to range from 29% to 60% (Lefevre et al., 1995), little is known about the combined effects of these diseases on cognitive control and emotion processing. The focus of this study was to investigate the interactions among attention, cognitive control, and emotion by examining the influence that the emotional valence of a stimulus exerts on processing cognitive conflict in individuals with alcoholism alone, HIV-1 infection alone, and both diseases compared with healthy control subjects.
Attention is not a unitary function (Asplund, Todd, Snyder, & Marois, 2010); rather, different component processes served by different neural systems interact for information focus, exchange, updating, and possible conflict and interference (Posner & Rothbart, 2007). Whereas automatic attention captured by emotional relevance or novelty is unconscious and stimulus driven (bottom-up) (Ohman, Lundqvist, & Esteves, 2001), voluntary attention is consciously controlled (top-down) and has been tested with cues that direct attention to a specific stimulus feature, location (Corbetta & Shulman, 2002).
A paradigm widely used to study cognitive control processes in clinical populations is the Stroop color-word interference task (Stroop, 1935). The traditional Stroop paradigm, in which the semantic meaning of words that are involuntarily and automatically processed, naturally override perceptual tags, such as the font color in which the word is written, and can be used to parse and manipulate processes of attention and cognitive conflict (MacLeod, 1991). Some studies of HIV-1 infected individuals (Martin et al., 1992) and alcoholics (Stormark et al., 2000) have shown enhanced Stroop color-word interference relative to controls; however, Stroop effects in HIV-1 infected individuals (Hinkin et al., 1999) and alcoholics (Dom, De Wilde, Hulstijn, van den Brink, & Sabbe, 2006) have not always been forthcoming.
Intersecting networks of frontal, limbic, and temporal brain regions subserve emotion perception and executive functioning. Compromise of these prefrontal cortical-limbic systems occurs in both alcoholism (Marinkovic et al., 2009) and HIV-1 infection (Ernst, Chang, Jovicich, Ames, & Arnold, 2002) and is linked to deficits in inhibitory control and emotional face processing (Langenecker et al., 2005). Converging evidence comes from neurophysiological studies reporting that impaired cognitive processing in alcoholism affected processing of emotional information such as the ability to detect emotion in another’s voice (Monnot, Nixon, Lovallo, & Ross, 2001) or to recognize facial expressions (Foisy et al., 2007). Although HIV-related neuropsychological impairment is generally mild, especially in medically asymptomatic infection, it most often involves attention (Becker, Lopez, Dew, & Aizenstein, 2004; Martin et al., 2004).
To study emotional responsiveness (automatic, or bottom-up processes) and cognitive control (voluntary, or top-down processes), we devised an emotional Stroop Match-to-Sample task, in which the subjects were asked to match the color of a cue to that of a target stimulus, which was either a word or a letter string. In word trials, color matching simultaneously required the inhibition of the words meaning for fast response execution. The word had either a positive or a negative emotional meaning to test whether cognitive control of (1) the word reading itself or (2) the word’s emotional content influenced color-matching performance differently in alcoholism, HIV-1 infection, and their comorbidity. Valence of emotion has been found to play a role in emotional Stroop processing (Kahan & Hely, 2008). Negative emotional expressions, including negative words, are assumed to trigger an automatic defense mechanism that interferes with concurrent cognitive processes (Pratto & John, 1991). In agreement with this notion, behavioral experiments on lexical decision-making and word naming found slowed responses to negative as compared to emotionally neutral and positive words (e.g., MacKay et al., 2004).
We further studied whether an emotional (angry or happy) face automatically captured attention and affected cue-target color matching performance differently in the four study groups. Perception of facial emotion has been conceptualized as a bottom-up process (Vuilleumier, Armony, Driver, & Dolan, 2001), and the cognitive evaluation and the regulatory control of emotion-related behavior as a top-down process (Hariri, Bookheimer, & Mazziotta, 2000). Emotional perception and the regulation of emotion are intertwined in a reciprocal manner (Quirk & Beer, 2006), making it difficult to isolate top-down influences from the bottom-up response engendered by the presentation of a face (Li et al., 2009).
The aim of the study was to investigate on a behavioral level whether co-morbid patients with HIV-1 infection and alcoholism would be more compromised than those with either disease alone and exhibit more difficulty than healthy controls in resolving conflict from emotional words. First, we hypothesized that all subjects would show Stroop effects with slowed responses to words than color strings. We next hypothesized that the groups would differ in the severity of the Stroop effect depending on attentional demand. Specifically, we expected that patient groups would be most compromised in Stroop conflict processing when the color cue misdirected attention in nonmatch trials, but not when the cue color correctly predicted the color of the Stroop word (color match) (Schulte, Müller-Oehring, Rosenbloom, Pfefferbaum, & Sullivan, 2005). Third, we hypothesized that Stroop performance would differ as a function of emotional valence with greater emotional Stroop effects, that is, more difficulty reducing interference, from negative than positive emotional word content (Dahl, 2001) and that this effect would be more salient in participants who show difficulty with emotion regulation such as in alcoholics (Clark et al., 2007) and HIV-1 infection (Novara et al., 2000). Specifically, we hypothesized that patients comorbid for alcoholism and HIV-1 would be more impaired in processing negative emotional Stroop content than patients with either disease alone and that patients with only one condition would be more impaired compared to healthy control subjects. Fourth, we hypothesized that emotionally salient stimuli that automatically capture attention (happy or angry faces) would interfere with color matching performance differently across the four groups, specifically for negative valence compared to positive valence. Here, we further expected that presenting emotional faces before Stroop words with matching content (e.g., angry face–word ANGRY) would enhance emotional responsiveness and Stroop interference from emotional words. Finally, we hypothesized that other measures of executive control, such as cognitive flexibility, would be related to the ability to resolve conflict from emotional words in the patient groups.
METHODS
Subjects
Four groups were tested: 20 patients with alcoholism alone (ALC), 20 patients with HIV-1 infection alone (HIV), 22 patients with ALC + HIV comorbidity, and 16 healthy controls (CTL). All participants gave written informed consent to participate in the study, which was approved by the institutional review boards at Stanford University School of Medicine and SRI International. All volunteers were screened by trained clinicians using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis (American Psychiatric Association, 1994) and medical history to exclude any with other lifetime Axis I diagnoses, current (past 3 months) non-alcohol drug abuse or dependence diagnosis, or use of drugs (excluding cannabis) in the past month. Subjects were screened for a history of head trauma, documented compound skull fracture, clear neurologic sequelae, or any disease with potential central nervous system involvement, such as stroke, multiple sclerosis, or epilepsy.
The study groups did not differ in age, gender, or verbal intelligence (ANART IQ, National Adult Reading Test; Nelson, 1982). All subjects were right handed as determined from an objective quantitative questionnaire (Crovitz & Zener, 1962). Socioeconomic status (SES) was determined using a 2-factor scale that includes both education and lifetime occupation (Hollingshead & Redlich, 1958) and can be considered a representative measure of the highest functioning achieved (Sassoon et al., 2007). Groups differed in SES (Hollingshead & Redlich, 1958), with HIV + ALC (p = .0001) and HIV (p = .039), but not ALC (p = .11), having a significantly lower SES than CTL. ALC + HIV had lower SES than ALC (p = .02) and HIV, as a trend (p = .07), whereas HIV and ALC did not differ from each other in SES (p = .60) (Table 1).
Table 1.
Demographic data (mean ± SD) and statistical results for four groups: Controls (CTL), alcoholics (ALC), HIV-1 infected individuals (HIV), and those with both diseases (ALC + HIV)
| CTL | ALC | HIV | HIV + ALC | χ2, F, t value | p | |
|---|---|---|---|---|---|---|
| n | 16 | 20 | 20 | 22 | ||
| Sex (women/men) | 8/8 | 10/10 | 15/5 | 13/9 | 3.32b | 0.35 |
| Ethnicity (n) | 15.07b | 0.09 | ||||
| Asian | 3 | 0 | 0 | 0 | ||
| African-American/black | 6 | 9 | 9 | 11 | ||
| Pacific Islander | 0 | 1 | 0 | 0 | ||
| Caucasian/white | 7 | 10 | 11 | 11 | ||
| Hispanice | 1 | 2 | 2 | 7 | ||
| Age (years) | 40.9 (11.7) | 45.7 (11.3) | 49 (6.3) | 46.9 (8.7) | 2.19c | 0.10 |
| SESa | 31.6 (13.2) | 38.1 (12) | 40 (11.7) | 46.5 (9.4) | 5.06c |
0.003 ALC + HIV > CTL = ALC; ALC = HIV; HIV > CTL |
| Verbal IQ (ANART) | 110.5 (13.4) | 105.6 (12.5) | 105.3 (11.1) | 102.6 (9.1) | 1.37c | 0.26 |
| Lifetime alcohol consumption (kg) | 24.3 (39) | 888.9 (619) | 57.9 (54) | 991.8 (1319) | 7.97c |
0.0001 ALC = ALC + HIV > HIV = CTL |
| Alcohol abstinence before the study (months) | — | 22 (31) | — | 15 (29) | 0.77d | 0.45 |
| CD4 (cell/mm3) | — | — | 558.2 (254) | 452.6 (209.5) | 1.47d | 0.15 |
| Age ALC onset (years) | — | 23 (9) | — | 22.8 (8) | 0.13d | 0.90 |
| Age HIV onset (years) | — | — | 34 (7) | 34 (10) | 0.19d | 0.85 |
| HART medication (n) | — | — | 16 | 20 | 1.02b | 0.31 |
| Karnofsky score (0–100) | 100 (0) | 100 (0) | 99.5 (2.2) | 99.1 (2.9) | 0.85c | 0.47 |
| BDI2 score | 2.6 (2.7) | 5.9 (4.3) | 12.5 (9.1) | 12.1 (9.1) | 7.59c |
0.0001 ALC + HIV = HIV > ALC = CTL |
| Other co-morbidity (n): | 0 | 6 | 5 | 14 | ||
| Hepatitis C | 0 | 0 | 4 | 8 | 13.57b | 0.004 |
| Other substance abuse | 0 | 4 | 0 | 7 | 12.18b | 0.007 |
| SCID mood diagnosis: | 2.78b | 0.43 | ||||
| Anxiety | 0 | 0 | 1 | 1 | ||
| Major depression | 0 | 2 | 0 | 2 |
Socioeconomic status (SES): higher scores represent lower SES (range, 11–77).
Pearson χ2.
Univariate analysis of variance: F value.
independent samples T-test: t value
Hispanic participants are included in ethnic categories above.
Lifetime alcohol consumption (in kilograms) was assessed using a semistructured interview (Pfefferbaum, Rosenbloom, Crusan, & Jernigan, 1988). Drinks of each type of alcoholic beverage were standardized to units containing approximately 13.6 g of absolute alcohol and summed over the lifetime. Groups differed significantly in lifetime alcohol consumption, with the two alcohol groups having consumed significantly more alcohol than the control and the HIV group, whereas ALC and ALC + HIV groups did not significantly differ from each other (Table 1). The two alcoholism groups did not differ in length of alcohol abstinence before study participation, and age at onset of alcoholism. The two HIV groups did not differ in age at onset of HIV-1 infection. From the 22 subjects in the ALC + HIV group, alcoholism preceded HIV-1 infection in 17 subjects, and HIV-1 infection preceded alcoholism in 3 subjects. Onset of alcoholism was unknown in one woman, and year of HIV-1 infection unknown in one man. The HIV and ALC + HIV groups did not differ in CD4 T-cell count, an index of disease severity (Table 1).
The Karnofsky scale, which ranged from 0 to 100 (Karnofsky, Abelmann, Craver, & Burchenal, 1948), estimated each patient’s HIV-infection disease severity in terms of daily functioning; no one scored below 80 (normal activity with effort and some signs of disease), 7.2% were scored 90 (normal activity; minor signs of disease), and 92.8% had no evidence of disease (score 100). Regarding HIV medication, 80% in the HIV group and 91% in the ALC + HIV group were taking highly active antiviral treatment (HAART). There was no significant difference in medication status between these two study groups (Table 1).
To address the potential contribution of depressive symptoms to our primary dependent measures, we used the Beck Depression Inventory (BDI-II), a quantitative measure of depressive symptoms (Beck, Steer, & Brown, 1996). BDI scores of 0–13 indicate minimal, 14–19 mild, 20–28 moderate, and 29–63 severe depressive symptoms. HIV and ALC + HIV groups scored on average within the minimal, non-clinical range. The scores were even lower for alcoholics and lowest in healthy controls. Groups differed significantly in BDI scores (Table 1); however, over all groups the BDI depression scores were relatively low. In the two HIV groups, number of BDI depressive symptoms correlated with the amount of lifetime alcohol consumption (HIV: rho = 0.54; p = .01; trend for ALC + HIV: rho = 0.35; p = .06; one-tailed), a relationship that was not observed in alcoholics (rho = 0.17, ns) or controls (rho = −0.23, ns). DSM-IV diagnoses assigned at the SCID interview indicated that 2 ALC and 2 ALC + HIV patients met criteria for current major depression. In addition, 4 HIV and 8 ALC + HIV tested positive for Hepatitis-C infection (HCV); 4 ALC and 7 ALC + HIV had also used other drugs than alcohol within the past year but not within the past 3 months before testing. All of these 11 subjects reported cocaine use, and 3 ALC + HIV had additionally used opioids and 1 ALC + HIV had also used amphetamines. Together, 6 ALC, 5 HIV, and 14 ALC + HIV had at least one other comorbidity, including mood disorder. Finally, all subjects took a breath-alcohol analyzer test to ensure abstinence from alcohol at the time of testing.
Data Acquisition and Analyses
The emotional Stroop Match-to-Sample task required subjects to match the color of an emotional word (HAPPY, ANGRY) or of a letter string (XXXXX) printed in red, green, or blue to that of a prior color cue (Figure 1). Cue and target colors matched in half of the trials. Participants pressed a YES-key for color matches and a NO-key for color nonmatches.
Fig. 1.

Emotional Stroop paradigm. The task was to match the color of a color cue to that of a target stimulus by pressing a YES-key for color matches and a NO-key for color nonmatches. The task had four color matching conditions: (1) “no face – letter string,” (2) “no face – emotional word,” (3) “face cue – letter string,” and (4) “face cue – emotional word.” In the face matching control condition “face cue – emotional word” subjects were asked to match the emotional face expression to the meaning of the word. Between cue and target presentation the word COLOR or the word FACE was briefly presented to instruct the subjects to either match colors of color cues and targets or the emotional content of faces and target words.
Face stimuli were selected from the MacBrain Face Stimulus Set (http://www.macbrain.org/resources.htm). Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development.
In the color match “letter string” condition, the target stimulus consisted of a neutral letter string XXXXX. In the color match “emotional word” condition, the target stimulus consisted of the word HAPPY or the word ANGRY. In half of these color match trials, an emotional face cue was presented between color cue and target stimulus to test how such emotional cueing would influence conflict resolution for emotional Stroop words. In color-match trials, emotional face cues and emotional Stroop words were always congruent, resulting in two combinations: “happy face–HAPPY” and “angry face–ANGRY.” Emotional face cues were also presented in combination with a letter string, that is, “happy face–XXXXX” and “angry face–XXXXX.” Between cue and target presentation the word COLOR or the word FACE was briefly presented to instruct the subjects to either match the color of a cue stimulus with the color of an emotional word or to match the emotional face expression with the meaning of the word by pressing a YES or NO key with their right hand. The face-match condition was designed as a control condition to ensure that subjects were able to match happy and angry faces correctly to emotional words. Conditions were administered in blocks of 4 trials; blocks were presented in pseudo-random order thereby mixing face-match and color-match conditions and ensuring the same number of set/rule switches for each subject. In total, 144 trials were presented, 48 for face-match and 96 for color-match with 24 trials in each of the four color-match conditions: (1) no face–letter string, (2) no face–emotional word, (3) face cue–letter string, and (4) face cue–emotional word. Number of correct responses, errors and reaction time data were recorded for each trial and subject. Subjects had to make at least 80% correct responses to be included for further analyses; subjects excluded on this basis were 2 controls, 3 ALC, 5 HIV, and 8 ALC + HIV, yielding group sizes of 16 CTL, 20 ALC, 20 HIV, and 22 ALC + HIV.
Statistical Analyses
Mean reaction times (RTs) for correct YES and NO responses and the numbers of errors were computed for each subject for each color-match condition. With exception for analyses involving set switching, RTs for face-match trials were not further analyzed. Analyses of variance (ANOVAs) for repeated measures were used to test for effects of group, emotional Stroop (letter string–emotional word), emotional face cue (no cue–face cue) and cue–target color match (match–nonmatch) in the emotional Stroop Match-to-Sample task (4 × 2 × 2 × 2 mixed factors). For further examination of the group-by-Stroop-by-cue-by-match interaction, we calculated (1) Stroop effects by subtracting emotional word from letter string trials for match and nonmatch conditions and for no-cue and emotional-cue conditions separately, and (2) cue effects by subtracting face-cue from no-cue trials for match and nonmatch conditions and for letter-string and emotional-word conditions separately. To test for the effect of emotional valence (happy–angry), Stroop and cue effects were calculated for both positive and negative emotional valence. The resulting emotional Stroop variables and emotional cue variables were subjected to ANOVAs testing for group differences on emotional valence on attention and Stroop conflict processing. A measure of effect size (partial eta-squared, ηp2) for significant effects is cited (0.01 ≅ small, 0.06 ≅ medium, 0.15 ≅ large). Significant group effects were followed up with post hoc least significant difference (LSD) tests. Over all ANOVAs and for within-group follow-up analyses, FDR thresholds for group–task interaction effects were calculated to correct for multiple comparisons (Benjamini, Drai, Elmer, Kafkafi, & Golani, 2001). Spearman’s rho correlation (two-tailed) examined the relationship between overall RTs and errors separately in each of the four study groups. Spearman’s rho correlation (one-tailed) was further used to test for relationships between demographic and clinical variables and emotional Stroop effects, based on the assumption that higher severity of illness would result in poorer performances. Applying family-wise Bonferroni correction for 4 comparisons of emotional Stroop valence effects (Stroop-angry match and nonmatch; Stroop-happy match and nonmatch), p-values ≤ .025 were considered significant. For all other statistical tests, alpha significance levels were set at p < .05 (SPSS 15.0).
RESULTS
Accuracy
The four groups differed significantly in number of errors (F(3,74) = 3.79; p = .014; ηp2 = .13), with ALC + HIV (9 ± 5.6 errors) having committed more errors than CTL (4 ± 3.5 errors; p = .007) and ALC (5 ± 4 errors; p = .012) but not more than HIV (8 ± 5 errors; p = .50; post hoc LSD test). There was a positive correlation between errors and overall RTs in ALC + HIV (rho = 0.50; p = .018) but not in ALC (rho = 0.37; p = .11), HIV (rho = 0.11; p = .65) and CTL (rho = 0.30; p = .25), suggesting an absence of a speed-accuracy trade-off (Salo, Henik, & Robertson, 2001).
Reaction Times Analyses
Emotional Stroop Match-to-Sample task performance
In an overall analysis, we first tested whether cognitive control of the word reading itself influenced color-matching performance differently in alcoholism, HIV-1 infection, and specifically their comorbidity compared to controls. Using a repeated measures ANOVA, we entered three within-subject factors that compared (1) color matching performance for words and letter strings trials (Stroop), irrespective of the Stroop word’s content, (2) trials with and without face cues (face cue), again irrespective of facial expression, and (3) color match and nonmatch trials (color-match); and group as between-subjects factor. A significant group effect (F(3,74) = 3.04; p = .034; ηp2 = .11) indicated overall longer RTs in ALC + HIV (p = .011) and HIV (p = .024) than CTL, and no difference in ALC and CTL (p = .39). As hypothesized, we found an effect for Stroop (F(1,74) = 40.66; p < .0001; ηp2 = .36), that is, longer RTs to words than letter strings. We further found a group-by-Stroop interaction with ALC + HIV showing greater Stroop effects than the other three groups (F(1,74) = 3.52; p = .019; ηp2 = .13) (Figure 2a). Significant effects of face-cue (F(1,74) = 27.16; p < .0001; ηp2 = .27), color-match (F(1,74) = 84.8; p < .0001; ηp2 = .53), and an face-cue–by–color-match interaction (F(1,74) = 19.5; p < .0001; ηp2 = .21) revealed that emotional face-cues slowed responses in non-match but not match conditions in all groups. Finally, using an analysis of covariance, we found that SES as covariate had no significant effect on emotional Stroop processing (F(1,71) = 0.44; p = .51; ηp2 = .006) but showed an interaction with Stroop, color matching, and face cueing effects (four-way interaction; F(1,71) = 5.21; p = .025; ηp2 = .07).
Fig. 2.
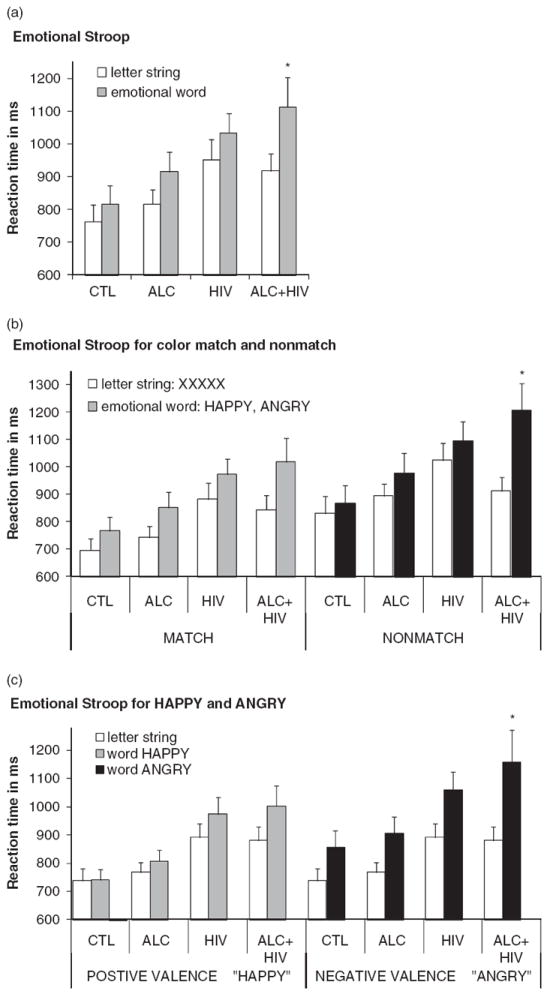
Emotional Stroop performance. Mean reaction times (RTs) and standard errors (SE) for each group and condition: Controls (CTL), alcoholics (ALC), HIV-1 infected individuals (HIV), and individuals with both diseases (ALC + HIV). (a) Emotional Stroop performance comparing cue-target color matching speed under letter string conditions and emotional word conditions (pooled over positive and negative emotional valence), (b) separately for cue-target color matches (MATCH) and nonmatches (NONMATCH), and (c) separately for positive (HAPPY) and negative (ANGRY) emotional valence.
*p < .05 group-by-emotional Stroop interaction: ALC + HIV had significantly longer RTs than the other three groups for emotional word trials (a), specifically when cue and target colors did not match (b), and when the emotional word content was negative (c).
Stroop for color match and nonmatch
We tested the hypothesis that patient groups, and especially co-morbid patients, would be most compromised in Stroop conflict processing when the color cue misdirected attention in nonmatch trials, but not when the cue color correctly predicted the color of the Stroop word (color-match). We conducted two separate ANOVAs for nonmatch and match conditions; each included group as between subjects factor, and Stroop (word–letter string) and face-cue (face–no face) as within-subjects factors. We found a significant group-by-Stroop interaction when the cue color did not match the Stroop stimulus color (F(1,74) = 3.89; p = .012; ηp2 = .14). Post hoc LSD test on the group-by-Stroop-nonmatch interaction (using difference RTs for Stroop-nonmatch = RT[word/nonmatch] − RT[letter string/nonmatch]) showed that ALC + HIV (p = .003), but not ALC (p = .44) or HIV (p = .56), had greater Stroop-nonmatch effects than CTL. Stroop effects did not differ between groups when cue and word colors matched (F(1,74) = 1.32; p = .28; ηp2 = .05) (Figure 2b).
Stroop for negative and positive emotional valence
We tested the hypotheses that Stroop interference depends on emotional valence, and that patients, especially comorbid patients, would show enhanced interference from negative (ANGRY) but not positive (HAPPY) word contents. For negative word content, an ANOVA with Stroop-angry (ANGRY–XXXXX) as within-subject factor and group as between-subjects factor revealed greater Stroop effects in ALC + HIV than HIV, ALC, or CTL (F(1,74) = 3.59; p = .018; ηp2 = .13). For positive word content, an ANOVA with Stroop-happy (HAPPY–XXXXX) as within-subject factor and group as between-subjects factor did not show such a group-by-Stroop interaction (F(1,74) = 1.68; p = .18; ηp2 = .06) (Figure 2c).
We next tested the hypothesis that negative emotion would interfere more with color matching performance than positive emotion, and that this effect would be most salient in comorbid participants. To do this, we computed Stroop effects by subtracting RTs to letter strings from RTs to words: (1) for negative valence (Stroop-angry = RT[ANGRY]−RT[XXXXX]) and (2) for positive valence (Stroop-happy = RT[HAPPY] − RT[XXXXX]), each separately for trials with and without face cues, and for each subject. An ANOVA with group as the between-subjects factor and Stroop-valence (Stroop-happy–-Stroop-angry), face-cue (face–no face), and color-match (match–nonmatch) as within-subject factors revealed greater Stroop-valence effects for ALC + HIV than CTL (p = .007), and a trend for HIV (p = .086), whereas ALC (p = .23) and HIV (p = .27) did not significantly differ from CTL (F(1,74) = 2.70; p = .05; ηp2 = .10). For all study groups, Stroop effects were greater for negative (ANGRY) than positive (HAPPY) words (F(1,74) = 16.7; p < .0001; ηp2 = .18) and greater for trials with than without emotional faces (F(1,74) = 9.47; p = .003; ηp2 = .11). The difference between Stroop effects for negative and positive emotion was attenuated when a face with matching emotion was presented before the Stroop word (happy face–word HAPPY, angry face–word ANGRY) (Stroop-valence-by-face-cue interaction: F(1,74) = 6.10; p = .016; ηp2 = .08).
Face cue effects for positive and negative emotion
We next tested the hypothesis that emotionally salient stimuli that automatically capture attention (happy or angry faces) would interfere with color-matching performance differently in CTL, HIV, ALC, and ALC + HIV, specifically for negative valence (angry faces) compared to positive valence (happy faces). To do this, we computed face-cue effects by subtracting RT(no-face) from RT(face-cue) trials, separately for happy and angry faces. An ANOVA with group as the between-subject factor, and face-cue-valence (angry–happy) and color-match (match–nonmatch) as within-subjects factors revealed greater cue-valence effects for angry than happy faces (F(1,74) = 6.83; p = .011; ηp2 = .11). Face-cue-valence effects differed between groups (group-by-face-cue-valence interaction: F(1,74) = 3.45; p = .021; ηp2 = .12) (Figure 3a). To explore this interaction, we conducted a series of within-group analyses, which revealed that this interaction was caused mainly by a disproportionate face-cue effect for angry compared to happy facial expressions in HIV (cue-valence effect: F(1,74) = 13.9, pFDRcorrected < .001; ηp2 = .42), an effect not seen in the other three groups (CTL: F(1,74) = 2.32; p = .15; ηp2 = .13; ALC: F(1,74) = .014; p = .91; ηp2 = .00; ALC + HIV: F(1,74) = .013; p = .91, ηp2 = .00).
Fig. 3.
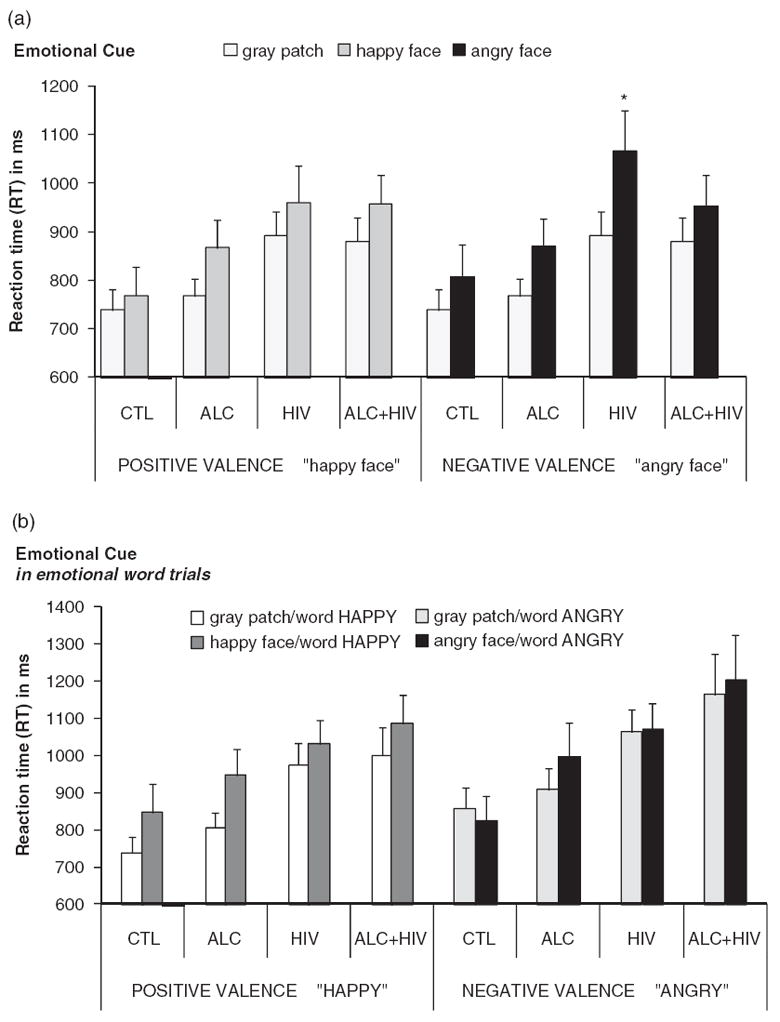
Face cue performance to angry and happy faces. Mean reaction times (RTs) and standard errors (SE) for each group and condition: Controls (CTL), alcoholics (ALC), HIV-1 infected individuals (HIV), and individuals with both diseases (ALC + HIV). Face cue performance comparing cue-target color matching time between “face cue” and “no face” (gray patch) conditions for both positive and negative facial expression, for (a) letter string, and (b) congruent word trials.
We then tested whether face-cue effects for negative and positive emotion would be influenced by congruent emotional word content. Again, we first computed emotional face-cue effects, now for word trials, by subtracting RT(no-face) from RT(face-cue) trials, separately for happy and angry faces. An ANOVA with group as between-subjects factor and face-cue-valence (angry–happy), for trials with congruent word content, revealed greater face-cue effects for happy than angry facial expressions (cue-valence effect: F(1,74) = 6.10; p = .016; ηp2 = .08) that did not differ between groups (group-by-face-cue-valence interaction: F(1,74) = 0.60; p = .62; ηp2 = .02) (Figure 3b).
Results endured when data analyses were performed with log-transformed data except for the overall enhanced Stroop effect in the comorbid group that did not reach statistical significance after log-transformation (one-tailed p = .055).
Correlation Analyses
Set switching between color and face matching
We further analyzed the effect of set/rule switching between color and face matching blocks on Stroop performance. To do this, we calculated the difference RTs between the last and first RT of each set/rule block, resulting in two difference RT values: (1) switch from face to color matching, and (2) switch from color to face matching. Specifically, we tested whether set/rule switching, that is, a measure of cognitive flexibility, was related to Stroop effects for negative (Stroop-angry) or positive (Stroop-happy) emotional valence. The Stroop effect for negative but not for positive valence was significantly related to cognitive flexibility, that is, switching from face-to-color match, in ALC + HIV, HIV, and ALC, but not in CTL (Figure 4). Thus, in all patient groups, and most pronounced in the two HIV groups, poorer cognitive flexibility was associated with negative Stroop-valence effects. We did not find any significant relationships between set/rule switch from color-to-face match with Stroop effects or overall task performance.
Fig. 4.
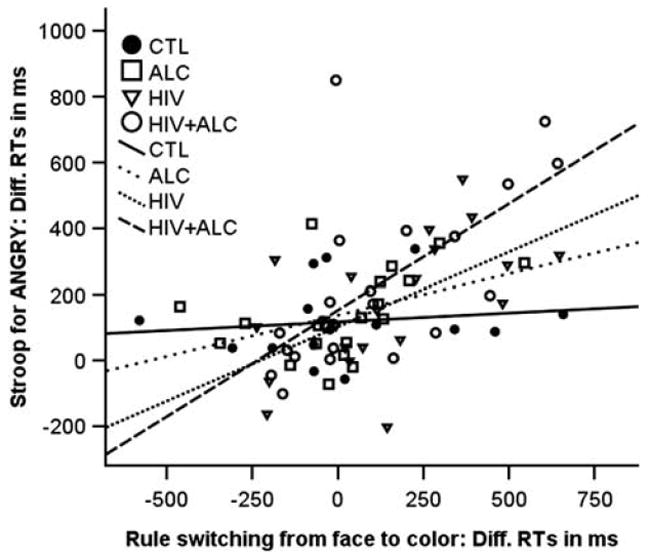
Correlations between switch costs and Stroop-angry for each group. Controls (CTL): Stroop-angry, rho = 0.15, ns; Stroop-happy, rho = −0.06, ns; alcoholics (ALC): Stroop-angry, rho = 0.48, p = .03; Stroop-happy, rho = −0.22, ns; HIV-1 infection (HIV): Stroop-angry, rho = 0.64, pFDRcorrected < .003; Stroop-happy, rho = 0.39, ns; both diseases (ALC + HIV): Stroop-angry, rho = 0.71, pFDRcorrected < .0001; Stroop-happy, rho = 0.43, p = .04.
Socioeconomic status (SES)
To test for the effect of SES on emotional Stroop performance, we examined the correlations between SES and the primary Stroop scores separately across the four groups. None of the correlations were significant.
Depressive symptoms (BDI)
We further tested whether emotional Stroop-valence effects (Stroop-happy, Stroop-angry) would correlate with an independent measure of emotionality, for example, BDI depressive symptoms. Given the slightly, although non-clinically, enhanced BDI scores in the two HIV groups, one would expect a relationship between Stroop valence effects and BDI scores in the two HIV groups but not in the ALC and CTL groups. In support of this prediction, more depressive symptoms (BDI) related to greater Stroop-valence effects in the HIV group (cued Stroop-happy-match: rho = 0.53; p = .008; trend for Stroop-angry-match: rho = 0.43; p = .03; Bonferroni correction requires p ≤ .025) and a trend in the ALC + HIV group (Stroop-angry-nonmatch: rho = 0.40; p = .034; Bonferroni correction requires p ≤ .025), but not in ALC and CTL (all ps > .05).
DISCUSSION
We investigated whether patients with HIV-1 infection with or without alcoholism and those with alcoholism alone exhibit more difficulty than healthy controls in resolving conflict from emotional words. In our emotional Stroop Match-to-Sample task, task-irrelevant happy and angry faces that automatically capture attention (Itti & Koch, 2000) were presented to study emotional responsiveness. Emotional words served to study top-down attentional control involved in the resolution of Stroop word conflict during color-match task performance.
Stroop Conflict
As expected, all groups showed Stroop effects, which were most pronounced in the comorbid ALC + HIV group. Specifically, ALC + HIV relative to the other three groups (HIV, ALC, and CTL) demonstrated enhanced interference and reduced conflict resolution when the cue color did not match the emotional Stroop stimulus color. However, ALC + HIV performed similarly to the other groups when cue and word colors matched. Thus, emotional Stroop Match-to-Sample performance depended on color-cue validity. In nonmatch trials, the Stroop word likely usurps more processing resources due to the mismatch of the color-cue and color naming of the word (Williams, Mathews, & MacLeod, 1996).
Stroop for Negative and Positive Valence
Consistent with our assumption, Stroop effects differed as a function of emotional valence with negative words enhancing the Stroop effect more than positive words, particularly in ALC + HIV. This suggests that negative emotional content consumes attentional capacity thereby slowing color matching (Dawkins & Furnham, 1989), consistent with the idea that greater cognitive effort is required to ignore negative emotions (Holmes, 1974).
Our findings in ALC + HIV are similar to those recently observed in a different ALC + HIV sample using a non-emotional Stroop Match-to-Sample task indicating compromise in executive control (Schulte et al., 2005). A combined effect of HIV and alcoholism on brain functions was further observed for associative learning, sustained attention (Sassoon et al., 2007) and episodic memory (Fama, Rosenbloom, Nichols, Pfefferbaum, & Sullivan, 2009). In our task, working memory processes may have played a role when the cue’s color information had to be held online for color matching with the upcoming Stroop stimulus. However, Fama et al. (2009) found deficits for immediate episodic memory in comorbid ALC + HIV patients relative to controls, whereas working memory functions were spared. This study adds to the evidence of a combined effect of HIV and alcoholism on certain brain functions. The compromise observed in ALC + HIV involved Stroop-negative-valence and Stroop-nonmatch effects; both conditions likely increase processing load and limit cognitive resources to resolve Stroop conflict. Together these results indicate a restriction of resources for cognitive control in ALC + HIV rather than a specific compromise in the processing of emotion.
Emotional Stroop Conflict and Set Switching
Subjects were further required to switch sets between color and face matching. Switching describes the ability to disengage from one cognitive set, that is, face matching to engage in a different one, that is, color matching. In ALC + HIV, HIV, and as a trend in ALC, we found that patients with higher switch costs from face to color matching had greater Stroop effects to negative emotional words than patients with lower switch costs. Switching is a controlled executive ability that requires cognitive flexibility and an invocation of an appropriate constellation of mental resources associated with frontal brain systems (Monsell, 2003). Resolving Stroop conflict from interfering word content requires inhibition of the prepotent response, that is, executive control, and has also been associated with frontal brain functions (e.g., Liu, Bai, & Zhang, 2008; Morishima, Okuda, & Sakai, 2010; Schulte et al., 2009; Vanderhasselt, De Raedt, & Baeken, 2009). The correlation between Stroop interference and set-switching costs, therefore, strengthens our conclusion that compromised executive control functions contribute to the observed abnormalities for emotional Stroop processing in the comorbid group. Furthermore, this relationship was observed only for Stroop interference from negative but not positive emotion, thereby supporting the position that negative emotions automatically bind more attentional resources (Duka & Townshend, 2004) and consequently result in higher executive control demands when negative emotional content interferes with task performance. In addition, deficits in cognitive flexibility may have exacerbated cognitive demands from negative word content when resolving Stroop conflict, specifically in the HIV groups. This is consistent with a recent study that identified switching impairments in HIV-infected individuals as major contributor to neuropsychological deficits in category fluency (Iudicello et al., 2008).
Emotional Face Cues
The presentation of emotional face cues prolonged color-matching responses in all groups. Patients with HIV without alcoholism showed disproportionately prolonged responses relative to the other three groups when processing angry face cues. Thus, the HIV group showed an attentional bias for emotionally salient negative stimuli, that is, angry faces automatically captured attention in HIV more than in the other groups. Neuroimaging evidence suggests that a distributed cortical network is associated with face processing involving both bottom-up and top-down mechanisms. Facial emotional perception has been closely linked to bottom-up processes and brain activation in the amygdala (Aggleton, 2000). Thus, we interpret the finding of a disproportionate “angry face cue effect” in HIV during simple color matching in letter string trials (angry face–XXXXX) as evidence for compromised bottom-up processes related to facial emotional perception. The higher responsiveness to negative facial affect in HIV may further reflect emotional perturbations, such as anxiety and depressive symptoms often reported in HIV infection (Bungener, Kosmadakis, Jouvent, & Widlocher, 1993). Cognitive evaluation and regulatory control of emotion-related behavior have been considered top-down processes associated with prefrontal cortex functions (Hariri et al., 2000). During emotional regulation, frontal cortex activity and amygdala activity are inversely related (Quirk & Beer, 2006), indicating reciprocal interdependence of bottom-up emotion and top-down control functions. This depiction comports with the finding that the presentation of the word ANGRY (providing a mechanism of executive control) eliminated the “angry-face-cue effect” in HIV (angry face–ANGRY). Thus, engagement of cognitive control processes (i.e., top-down) to resolve Stroop conflict from the word ANGRY likely attenuated the “angry-face-cue effect” and regulated emotional perception in HIV.
DIFFERENTIAL DIAGNOSTIC EFFECTS ON COGNITIVE CONTROL AND EMOTION
HIV—alcoholism comorbidity
The ALC + HIV group showed enhanced Stroop effects, specifically for the more difficult nonmatch condition and for negative emotional word content. At the same time, the ALC + HIV group showed normal responses to emotional faces.
Marinkovic et al. (2009) previously reported impaired decision-making for neutral, that is, emotionally ambiguous faces and reduced neural responsiveness of the amygdala to emotional expressions in alcoholics. However, when unambiguous emotional faces were presented in color-match trials that did not require a decision or categorization of facial expressions, we found similar effects for emotional faces in ALC + HIV and controls. Thus, ALC + HIV comorbidity neither reduced nor enhanced an automatic attentional bias to emotional faces compared with controls. Rather alcoholism with HIV-1 comorbidity affected higher-order executive control processes and decisions requiring decoding of emotion (for alcoholism: Foisy et al., 2007; Kornreich et al., 2001; Philippot et al., 1999; for HIV: Clark, Cohen, Westbrook, Devlin, & Tashima, 2010). We speculate that enhanced Stroop effects in ALC + HIV to negative emotion and to color-nonmatch conditions reflect compromise of fronto-parietal network functions involved in top-down attentional control for feature selection and the inhibition of task-irrelevant negative emotions. This interpretation is consistent with neuroimaging studies that have associated inhibitory control functions with frontal brain regions (e.g., Whalen et al., 1998). Thus, compromise of fronto-parietal attention systems that closely interact with frontal-subcortical emotion systems (Phan et al., 2005) may constitute the neural basis for cognitive impairment and emotional dysregulation (Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007), especially in those alcoholics who are comorbid with HIV.
HIV-1 infection
The finding of a greater attentional bias for emotionally salient negative stimuli such as angry faces in the HIV than in the ALC and ALC + HIV was unexpected. It could be interpreted as disturbance in stimulus-driven processing of emotionally salient stimuli. This processing bias may reflect enhanced responsiveness of limbic system functions involved in attentional capture and automatic alertness to emotionally negative cues (Cole et al., 2007), while fronto-parietal attention network functions appear intact in HIV (Schulte et al., 2005). It may also reflect an enhanced sensitivity to emotional situations and vulnerability in HIV-1 infected individuals without alcoholism (Clark et al., 2010; Pukay-Martin, Cristiani, Saveanu, & Bornstein, 2003), whereas neuroadaptation to stressful situations in alcoholics (Heilig & Koob, 2007) may dampen responsiveness to emotionally negative stimuli (Gilman & Hommer, 2008), possibly moderating emotion effects in ALC and ALC + HIV in an emotional Stroop Match-to-Sample task.
Alcoholism
We had expected to find effects indicative of emotion processing deficits in alcoholism, but patients with alcoholism alone performed at comparable level to controls in our emotion Stroop Match-to-Sample task. In a previous study, deficits in affective processing in alcoholics became evident in ambiguous situations in which no additional cues could be used for the interpretation of emotion (Uekermann, Daum, Schlebusch, & Trenckmann, 2005). Similarly, Jung, Kim, Kim, and Namkoong (2009) found altered behavioral responses in alcoholics in an emotional discrimination task when judging neutral words and pictures as “good,” “bad,” or “neither.” One possible explanation for the “no effect” in alcoholics is that our emotional Stroop Match-to-Sample task did not use emotionally ambiguous stimuli. Another possibility is that more subtle compromise in emotion processing and cognitive control may have gone undetected compared to the larger compromise in HIV and ALC + HIV groups.
Even though the total study sample included 78 subjects, subgroup samples were relatively small, thereby limiting the interpretation of our results for population inference. Furthermore, attention was undoubtedly involved in emotional Stroop conflict resolution but not manipulated to parse it from emotion effects, leaving the possibility that some of the observed effects were due to group differences in some aspect of attention that were not assessed in this study. In addition, our data indicated a modest modulating influence of socioeconomic status on the interaction between Stroop, color match and face cue effects over all groups. Thus, some of the observed effects on cognitive control, attention and emotion regulation may echo the highest level of functioning study participants achieved in their daily life, and may provide an explanation for group difference in SES or vice versa.
Another limitation of this study is that 29% of the patients in the two HIV groups also had hepatitis C infection (HCV), and that 26% of patients in the two ALC groups had used other drugs in addition to alcohol. These other comorbidities may have contributed to the observed compromise in emotional Stroop and face cue processing in the patient groups. Nevertheless, we believe that these comorbidities are representative of HIV and alcoholism patient samples, as has been reported previously (e.g., Bräu et al., 2002; Rosenbloom et al., 2007). For example, Fuller, Loftis, Rodriguez, McQuesten, and Hauser (2009) reported in his review article that 15 to 37% of HIV-1 infected people also have HCV.
Here, we observed that the slightly elevated depressive symptoms scores in the two HIV groups were related to alcohol consumption, a relationship that was not observed in patients with alcoholism alone. This suggests that in patients with HIV-1 infection, alcohol consumption may aggravate HIV-related feelings of physical weakness and fatigue, which could consequently be reflected in higher depressive symptoms scores such as loss of energy, appetite, concentration difficulty, tiredness, or fatigue.
Analogous to that reported in patients with a DSM-IV diagnosis of Major Depressive Disorder (MDD) (Mitterschiffthaler et al., 2008), depressive symptoms in HIV and ALC + HIV patients not meeting criteria for MDD correlated with greater emotional Stroop effects. The relationship between emotional Stroop effects and an independent measure of emotional dysregulation (enhanced BDI score) provides an external validation for the sensitivity of the emotional component of the Stroop Match-to-Sample task. Although MDD was not tested in the current study, we speculate that the processes assessed with the emotional Stroop paradigm might be appropriate to examine cognitive and emotional processing differences that accompany MDD. Similar deficits in face perception and executive function have been observed in patients with depression (Langenecker et al., 2005), alcoholism (Maurage et al., 2008), and HIV infection (Cruess et al., 2003). Thus, it remains to future studies to determine whether the observed interrelatedness of emotional Stroop processing and depressive symptoms reflects focal or distributed compromise in frontal, limbic, and temporal brain networks subserving cognition and emotion functions in the respective patient groups.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants AA018022, AA017347, and AA017168. The authors thank Margaret J. Rosenbloom, M.A., for comments on this manuscript. We also thank Stephanie Sassoon, Ph.D., Megan Thompson, B.A., and Crystal Caldwell for help with recruiting and screening study participants and assistance in data collection. The information in this manuscript and the manuscript itself are new and original and has never been published either electronically or in print.
Footnotes
There are no financial or other relationships that could be interpreted as a conflict of interest affecting this manuscript.
References
- Aggleton JP. The amygdala: A functional analysis. xiv. New York: Oxford University Press; 2000. p. 690. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nature Neuroscience. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Shanley JD. ASPD blunts the effects of HIV and antiretroviral treatment on event-related brain potentials. Neuropsychobiology. 2006;53:17–25. doi: 10.1159/000089917. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(Suppl. 1):S11–S18. [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Bräu N, Bini EJ, Shahidi A, Aytaman A, Xiao P, Stancic S, Paronetto F, et al. Prevalence of hepatitis C and coinfection with HIV among United States veterans in the New York City metropolitan area. The American Journal of Gastroenterology. 2002;97:2071–2078. doi: 10.1111/j.1572-0241.2002.05924.x. [DOI] [PubMed] [Google Scholar]
- Bungener C, Kosmadakis CS, Jouvent R, Widlocher D. Emotional and psychopathological disturbances in HIV-infection. Progress in Neuropsychopharmacology & Biological Psychiatry. 1993;17:927–937. doi: 10.1016/0278-5846(93)90020-s. [DOI] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Westbrook ML, Devlin KN, Tashima KT. Facial emotion recognition impairments in individuals with HIV. Journal of the International Neuropsychological Society. 2010;16:1127–1137. doi: 10.1017/S1355617710001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21:346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MA, Castellon SA, Perkins AC, Ureno OS, Robinet MB, Reinhard MJ, Hinkin CH, et al. Relationship between psychiatric status and frontal-subcortical systems in HIV-infected individuals. Journal of the International Neuropsychological Society. 2007;13:549–554. doi: 10.1017/S135561770707066X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Cruess DG, Petitto JM, Leserman J, Douglas SD, Gettes DR, Ten Have TR, Evans DL. Depression and HIV infection: Impact on immune function and disease progression. CNS Spectrums. 2003;8:52–58. doi: 10.1017/s1092852900023452. [DOI] [PubMed] [Google Scholar]
- Dahl M. Asymmetries in the processing of emotionally valenced words. Scandinavian Journal of Psychology. 2001;42:97–104. doi: 10.1111/1467-9450.00218. [DOI] [PubMed] [Google Scholar]
- Dawkins K, Furnham A. The colour naming of emotional words. British Journal of Psychology. 1989;80:383–389. [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, van den Brink W, Sabbe B. Behavioural aspects of impulsivity in alcoholics with and without a cluster-B personality disorder. Alcohol and Alcoholism. 2006;41:412–420. doi: 10.1093/alcalc/agl030. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176:353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan EV. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: Baseline and 1-year follow-up examinations. Alcoholism, Clinical and Experimental Research. 2009;33:1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisy ML, Kornreich C, Petiau C, Parez A, Hanak C, Verbanck P, Philippot P, et al. Impaired emotional facial expression recognition in alcoholics: Are these deficits specific to emotional cues? Psychiatric Research. 2007;150:33–41. doi: 10.1016/j.psychres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Fuller BE, Loftis JM, Rodriguez VL, McQuesten MJ, Hauser P. Psychiatric and substance use disorders comorbidities in veterans with hepatitis C virus and HIV coinfection. Current Opinion in Psychiatry. 2009;22:401–408. doi: 10.1097/YCO.0b013e32832cadb9. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addiction Biology. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Granholm E, Siegle G. Computerized and traditional Stroop task dysfunction in HIV-1 infection. Neuropsychology. 1999;13:306–316. doi: 10.1037//0894-4105.13.2.306. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social class and mental illness: A community sample. New York: John Wiley and Sons; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DS. Investigations of repression: Differential recall of material experimentally or naturally associated with ego threat. Psychological Bulletin. 1974;81:632–653. doi: 10.1037/h0036950. [DOI] [PubMed] [Google Scholar]
- Hutton HE, Lyketsos CG, Zenilman JM, Thompson RE, Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transmitted disease clinic. American Journal of Psychiatry. 2004;161:912–914. doi: 10.1176/appi.ajp.161.5.912. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Research. 2000;40:1489–1506. doi: 10.1016/s0042-6989(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Weber E, Dawson MS, Scott JC, Carey CL, Grant I HIV Neurobehavioral Research Center (HNRC) Group. Cognitive mechanisms of switching in HIV-associated category fluency deficits. Journal of Clinical and Experimental Neuropsychology. 2008;30:797–804. doi: 10.1080/13803390701779578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YC, Kim NW, Kim JJ, Namkoong K. Distinct affective processing of emotionally stimulating written words and pictures in patients with alcohol dependence. Psychiatry Research. 2009;30:267–270. doi: 10.1016/j.psychres.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Kahan TA, Hely CD. The role of valence and frequency in the emotional Stroop task. Psychonomic Bulletin & Review. 2008;15:956–960. doi: 10.3758/PBR.15.5.956. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of nitrogen mustards in the palliative treatment of cancer. Cancer. 1948;1:634–656. [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Hess U, Noël X, Streel E, Verbanck P, et al. Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. Journal of Studies on Alcohol. 2001;62:533–542. doi: 10.15288/jsa.2001.62.533. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology. 2005;27:320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Lefevre F, O’Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J. Alcohol consumption among HIV-infected patients. Journal of General Internal Medicine. 1995;10:458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- Leigh BC, Stall R. Substance use and risky sexual behavior for exposure to HIV. Issues in methodology, interpretation, and prevention. American Psychologist. 1993;48:1035–1045. doi: 10.1037//0003-066x.48.10.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu J, Liang J, Zhang H, Zhao J, Huber DE, Shi G, et al. A distributed neural system for top-down face processing. Neuroscience Letters. 2009;451:6–10. doi: 10.1016/j.neulet.2008.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Bai J, Zhang D. Cognitive control explored by linear modelling behaviour and fMRI data during Stroop tasks. Physiological Measurement. 2008;29:703–710. doi: 10.1088/0967-3334/29/7/001. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Shafto M, Taylor JK, Marian DE, Abrams L, Dyer JR. Relations between emotion, memory, and attention: Evidence from taboo Stroop, lexical decision, and immediate memory tasks. Memory & Cognition. 2004;32:474–488. doi: 10.3758/bf03195840. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Harris GJ, et al. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcoholism, Clinical and Experimental Research. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Novak RM, Fendrich M, Vassileva J, Gonzalez R, Grbesic S, Sworowski L, et al. Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. Journal of the International Neuropsychological Society. 2004;10:298–300. doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- Martin EM, Robertson LC, Edelstein HE, Jagust WJ, Sorensen DJ, San Giovanni D, Chirurgi VA. Performance of patients with early HIV-1 infection on the Stroop Task. Journal of Clinical and Experimental Neuropsychology. 1992;14:857–868. doi: 10.1080/01688639208402867. [DOI] [PubMed] [Google Scholar]
- Maurage P, Philippot P, Joassin F, Pauwels L, Pham T, Prieto EA, Campanella S, et al. The auditory-visual integration of anger is impaired in alcoholism: An event-related potentials study. Journal of Psychiatry & Neuroscience: JPN. 2008;33:111–122. [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Williams SC, Walsh ND, Cleare AJ, Donaldson C, Scott J, Fu CH. Neural basis of the emotional Stroop interference effect in major depression. Psychological Medicine. 2008;38:247–256. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- Monnot M, Nixon S, Lovallo W, Ross E. Altered emotional perception in alcoholics: Deficits in affective prosody comprehension. Alcoholism, Clinical and Experimental Research. 2001;25:362–369. [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Okuda J, Sakai K. Reactive mechanism of cognitive control system. Cerebral Cortex. 2010;20:2675–2683. doi: 10.1093/cercor/bhq013. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART): Test manual. Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- Novara C, Casari S, Compostella S, Dorz S, Sanavio E, Sica C. Coping and cognitive processing style in HIV-positive subjects. Psychotherapy and Psychosomatics. 2000;69:316–321. doi: 10.1159/000012414. [DOI] [PubMed] [Google Scholar]
- Ohman A, Lundqvist D, Esteves F. The face in the crowd revisited: A threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80:381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL. Brain CT changes in alcoholics: Effects of age and alcohol consumption. Alcoholism, Clinical and Experimental Research. 1988;12:81–87. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Philippot P, Kornreich C, Blairy S, Baert I, Den Dulk A, Le Bon O, Verbanck P, et al. Alcoholics’ deficits in the decoding of emotional facial expression. Alcoholism, Clinical and Experimental Research. 1999;23:1031–1038. [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Pratto F, John OP. Automatic vigilance: The attention-grabbing power of negative social information. Journal of Personality and Social Psychology. 1991;61:380–391. doi: 10.1037//0022-3514.61.3.380. [DOI] [PubMed] [Google Scholar]
- Pukay-Martin ND, Cristiani SA, Saveanu R, Bornstein RA. The relationship between stressful life events and cognitive function in HIV-infected men. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:436–441. doi: 10.1176/jnp.15.4.436. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sullivan EV, Sassoon SA, O’Reilly A, Fama R, Kemper CA, Pfefferbaum A, et al. Alcoholism, HIV infection, and their comorbidity: Factors affecting self-rated health-related quality of life. Journal of Studies on Alcohol and Drugs. 2007;68:115–125. doi: 10.15288/jsad.2007.68.115. [DOI] [PubMed] [Google Scholar]
- Salo R, Henik A, Robertson LC. Interpreting Stroop interference: An analysis of differences between task versions. Neuropsychology. 2001;15:462–471. doi: 10.1037//0894-4105.15.4.462. [DOI] [PubMed] [Google Scholar]
- Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the digit symbol test: Differential deficits in alcoholism, HIV infection, and their comorbidity. Alcoholism, Clinical and Experimental Research. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: Evidence from a Stroop Match-to-Sample task. Biological Psychiatry. 2005;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Vinco S, Hoeft F, Pfefferbaum A, Sullivan EV. Double dissociation between action-driven and perception-driven conflict resolution invoking anterior versus posterior brain systems. Neuroimage. 2009;48:381–390. doi: 10.1016/j.neuroimage.2009.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormark KM, Laberg JC, Nordby H, Hugdahl K. Alcoholics’ selective attention to alcohol stimuli: Automated processing? Journal of Studies on Alcohol. 2000;61:18–23. doi: 10.15288/jsa.2000.61.18. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;12:643–662. [Google Scholar]
- Uekermann J, Daum I, Schlebusch P, Trenckmann U. Processing of affective stimuli in alcoholism. Cortex. 2005;4:189–194. doi: 10.1016/s0010-9452(08)70893-1. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R, Baeken C. Dorsolateral prefrontal cortex and Stroop performance: Tackling the lateralization. Psychonomic Bulletin & Review. 2009;16:609–612. doi: 10.3758/PBR.16.3.609. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]


