Abstract
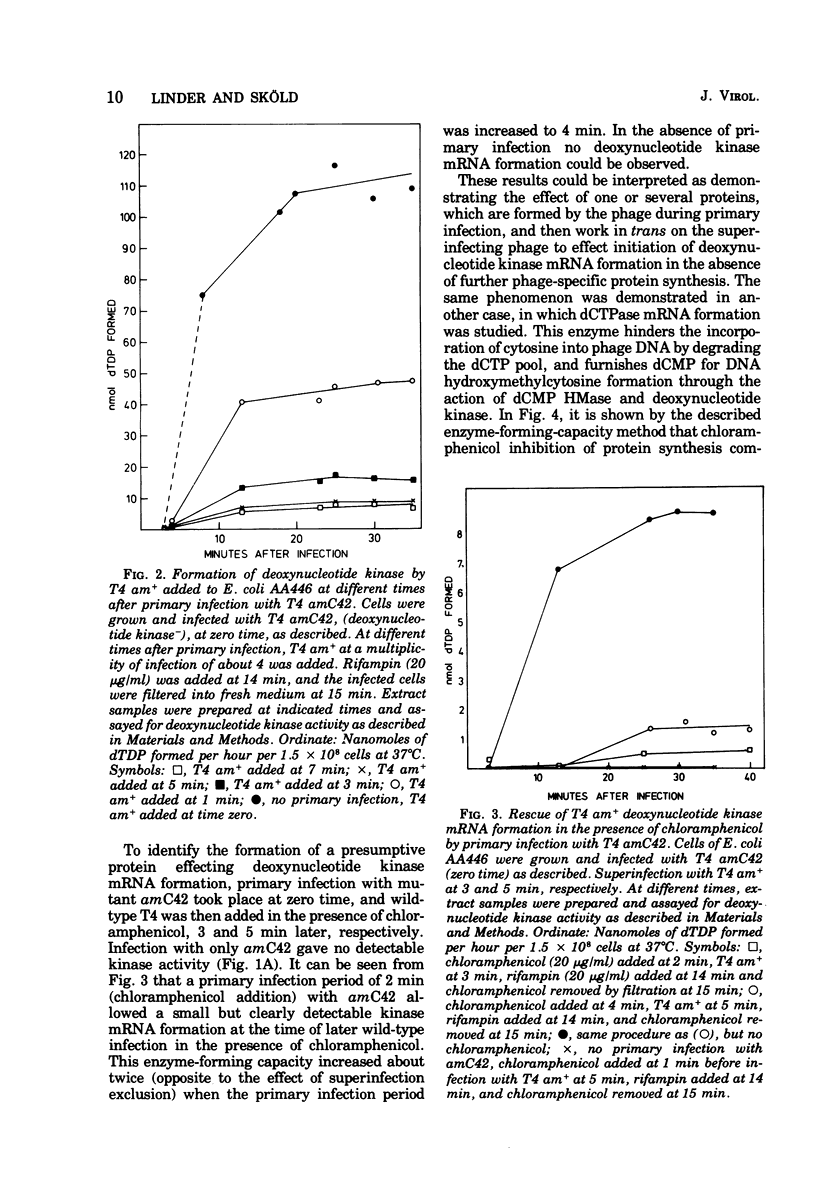
Two forms of prereplicative phage RNA can be discerned in Escherichia coli early after infection with bacteriophage T4, immediate early and delayed early RNA. The transition from immediate early to delayed early RNA synthesis is inhibited by chloramphenicol. The present work presents evidence for the existence of a phage-specific protein, which effects this transition. Delayed early RNA formation was measured by a cistron-specific enzyme-forming-capacity method, in which RNA synthesized in the presence of chloramphenicol was allowed to express itself into enzyme activity after (i) the addition of rifampin to inhibit further transcription and (ii) subsequent removal of chloramphenicol. As representatives of delayed early transcription, the two phage-specific enzymes dCTPase and deoxynucleotide kinase were chosen. Primary infection with a phage mutant defective in one of these two enzymes was found to induce a diffusible factor, which in the presence of chloramphenicol could effect the formation of delayed early RNA corresponding to the missing enzyme, upon superinfection with wild-type phage. The activity of this factor, acting in trans, was abolished by the amino acid analogue ethionine. Mutation in the suA gene of the host did not relieve phage of the apparent need for protein synthesis in the transition from immediate early to delayed early phage RNA synthesis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black L. W., Gold L. M. Pre-replicative development of the bacteriophage T4: RNA and protein synthesis in vivo and in vitro. J Mol Biol. 1971 Sep 14;60(2):365–388. doi: 10.1016/0022-2836(71)90300-7. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. The enzymology of virus-infected bacteria. X. A biochemical-genetic study of the deoxynucleotide kinase induced by wild type and amber mutants of phage T4. J Biol Chem. 1967 Jun 25;242(12):2877–2885. [PubMed] [Google Scholar]
- EDLIN G. GENE REGULATION DURING BACTERIOPHAGE T4 DEVLOPMENT. I. PHENOTYPIC REVERSION OF T4 AMBER MUTANTS BY 5-FLUOROURACIL. J Mol Biol. 1965 Jun;12:363–374. doi: 10.1016/s0022-2836(65)80260-1. [DOI] [PubMed] [Google Scholar]
- Goldman E., Lodish H. F. T4 phage and T4 ghosts inhibit f2 phage replication by different mechanisms. J Mol Biol. 1973 Feb 25;74(2):151–161. doi: 10.1016/0022-2836(73)90104-6. [DOI] [PubMed] [Google Scholar]
- Grasso R. J., Buchanan J. M. Synthesis of early RNA in bacteriophage T4-infected Escherichia coli B. Nature. 1969 Nov 29;224(5222):882–885. doi: 10.1038/224882a0. [DOI] [PubMed] [Google Scholar]
- Holmes G. E. Suppression of phage nonsense and temperature-sensitive mutants by an suA mutant E. coli. Nature. 1974 Jul 5;250(461):73–75. doi: 10.1038/250073a0. [DOI] [PubMed] [Google Scholar]
- Jayaraman R. Transcription of bacteriophage T4 DNA by Escherichia coli RNA polymerase in vitro: identification of some immediate-early and delayed-early genes. J Mol Biol. 1972 Sep 28;70(2):253–263. doi: 10.1016/0022-2836(72)90537-2. [DOI] [PubMed] [Google Scholar]
- Lembach K. J., Buchanan J. M. The relationship of protein synthesis to early transcriptive events in bacteriophage T4-infected Escherichia coli B. J Biol Chem. 1970 Apr 10;245(7):1575–1587. [PubMed] [Google Scholar]
- Linder C. H., Fast R. Regulation of early mRNA synthesis after bacteriophage T4 infection of Escherichia coli. J Virol. 1975 Sep;16(3):463–469. doi: 10.1128/jvi.16.3.463-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Ratner D. Evidence that mutations in the suA polarity suppressing gene directly affect termination factor rho. Nature. 1976 Jan 15;259(5539):151–153. doi: 10.1038/259151a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- STRETTON A. O., BRENNER S. MOLECULAR CONSEQUENCES OF THE AMBER MUTATION AND ITS SUPPRESSION. J Mol Biol. 1965 Jun;12:456–465. doi: 10.1016/s0022-2836(65)80268-6. [DOI] [PubMed] [Google Scholar]
- Sakiyama S., Buchanan J. M. Control of the synthesis of T4 phage deoxynucleotide kinase messenger ribonucleic acid in vivo. J Biol Chem. 1972 Dec 10;247(23):7806–7814. [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Control of gene function in bacteriophage T4. IV. Post-transcriptional shutoff of expression of early genes. J Virol. 1973 Sep;12(3):538–547. doi: 10.1128/jvi.12.3.538-547.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]
- Vallée M., De Lapeyrière O. The role of the genes imm and s in the development of immunity against T4 ghosts and exclusion of superinfecting phage in Escherichia coli infected with T4. Virology. 1975 Sep;67(1):219–233. doi: 10.1016/0042-6822(75)90419-5. [DOI] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. C., Greenberg G. R. Tetrahydrofolate-dependent labilization of the hydrogen atom on carbon 5 of 5'-deoxycytidylate, a step in the deoxycytidylate hydroxymethylase reaction. J Biol Chem. 1967 Mar 25;242(6):1307–1313. [PubMed] [Google Scholar]



