Abstract
A pET based expression system for the production of recombinant human growth hormone (hGH) directed to the Escherichia coli periplasmic space was developed. The pET22b plasmid was used as a template for creating vectors that encode hGH fused to either a pelB or ompA secretion signal under control of the strong bacteriophage T7 promoter. The pelB- and ompA-hGH constructs expressed in BL21 (DE3)-RIPL E. coli are secreted into the periplasm which facilitates isolation of soluble hGH by selective disruption of the outer membrane. A carboxy-terminal poly-histidine tag enabled purification by Ni2+ affinity chromatography with an average yield of 1.4 mg/L culture of purified hGH, independent of secretion signal. Purified pelB- and ompA-hGH are monomeric based on size exclusion chromatography with an intact mass corresponding to mature hGH indicating proper cleavage of the signal peptide and folding in the periplasm. Both pelB- and ompA-hGH bind the hGH receptor with high affinity and potently stimulate Nb2 cell growth. These results demonstrate that the pET expression system is suitable for the rapid and simple isolation of bioactive, soluble hGH from E. coli.
Keywords: Human growth hormone, pET system, periplasm, Escherichia coli, Nb2
Introduction
Genetic engineering has enabled the production of numerous recombinant proteins in large quantities in effect transforming the biotechnology industry by enabling the cost effective manufacture of human proteins [1–3]. The pET expression system pioneered by Studier and Moffat [4] and commercialized by Novagen is one of the most widely used systems for recombinant protein production in E. coli. The multitude of commercially available vectors, E. coli strains, and related products enables expression and purification of a wide variety of foreign proteins. Of particular interest for the expression of disulfide bonded proteins is a family of pET vectors containing the N-terminal pelB secretion signal, which directs synthesized polypeptides to the E. coli periplasm [5]. Disulfide oxidoreductases and isomerases located in the E. coli periplasm catalyze the formation of disulfide bonds enabling the accumulation of properly folded, soluble protein making the periplasm an ideal compartment for expression of certain therapeutic proteins [6].
Human growth hormone (hGH) is a 191 amino acid, disulfide-linked, pituitary-derived protein that regulates a number of metabolic processes involved in growth and development [7]. E. coli derived recombinant hGH is approved for the treatment of multiple human diseases and new indications, treatment modalities, and novel delivery systems represent an active area of research in growth hormone based therapy [8–15]. The lack of glycosylation makes E. coli an ideal host for hGH production, which can be achieved in the cytoplasm [16–19] or periplasm [20–26]. Over-expression of hGH in the cytoplasm results in the formation of insoluble aggregates, or inclusion bodies. The isolation of hGH from inclusion bodies requires a re-folding step to obtain soluble protein that adds process complexity and contributes to reduced yields. Recombinant hGH directed to the periplasm can be easily isolated in its native state by selective disruption of the E. coli outer membrane resulting in a reduction in processing steps, complexity, and time.
There are a number of reports on the periplasmic expression of recombinant human growth hormone [20–26]; however, each uses custom prepared vectors, a range of E. coli strains, expression conditions, and purification schemes making replication difficult for the academic laboratory. We describe simple methods for the expression, purification, and characterization of recombinant hGH produced at the shake flask level using the pET expression system. All components necessary for cloning, expression, and purification are commercially available. This process results in an average yield of 1.4 mg/L culture of purified protein from ~ 10 – 15 g wet cells. The recombinant hGH isolated using this system is soluble, monomeric, binds the hGH receptor with high affinity, and potently stimulates cell growth comparable to pharmaceutical grade hGH. Thus, the pET expression system provides a rapid and economical method for production of recombinant human growth hormone in E. coli.
Material and Methods
Materials
Ampicillin, Terrific Broth (TB), 3,3′,5,5′-tetramethylbenzidine (TMB), and all buffer salts were purchased from Sigma-Aldrich (St. Louis, MO). Nickel Sepharose high performance resin prepacked in 5 mL HiTrap columns (HisTrap FF), PD-10 desalting columns, and Superdex 75 size exclusion chromatography (SEC) column were purchased from GE Healthcare (Piscataway, NJ). Complete EDTA-free protease inhibitor cocktail tablets and isopropyl -D-1-thiogalactopyranoside (IPTG) were purchased from Roche Diagnostics (Indianapolis, IN). Steri-cup 0.45 μm vacuum filters and Amicon 10 kDa MWCO spin filters were from Millipore (Billerica, MA). TEV-TROPIN (Teva Pharmaceuticals; North Wales, PA) was obtained from the UCSF pharmacy. Anti-human growth hormone antibodies and human growth hormone receptor Fc fusion (hGHR-Fc) were purchased from R&D systems (Minneapolis, MN). The bacterial expression vector pET22b was purchased from Novagen (San Diego, CA). All restriction enzymes and buffers used for cloning were purchased from New England Biolabs (Beverly, MA) and all primers were purchased from IDT (San Diego, CA).
E. coli expression vectors
Human growth hormone cDNA was purchased from OpenBiosystems (Huntsville, AL) and maintained in the vector pCR4-TOPO. The gene encoding hGH was PCR amplified from pCR4-TOPO with primers designed to incorporate 5′ NcoI and 3′ XhoI restriction sites. All PCR reactions were performed with Phusion DNA polymerase (New England BioLabs) as follows: 98 °C 1 min; 98 °C 15 sec, 69 °C 30 sec, 72 °C 15 sec, repeat for 35 cycles; 72 °C 10 min, 4 °C hold. The resulting PCR product was purified and restriction cloned into the NcoI and XhoI sites of the bacterial expression vector pET22b (Novagen; San Diego, CA). The resulting vector, pET22b_pelB_hGH, encodes hGH containing a N-terminal pelB leader sequence to enable periplasmic secretion via the Sec translocation machinery and a C-terminal poly-histidine tag for purification by immobilized metal affinity chromatography (IMAC). The corresponding vector, pET22b_ompA_hGH was generated by PCR amplification of the gene encoding hGH from pCR4-TOPO with primers designed to incorporate a 5′ NdeI site followed by the ompA signal sequence and a 3′ XhoI site. The resulting PCR product was restriction cloned into the NdeI and XhoI sites of pET22b. The resulting vector encodes hGH containing a N-terminal ompA leader sequence to enable periplasmic secretion and a C-terminal poly-histidine tag for purification. All plasmids were confirmed by DNA sequencing. A complete list of primers used in this study is provided in Table 1.
Table 1.
Primers used in this study
| Vector | Primer Pair | Restriction Site |
|---|---|---|
| pET22b-pelB hGH | F = 5′-GATGGCCATGGGCTTCCCAACCATTCCCTTATC-3′ | NcoI |
| R = 5′-GGTGCTCGAGGCTGCCGCGCGGCACCAGGAAGCCACAGCTGCCCTC-3′ | XhoI | |
| pET22b-ompA hGH | F = 5′-CATACATATGAAAAAAACCGCGATTGCGATTGCGGTGGCGTTAGCGGGCTTTGCGACCGTGGCGCAGGCGTTCCCAACCATTCCCTTATC-3′ | NdeI |
| R = 5′-GGTGCTCGAGGCTGCCGCGCGGCACCAGGAAGCCACAGCTGCCCTC-3′ | XhoI |
Protein expression and purification
Expression of hGH was carried out in BL21-Codon Plus (DE3)-RIPL E. coli cells (Stratagene; La Jolla, CA) harboring the pET22b-hGH vectors described above. A single colony was selected and cultured overnight at 37 °C in 10 mL of terrific broth (TB) containing 100 μg/mL ampicillin. The 10 mL overnight E. coli culture was used to inoculate a 1L culture of TB containing 100 μg/mL ampicillin. Cells were cultured at 37 °C until OD600 = 0.7 – 0.9. Protein expression was induced by addition of 0.1 mM IPTG and cultured for an additional 16 – 18 hrs at 25 °C. Cells were harvested by centrifugation (7650 g for 30 min) and the periplasmic E. coli fraction was extracted via osmotic shock as previously described [27, 28]. Briefly, harvested cells were suspended in a hypertonic solution of 30 mM Tris, 20% w/v sucrose, 1 mM ETDA, pH 8 (25 mL) and incubated for 30 min at 4 °C. Cells were centrifuged and the supernatant collected. Cells were re-suspended in a hypotonic solution of 5 mM MgSO4 (25mL) and incubated for 30 min at 4 °C followed by an additional centrifugation. The supernatant from the hypotonic solution was combined with the supernatant from the hypertonic solution, centrifuged to remove debris, and dialyzed against D-PBS overnight at 4 °C.
The periplasmic solution containing soluble hGH was clarified over a 0.45 μm filter and purified by Ni2+ affinity chromatography as follows. A 5mL HisTrap FF column charged with Ni2+ was equilibrated with 20 mM Tris, 300 mM NaCl, 40 mM imidazole, pH 8. The clarified osmotic shock fluid was loaded onto the HisTrap FF column and washed with equilibration buffer for 8 column volumes (CV). Bound protein was eluted with 3 CVs of 20 mM Tris, 300 mM NaCl, 500 mM imidazole, pH 8. Fractions with an absorbance at 280 nm greater than 0.05 were pooled, concentrated and buffer exchanged into D-PBS, and subject to analysis by reducing and non-reducing SDS-PAGE. Protein concentrations were measured based on absorbance at 280 nm assuming an extinction coefficient of 17670 M−1 m−1 predicted based on the mature hGH amino acid sequence using ExPASy ProtParam tool (www.expasy.org). The hGHs characterized in this study contain a carboxy terminal poly-histidine tag with the exception of TEV-TROPIN. The amino acid sequence of the tag appended to the carboxy-terminus of hGH is LVPRGSLEHHHHHH.
Matrix-assisted laser desorption and ionization (MALDI)-time of flight (TOF) mass spectrometry
The intact mass of purified hGH was determined by MALDI-TOF mass spectrometry. Purified hGHs and TEV-TROPIN were desalted using C4 ZipTips (Millipore; Billerica, MA) per the manufactures recommended protocol and eluted with 4 μL of 75% acetonitrile, 0.1% TFA in water. Desalted proteins (1 μL) were mixed with 1 μL of a saturated solution of sinapinic acid (SA) and spotted on top of a pre-formed layer of SA matrix. Mass spectra were obtained on a Microflex LT mass spectrometer (Bruker Daltonics; Billerica, MA) operated in linear, positive mode at a laser frequency of 60 Hz (100 shots total). The spectra were calibrated using the protein Standard II from Bruker-Daltonics. Mass spectra were analyzed with the FLEX Analysis software (Bruker Daltonics).
Size exclusion chromatography
The size and composition of purified hGH was analyzed by size exclusion chromatography on a Dionex FPLC equipped with a Superdex 75 column (GE Healthcare). The column was operated at a flow rate of 0.5 mL/min in D-PBS and the eluate was monitored at 280 nm. Column calibration was performed using gel filtration standards (Bio-Rad; Hercules, CA) containing bovine thyroglobulin (670 kDa), bovine -globulin (158 kDa), chicken ovalbumin (44 kDa), horse myoglobin (17 kDa), and vitamin B12 (1.35 kDa) and plotted as Log10 molecular weight, in kilo-daltons, versus retention time. The standard curve was used to estimate the molecular weight of purified hGH.
In vitro receptor binding assay
An enzyme-linked immunosorption assay (ELISA) was used to evaluate binding of hGH to its receptor [8, 29] with minor modifications. The wells of a Costar 3690 plate were coated with 25 ng of recombinant human growth hormone receptor Fc fusion (hGHR-Fc; R&D Systems, Minneapolis, MN) from a 0.63 μg/mL solution in D-PBS overnight at 4 °C. Wells were washed 3 times with 150 μL of PBS-T (PBS, 0.05% Tween-20) and blocked with 200 μL of a 2% BSA solution in PBS-T. Serial dilutions of purified hGH in blocking buffer were incubated with hGHR-Fc coated wells, washed 3 times with PBS-T and probed with a 1:500 dilution of biotinylated anti-hGH antibody (R&D Systems). After 3 washes with PBS-T, wells were incubated with a 1:2000 dilution of streptavidin-horseradish peroxidase (HRP), washed 6 times with PBS-T, and bound hGH was detected by addition of the HRP substrate TMB. Color was developed for 5 minutes, quenched with a 1M sulfuric acid solution and absorbance was measured at 450 nm with blank subtraction at 550 nm. All incubations were for 1 hr at room temperature in a 40 μL volume unless otherwise noted. Antibody and streptavidin-HRP dilutions were prepared in 1/5 strength blocking buffer.
Nb2 cell proliferation assay
Nb2 cells were obtained from the UCSF cell culture facility and cultured in suspension in Fischer’s medium containing L-glutamine supplemented with 10% horse serum, 10% FBS, 0.055 mM 2-mercaptoethanol, 0.075% sodium bicarbonate, and 1% penicillin/streptomycin. Cells were collected by centrifugation, re-suspended in medium lacking FBS (assay medium), seeded at a density of 4000 cells per well (in 100 μL) in a 96-well tissue culture plate, and serum starved for 24 hrs. Nb2 cell growth was stimulated by addition of hGH diluted in assay medium (100 μL) to a final concentration between 0.1 pM and 500 pM in a final volume of 200 μL. Cells were cultured for an additional 3 days at 37 °C in a CO2 controlled and humidified incubator then counted on a FACS Array cell sorter (BD Biosciences; San Jose, CA). Cell counts for each test population were derived after gating for live cells based on the forward scatter (FSC) and side scatter (SSC). Data were analyzed using FlowJo (Tree Star Inc.; Ashland, OR) and all incubations were done in triplicate with data represented as mean ± SD.
Affinity measurements by surface plasmon resonance
SPR measurements were obtained using a BIAcore T100 instrument (BIAcore Inc.; Piscataway, NJ). The hGHR-Fc fusion protein (R&D Systems) was captured on a CM5 sensor chip by amine coupling at pH 4.5 to a final immobilization density of ~ 680 resonance units (RU). Un-reacted sites were blocked with 1M ethanolamine. A control flow cell without immobilized hGHR-Fc was prepared for reference subtraction. Dilutions of hGH in running buffer [10 mM Hepes, 150 mM NaCl, 0.005% Tween 20, pH 7.4 (HBS-T) or 50 mM phosphate, 100 mM NaCl, 0.01% Tween 20, pH 6] were injected over the chip for 90 sec followed by a 200 sec dissociation in running buffer. The chip was regenerated with a 30 sec injection of 10 mM glycine, pH 2.5 and two 45 sec injections of HBS-T. The flow rate used for all methods was 30 L/min. Binding affinities were derived by analysis of the generated sensograms using the Biacore T100 evaluation software. The equilibrium RU observed for each injection was plotted against protein concentration and fit to a steady-state affinity model included in the evaluation software for determination of the equilibrium binding affinity (KD).
Results and Discussion
Construction of pET22b hGH expression vectors
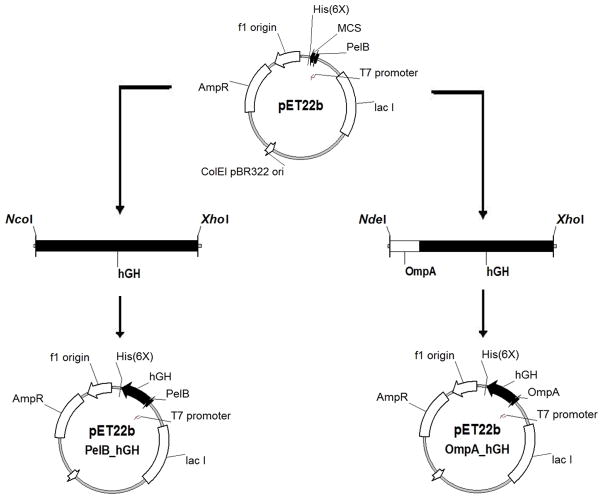
The plasmid pET22b (Novagen) was used as a template to construct vectors that target hGH to the E. coli periplasm. The plasmid contains a T7 promoter, lacI gene, N-terminal pelB signal sequence for periplasmic localization, multiple cloning sites, and an optional C-terminal poly-histidine tag. The pET22b-pelB hGH secretion vector was created by PCR amplification of the gene encoding hGH with primers (Table 1) that add a 5′ NcoI and 3′ XhoI restriction site and restriction cloned into the NcoI and XhoI sites of pET22b (Figure 1). The resulting pET22b-pelB hGH vector encodes a N-terminal pelB sequence in frame with the hGH sequence followed by a poly-histidine tag. The pET22b-ompA hGH secretion vector was created by PCR amplification of the gene encoding hGH with primers that add a 5′ NdeI site followed by the ompA signal sequence and a 3′ XhoI site. The ompA-hGH PCR product was subsequently restriction cloned into the NdeI and XhoI sites of pET22b resulting in replacement of the pelB sequence with ompA. The resulting pET22b-ompA hGH vector encodes a N-terminal ompA sequence in frame with hGH followed by a poly-histidine tag.
Figure 1. Schematic depicting the construction of pET22b-pelB hGH (left) and pET22b-ompA hGH (right) expression vectors.
The gene encoding hGH was PCR amplified as described in Materials and Methods and restriction cloned into the pET22b E. coli expression vector.
Protein expression, purification and characterization
Vectors encoding hGH with either the pelB or ompA secretion signal were transformed into BL21-Codon Plus (DE3)-RIPL E. coli cells for recombinant protein production. This E. coli strain carries a chromosomal copy of the T7 RNA polymerase gene under control of the lacUV5 promoter. IPTG addition induces expression of T7 RNA polymerase resulting in transcription of the hGH gene under control of the T7 promoter in cells harboring the pET22b-hGH vectors. The N-terminal pelB and ompA secretion signals target the translated polypeptide in its unfolded state to the E. coli periplasm via the Sec-dependent transport pathway [5]. The signal peptide is cleaved and the protein folds in the periplasm with the help of chaperones and disulfide bond isomerases. Although the pelB and ompA leader sequences are removed upon secretion to the periplasm, the terms pelB-hGH and ompA-hGH are used throughout to indicate the hGH described was produced from either pET22b_pelB_hGH or pET22b_ompA_hGH vectors, respectively. It is expected that an amino-terminal methionine or phenylalanine is generated upon cleavage of the pelB or ompA secretion signals, respectively, resulting in the mature hGH sequences. Both pelB- and ompA-hGH were purified from osmotic shock fluid containing E. coli periplasmic proteins. Purification was facilitated by the C-terminal poly-histidine tag which enabled separation of soluble, periplasmic hGH from contaminating E. coli cell proteins by Ni2+ affinity chromatography. No distinct over-expression of hGH was observed in the lysate or periplasmic fraction by SDS-PAGE; therefore, we report yields as the amount of hGH recovered after the Ni2+ affinity chromatography step. Yields after purification ranged from 0.64 to 2.57 mg/L culture (0.39 – 1.13 mg/L/OD600) for pelB-hGH and 0.32 to 2.29 mg/L culture (0.24 – 1.37 mg/L/OD600) for ompA-hGH from ~ 10 – 15 grams of wet cells. The variability in yield from batch to batch is likely due to colony selection as each round of expression and purification started with a colony selected from freshly transformed E. coli. It is therefore important to screen for high-expressing colonies to maximize yield of hGH using the pET expression system. It is difficult to compare yields to previous reports given the variation in expression systems, scale, and analytical methods; however, based on yields summarized by Soares et al. [20] the pET system is within the reported range, although notably lower than yields from industrial expression systems [21, 22], as expected.
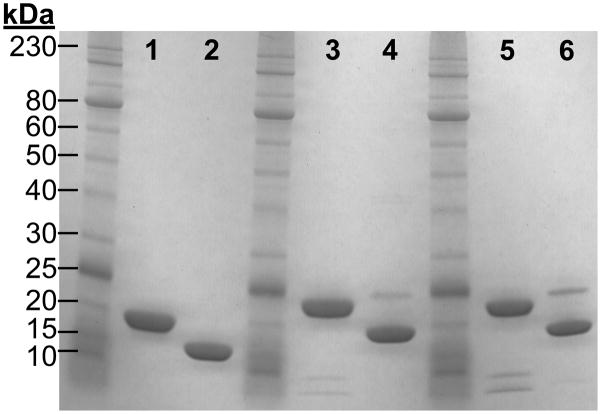
Purified pelB- and ompA-hGH migrate at approximately ~ 24 kDa under reducing and ~ 19 kDa under non-reducing SDS-PAGE (Figure 2). The observed shift in electrophoreic mobility is consistent with that of pharmaceutical grade recombinant hGH (TEV-TROPIN) and indicates the formation of native disulfide bonds resulting in a more compact tertiary structure and faster migration under non-reducing conditions. There are no detectable high molecular weight aggregates by SDS-PAGE; however, a low proportion of an ~ 25 kDa band is present in both the pelB- and ompA-hGH preparations under non-reducing conditions. Upon reduction the 25 kDa species migrates as two low molecular weight fragments suggesting that the 25 kDa species is the result of a previously characterized endoproteolytic cleavage between Thr-142 and Tyr-143 of hGH during culture [25]. This “two chain” hGH is commonly observed in periplasmic preparations and has equivalent biological activity as non-cleaved hGH in both the hyphopysectomized rat weight-gain and Nb2 cell growth assay [21, 25, 30]. Further optimization of culture conditions such as pH, temperature, cation concentration, etc. may reduce proteolytic cleavage and improve product quality.
Figure 2. Reducing and non-reducing SDS-PAGE analysis of recombinant hGH.
Reduced TEV-TROPIN (lane 1), non-reduced TEV-TROPIN (lane 2), reduced pelB-hGH (lane 3), non-reduced pelB-hGH (lane 4), reduced ompA-hGH (lane 5), non-reduced ompA-hGH (lane 6). 7.5 μg of protein were loaded in each lane.
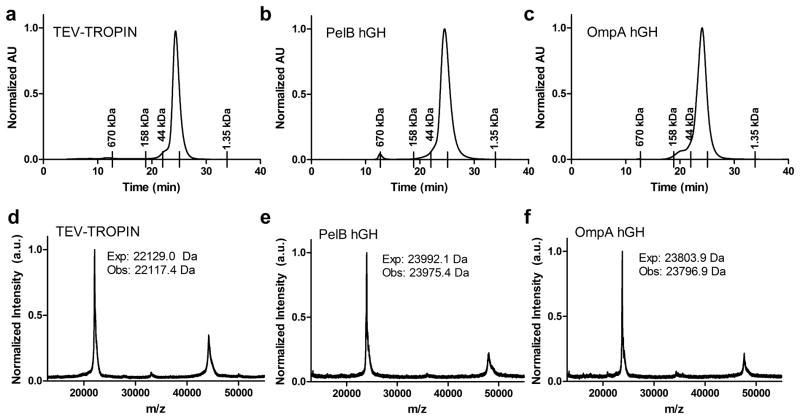
Affinity purified hGHs were analyzed by size exclusion chromatography under non-denaturing conditions (Figure 3a–c). All proteins migrate as monomers with a predicted molecular weight of ~ 23 kDa, ~ 22 kDa, and ~ 25 kDa for TEV-TROPIN, pelB-hGH, and ompA-hGH, respectively. Intact mass was further confirmed by MALDI-TOF MS (Figure 3d–f). The observed molecular weights are in agreement with the expected molecular weights of mature hGH indicating proper cleavage of the pelB and ompA signal peptides upon secretion into the E. coli periplasm. A peak corresponding to the mass of hGH dimer is present by MALDI-TOF MS analysis in all hGH samples, including TEV-TROPIN, that is not detectable by SDS-PAGE or size exclusion chromatography. This likely represents a very small fraction of the total protein that is detectable due to the sensitivity of MALDI-TOF MS and may be exaggerated due to ionization differences between monomer and dimer.
Figure 3. Size exclusion chromatography (a–c) and MALF-TOF (d–f) analysis of TEV-TROPIN (a, d), pelB-hGH (b, e), and ompA-hGH (c, f).
The retention time of the SEC calibration standards are indicated by tick marks on the x-axis. The standards include thyroglobulin 670 kDa, gamma-globulin 158 kDa, ovalbumin 44kDa, myoglobin 17 kDa, and vitamin B12 1.35 kDa. The unlabeled tick under the hGH peak in each panel corresponds to the retention time of the 17 kDa myoglobin standard. Obs: = Observed, Exp: = Expected.
Affinity measurement by surface plasmon resonance and ELISA
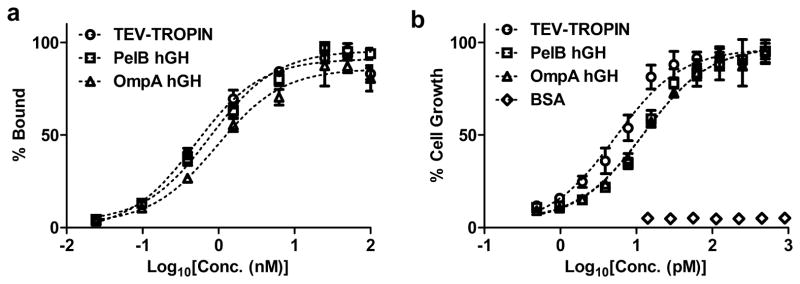
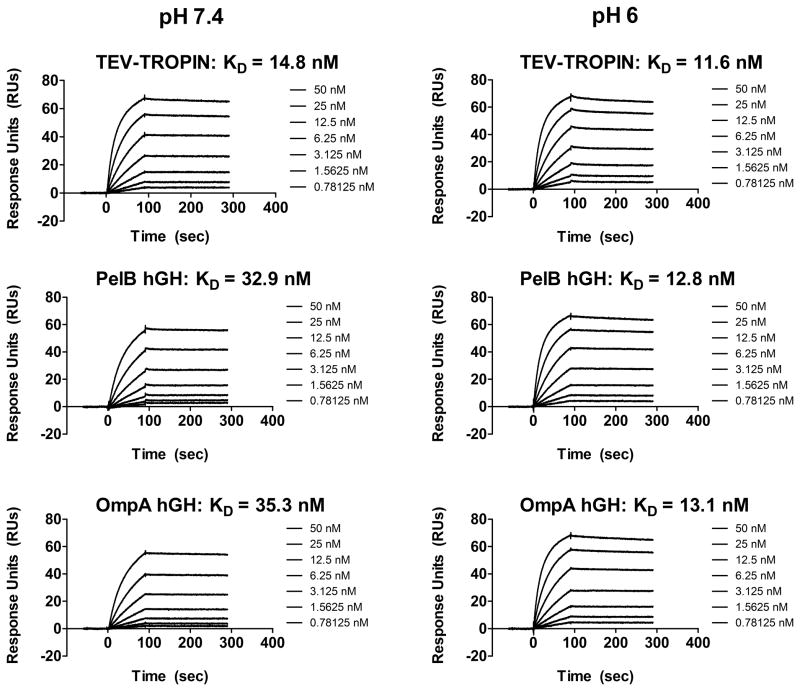
We characterized binding between purified hGHs and the hGH receptor (hGH-R) by surface plasmon resonance and ELISA. One hGH molecule contains two hGH-R binding sites allowing the formation of a 2:1 complex between hGH-R and hGH [31, 32]. Both pelB- and ompA-hGH bind the hGH-R with similar affinity (Figure 4a and Table 2) indicating the choice of secretion sequence for periplasmic targeting does not influence receptor binding. The affinity of pelB- and ompA-hGH by SPR and ELISA is slightly lower than that of TEV-TROPIN likely due to the C-terminal poly-histidine tag given alternative peptide fusions to the carboxy-terminus of hGH also have reduced potency [33]. Analysis of the SPR sensograms indicates the slight reduction in affinity at pH 7.4 is mainly attributed to a reduction in the rate of association (Figure 5). We also evaluated whether binding of hGH to the hGH-R is affected by pH. A slight increase in affinity is observed at pH 6 compared to pH 7.4 for pelB- and ompA-hGH, whereas the affinity of TEV-TROPIN does not change with pH (Figure 5 and Table 2). Again, this slight increase in affinity at pH 6 may be attributed to the protonated histidine residues in the poly-histidine tag given there is no difference in affinity as a function of pH for TEV-TROPIN. Therefore, for certain applications it may be necessary to remove the poly-histidine tag prior to evaluation.
Figure 4. In vitro hGH receptor binding ELISA (a) and Nb2 cell potency bioassay (b).
Each data point represent the mean (n=3) and error bars indicate standard deviation (s.d.). The dashed line indicates data fit to a log(agonist) vs. response model using Prism for derivation of KD and EC50. Data points were normalized to the maximum observed hGH receptor binding (a) or Nb2 cell count (b) and plotted as percentage of maximum binding or growth. The data shown in (a) and (b) are representative of at least 3 independent protein preparations.
Table 2.
Binding affinity to hGH receptor measured by SPR and ELISA and in vitro hGH potency in Nb2 cell growth bioassay
| Molecule | KD (SPR)* pH 7.4, (nM) | KD (SPR)* pH 6, (nM) | KD (ELISA) pH 7.4, (nM) | EC50 (pM) |
|---|---|---|---|---|
| TEV-TROPIN | 14.8 | 11.6 | 0.52 ± 0.05 | 10.2 ± 4.8 |
| PelB hGH | 32.9 | 12.8 | 0.81 ± 0.28 | 23.7 ± 9.9 |
| OmpA hGH | 35.3 | 13.1 | 1.17 ± 0.38 | 21.6 ± 12.7 |
Data were fit to a steady state affinity model for derivation of apparent KD.
Figure 5. Surface plasmon resonance sensograms of hGH binding to immobilized hGH-R.
Increasing concentrations of TEV-TROPIN, pelB-hGH, or ompA-hGH (0.78 nM to 50 nM) were injected over immobilized hGHR-Fc at pH 7.4 (left) or pH 6 (right) as described in Materials and Methods. The resulting sensograms were fit to a steady state affinity model for derivation of KD. All data were baseline-adjusted and reference cell-subtracted.
Nb2 cell bioassay for growth hormone activity
The Nb2 rat node lymphoma cell line was used to quantify the in vitro bioactivity of purified hGH [34]. Nb2 cell growth is dependent on mammalian lactogens, such as prolactin and hGH, and is a useful tool for quantifying the potency of recombinant hGH [35]. Nb2 cells starved of FBS undergo cell cycle arrest which can be rescued by addition of exogenous hGH. The ability of hGH to elicit a biological response (e.g. cell growth) is dependent on hGH induced receptor dimerization. Each hGH contains two separate hGH-R binding sites. hGH first binds the hGH-R using site 1 with subsequent binding to a second hGH-R using site 2 [31]. Sequential binding and receptor dimerization make hGH a potent agonist whereas dimerization blockade through site 2 mutagenesis or high hGH concentrations that saturate hGH receptors with site 1 binders act as antagonists [36]. TEV-TROPIN, pelB-, and ompA-hGH stimulate Nb2 cell growth in a dose-dependent manner with an EC50 of 10.2 pM, 23.7 pM, and 21.6 pM, respectively, whereas BSA has no effect on cell growth at concentrations up to 14 nM (Figure 4b and Table 2). The data indicate that pelB- and ompA-hGH contain 2 functional hGH-R binding sites and form the appropriate receptor-hormone complex necessary for potent growth stimulation.
Conclusions
In summary, we report a simple system for production of recombinant human growth hormone directed to the E. coli periplasm via the pET based expression platform to yield soluble, properly folded hGH containing a C-terminal poly-histidine tag. The hGH isolated by a single affinity chromatography step is monomeric, binds the hGH receptor with high affinity, and potently stimulates cell growth with only a slight reduction in potency compared to pharmaceutical grade recombinant hGH. The amount of hGH recovered per batch is suitable for in vitro and in vivo studies. This system will be useful for the average research laboratory wishing to produce material to study hGH biology or design novel hGH variants and delivery systems.
Highlights.
Human growth hormone was produced in E. coli using the pET expression system
hGH was isolated in its properly folded conformation from the E. coli periplasm
A single step purification yields bioactive hGH
Acknowledgments
This work is funded in part by NIH 1 R21 EB015520-01, NIH 3 R01 EB003008-07S1, and NIH TG T32 GM007175. J.S. acknowledges past and current fellowship support from the American Foundation for Pharmaceutical Education (AFPE), UCSF Graduate Dean’s Chancellor’s Fellowship, and the Pharmaceutical Research and Manufacturers of America (PhRMA) foundation.
Footnotes
Conflict of Interest. The authors declare no conflict of interest.
Contributions. J.S. designed research, executed experiments, contributed new reagents, analyzed data, and wrote the manuscript. F.S. reviewed data, and revised the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jonathan T. Sockolosky, Email: jonathan.sockolosky@ucsf.edu.
Francis C. Szoka, Email: szoka@cgl.ucsf.edu.
References
- 1.Itakura K, Hirose T, Crea R, Riggs AD, Heyneker HL, Bolivar F, Boyer HW. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977;198:1056–63. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- 2.Morrow JF, Cohen SN, Chang AC, Boyer HW, Goodman HM, Helling RB. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:1743–7. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SN, Chang AC, Boyer HW, Helling RB. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci USA. 1973;70:3240–4. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–30. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 5.Yoon SH, Kim SK, Kim JF. Secretory production of recombinant proteins in Escherichia coli. Recent Pat Biotechnol. 2010;4:23–9. doi: 10.2174/187220810790069550. [DOI] [PubMed] [Google Scholar]
- 6.Berkmen M. Production of disulfide-bonded proteins in Escherichia coli. Protein Expres Purif. 2012;82:240–51. doi: 10.1016/j.pep.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JL, Geething NC, Moore JA, Rogers BC, Spink BJ, Wang C-W, Alters SE, Stemmer WPC, Schellenberger V. A novel long-acting human growth hormone fusion protein (VRS-317): enhanced in vivo potency and half-life. J Pharm Sci. 2012;101:2744–54. doi: 10.1002/jps.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Illum L, Jordan F, Lewis AL. CriticalSorb™: A novel efficient nasal delivery system for human growth hormone based on Solutol HS15. J Control Release. 2012;162:194–200. doi: 10.1016/j.jconrel.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 10.de Schepper J, Rasmussen MH, Gucev Z, Eliakim A, Battelino T. Long-acting pegylated human GH in children with GH deficiency: a single-dose, dose-escalation trial investigating safety, tolerability, pharmacokinetics and pharmacodynamics. Eur J Endocrinol. 2011;165:401–9. doi: 10.1530/EJE-11-0536. [DOI] [PubMed] [Google Scholar]
- 11.Péter F, Bidlingmaier M, Savoy C, Ji H-J, Saenger PH. Three-year efficacy and safety of LB03002, a once-weekly sustained-release growth hormone (GH) preparation, in prepubertal children with GH deficiency (GHD) J Clin Endocr Metab. 2012;97:400–7. doi: 10.1210/jc.2011-2234. [DOI] [PubMed] [Google Scholar]
- 12.Amet N, Wang W, Shen W-C. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release. 2010;141:177–82. doi: 10.1016/j.jconrel.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk J. Indications for growth hormone therapy in children. Arch Dis Child. 2012;97:63–8. doi: 10.1136/adc.2010.186205. [DOI] [PubMed] [Google Scholar]
- 14.Cuatrecasas G, Alegre C, Fernandez-Solà J, Gonzalez MJ, Garcia-Fructuoso F, Poca-Dias V, Nadal A, Cuatrecasas G, Navarro F, Mera A, Lage M, Peinó R, Casanueva F, Liñan C, Sesmilo G, Coves MJ, Izquierdo JP, Alvarez I, Granados E, Puig-Domingo M. Growth hormone treatment for sustained pain reduction and improvement in quality of life in severe fibromyalgia. Pain. 2012;153:1382–9. doi: 10.1016/j.pain.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Sockolosky JT, Tiffany MR, Szoka FC. Engineering neonatal Fc receptor-mediated recycling and transcytosis in recombinant proteins by short terminal peptide extensions. Proc Natl Acad Sci USA. 2012;109:16095–16100. doi: 10.1073/pnas.1208857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patra AK, Mukhopadhyay R, Mukhija R, Krishnan A, Garg LC, Panda AK. Optimization of inclusion body solubilization and renaturation of recombinant human growth hormone from Escherichia coli. Protein Expres Purif. 2000;18:182–92. doi: 10.1006/prep.1999.1179. [DOI] [PubMed] [Google Scholar]
- 17.Peterson FC, Anderson PJ, Berliner LJ, Brooks CL. Expression, folding, and characterization of small proteins with increasing disulfide complexity by a pT7-7-derived phagemid. Protein Expres Purif. 1999;15:16–23. doi: 10.1006/prep.1998.0984. [DOI] [PubMed] [Google Scholar]
- 18.Singh SM, Sharma A, Panda AK. High throughput purification of recombinant human growth hormone using radial flow chromatography. Protein Expres Purif. 2009;68:54–9. doi: 10.1016/j.pep.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Mukhija R, Rupa P, Pillai D, Garg LC. High-level production and one-step purification of biologically active human growth hormone in Escherichia coli. Gene. 1995;165:303–6. doi: 10.1016/0378-1119(95)00525-b. [DOI] [PubMed] [Google Scholar]
- 20.Soares CRJ, Gomide FIC, Ueda EKM, Bartolini P. Periplasmic expression of human growth hormone via plasmid vectors containing the lambdaPL promoter: use of HPLC for product quantification. Protein Eng. 2003;16:1131–8. doi: 10.1093/protein/gzg114. [DOI] [PubMed] [Google Scholar]
- 21.Hsiung HM, Mayne NG, Becker GW. High–level expression, efficient secretion and folding of human growth hormone in Escherichia coli. Bio/Technology. 1986;4:991–995. doi: 10.1016/0014-5793(86)81403-x. [DOI] [PubMed] [Google Scholar]
- 22.Chang CN, Rey M, Bochner B, Heyneker H, Gray G. High-level secretion of human growth hormone by Escherichia coli. Gene. 1987;55:189–96. doi: 10.1016/0378-1119(87)90279-4. [DOI] [PubMed] [Google Scholar]
- 23.Gray GL, Baldridge JS, McKeown KS, Heyneker HL, Chang CN. Periplasmic production of correctly processed human growth hormone in Escherichia coli: natural and bacterial signal sequences are interchangeable. Gene. 1985;39:247–54. doi: 10.1016/0378-1119(85)90319-1. [DOI] [PubMed] [Google Scholar]
- 24.Ghorpade A, Garg LC. Efficient processing and export of human growth hormone by heat labile enterotoxin chain B signal sequence. FEBS Lett. 1993;330:61–65. doi: 10.1016/0014-5793(93)80920-p. [DOI] [PubMed] [Google Scholar]
- 25.Chang JY, Pai RC, Bennett WF, Bochner BR. Periplasmic secretion of human growth hormone by Escherichia coli. Biochem Soc T. 1989;17:335–7. doi: 10.1042/bst0170335. [DOI] [PubMed] [Google Scholar]
- 26.Teresa M, Ribela CP, Camargo IM, Oliveira JE, Bartolini P. Single-step purification of recombinant human growth hormone (hGH) directly from bacterial osmotic shock fluids, for the purpose of (125)I-hGH preparation. Protein Expres Purif. 2000;18:115–20. doi: 10.1006/prep.1999.1184. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Marks JD. Selection of phage antibody libraries for binding and internalization into mammalian cells. In: Kontermann R, Dubel S, editors. Antibody Engineering. Springer; New York: 2010. pp. 183–195. [Google Scholar]
- 28.Koshland D, Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980;20:749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- 29.Schellenberger V, Wang C, Geething NC, Spink BJ, Campbell A, To W, Scholle MD, Yin Y, Yao Y, Bogin O, Cleland JL, Silverman J, Stemmer WPC. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat Biotechnol. 2009;27:1186–1190. doi: 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- 30.Canova-Davis E, Baldonado IP, Moore JA, Rudman CG, Bennett WF, Hancock WS. Properties of a cleaved two-chain form of recombinant human growth hormone. Int J Pept Prot Res. 2009;35:17–24. doi: 10.1111/j.1399-3011.1990.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham B, Ultsch M, De Vos A, Mulkerrin M, Clauser K, Wells J. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991;254:821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- 32.de Vos A, Ultsch M, Kossiakoff A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255:306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 33.Langenheim JF, Chen WY. Improving the pharmacokinetics/pharmacodynamics of prolactin, GH, and their antagonists by fusion to a synthetic albumin-binding peptide. J Endocrinol. 2009;203:375–87. doi: 10.1677/JOE-09-0211. [DOI] [PubMed] [Google Scholar]
- 34.Gout PW, Beer CT, Noble RL. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980;40:2433–6. [PubMed] [Google Scholar]
- 35.Tanaka T, Shiu RP, Gout PW, Beer CT, Noble RL, Friesen HG. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J Clin Endocr Metab. 1980;51:1058–63. doi: 10.1210/jcem-51-5-1058. [DOI] [PubMed] [Google Scholar]
- 36.Fuh G, Cunningham BC, Fukunaga R, Nagata S, Goeddel DV, Wells JA. Rational design of potent antagonists to the human growth hormone receptor. Science. 1992;256:1677–80. doi: 10.1126/science.256.5064.1677. [DOI] [PubMed] [Google Scholar]