Abstract
Parkinson’s disease (PD) is a devastating neurological condition that affects about 1% of people older than 65 years of age. In PD, dopaminergic neurons in the midbrain slowly accumulate cytoplasmic inclusions (Lewy bodies, LBs) of the protein alpha-synuclein (α-syn) and then gradually lose function and die off. Cell death is thought to be causally linked to the aggregation/fibrillization of α-syn. This review focuses on new findings about the structure of α-syn, about how α-syn cooperates with Hsp70 and Hsp40 chaperones to promote neurotransmitter release, and about cell-to-cell transfer of pathogenic forms of α-syn and how Hsp70 might protect against this disease process.
Keywords: alpha-synuclein, cysteine string protein, Hsp40, Hsp70, molecular chaperone, neurodegeneration, Parkinson’s disease
Introduction
Alpha-synuclein (α-syn) is a neuronal protein that has been linked to several neurodegenerative diseases, referred to as synucleinopathies, such as Parkinson’s disease (PD), dementia with Lewy bodies, multiple system atrophy and neurodegeneration with brain iron accumulation type 1 [1–3]. The common feature of these diseases is that intracellular inclusions of α-syn form in neurons in an age-dependent process. In sporadic PD, alterations in α-syn homeostasis with age lead to α-syn inclusions (Lewy bodies [LB] and Lewy neurites [LN]) and the selective degeneration of dopaminergic neurons in the substantia nigra, which leads to postural instability, slowness of movement, and tremors. In familial PD, that is, for individuals who possess mutations (A30P, A53T or E46K) [4–6] or multiple copies of the α-syn gene [7], LB pathology and the age of disease onset are earlier, i.e. 30 year versus 65 year for sporadic. Other than replenishing dopamine, no other therapeutic strategies can thwart this disease.
The Structure and Function of α-Syn
α-Syn has long been considered as an intrinsically unfolded monomeric protein, but in light of new findings, this classification may no longer be valid. First, it is important to note the basic sequence elements of this protein. (i) The N-terminal domain (residues 1–65), which contains hydrophobic and cationic residues, can adopt an α-helical conformation and interact with the surface of micelles and membranes that are enriched in anionic phospholipids [8]. (ii) The hydrophobic NAC domain (residues 66–95) controls the self-association of this protein. Because this domain is often found in Alzheimer’s disease (AD) plaques, it was named the “non-Aβ component of Alzheimer’s disease amyloid plaque” [9]. Fibers composed of the NAC domain can infect and kill healthy cells [10]. (iii) The C-terminal domain (residues 96–140) is unstructured and contains acidic amino acids and serine and tyrosine residues, which are subject to phosphorylation. The C-terminal domain may have chaperone activity [11]. α-Syn shares sequence similarity with 14-3-3 proteins [12] and a lipid binding motif found in a family of lipid binding proteins [13].
Recombinant human α-syn purified from E. coli using denaturing conditions is an intrinsically unfolded monomeric protein [14] that has a propensity to adopt an α-helical structure when bound to the surface of micelles or liposomes [15] (Fig. 1), and under certain conditions it slowly forms soluble aggregates and insoluble β-amyloid fibers [16–18].
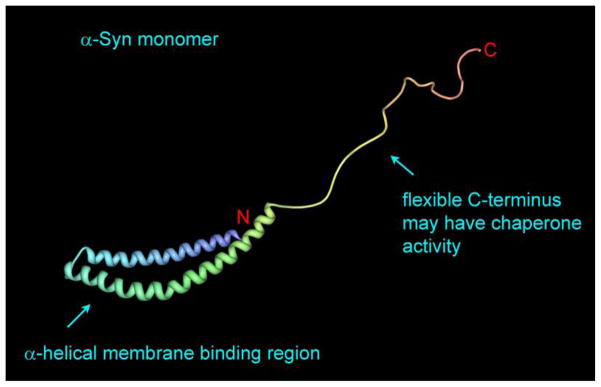
Fig. 1.
Monomeric α-syn bound to a micelle. The N-terminal residues (1–95) form two α-helices that adopt a hairpin-like structure, while the C-terminal residues are unstructured. Protein data bank file: 1XQ8 [32].
Human α-syn purified from red blood cells, or even from E. coli, using non-denaturing conditions is not a monomer [19,20]. Instead, it is a tetramer—a four-helix—bundle that resists aggregation. Tetrameric α-syn binds to phospholipid membranes with higher affinity than monomers, and, the tetramers fail to form fibers over a 10 d incubation period, whereas the monomers do. This raises the possibility that conditions exist in cells that break up the tetramers, thereby releasing monomers that may slowly aggregate and fibrillize (Fig. 2). These early studies of monomeric α-syn gave important insight into the self-association behavior of the monomer. The new studies are causing a paradigm shift that should result in a deeper understanding of the cellular events that lead α-syn to aggregate and fibrillize.
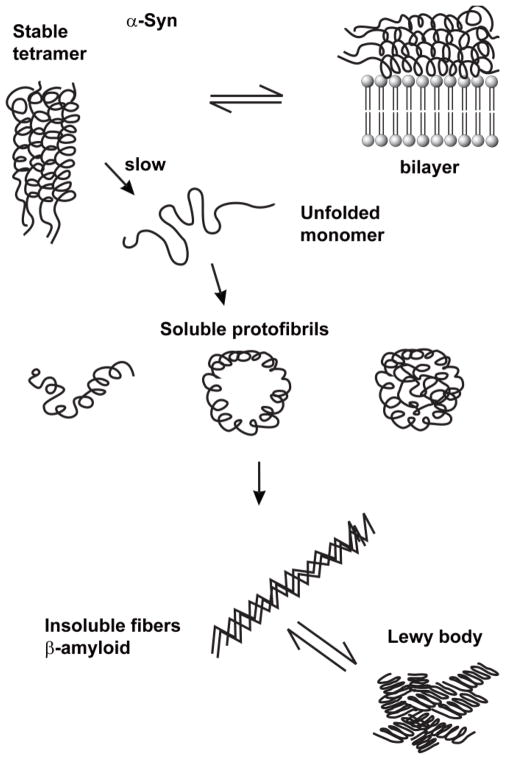
Fig. 2.
Model for α-syn aggregate/fibril formation. The α-syn tetramer is thermodynamically favored inside cells because of the high expression level of this protein. The tetramers could dissolve into unfolded monomers because of post-translational modifications, dilution, or unknown factors. Unfolded monomers can aggregate into soluble high molecular mass protofibrils and insoluble β-amyloid fibers. Emerging evidence indicates that a pathogenic conformation of α-syn is transmitted from cell-to-cell, and this transmission causes disease spread through the brain. For simplicity, in Figs. 2–4 the α-syn tetramer is depicted as a four-helix bundle where each monomer is an α-helix with a flexible tail. In the physiological α-syn tetramer, each monomer has been suggested to adopt the hair-pin-like structure shown in Fig. 1 [20].
A non-essential protein that is highly expressed in neurons, α-syn localizes to the nuclear membrane and presynaptic nerve terminals [21], where it likely functions in synaptic vesicle recycling and neurotransmitter release [11]. Insights into α-syn biology and pathobiology have come about from overexpression and knockout studies. Studies have shown that at elevated levels α-syn inhibits vesicle trafficking [22], the proteasome [23], and even pokes holes in membranes [18,24]. Knockout studies in the mouse and rat have probed the effect of loss of function. In the mouse, although knocking out α-syn fails to produce neurodegeneration [25–28], compared to control animals, reductions occur in the level of striatal dopamine [29] and the pool of undocked synaptic vesicles [27]. In the rat, virus-mediated RNAi-mediated silencing of α-syn results in a rapid degeneration of dopaminergic neurons compared to control animals, suggesting that normal levels of α-syn are required for the viability of these neurons [30]. The authors of this latter study cautioned that a therapeutic approach based on RNAi-mediated silencing of α-syn in humans could backfire and exacerbate neurodegeneration. Taken together, α-syn overexpression and knockout studies support a U-shaped toxicity curve: at the optimal level, α-syn is non-toxic and carries out its normal function, whereas very low or very high levels trigger synaptic dysfunction and neurodegeneration. One way to look at such a U-shaped toxicity curve is that a loss-of-function occurs at low or zero levels of α-syn, whereas a toxic gain-of-function occurs at high levels. Of course, an elevated level might promote self-association, which in turn could decrease the level of the active α-syn species (whether monomer or tetramer) at presynaptic terminals, resulting in, oddly, a loss-of-function phenotype.
Expressed in many types of cells other than neurons, α-syn is abundant even in red blood cells (RBCs) [31], although no one knows why. Possible functions of α-syn in RBCs, which have no nucleus, no DNA, no RNA, no protein synthesis, and no mitochondria, are: (i) A tether – α-Syn binds membranes via its N-terminal α-helical domain [32], whereas its flexible C-terminal tail, which does not bind the membrane, is free to bind other proteins like a chaperone (Fig. 1). It’s easy to imagine one end of an α-syn molecule tightly bound to the plasma membrane while the other end grabs a cytosolic protein. A tether function could enable a soluble protein to modulate the activity of a membrane-bond protein. A possible tether function has been reported for α-syn (see “Deletion of the Hsp40 Cysteine String Protein-α (CSPα) induces neurodegeneration; α-Syn reverses this effect”). (ii) A regulator of membrane fluidity – The avid binding of α-syn to the inner leaflet of the plasma membrane could alter the membrane fluidity in such a way as to increase the mechanical strength and even the lifetime of RBCs. An interesting parallel exists between α-syn and Hsp12, which is a small heat shock protein (109 amino acids) expressed by the yeast S. cerevisiae and many other fungi [33,34]. It was recently reported that Hsp12, which is induced up to 100-fold upon heat shock, is an intrinsically unfolded chaperone that folds into an alpha-helical conformation upon binding to biological membranes [35]. A variety of experimental techniques showed that Hsp12 stabilizes the yeast plasma membrane and protects cells from high temperature stress. Hsp12 even has weak homology (< 20%) to α-syn, although there is no evidence that Hsp12 forms pathological oligomeric structures like α-syn. Perhaps in some types of cells, such as RBCs, α-syn is a chaperone that stabilizes the plasma membrane against disruption by various stresses. (iii) An anti-oxidant – RBCs have evolved to hold large numbers of hemoglobin molecules. It is noteworthy that the a-syn gene and three heme biosynthetic genes ALAS2, FECH, and BLVRB show highly correlated gene expression profiles [36], suggesting that α-syn plays some role in heme metabolism. Maybe α-syn inhibits oxidative stress during heme and hemoglobin synthesis in RBC progenitor cells and this function is also required in RBCs. Perhaps not surprising, antioxidant functions of α-syn have been reported [30,37–43].
Molecular chaperones
Cells expend considerable amounts of ATP to synthesize the multitude of proteins that constitute its proteome. To prevent the loss of the proteins due to inefficient folding, cells have evolved an amazing set of proteins, first dubbed ‘heat shock proteins’ (HSP) and now referred to as ‘molecular chaperones’, that help fold newly synthesized proteins, transport proteins into organelles, inhibit and reverse misfolding and aggregation, and degrade proteins that cannot be massaged back into shape [44–46]. Because of the complex organelle structure of the typical eukaryotic cells, not only does the cytosol contain molecular chaperones, but most organelles and compartments, such as nerve terminals, contain specialized chaperones and co-chaperones that participate in maintaining the protein homeostasis of each organelle and compartment. This review focuses on the role membrane-associated molecular chaperones play in maintaining α-syn homeostasis.
Many neurodegenerative diseases are ‘proteinopathies’ because toxic protein aggregates and fibers accumulate in age-dependent process. Such diseases include Alzheimer’s, Huntington’s, Parkinson’s, amyotropic lateral sclerosis, numerous ataxias, and prion diseases [1,47–50]. The accumulation of insoluble protein aggregates with age implies a failure of the cellular protein quality control system, which consists of molecular chaperones, the ubiquitin-proteasome system and the autophagy-lysosome system [51,52]. Neurons are particularly susceptible to aggregate formation, I suggest, for the following reasons. First, the high metabolic rate of neurons is conducive to protein aggregation. Neurons synthesize and consume more ATP per cell than most other types of cells [53]. Oxidative phosphorylation, which is responsible for most of the ATP synthesis in neurons, generates reactive oxygen species (ROS), which indiscriminately oxidizes lipids, DNA and proteins. Oxidized proteins often aggregate [54], and such aggregates may resist degradation by the proteasome and lysosome. Second, neurons are non-dividing cells that cannot dilute away protein aggregates by stealthily passing them to daughter cells. Aggregates that resist elimination have nowhere else to go; thus, they coalesce into inclusions (Lewy bodies), which may or may not be toxic per se, but certainly they can sequester essential proteins [55]. Third, neurotransmitter release at nerve terminals occurs on a millisecond time scale, with hundreds or thousands of events occurring per minute. For instance, dopaminergic neurons rapidly release dopamine into the synapase by a complex process involving exocytosis of dopamine-containing vesicles in coordination with Ca+2 influx through the L-type calcium channels. This rapid neurotransmitter release (and reuptake), which involves numerous proteins, protein conformation changes, and reversible associations with membranes, has the potential to run amok if essential factors misfold or aggregate [56,57]. As shown below, α-syn together with Hsc70/Hsp70 and two co-chaperones (cysteine string protein-α, CSPα, and small glutamine-rich TPR protein, SGT) are a chaperone machine that functions to maintain efficient neurotransmitter release at nerve terminals. (TPR stands for tetratricopeptide repeat, which is a protein-protein interaction domain.) Chaperone machine failure results in inefficient neurotransmitter release followed by gradual neurodegeneration. In general, by dissolving away harmful aggregates or inhibiting their formation, molecular chaperones are instrumental to maintaining protein homeostasis in all types of cells and especially neurons [58,59].
Hsp70 Protects from α-Syn-Induced Neurodegeneration
The fruit fly (Drosophila) has been an invaluable organism to study PD [60]. In addition to analyzing the effect of the directed expression of human α-syn on dopaminergic neurons, retinal degeneration and movement defects can also be analyzed in flies. This was the first model organism in which the importance of molecular chaperones, specifically Hsp70, vis-à-vis α-syn-induced neurodegeneration was illuminated. Bonini and colleagues showed that human wild-type α-syn triggers an age-dependent degeneration of dopaminergic neurons compared to control flies, which was reversed by the co-expression of human Hsp70 [61,62]. Interestingly, transgenic flies exhibited α-syn inclusions, and these inclusions were still evident when human Hsp70 was co-expressed. Note that disrupting the function of the fly Hsp70 (called Hsc4) also accelerated neurodegeneration with or without the human α-syn transgene [63]. This landmark study revealed that Hsp70 overexpression protects cells from α-syn toxicity, that α-syn inclusions are inert, and that the toxic species is probably soluble. Subsequent studies began to decipher the molecular details by which Hsp70/Hsc70 protects cells from α-syn-induced neurodegeneration [64–72].
Deletion of the Hsp40 Cysteine String Protein-α (CSPα) induces neurodegeneration; α-Syn reverses this effect
Overexpression of Hsp70/Hsc70 chaperones is almost universally protective against toxic aggregates and filamentous proteins [61,66,68,73,74]; whereas, knocking out a chaperone rarely causes aggregate formation and cell death. Knocking out the chaperone CSPα is unique in that it causes neurodegeneration in several organisms. CSPα is a member of the Hsp40 family, which, via palmitoylation of its cysteine residues [75], localizes to the outer leaflet of presynaptic vesicles where it functions as a co-chaperone for Hsc70. There are several excellent reviews on J proteins [76–80] in general and neural J proteins [81–83] specifically.
Deletion of the csp gene in flies causes defects in synaptic transmission, which leads to paralysis and premature death [84]. Only 4% of flies with csp deleted reached adulthood at 25°C, whereas none reached adulthood at 29°C. Flies that survived to adulthood and were then exposed to 29°C became paralyzed and rapidly died. Electron microscopy analysis of the lamina region of photoreceptor terminals revealed that synaptic vesicles were visible in wild-type but not the csp mutant. The authors of the study concluded that CSP protein has a role in neurotransmitter release. The temperature dependence of the lethality was suggested to indicate that CSP is essential at high temperatures, like an inducible chaperone. This work was followed by three elegant studies from the same lab that illuminated the functions of CSP and α-syn [11,85,86].
First, deletion of CSPα also causes rapid and progressive neurodegeneration in mice [85]. Analysis of tissue extracts from CSPs knockout (KO) mice revealed that deletion of CSPα significantly decreased the levels of the plasma membrane SNARE protein (SNARE, soluble NSF [N-ethylmaleimide–sensitive factor] attachment protein receptor) called SNAP-25, Hsp70, Hsc70 and even α-syn. SNAREs catalyze vesicle-membrane fusion events in cells (for reviews on SNARE proteins, see [56,57,87]). The decrease in the levels of these four proteins upon CSPs deletion suggests a functional link between these proteins. The striking finding was that transgenic overexpression of human wild-type α-syn abolished the lethal neurodegeneration in CSPα KO mice. α-Syn overexpression promoted SNARE complex assembly, increased the levels of Hsp70 and Hsc70, but failed to increase the level of SNAP-25 [85]. The ability of three different human α-syn species to block neurodegeneration in CSPα KO mice was evaluated and gave insight into the mechanism of protection. Given that wild-type α-syn and A53T avidly bind membranes, whereas A30P does not [88], it was interesting that α-syn and A53T blocked neurodegeneration in the CSPα KO mice whereas A30P did not. An important conclusion was that the membrane-binding ability of α-syn, which is mediated by the N-terminal domain, is necessary to rescue neurodegeneration in CSPα KO mice
Second, using the mouse model to probe the mechanism by which α-syn in functionally compensates for the loss of CSPα, it was discovered that the flexible C-terminal domain of α-syn is also required to rescue neurodegeneration caused by deletion of CSPα [11]. It turns out that the C-terminus of α-syn binds to the SNARE-protein synaptobrevin-2/vesicle-associated membrane protein 2 (VAMP2), which is lodged in the synaptic vesicle membrane. One model is that α-syn, which is bound to the surface of a synaptic vesicle, acts as a non-classical chaperone that maintains synaptobrevin-2 in an active conformation that enables a synaptic vesicle to fuse with the presynaptic membrane. Alternatively, α-syn could tether a presynaptic vesicle to the presynaptic membrane. In this model, α-syn is bound to the presynaptic membrane while its C-terminal domain is bound to vesicular synaptobrevin-2. Whether α-syn is a chaperone or a tether will await further experimentation.
Third, digging deeper into the role of CSPα in synaptic transmission, it was revealed that SNAP-25 is a client of the CSPα-Hsc70-SGT chaperone machine [86]. In addition to the decrease in SNAP-25 level in CSPα KO mice, knocking out CSPα triggers an increase (40%) in ubiquitinated forms of SNAP-25 and Hsc70, but not other neuronal proteins. This suggests that SNAP-25 is destabilized and subject to degradation by the proteasome when CSPα is deleted. In support of this hypothesis, the CSPα-Hsc70-SGT chaperone complex binds to monomeric SNAP-25 but not to assembled SNARE complexes containing SNAP-25. In the absence of CSPα, apparently a larger fraction of SNAP-25 molecules adopt an off-pathway, inactive, and potentially toxic conformation (SNAP-25*) that is tagged with ubiquitin for degradation. The CSPα-Hsc70-SGT chaperone machine facilitates the folding of SNAP-25, which minimizes its degradation, and thus promotes SNARE complex assembly and membrane fusion. The function of α-syn may be to maintain SNARE-complex assembly in a presynaptic terminal during aging.
Cell-to-cell Transfer of Chaperones and α-Syn
In the 1980’s, studies were published showing that cells release Hsc70/Hsp70 molecules in response to heat shock, and that the released chaperones are taken up by neighboring cells [89,90]. Hsp70 was even found to form ion channels in membranes [91,92]. Although the mechanisms of chaperone release have not been fully elucidated, three mechanisms have been proposed [93,94]: (i) Chaperones are released from dead cells. (ii) Chaperones are released from living cells via exosomes, which are small (50 nm – 100 nm) vesicles that arise from multivesicular bodies within cells [95]. (iii) Chaperones escape living cells by forming pores in the cell membrane [92]. Whatever the mechanism, the function of extracellular Hsc70/Hsp70 could depend on the specific compartment, but ideas include priming or modulating the immune response [96,97], killing tumor cells [98], signaling stress, and even, more recently, inhibiting the conversion of α-syn into toxic conformers in the extracellular milieu in the brain [99]. Mechanisms (i–iii) probably also govern the release of α-syn from cells [24,100–103].
The idea of PD spread, i.e., the cell-to-cell transfer of α-syn, came from a postmortem analysis of the brains of PD subjects and age-matched healthy controls. Braak and colleagues found that sporadic PD progresses in six distinct stages [104]. In the earliest pre-symptomatic stage, LBs immune-reactive for α-syn and ubiquitin form in the anterior olfactory bulb and the vagus nerve. The LB pathology slowly spreads up the brain stem in to cortical areas, ultimately affecting sensory and motor areas of the brain. Why the olfactory bulb and enteric nerves are particularly susceptible to LB formation is not known. One possibility is that an inhalant or a food triggers LB formation. For reviews on PD spread see [105–108].
Some individuals with severe PD received fetal stem cell transplants in order to slow down the progressive loss of striatal dopamine. Postmortem analysis of such subjects revealed an amazing finding. It was discovered that 11–16 years after transplantation the grafted neurons had LB-like inclusions that stained positively for α-syn and ubiquitin [109,110]. In contrast, in PD patients who had received similar grafts, but who had died much sooner after transplantation (18 months), the grafts showed no signs of PD. One explanation is that dying neurons release a toxic form of α-syn, which is subsequently taken up by healthy neighboring cells. Inside the healthy cells, the toxic conformers act as a template to convert endogenous, non-toxic α-syn into a toxic, self-propagating conformation. The results support the hypothesis that, like the human prion protein, α-syn can adopt two very different conformations, i.e., one is non-infectious, the other is infectious. The non-infectious conformation is probably the stable, α-helical tetramer, whereas the infectious conformation probably has solvent-exposed β-sheets.
If PD is transmissible, then pathogenic forms of α-syn that are secreted by infected cells should be taken up by healthy cells and trigger their demise. Using a variety of clever techniques that monitor uptake of exogenous α-syn by healthy cells, several labs have verified this hypothesis [10,99,111,112]. Fragments of preformed α-syn fibrils are endocytosed by primary mouse neurons in culture, and these fibrils trigger soluble endogenous α-syn to form LB and LN-like inclusions in various parts of the neurons [10]. These inclusions stain for α-syn, phosphorylated α-syn and ubiquitin. The NAC domain is the minimum fragment that propagates LB pathology. Strikingly, several days after the fibrils were added to the culture medium, decrements in the levels of SNARE proteins (VAMP2, SNAP-25, and CSPα) occur, with resultant loss of neuronal function and ultimately cell death. These SNARE proteins are either degraded when endogenous α-syn aggregates or they are sequestered in the LBs and LNs. These experimental systems will be extremely powerful to unraveling the mechanism of PD spread.
One model for cell-to-cell transfer of α-syn is that dysfunctional cells secrete an infectious form of α-syn that then propagates to other cells. Another model is that normally functioning cells occasionally, or rarely, secrete the non-infectious, tetrameric α-syn, which causes problems as follows. I propose that the concentration of α-syn is so high inside cells that the tetramer is thermodynamically favored. If α-syn tetramers are released by a small fraction of healthy cells, then because of a dilution effect the thermodynamically favored species in the extracellular milieu is the monomer, which is prone to aggregate/fibrillize. Of course, most, but not all, monomers will be degraded by extracellular proteases. But, over time, small quantities of infectious α-syn seeds could form, which could then infect, propagate in, and kill healthy neighboring cells (Fig. 3). In short, the non-infectious α-syn tetramer should be favored inside neurons, whereas the potentially toxic monomer should be favored outside neurons. Keeping α-syn inside and preventing seed formation outside neurons are potential therapeutic approaches.
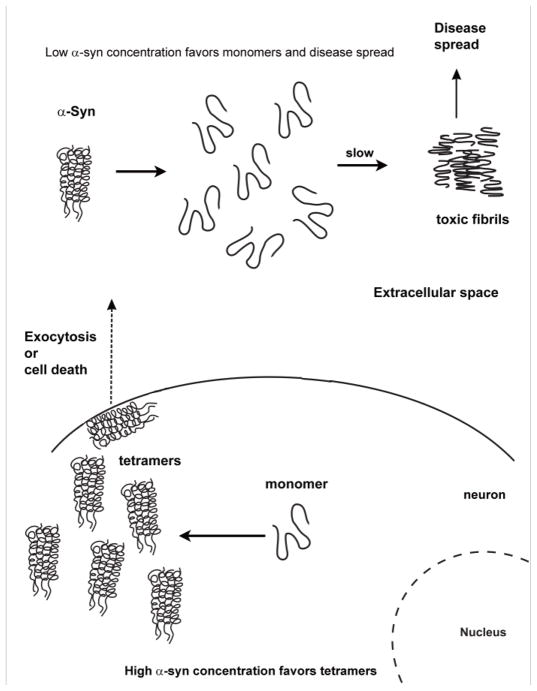
Fig. 3.
Model of α-syn toxicity and disease spread. The model stipulates that a high concentration of α-syn inside cells favors the non-infectious tetramer whereas a low concentration outside the cells favors the potentially toxic monomer. Probably most of the monomeric α-syn in the extracellular space is degraded by proteases; nevertheless, in time, toxic α-syn conformations can form and infect neighboring cells.
The potential of chaperones, especially Hsp70, to protect neurons from toxic extracellular seeds of α-syn is best illustrated by a recent study that showed that Hsp70 can inhibit the cytotoxicity of extracellular α-syn. Using a novel assay to detect α-syn secreted by H4 neuroglioma cells into the tissue culture medium (CM), it was shown that H4 cells secrete toxic oligomeric forms that can be taken up by neighboring cells [99]. When Hsp70 was overexpressed the level of α-syn oligomers in the CM was decreased by 64%, although the level of intracellular α-syn was unaffected. This means that intracellular Hsp70 inhibits the formation and release of toxic oligomeric forms of α-syn. Another possibility is that, when overexpressed, Hsp70 is secreted into the CM and that extracellular Hsp70 also inhibits the formation of toxic α-syn oligomers in the extracellular milieu. This possibility was tested using an enzyme-linked immunosorbent assay to detect Hsp70. It was discovered that H4 cells co-overexpressing Hsp70 and α-syn showed significant levels of Hsp70 in the CM compared to control cells in which these proteins were not overexpressed. Additional controls ruled out the release of Hsp70 by dead cells. This raises the possibility that Hsp70 and α-syn are secreted together. An issue is whether ATP and co-chaperones are secreted with Hsp70; for it would be unusual for Hsp70 to perform work on substrates without ATP and co-chaperone help. Overall, this study showed that Hsp70, whether intracellular or extracellular, can inhibit the formation of toxic conformations of α-syn that can infect neighboring healthy cells (Fig. 4). Therapeutic approaches that increase Hsp70 levels in the brain are one way to inhibit the formation of toxic seed-like conformations of α-syn.
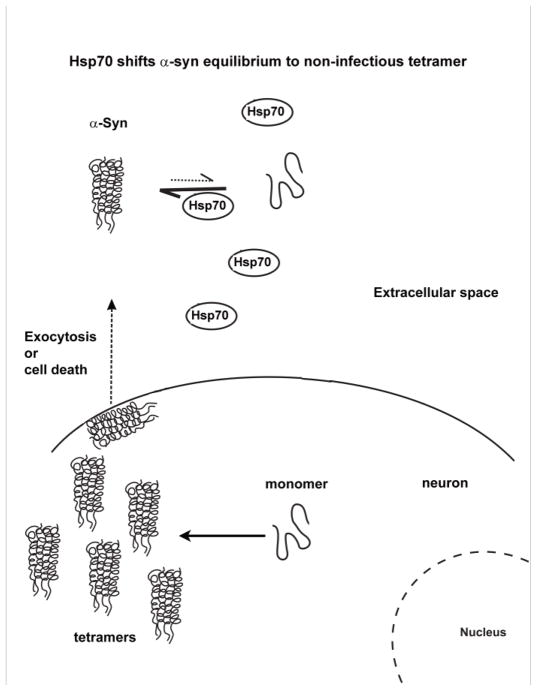
Fig. 4.
Hsp70 protects against toxic extracellular forms of α-syn. Hsp70/Hsc70 chaperones are known to be secreted, perhaps via exosomes, from a variety of cells in response to stress. Hsp70/Hsc70 chaperones protect against α-syn-induced cell death in a variety of organisms, and Hsp70 was recently shown to reduce the infectivity of extracellular α-syn. The figure illustrates that in the extracellular milieu Hsp70 shifts the α-syn equilibrium to the non-infectious tetramer.
Summary
The major recent findings in the PD field were that α-syn is a stable tetramer, the CSPα-Hsc70-SGT chaperone machine, in concert with α-syn, promotes neurotransmitter release, and α-syn can infect and kill healthy cells. With respect to chaperones, it will be interesting to see whether α-syn tetramers form spontaneously or whether chaperone help is required. Given that Hsp70 protects cells against α-syn in so many models, drugs that increase Hsp70 levels or drugs that mimic the action of Hsp70s have great potential to inhibit PD spread. The mechanisms by which cells secrete chaperones and α-syn need to be elucidated.
Acknowledgments
This work was supported by the NIH grant NS057656.
References
- 1.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 2.Lee VM, Trojanowski JQ. Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Breydo L, Wu JW, Uversky VN. alpha-Synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta. 2011;1822:261–285. doi: 10.1016/j.bbadis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 5.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 6.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 7.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 8.Zhu M, Li J, Fink AL. The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003;278:40186–40197. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 9.Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, et al. alpha-Synuclein shares physical and functional homology with 14–3–3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharon R, Goldberg MS, Bar-Josef I, Betensky RA, Shen J, et al. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc Natl Acad Sci U S A. 2001;98:9110–9115. doi: 10.1073/pnas.171300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 15.Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC. A broken alpha -helix in folded alpha -Synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 16.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 17.Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 18.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 19.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, et al. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, et al. Aggregated and monomeric alpha-synuclein bind to the S6’ proteasomal protein and inhibit proteasomal function. J Biol Chem. 2003;278:11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- 24.Anguiano M, Nowak RJ, Lansbury PT., Jr Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 25.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 26.Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, et al. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc. Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Wandi A, Ninkina N, Millership S, Williamson SJ, Jones PA, et al. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol Aging. 2010;31:796–804. doi: 10.1016/j.neurobiolaging.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorbatyuk OS, Li S, Nash K, Gorbatyuk M, Lewin AS, et al. In vivo RNAi-mediated alpha-synuclein silencing induces nigrostriatal degeneration. Mol Ther. 2010;18:1450–1457. doi: 10.1038/mt.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbour R, Kling K, Anderson JP, Banducci K, Cole T, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5:55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 32.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 33.Mtwisha L, Brandt W, McCready S, Lindsey GG. HSP 12 is a LEA-like protein in Saccharomyces cerevisiae. Plant Mol Biol. 1998;37:513–521. doi: 10.1023/a:1005904219201. [DOI] [PubMed] [Google Scholar]
- 34.Motshwene P, Karreman R, Kgari G, Brandt W, Lindsey G. LEA (late embryonic abundant)-like protein Hsp 12 (heat-shock protein 12) is present in the cell wall and enhances the barotolerance of the yeast Saccharomyces cerevisiae. Biochem J. 2004;377:769–774. doi: 10.1042/BJ20031301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welker S, Rudolph B, Frenzel E, Hagn F, Liebisch G, et al. Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function. Mol Cell. 2010;39:507–520. doi: 10.1016/j.molcel.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Scherzer CR, Grass JA, Liao Z, Pepivani I, Zheng B, et al. GATA transcription factors directly regulate the Parkinson’s disease-linked gene alpha-synuclein. Proc Natl Acad Sci U S A. 2008;105:10907–10912. doi: 10.1073/pnas.0802437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu M, Qin ZJ, Hu D, Munishkina LA, Fink AL. Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry. 2006;45:8135–8142. doi: 10.1021/bi052584t. [DOI] [PubMed] [Google Scholar]
- 39.Bayir H, Kapralov AA, Jiang J, Huang Z, Tyurina YY, et al. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome C: protection against apoptosis versus delayed oxidative stress in Parkinson disease. J Biol Chem. 2009;284:15951–15969. doi: 10.1074/jbc.M900418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Sanchez F, Milan M, Buendia P, Cano-Jaimez M, Ambrosio S, et al. Prosurvival effect of human wild-type alpha-synuclein on MPTP-induced toxicity to central but not peripheral catecholaminergic neurons isolated from transgenic mice. Neuroscience. 2010;167:261–276. doi: 10.1016/j.neuroscience.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Cano-Jaimez M, Perez-Sanchez F, Milan M, Buendia P, Ambrosio S, et al. Vulnerability of peripheral catecholaminergic neurons to MPTP is not regulated by alpha-synuclein. Neurobiol Dis. 2010;38:92–103. doi: 10.1016/j.nbd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Davies P, Moualla D, Brown DR. Alpha-synuclein is a cellular ferrireductase. PLoS ONE. 2011;6:e15814. doi: 10.1371/journal.pone.0015814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Lee YJ, Liou LC, Ren Q, Zhang Z, et al. Alpha-synuclein functions in the nucleus to protect against hydroxyurea-induced replication stress in yeast. Hum Mol Genet. 2011;20:3401–3414. doi: 10.1093/hmg/ddr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 45.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 46.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 48.Selkoe DJ. Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2011;3:a004457. doi: 10.1101/cshperspect.a004457. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 50.Colby DW, Prusiner SB. Prions. Cold Spring Harb Perspect Biol. 2011;3:1–22. doi: 10.1101/cshperspect.a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClellan AJ, Tam S, Kaganovich D, Frydman J. Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol. 2005;7:736–741. doi: 10.1038/ncb0805-736. [DOI] [PubMed] [Google Scholar]
- 52.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 53.Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- 54.Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 55.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, et al. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 56.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 57.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witt SN. Hsp70 molecular chaperones and Parkinson’s disease. Biopolymers. 2010;93:218–228. doi: 10.1002/bip.21302. [DOI] [PubMed] [Google Scholar]
- 59.Witt SN, editor. Protein Chaperones and Protection from Neurodegenerative Diseases. Hoboken, N.J: John Wiley & Sons, Inc; 2011. pp. 1–427. [Google Scholar]
- 60.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 61.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 62.Auluck PK, Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002;8:1185–1186. doi: 10.1038/nm1102-1185. [DOI] [PubMed] [Google Scholar]
- 63.Bronk P, Wenniger JJ, Dawson-Scully K, Guo X, Hong S, et al. Drosophila Hsc70–4 is critical for neurotransmitter exocytosis in vivo. Neuron. 2001;30:475–488. doi: 10.1016/s0896-6273(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y, Gu G, Goodlett DR, Zhang T, Pan C, et al. Analysis of alpha-synuclein-associated proteins by quantitative proteomics. J Biol Chem. 2004;279:39155–39164. doi: 10.1074/jbc.M405456200. [DOI] [PubMed] [Google Scholar]
- 65.Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 Reduces alpha-Synuclein Aggregation and Toxicity. J Biol Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- 66.Flower TR, Chesnokova LS, Froelich CA, Dixon C, Witt SN. Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson’s disease. J Mol Biol. 2005;351:1081–1100. doi: 10.1016/j.jmb.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 67.Auluck PK, Meulener MC, Bonini NM. Mechanisms of Suppression of {alpha}-Synuclein Neurotoxicity by Geldanamycin in Drosophila. J Biol Chem. 2005;280:2873–2878. doi: 10.1074/jbc.M412106200. [DOI] [PubMed] [Google Scholar]
- 68.Dong Z, Wolfer DP, Lipp HP, Bueler H. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Mol Ther. 2005;11:80–88. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 69.Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem. 2005;280:14733–14740. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- 70.Shin Y, Klucken J, Patterson C, Hyman BT, McLean PJ. The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysosomal pathways. J Biol Chem. 2005;280:23727–23734. doi: 10.1074/jbc.M503326200. [DOI] [PubMed] [Google Scholar]
- 71.Huang C, Cheng H, Hao S, Zhou H, Zhang X, et al. Heat shock protein 70 inhibits alpha-synuclein fibril formation via interactions with diverse intermediates. J Mol Biol. 2006;364:323–336. doi: 10.1016/j.jmb.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 72.Luk KC, Mills IP, Trojanowski JQ, Lee VM. Interactions between Hsp70 and the hydrophobic core of alpha-synuclein inhibit fibril assembly. Biochemistry. 2008;47:12614–12625. doi: 10.1021/bi801475r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adachi H, Katsuno M, Minamiyama M, Sang C, Pagoulatos G, et al. Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J Neurosci. 2003;23:2203–2211. doi: 10.1523/JNEUROSCI.23-06-02203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, et al. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci. 2005;25:9735–9745. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gundersen CB, Mastrogiacomo A, Faull K, Umbach JA. Extensive lipidation of a Torpedo cysteine string protein. J Biol Chem. 1994;269:19197–19199. [PubMed] [Google Scholar]
- 76.Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63:2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craig EA, Huang P, Aron R, Andrew A. The diverse roles of J-proteins, the obligate Hsp70 co-chaperone. Rev Physiol Biochem Pharmacol. 2006;156:1–21. doi: 10.1007/s10254-005-0001-0. [DOI] [PubMed] [Google Scholar]
- 78.Cyr DM, Langer T, Douglas MG. DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 79.Cheetham ME, Caplan AJ. Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:sfaeod>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walsh P, Bursac D, Law YC, Cyr D, Lithgow T. The J-protein family: modulating protein assembly, disassembly and translocation. EMBO Rep. 2004;5:567–571. doi: 10.1038/sj.embor.7400172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chamberlain LH, Burgoyne RD. Cysteine-string protein: the chaperone at the synapse. J Neurochem. 2000;74:1781–1789. doi: 10.1046/j.1471-4159.2000.0741781.x. [DOI] [PubMed] [Google Scholar]
- 82.Gibbs SJ, Braun JE. Emerging roles of J proteins in neurodegenerative disorders. Neurobiol Dis. 2008;32:196–199. doi: 10.1016/j.nbd.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 83.Zhao X, Braun AP, Braun JE. Biological roles of neural J proteins. Cell Mol Life Sci. 2008;65:2385–2396. doi: 10.1007/s00018-008-8089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zinsmaier KE, Eberle KK, Buchner E, Walter N, Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 1994;263:977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]
- 85.Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 86.Sharma M, Burre J, Sudhof TC. CSPalpha promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol. 2011;13:30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- 87.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tytell M, Greenberg SG, Lasek RJ. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986;363:161–164. doi: 10.1016/0006-8993(86)90671-2. [DOI] [PubMed] [Google Scholar]
- 90.Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- 91.Alder GM, Austen BM, Bashford CL, Mehlert A, Pasternak CA. Heat shock proteins induce pores in membranes. Biosci Rep. 1990;10:509–518. doi: 10.1007/BF01116611. [DOI] [PubMed] [Google Scholar]
- 92.Arispe N, De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem. 2000;275:30839–30843. doi: 10.1074/jbc.M005226200. [DOI] [PubMed] [Google Scholar]
- 93.Multhoff G, Hightower LE. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones. 1996;1:167–176. doi: 10.1379/1466-1268(1996)001<0167:cseohs>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: A form of communication during injury, infection, and cell damage. Cell Stress and Chaperones. 2011;16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 96.Srivastava PK. Heat shock proteins in immune response to cancer: the Fourth Paradigm. Experientia. 1994;50:1054–1060. doi: 10.1007/BF01923461. [DOI] [PubMed] [Google Scholar]
- 97.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 98.Schilling D, Gehrmann M, Steinem C, De Maio A, Pockley AG, et al. Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J. 2009;23:2467–2477. doi: 10.1096/fj.08-125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, et al. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Sugeno N, et al. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J Neurochem. 2006;97:1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- 101.van Rooijen BD, Claessens MM, Subramaniam V. Membrane Permeabilization by Oligomeric alpha-Synuclein: In Search of the Mechanism. PLoS ONE. 2010;5:e14292. doi: 10.1371/journal.pone.0014292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 105.Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson’s disease pathology spread. Nat Rev Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- 106.Del Tredici K, Braak H. A not entirely benign procedure: progression of Parkinson’s disease. Acta Neuropathol. 2008;115:379–384. doi: 10.1007/s00401-008-0355-5. [DOI] [PubMed] [Google Scholar]
- 107.Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 108.Steiner JA, Angot E, Brundin P. A deadly spread: cellular mechanisms of alpha-synuclein transfer. Cell Death Differ. 2011;18:1425–1433. doi: 10.1038/cdd.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 110.Li JY, Englund E, Holton JL, Soulet D, Hagell P, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 111.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]