Abstract
The p53 tumor suppressor is activated in response to cellular stresses to induce cell cycle arrest, cellular senescence, and apoptosis. The p53 gene is inactivated by mutations in more than 50% of human tumors. In addition, tumor cells dampen p53 activities via overexpression of p53 negative regulators, in particular two structurally related proteins Mdm2 and Mdm4. And yet, Mdm2 and Mdm4 possess p53-independent activities, which also contribute to tumor formation and progression. Given that Mdm2 and Mdm4 inhibit p53 activities to promote tumor development, small molecules and peptides were developed to abrogate the inhibition of p53 by Mdm proteins. Anti-tumor activities of these molecules have already been confirmed in preclinical studies and early-phase clinical trials. These research endeavors and clinical advances constitute the main focus of this review.
Background
p53, the most frequently inactivated gene in human cancers, is mutated in over half of human tumors. In response to various extra- and intra-cellular stresses including but not limited to oncogene activation, DNA damage and hypoxia, p53 functions as a transcriptional factor and transactivates a set of genes engaged in multiple cellular processes such as cell cycle arrest, cellular senescence, energy metabolism, and apoptosis. p53 signaling is also alternatively inactivated by high levels of p53 inhibitors. Suppression of p53 activities is largely attributed to two major negative regulators of p53, Mdm2 and Mdm4 (reviewed in 1). Different mechanisms increase the levels of Mdm2 and/or Mdm4 in a large number of tumors that carry wild-type p53 alleles. These observations suggest that upregulation of Mdm2 and/or Mdm4 serves as an alternate means of inactivating the p53 pathway, further highlighting that silencing p53 signaling is a more common event in tumorigenesis than previously thought.
In this review, we will discuss the regulation of p53 by the Mdm proteins, p53-independent oncogenic functions of Mdm2 and Mdm4 as well as potential pharmaceuticals targeting Mdm2 and Mdm4 in cancer treatment.
Regulation of p53 Functions by Mdm2 and Mdm4
The murine double minute 2 (Mdm2) was first identified as one of the genes that were amplified on double minute chromosomes in transformed mouse NIH-3T3 fibroblasts (2). Early studies demonstrated that Mdm2 could bind the p53 transactivation domain and repress its transcriptional activity (3, 4). Additionally, Mdm2 functions as an E3 ubiquitin-ligase, mediates ubiquitination of p53, and targets it for degradation (reviewed in 1). By exploiting the beauty of genetic models, two research groups independently showed that biallelic deletion of p53 rescued the embryonic lethal phenotypes in Mdm2-deficient embryos (5, 6). Deletion of Mdm2 in specific types of cells such as neuronal progenitors and cardiomyocytes also leads to p53-dependent embryonic lethal phenotypes (7-9). These in vitro and in vivo data together suggest an essential function of Mdm2 as a negative regulator of p53 in numerous cell types.
Mdm4 was originally discovered as a p53-interacting protein through screening of a mouse embryo cDNA expression library (10). The Mdm4 and Mdm2 proteins are very similar at the primary structural level (10, 11). The p53-binding domains at the N-termini of Mdm4 and Mdm2 show very high structural and functional similarities (10). The p53-binding domain of Mdm4, like that of Mdm2, interacts with the transactivation domain of p53 to represses its transcriptional activity (10). Another prominent conserved domain between Mdm4 and Mdm2 is the RING-finger domain at the C-termini of both proteins (10, 11). The RING domain of Mdm2 is responsible for its E3 ubiquitin ligase function (12); however, the Mdm4 RING domain lacks E3 ligase activity (reviewed in 1). Mice with Mdm4 RING domain alterations recently revealed that interaction of Mdm2 with Mdm4 through this RING domain is required for modulating p53 activities in embryonic stages but dispensable for Mdm2 and p53 stabilization in the adult mouse (13, 14).
Loss of Mdm4 in mice also results in embryonic lethality, which is completely rescued by concomitant p53 loss (15-17). These data indicate that Mdm4 negatively regulates p53 activity in vivo. Deletion of Mdm4 in mouse erythroid progenitors, embryonic neuronal progenitors, cardiomyocytes, and intestinal epithelia leads t o distinct pathological phenotypes, compared with that of Mdm2 (18-22). Deletion of both Mdm2 and Mdm4 in mouse embryonic neuronal progenitors results in a more severe phenotype compared with deletion of either gene alone (8). All Mdm loss-of-function phenotypes are rescued by deletion of p53 (7, 8, 18-20). Together, these data suggest that Mdm2 and Mdm4 function in a non-overlapping manner to suppress p53 activities in vivo. Intriguingly, overexpression of Mdm2 also rescues the Mdm4−/− embryonic lethality (23), implying that high levels of Mdm2 can compensate for loss of Mdm4 possibly through inhibition of p53 activities.
p53-independent Functions of Mdm2 and Mdm4
Although the major function of Mdm2 is to suppress p53 activities, emerging evidence has identified p53-independent roles of Mdm2 in tumor formation and progression. Early studies showed that p53−/− mice carrying an Mdm2 transgene developed a higher percentage of sarcomas compared to p53−/− mice (24), supporting the p53-independent functions of Mdm2 in tumorigenesis. p53-independent activities of Mdm2 include regulation of genomic instability, apoptosis and metastasis.
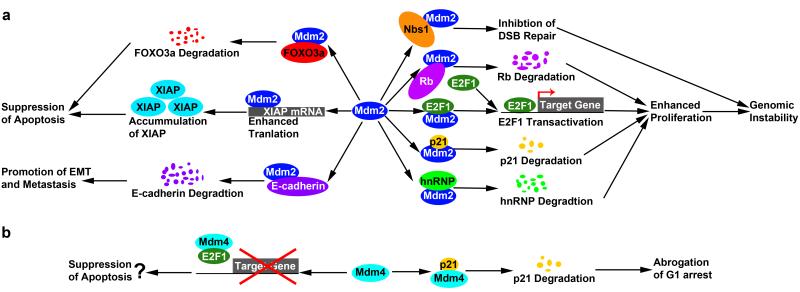
Elevated genomic instability is a hallmark of cancers (reviewed in 25). Ectopic expression of Mdm2 in murine mammary epithelial cells and murine embryonic fibroblasts (MEFs) increases ploidy and chromosome/chromatid breaks regardless of p53 status (reviewed in 26), suggesting that Mdm2 promotes genomic instability independently of p53. Mdm2 induces genomic instability likely through inhibiting DNA damage repair and suppressing cell cycle arrest. The repair of DNA double strand breaks (DSBs) caused by ionizing radiation (IR) requires the Mre11 complex, composed of Mre11, Rad50 and Nbs1 (27). Mdm2 directly binds and co-localizes with Nbs1 at DNA damage sites after IR treatment, and in turn inhibits DNA DSB repair (28) (Figure 1a). The p53-independent functions of Mdm2 in suppressing cell cycle arrest involve the direct interaction with Retinoblastoma protein (Rb) and E2F transcription factors (Figure 1a). The Rb protein interacts with several E2Fs, suppresses E2F-mediated transcription of cell-cycle essential genes, and therefore causes cell cycle arrest especially at the G1/S transition (reviewed in 29). Loss of Rb activity leads to genomic instability and tumorigenesis due to defective cell proliferation and chromosome mis-segregation (30). Mdm2 compromises Rb-mediated G1 arrest through multiple mechanisms: 1) Mdm2 directly binds Rb protein, suppresses the interaction between Rb and E2F1, and induces activation of E2F1; and 2) Mdm2 targets Rb for ubiquitin-dependent and -independent degradation (reviewed in 26) (Figure 1a). In addition, Mdm2 also directly binds E2F1 and activates its transcriptional activities (31) (Figure 1a). Moreover, Mdm2 targets other cell cycle inhibitors such as p21 and hnRNP K for proteasomal degradation, and therefore promotes cell proliferation (reviewed in 22, 26) (Figure 1a). Notably, p21 is involved in maintaining genomic stability (32), and loss of p21 induces aneuploidy, chromosomal aberrations, and accelerated tumor onset in mice (33). These data suggest degradation of p21 by Mdm2 contributes to genomic aberrations (Figure 1a). Thus, Mdm2 binds and alters the activities of several proteins involved in DNA repair and transition through the cell cycle impacting ploidy and other chromosomal abnormalities.
Figure 1. Schematic representation of p53-independent functions of Mdm2 and Mdm4.
a. Mdm2 interacts with multiple factors to affect genomic instability, apoptosis and metastasis. hnRNP: hnRNP K protein. b. Mdm4 interacts with p21 and E2F1 to regulate cell cycle arrest and apoptosis.
Additional data indicate a role of Mdm2 in inhibition of apoptosis independently of p53. FOXO3a, a forkhead transcription factor, induces apoptosis and decreases tumorigenicity of breast cancer cells (34). And inverse correlation between Mdm2 and FOXO3a expression exists in human breast cancer samples, due to Mdm2-mediated degradation of FOXO3a (34) (Figure 1a), indicating that Mdm2 may counteract FOXO3a-mediated apoptosis during tumor development. Unexpectedly, Mdm2 was also reported to suppress apoptosis via a different pathway by upregulating the anti-apoptotic protein XIAP through translational regulation (35) (Figure 1a). Upon DNA damage, cytoplasmic Mdm2 binds the internal ribosome entry site at the XIAP 5′-UTR and enhances the translation of XIAP mRNA, which may confer resistance to radiation-induced apoptosis in tumor cells (35). The mechanism by which Mdm2 regulates this process is unknown.
Additional p53-independent functions of Mdm2 include the ability to promote epithelial-to-mesenchymal transition (EMT) and metastasis (Figure 1a). The EMT effector TGF-β1 induces Mdm2 transcription via activation of Smad3 in murine mammary epithelial cells (36). Histopathological analyses of human breast cancers indicate that activation of Smad3 and overexpression of Mdm2 coexist in 65% of late-stage carcinomas and are strongly correlated to metastatic phenotypes in breast cancer patients (36), suggesting an important role of Mdm2 overexpression in metastasis. A mechanism by which Mdm2 promotes EMT and metastasis is through E-cadherin. Mdm2 binds E-cadherin, which targets it for degradation thus inducing EMT (37) (Figure 1a). Overexpression of Mdm2 in breast cancer cells disrupts cell-cell contacts, enhances cell motility, and promotes cell invasiveness (37). High Mdm2 levels are also strongly associated with low E-cadherin levels in human metastatic breast cancer specimens (37). Thus, a series of experiments in breast cancers indicate high levels of Mdm2 correlate with metastasis.
Data for p53-independent functions of Mdm4 are by far more limited. Mdm4 was found to interact with p21 and target it for proteasomal degradation independently of ubiquitination (38) (Figure 1b). Degradation of p21 mediated by Mdm4 is independent of but in cooperation with Mdm2 and leads to abrogation of G1 cell cycle arrest (38). Surprisingly, loss of Mdm4 in p53-null cells leads to multipolar spindle formation, enhanced loss of chromosomes, elevated proliferation potentials and increased spontaneous tumor transformation as compared to p53−/− cells (39). In addition, deletion of Mdm4 results in accelerated tumorigenesis in both p53+/− and p53−/− mice (39). Since Mdm4 forms heterodimers with Mdm2 (reviewed in 1), loss of Mdm4 may promote interaction of Mdm2 with other proteins such as Rb and p21 and thus enhances tumorigenesis. Moreover, Mdm4 represses E2F1 transactivation though either disrupting E2F1-DNA binding (40) or altering localization of E2F1 transcription complex (41) (Figure 1b). Overexpression of E2F1 promotes G1/S transition in cells, and is associated with tumorigenesis (42). However, upregulation of E2F1 also triggers both p53-dependent and -independent apoptosis (reviewed in 43). These data together suggest that upregulation of Mdm4 that is present in many human tumors (44), in addition to suppressing p53 activity, may represent a mechanism for tumor cells to overcome apoptosis induced by elevated E2F1 levels.
Elevated Levels of Mdm Proteins in Human Tumors
The functions of Mdm2 and Mdm4 including p53 inhibition and p53-independent activities all suggest important roles in tumorigenesis and tumor progression. High levels of Mdm2 are commonly observed in human cancers including sarcomas, gliomas, hematological malignancies, melanomas, and carcinomas (reviewed in 45). By analyzing gene amplification and overexpression status of Mdm2 in human soft tissue tumors, Patterson et al (46) demonstrated that high levels of Mdm2 detected by immunohistochemistry occurred in tumors with Mdm2 gene amplification, but were also found in tumors without Mdm2 gene amplification (46). This observation indicates that Mdm2 gene amplification indeed results in its overexpression, but other mechanisms may also confer Mdm2 upregulation. Extensive studies have indicated that upregulation of Mdm2 results from elevated transcription, increased mRNA stability, enhanced translation and altered post-translational modification (reviewed in 22, 47). In addition, these mechanisms are associated with upregulation of Mdm2 in tumors.
High levels of Mdm4 are also found in a variety of human cancers. Mdm4 upregulation in malignancies is mostly ascribed to Mdm4 gene amplification (reviewed in 44). Gene amplification and consequent upregulation of Mdm4 are reported in multiple types of human tumors including glioma, soft tissue sarcoma, head and neck squamous carcinoma, retinoblastoma, melanoma, and breast cancer (reviewed in 44). To date, information regarding transcriptional regulation of Mdm4 remains extremely limited. Stimulation with mitogens, e.g. IGF-1, leads to phosphorylation of mitogen-activated protein kinase (MAPK) ERK and thus activates transcriptional factors c-Ets-1 and Elk-1, which bind the Mdm4 promoter and induce Mdm4 expression (48). Human colon tumors that are stained positive for phosphorylated ERK are two-fold more likely to have high levels of Mdm4 (48), implying a role of Mdm4 overexpression stimulated by the MAPK in tumor development. Mdm4-S, the first Mdm4 transcript variant identified in human and mouse cells, produces an Mdm4 isoform containing the p53-binding domain but missing the negative regulatory sites, which is more stable and oncogenic than full-length Mdm4 (reviewed in 49). Overexpression of Mdm4-S has been found in several types of cancers, and is associated with decreased survival and poor prognosis for cancer patients (reviewed in 44, 49), indicating a role of Mdm4-S in tumor development.
A number of studies have modeled Mdm2 and Mdm4 overexpression in vivo using genetically engineered mice. Transgenic mice overexpressing Mdm2/Mdm4 either globally or in specific tissues/cell types all develop spontaneous tumors (24, 50-54). A T to G single nucleotide polymorphism (SNP309) in the human Mdm2 P2 promoter creates an additional binding site for the transcriptional activator Sp1, leads to elevated Mdm2 expression, and attenuates p53 signaling (55). An association between SNP309 and earlier tumor onset is revealed in both hereditary and sporadic cancer patients (55). A knock-in mouse with a humanized Mdm2 intron 1 harboring SNP309 was generated in our laboratory and develops multiple types of malignancies with accelerated onset in both p53 wild-type and p53 mutant backgrounds, providing causal evidence for the role SNP309 in tumorigenesis (54). Approximately 65% of human retinoblastomas undergo gene amplification of Mdm4 and 10% have extra copies of Mdm2, but no p53 mutations or defects downstream to p53 are found in the retinoblastomas caused by Rb loss (56), suggesting that p53 is inactivated in retinoblastomas via overexpression of p53 negative regulators. A very low retinoblastoma penetrance is observed in mice with loss of Rb family genes Rb and p107 in retina (56), but ectopic expression of Mdm4 in retina significantly promotes retinoblastoma development in these mice with Rb and p107 loss (56). In addition, the treatment of mice with p53-activating drugs leads to growth suppression of Mdm4-overepressing retinoblastomas (56), suggesting that p53 remains intact in these tumors. Therefore, this retinoblastoma mouse model recapitulates human defects and emphasizes the causative role of Mdm4 overexpression in retinoblastoma formation. In a recent study, Mdm4 is overexpressed in 65% of human melanoma samples examined, and p53 mutations are rare among these melanoma cases (57). Specific overexpression of Mdm4 in the murine melanocytes enhances tumorigenesis of Nras-induced melanoma in both p53 wild-type and heterozygous backgrounds, while knockdown of Mdm4 in human melanoma cells leads to increased cell death but reduced cell proliferation and oncogenic ability (57). However, the p53 status in these mouse melanomas has not been examined. Nevertheless, this melanoma mouse model elucidates the tumorigenic roles of Mdm4 overexpression in melanoma formation in vivo.
Clinical-Translational Advances: Targeting Mdm Proteins in Cancer Therapy
Clearly, Mdm proteins are present at high levels and inhibit p53 activities in many human cancers. Thus, disrupting the Mdm-p53 interaction to reactivate p53 is a valuable therapeutic strategy for tumor treatment.
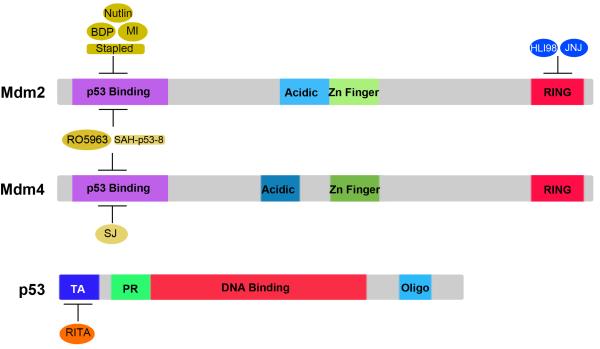
Several strategies have been used to develop small molecules to block Mdm2-p53 interaction. Using structure based design and high throughput screening, several groups independently developed distinct small molecules including Nutlins (58), benzodiazepinediones (59) and MI-63/MI-219 (60, 61) (Figure 2) that bind Mdm2 in its p53-binding pocket and disrupt Mdm2-p53 interaction (58-61). Treatment with these small molecules leads to elevated p53 protein levels, induction of p53 target gene expression, decrease in cell proliferation, upregulation of apoptosis, and reduced tumorigenic ability in a variety of tumor cell lines carrying wild-type but not mutant p53 (58-61). Notably, no significant weight loss or any other gross abnormalities is observed in the mice undergoing Nutlin-3 or MI-219 treatment (58, 61). Another small molecule RITA (Figure 2) binds the p53 N-terminus instead of Mdm2 and blocks its interaction with Mdm2 (62). Treatment of cells with RITA results in similar effects as treatment with Nutlins or MI-219 (62). A novel strategy using stapled peptides is also applied to block interaction between Mdm2 and p53 (63). These small synthetic peptides, locked into certain folded shapes by an all-hydrocarbon crosslink, are optimized for protein interaction and are resistant to proteolysis (63). Stapled peptides SAH-p53s (Figure 2) compete with p53 for Mdm2 binding, reactivate p53, and lead to apoptosis in cancer cells with high levels of Mdm2 (63). Yang et al demonstrated that HLI98 (Figure 2), a family of small molecules inhibiting Mdm2, could bind Mdm2 at the C-terminus and compromise its E3 ubiquitin ligase activity (64). HLI98 treatment suppresses degradation of p53 and induces p53-dependent apoptosis in tumor cells (64). A different small molecule, JNJ-26854165 (Figure 2), reactivates p53 by inhibiting Mdm2 E3 activity and inhibits tumor growth in mouse xenografts modeling multiple types of human cancers (65). Since p53-independent G2 arrest was observed in tumor cells upon HLI98 treatment (64), small molecules that bind the Mdm2 RING domain may also inhibit p53-independent functions of Mdm2.
Figure 2. Schematic representation of the targeting sites of Mdm protein antagonists on Mdm2, Mdm4 and p53.
BDP: benzodiazepinedione; MI: MI-63/219; Stapled: SAH-p53 Stapled peptides; JNJ: JNJ-26854165; SJ: SJ-172550; p53 Binding: p53 binding domain; Acidic: Acidic domain; Zn Finger: Zn finger domain; RING: RING domain; TA: transactivation domain; PR: proline-rich domain; DNA Binding: DNA binding domain; oligo: oligomerization domain.
The small molecule inhibitors of Mdm2 described above show very low affinity for Mdm4 binding. But high levels of Mdm4 attenuate the apoptotic response to the small molecules targeting Mdm2-p53 interaction e.g. Nutlin-3a and MI-219 in tumor cells (57, 61, 66, 67). Given the importance of Mdm4 in inhibition of p53 and its presence at high levels in a variety of human tumors such as retinoblastomas and melanomas, efforts have recently been dedicated to the development of Mdm4 inhibitors. A small molecule SJ-172550 (Figure 2) targets p53-Mdm4 binding (68). A benzofuroxan derivative XI-006 suppresses the transcription of Mdm4 (69). Both induce p53-dependent apoptosis, and act additively with Nutlin-3a in human cancer cell lines, which retain wild-type p53 but overexpress Mdm4 (68, 69). These observations suggest that an inhibitor against both Mdm2 and Mdm4 may promise a greater potential for anti-tumor activities. A stapled peptide SAH-p53-8 (Figure 2) binds both Mdm2 and Mdm4 and disrupts their interaction with p53 (67). Treatment of SAH-p53-8 reduces the viability of wild-type p53-carrying cancer cells that also contain high levels of Mdm2 and/or Mdm4 proteins (67). Of note, wild-type p53-carrying cancer cells with high levels of Mdm4 show minimal response to Nutlin-3 treatment (67). Recently, a dual Mdm2/Mdm4 antagonist RO-5963 (Figure 2) was identified that blocks p53 interaction with both Mdm2 and Mdm4 by inducing formation of homo- or hetero-dimers between Mdm proteins through their p53-binding domains (70). In the p53 wild-type but not mutant tumor cells, the dual antagonist induces apoptotic activity even in the presence of high levels of Mdm4, while Nutlin-3a induces a much weaker apoptotic response (70).
To date, a phase I clinical study of JNJ-26854165 (NCT00676910) to determine safety and dosing in patients with advanced stage or refractory solid tumors has been completed. Although JNJ-26854165 was well tolerated in these patients, no objective responses were observed (71). Several phase I studies of a Nutlin family member RG7112 in patients with liposarcomas (prior to debulking surgery), solid tumors, hematologic neoplasms and soft tissue sarcomas (NCT01143740, NCT01164033, NCT00559533, NCT00623870 and NCT01605526) have been completed or are in progress. Acceptable safety profiles and favorable clinical outcomes including complete remission, induction of apoptosis and decrease in proliferation were seen in patients treated with RG7112 for leukemias, lymphomas, sarcomas and other solid tumors (72-76). Further studies are needed to verify the efficacy of this agent long term in cancer treatment.
Despite these initial outcomes, concerns arise for potential adverse effects of Mdm2/Mdm4 inhibitor treatment of cancer patients. Genetically restoring p53 activity in mice in the absence of Mdm2 leads to ablation of the classically radiosensitive tissues including bone marrow, spleen, thymus and intestine, which results in 100% fatality of mice within 5-6 days (77). In addition, mice heterozygous for Mdm2 or Mdm4, although normal, are sensitive to low dose IR treatment (78), suggesting that the haploinsufficiency of Mdm2 and Mdm4 in response to DNA damage is detrimental for the organism. These two observations above question whether p53 activated by Mdm2/Mdm4 inhibitors will be toxic to normal tissues especially under stressed conditions. Xenograft models indicate that the tumor-suppressing doses of Nutlin-3, MI-219 and SAH-p53-8 are well tolerated in mice, leading to no significant weight loss or any gross abnormalities (58, 61, 67). Moreover, the treatment with Nutlin-3 in combination of the therapeutic DNA-damaging agent topotecan results in regression of retinoblastomas without any obvious side-effects in mice (56), implying that Mdm protein inhibitors may not be toxic to normal tissues even when combined with other therapeutic agents in patients. However, these data should be cautiously interpreted, as these preclinical studies in mice were short-term ranging from 6 days to 20 days. It still needs to be examined whether prolonged administration of Mdm inhibitors together with other anti-tumor agents will be detrimental to patients.
Other studies have demonstrated the interaction of Mdm2 with mutant p53 (79, 80). Disruption of this interaction causes stabilization of mutant p53 (79, 80), which has been shown to have gain-of-function metastatic activities (81). Treatment with Mdm2 inhibitors might lead to unfavorable outcomes in patients with even one allele of mutant p53. Therefore, it is extremely important that Mdm2 inhibitor treatment should be given to patients with tumors carrying only wild-type and not mutant p53. Moreover, Nutlin-3 treatment of SJSA-1 tumor cells (p53 wild-type) leads to selection for p53 mutations and subsequent resistance to Nutlin-3 treatment (82). In addition, selected p53 mutants may lead to more adverse effects, if mutant p53 accumulates and exerts gain-of-function activities in the resistant cells. Thus, combinational therapies and inter-treatment evaluation of p53 status should be a standard of care for cancer patients administered Mdm inhibitors. Lastly, accumulation of Mdm2 proteins are observed when Mdm2/Mdm4 inhibitors are applied to normal and tumor cells (58-60, 62, 64, 66, 69, 70), and accumulated Mdm2 can execute p53-independent oncogenic functions as discussed in this review. Therefore, administration of Mdm2/Mdm4 inhibitors to cancer patients might induce oncogenic side-effects, which may be evident only after a long latency. Thus, studies with long-term follow-up will be necessary to evaluate the safety of these inhibitors.
Concluding Remarks
Studies of Mdm2 and Mdm4 have long been focused on their roles as the negative regulators of the p53 tumor suppressor. Small molecules and synthetic peptides antagonizing Mdm2 and/or Mdm4 reactivate p53 in preclinical studies and have produced favorable outcomes in cancer patients in early-phase clinical trials. And yet, accumulating evidence has implicated p53-independent functions of Mdm2 and Mdm4, which accelerate tumorigenesis and tumor progression. However, it remains largely unclear whether inhibiting the binding of Mdm proteins to p53 compromises their p53-independent functions. Therefore, mouse models carrying mutations that will abrogate or enhance the p53-independent functions of Mdm proteins may serve as valuable tools to examine the relationship between these functions and tumor phenotypes. Furthermore, drugs targeting these p53-independent activities of Mdm2 and Mdm4 may potentiate the effectiveness of pharmaceuticals targeting Mdm2 and Mdm4 to reactivate p53 in cancer treatment.
Acknowledgements
We are thankful to Drs. Shunbin Xiong and Vinod Pant for critical reading of the manuscript. These studies are supported by NIH grant CA47296 to G.L. and M. D. Anderson CPRIT training grant and Hearst Foundation Scholarship to Q.L.
Footnotes
Authorship
Contribution: G.L. outlined the review; Q.L. and G.L. wrote the manuscript.
Conflict-of-Interest Disclosure: The authors declare no competing financial interests.
References
- 1.Marine JC, Francoz S, Maetens M, Wahl G, Toledo F, Lozano G. Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell death and differentiation. 2006;13:927–34. doi: 10.1038/sj.cdd.4401912. [DOI] [PubMed] [Google Scholar]
- 2.Fakharzadeh SS, Trusko SP, George DL. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. The EMBO journal. 1991;10:1565–9. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–60. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 4.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–45. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 5.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–8. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 6.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–6. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 7.Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Molecular and cellular biology. 2006;26:192–8. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong S, Van Pelt CS, Elizondo-Fraire AC, Liu G, Lozano G. Synergistic roles of Mdm2 and Mdm4 for p53 inhibition in central nervous system development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3226–31. doi: 10.1073/pnas.0508500103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francoz S, Froment P, Bogaerts S, De Clercq S, Maetens M, Doumont G, et al. Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3232–7. doi: 10.1073/pnas.0508476103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. The EMBO journal. 1996;15:5349–57. [PMC free article] [PubMed] [Google Scholar]
- 11.Shvarts A, Bazuine M, Dekker P, Ramos YF, Steegenga WT, Merckx G, et al. Isolation and identification of the human homolog of a new p53-binding protein, Mdmx. Genomics. 1997;43:34–42. doi: 10.1006/geno.1997.4775. [DOI] [PubMed] [Google Scholar]
- 12.Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. The Journal of biological chemistry. 2000;275:8945–51. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 13.Pant V, Xiong S, Iwakuma T, Quintas-Cardama A, Lozano G. Heterodimerization of Mdm2 and Mdm4 is critical for regulating p53 activity during embryogenesis but dispensable for p53 and Mdm2 stability. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11995–2000. doi: 10.1073/pnas.1102241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L, Yan Z, Liao X, Li Y, Yang J, Wang ZG, et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12001–6. doi: 10.1073/pnas.1102309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Jochemsen AG, et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nature genetics. 2001;29:92–5. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- 16.Finch RA, Donoviel DB, Potter D, Shi M, Fan A, Freed DD, et al. mdmx is a negative regulator of p53 activity in vivo. Cancer research. 2002;62:3221–5. [PubMed] [Google Scholar]
- 17.Migliorini D, Lazzerini Denchi E, Danovi D, Jochemsen A, Capillo M, Gobbi A, et al. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Molecular and cellular biology. 2002;22:5527–38. doi: 10.1128/MCB.22.15.5527-5538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong S, Van Pelt CS, Elizondo-Fraire AC, Fernandez-Garcia B, Lozano G. Loss of Mdm4 results in p53-dependent dilated cardiomyopathy. Circulation. 2007;115:2925–30. doi: 10.1161/CIRCULATIONAHA.107.689901. [DOI] [PubMed] [Google Scholar]
- 19.Valentin-Vega YA, Okano H, Lozano G. The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell death and differentiation. 2008;15:1772–81. doi: 10.1038/cdd.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentin-Vega YA, Box N, Terzian T, Lozano G. Mdm4 loss in the intestinal epithelium leads to compartmentalized cell death but no tissue abnormalities. Differentiation; research in biological diversity. 2009;77:442–9. doi: 10.1016/j.diff.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maetens M, Doumont G, Clercq SD, Francoz S, Froment P, Bellefroid E, et al. Distinct roles of Mdm2 and Mdm4 in red cell production. Blood. 2007;109:2630–3. doi: 10.1182/blood-2006-03-013656. [DOI] [PubMed] [Google Scholar]
- 22.Marine J-C. Chapter 3 - MDM2 and MDMX in Cancer and Development. In: Michael AD, editor. Current Topics in Developmental Biology. Academic Press; 2011. pp. 45–75. [DOI] [PubMed] [Google Scholar]
- 23.Steinman HA, Hoover KM, Keeler ML, Sands AT, Jones SN. Rescue of Mdm4-deficient mice by Mdm2 reveals functional overlap of Mdm2 and Mdm4 in development. Oncogene. 2005;24:7935–40. doi: 10.1038/sj.onc.1208930. [DOI] [PubMed] [Google Scholar]
- 24.Jones SN, Hancock AR, Vogel H, Donehower LA, Bradley A. Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15608–12. doi: 10.1073/pnas.95.26.15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nature reviews Molecular cell biology. 2010;11:220–8. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 26.Bouska A, Eischen CM. Murine double minute 2: p53-independent roads lead to genome instability or death. Trends in biochemical sciences. 2009;34:279–86. doi: 10.1016/j.tibs.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nature reviews Molecular cell biology. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alt JR, Bouska A, Fernandez MR, Cerny RL, Xiao H, Eischen CM. Mdm2 binds to Nbs1 at sites of DNA damage and regulates double strand break repair. The Journal of biological chemistry. 2005;280:18771–81. doi: 10.1074/jbc.M413387200. [DOI] [PubMed] [Google Scholar]
- 29.Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes & development. 2000;14:2393–409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- 30.Manning AL, Dyson NJ. pRB, a tumor suppressor with a stabilizing presence. Trends in cell biology. 2011;21:433–41. doi: 10.1016/j.tcb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin K, Trouche D, Hagemeier C, Sorensen TS, La Thangue NB, Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995;375:691–4. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- 32.Begus-Nahrmann Y, Lechel A, Obenauf AC, Nalapareddy K, Peit E, Hoffmann E, et al. p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nature genetics. 2009;41:1138–43. doi: 10.1038/ng.426. [DOI] [PubMed] [Google Scholar]
- 33.Barboza JA, Liu G, Ju Z, El-Naggar AK, Lozano G. p21 delays tumor onset by preservation of chromosomal stability. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19842–7. doi: 10.1073/pnas.0606343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nature cell biology. 2008;10:138–48. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu L, Zhu N, Zhang H, Durden DL, Feng Y, Zhou M. Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer cell. 2009;15:363–75. doi: 10.1016/j.ccr.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araki S, Eitel JA, Batuello CN, Bijangi-Vishehsaraei K, Xie XJ, Danielpour D, et al. TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. The Journal of clinical investigation. 2010;120:290–302. doi: 10.1172/JCI39194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JY, Zong CS, Xia W, Wei Y, Ali-Seyed M, Li Z, et al. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Molecular and cellular biology. 2006;26:7269–82. doi: 10.1128/MCB.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin Y, Zeng SX, Sun XX, Lee H, Blattner C, Xiao Z, et al. MDMX promotes proteasomal turnover of p21 at G1 and early S phases independently of, but in cooperation with, MDM2. Molecular and cellular biology. 2008;28:1218–29. doi: 10.1128/MCB.01198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matijasevic Z, Steinman HA, Hoover K, Jones SN. MdmX promotes bipolar mitosis to suppress transformation and tumorigenesis in p53-deficient cells and mice. Molecular and cellular biology. 2008;28:1265–73. doi: 10.1128/MCB.01108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strachan GD, Jordan-Sciutto KL, Rallapalli R, Tuan RS, Hall DJ. The E2F-1 transcription factor is negatively regulated by its interaction with the MDMX protein. Journal of cellular biochemistry. 2003;88:557–68. doi: 10.1002/jcb.10318. [DOI] [PubMed] [Google Scholar]
- 41.Wunderlich M, Ghosh M, Weghorst K, Berberich SJ. MdmX represses E2F1 transactivation. Cell Cycle. 2004;3:472–8. [PubMed] [Google Scholar]
- 42.Crosby ME, Almasan A. Opposing roles of E2Fs in cell proliferation and death. Cancer biology & therapy. 2004;3:1208–11. doi: 10.4161/cbt.3.12.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanelle J, Putzer BM. E2F1-induced apoptosis: turning killers into therapeutics. Trends in molecular medicine. 2006;12:177–85. doi: 10.1016/j.molmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Markey MP. Regulation of MDM4. Frontiers in bioscience : a journal and virtual library. 2011;16:1144–56. doi: 10.2741/3780. [DOI] [PubMed] [Google Scholar]
- 45.Onel K, Cordon-Cardo C. MDM2 and prognosis. Molecular cancer research: MCR. 2004;2:1–8. [PubMed] [Google Scholar]
- 46.Patterson H, Barnes D, Gill S, Spicer J, Fisher C, Thomas M, et al. Amplification and Over-Expression of the MDM2 Gene in Human Soft Tissue Tumours. Sarcoma. 1997;1:17–22. doi: 10.1080/13577149778434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley MF, Lozano G. The Many Faces of MDM2 Binding Partners. Genes & Cancer. 2012 doi: 10.1177/1947601912455322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilkes DM, Pan Y, Coppola D, Yeatman T, Reuther GW, Chen J. Regulation of MDMX expression by mitogenic signaling. Molecular and cellular biology. 2008;28:1999–2010. doi: 10.1128/MCB.01633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mancini F, Di Conza G, Moretti F. MDM4 (MDMX) and its Transcript Variants. Current genomics. 2009;10:42–50. doi: 10.2174/138920209787581280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundgren K, Montes de Oca Luna R, McNeill YB, Emerick EP, Spencer B, Barfield CR, et al. Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes & development. 1997;11:714–25. doi: 10.1101/gad.11.6.714. [DOI] [PubMed] [Google Scholar]
- 51.Ganguli G, Abecassis J, Wasylyk B. MDM2 induces hyperplasia and premalignant lesions when expressed in the basal layer of the epidermis. The EMBO journal. 2000;19:5135–47. doi: 10.1093/emboj/19.19.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alkhalaf M, Ganguli G, Messaddeq N, Le Meur M, Wasylyk B. MDM2 overexpression generates a skin phenotype in both wild type and p53 null mice. Oncogene. 1999;18:1419–34. doi: 10.1038/sj.onc.1202448. [DOI] [PubMed] [Google Scholar]
- 53.Xiong S, Pant V, Suh YA, Van Pelt CS, Wang Y, Valentin-Vega YA, et al. Spontaneous tumorigenesis in mice overexpressing the p53-negative regulator Mdm4. Cancer research. 2010;70:7148–54. doi: 10.1158/0008-5472.CAN-10-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Post SM, Quintas-Cardama A, Pant V, Iwakuma T, Hamir A, Jackson JG, et al. A high-frequency regulatory polymorphism in the p53 pathway accelerates tumor development. Cancer cell. 2010;18:220–30. doi: 10.1016/j.ccr.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C, et al. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444:61–6. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- 57.Gembarska A, Luciani F, Fedele C, Russell EA, Dewaele M, Villar S, et al. MDM4 is a key therapeutic target in cutaneous melanoma. Nature medicine. 2012;18:1239–47. doi: 10.1038/nm.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 59.Grasberger BL, Lu T, Schubert C, Parks DJ, Carver TE, Koblish HK, et al. Discovery and cocrystal structure of benzodiazepinedione HDM2 antagonists that activate p53 in cells. Journal of medicinal chemistry. 2005;48:909–12. doi: 10.1021/jm049137g. [DOI] [PubMed] [Google Scholar]
- 60.Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, et al. Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2-p53 interaction. Journal of medicinal chemistry. 2006;49:3432–5. doi: 10.1021/jm051122a. [DOI] [PubMed] [Google Scholar]
- 61.Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3933–8. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nature medicine. 2004;10:1321–8. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 63.Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL. Reactivation of the p53 tumor suppressor pathway by a stapled p53 peptide. Journal of the American Chemical Society. 2007;129:2456–7. doi: 10.1021/ja0693587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Ludwig RL, Jensen JP, Pierre SA, Medaglia MV, Davydov IV, et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer cell. 2005;7:547–59. doi: 10.1016/j.ccr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 65.Arts J, Page M, Valckx A, Blattner C, Kulikov R, Floren W, et al. JNJ-26854165 - a novel hdm2 antagonist in clinical development showing broad-spectrum preclinical antitumor activity against solid malignancies. AACR Meeting Abstracts. 2008;2008:1592. [Google Scholar]
- 66.Patton JT, Mayo LD, Singhi AD, Gudkov AV, Stark GR, Jackson MW. Levels of HdmX expression dictate the sensitivity of normal and transformed cells to Nutlin-3. Cancer research. 2006;66:3169–76. doi: 10.1158/0008-5472.CAN-05-3832. [DOI] [PubMed] [Google Scholar]
- 67.Bernal F, Wade M, Godes M, Davis TN, Whitehead DG, Kung AL, et al. A stapled p53 helix overcomes HDMX-mediated suppression of p53. Cancer cell. 2010;18:411–22. doi: 10.1016/j.ccr.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reed D, Shen Y, Shelat AA, Arnold LA, Ferreira AM, Zhu F, et al. Identification and characterization of the first small molecule inhibitor of MDMX. The Journal of biological chemistry. 2010;285:10786–96. doi: 10.1074/jbc.M109.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Ma X, Ren S, Buolamwini JK, Yan C. A small-molecule inhibitor of MDMX activates p53 and induces apoptosis. Molecular cancer therapeutics. 2011;10:69–79. doi: 10.1158/1535-7163.MCT-10-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graves B, Thompson T, Xia M, Janson C, Lukacs C, Deo D, et al. Activation of the p53 pathway by small-molecule-induced MDM2 and MDMX dimerization. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11788–93. doi: 10.1073/pnas.1203789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabernero J, Dirix L, Schoffski P, Cervantes A, Capdevila J, Baselga J, et al. Phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of HDM-2 antagonist JNJ-26854165 in patients with advanced refractory solid tumors. Journal of Clinical Oncology. 2009:27. [Google Scholar]
- 72.Andreeff M, Kojima K, Padmanabhan S, Strair R, Kirschbaum M, Maslak PG, et al. A Multi-Center, Open-Label, Phase I Study of Single Agent RG7112, A First In Class p53-MDM2 Antagonist, In Patients with Relapsed/Refractory Acute Myeloid and Lymphoid Leukemias (AML/ALL) and Refractory Chronic Lymphocytic Leukemia/Small Cell Lymphocytic Lymphomas (CLL/SCLL); 52nd ASH Annual Meeting and Exposition; 2010. [Google Scholar]
- 73.Andreeff M, Kojima K, Ruvolo V, Younes A, Wei W, Konopleva M, et al. Pharmacodynamic Biomarkers in the Phase 1 Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia; 53rd ASH Annual Meeting and Exposition; 2011. [Google Scholar]
- 74.Ray-Coquard IL, Blay J, Italiano A, Cesne AL, Penel N, Zhi J, et al. Neoadjuvant MDM2 antagonist RG7112 for well-differentiated and dedifferentiated liposarcomas (WD/DD LPS): A pharmacodynamic (PD) biomarker study. Journal of Clinical Oncology. 2011:29. [Google Scholar]
- 75.Beryozkina A, Nichols GL, Reckner M, Vassilev LT, Rueger R, Jukofsky L, et al. Pharmacokinetics (PK) and pharmacodynamics (PD) of RG7112, an oral murine double minute 2 (MDM2) antagonist, in patients with leukemias and solid tumors. Journal of Clinical Oncology. 2011:29. [Google Scholar]
- 76.Kurzrock R, Blay J-Y, Nguyen BB, Wagner AJ, Maki RG, Schwartz GK, et al. A phase I study of MDM2 antagonist RG7112 in patients (pts) with relapsed/refractory solid tumors. Journal of Clinical Oncology. 2012:29. [Google Scholar]
- 77.Ringshausen I, O’Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer cell. 2006;10:501–14. doi: 10.1016/j.ccr.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Terzian T, Wang Y, Van Pelt CS, Box NF, Travis EL, Lozano G. Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development. Molecular and cellular biology. 2007;27:5479–85. doi: 10.1128/MCB.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes & development. 2008;22:1337–44. doi: 10.1101/gad.1662908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lukashchuk N, Vousden KH. Ubiquitination and degradation of mutant p53. Molecular and cellular biology. 2007;27:8284–95. doi: 10.1128/MCB.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lozano G. The oncogenic roles of p53 mutants in mouse models. Current opinion in genetics & development. 2007;17:66–70. doi: 10.1016/j.gde.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 82.Aziz MH, Shen H, Maki CG. Acquisition of p53 mutations in response to the non-genotoxic p53 activator Nutlin-3. Oncogene. 2011;30:4678–86. doi: 10.1038/onc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]