Abstract
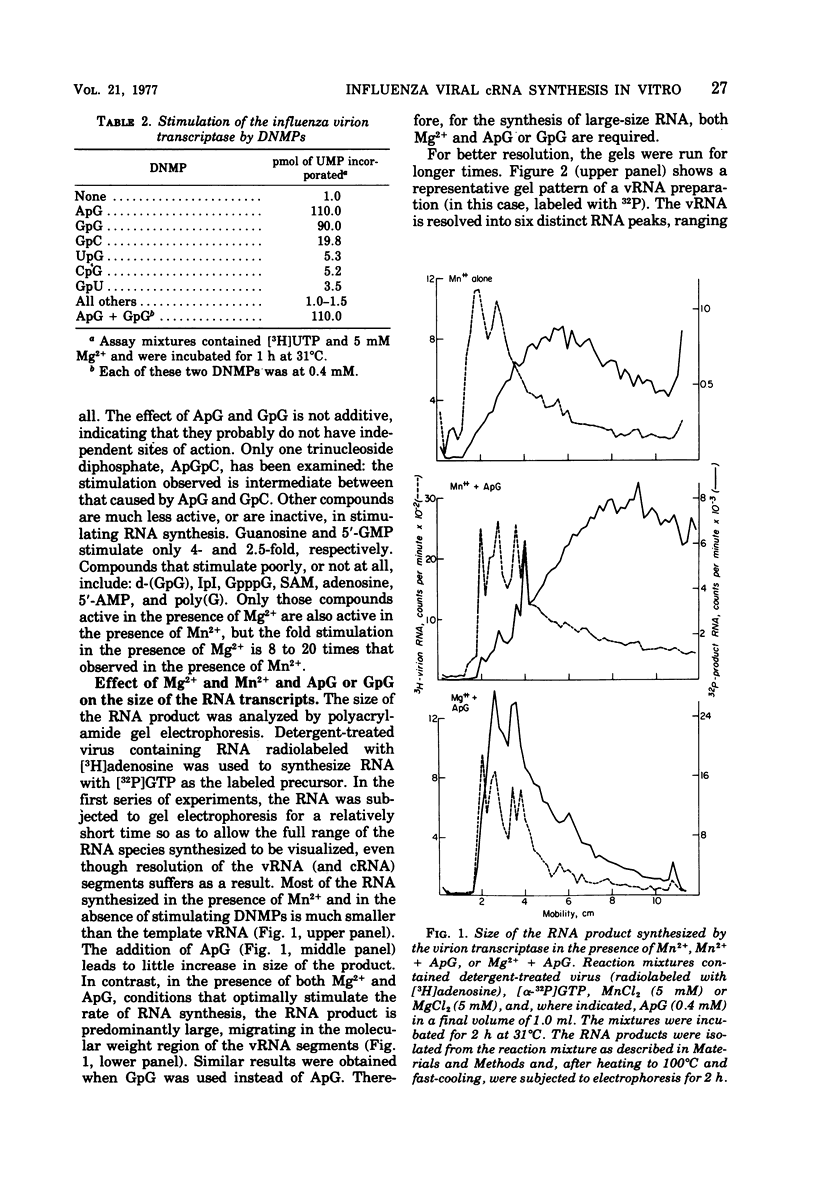
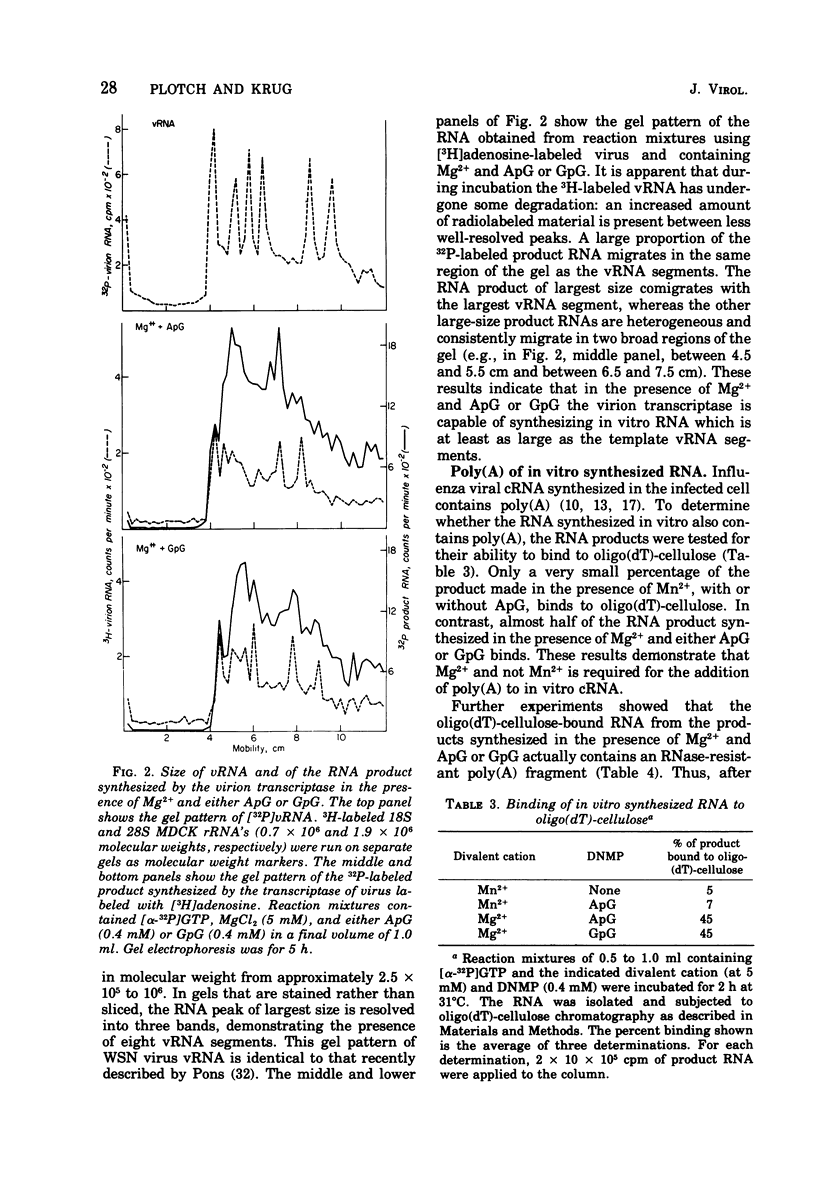
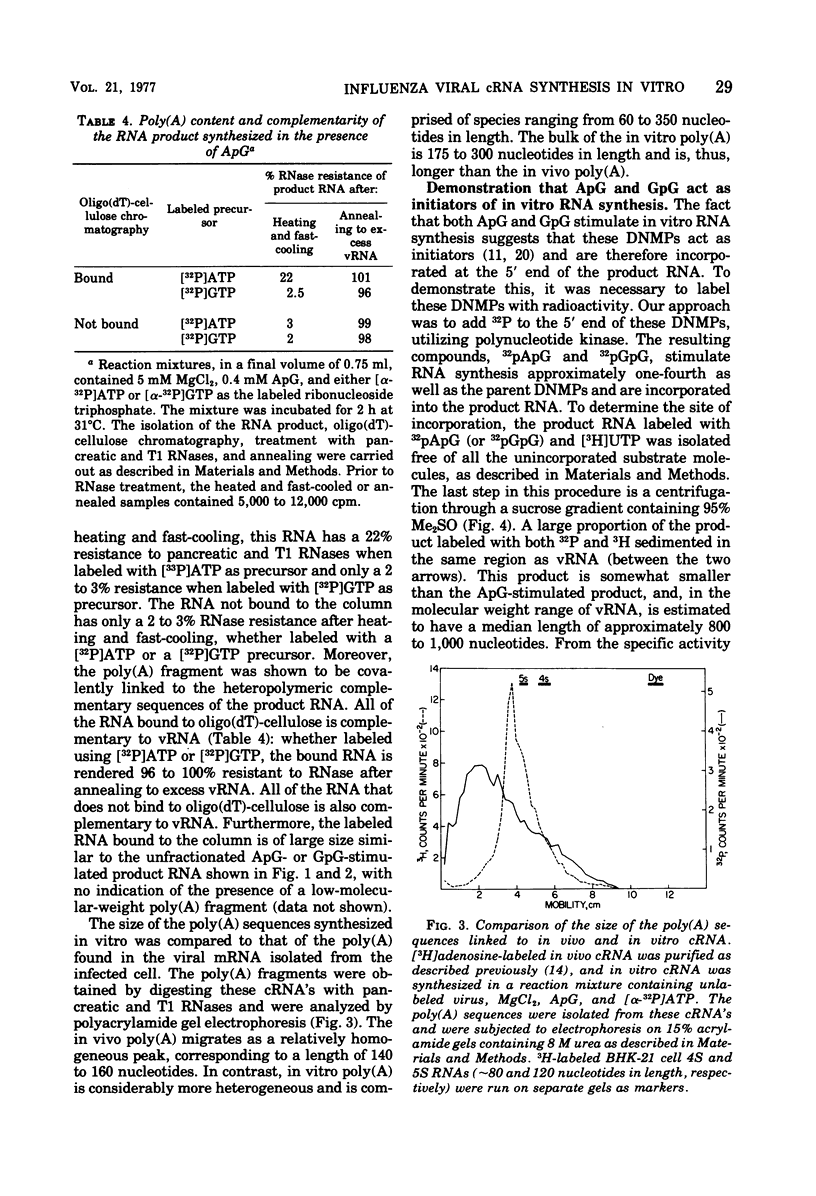
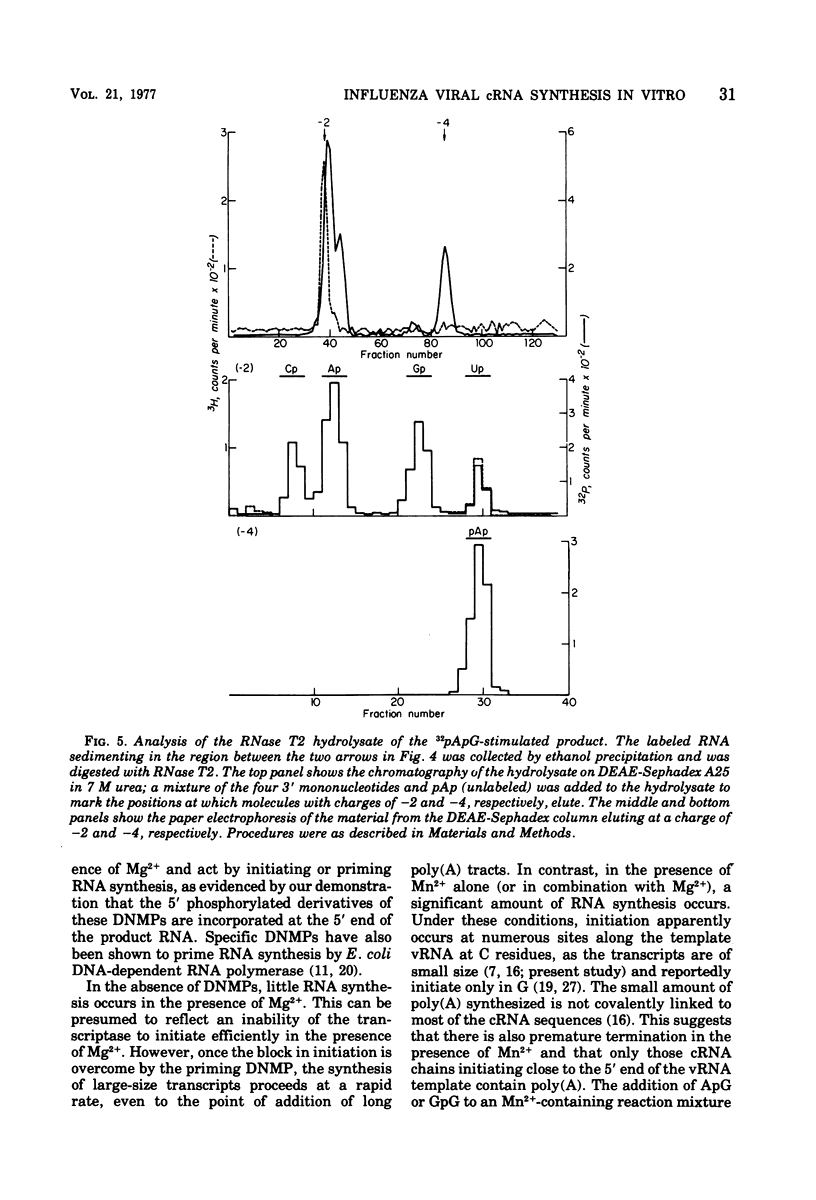
The influenza virion transcriptase is capable of synthesizing in vitro complementary RNA (cRNA) that is similar in several characteristics to the cRNA synthesized in the infected cell, which is the viral mRNA. Most of the in vitro cRNA is large (approximately 2.5 X 10(5) to 10(6) daltons), similar in size to in vivo cRNA. The in vitro transcripts initiate in adenosine (A) or guanosine (G) at the 5' end, as also appears to be the case with in vivo cRNA (R.M. Krug et al., 1976). The in vitro transcripts contain covalently linked polyadenylate [poly(A)] sequences, which are longer and more heterogeneous than the poly(A) sequences found on in vivo cRNA. The synthesis in vitro of cRNA with these characteristics requires both the proper divalent cation, Mg2+, and a specific dinulceside monophosphage (DNMP), ApG or GpG. These DNMPs stimulate cRNA synthesis about 100-fold in the presence of Mg2+ and act as primers to initiate RNA chains, as demonstrated by the fact that the 5'-phosphorylated derivatives of these DNMP's, 32pApG or 32pGpG, are incroporated at the 5' end of the product RNA. The RNA synthesized in vitro differs from in vivo cRNA in that neither capping nor methylation of the in vitro transcripts has been detected. The virion does contain a methylase activity, as shown by its ability to methylate exogenous methyl-deficient Escherichia coli tRNA.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. The nature of the RNA products synthesized in vitro by subviral components of visicular stomatitis virus. Virology. 1976 May;71(1):230–241. doi: 10.1016/0042-6822(76)90108-2. [DOI] [PubMed] [Google Scholar]
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Primary transcription of the influenza virus genome in permissive cells. Virology. 1973 Dec;56(2):646–651. doi: 10.1016/0042-6822(73)90067-6. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Transcriptase activity and genome composition of defective influenza virus. J Virol. 1976 Apr;18(1):365–369. doi: 10.1128/jvi.18.1.365-369.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. II. Nature of the in vitro polymerase product. J Virol. 1971 Jul;8(1):74–80. doi: 10.1128/jvi.8.1.74-80.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow N. L., Simpson R. W. RNA-dependent RNA polymerase activity associated with virions and subviral particles of myxoviruses. Proc Natl Acad Sci U S A. 1971 Apr;68(4):752–756. doi: 10.1073/pnas.68.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Stone H. O. Isolation of a transcriptive complex from Newcastle disease virions. J Virol. 1976 Sep;19(3):1080–1089. doi: 10.1128/jvi.19.3.1080-1089.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J. Cell-free translation of influenza virus mRNA. J Virol. 1976 May;18(2):604–618. doi: 10.1128/jvi.18.2.604-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey K. M., Jurmark B. S., So A. G. Determination of nucleotide sequences at promoter regions by the use of dinucleotides. Biochemistry. 1971 Dec 21;10(26):4970–4975. doi: 10.1021/bi00802a021. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Martin S. A., Paoletti E., Moss B. Modification of the 5'-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Influenza viral messenger RNA. Virology. 1974 Nov;62(1):38–45. doi: 10.1016/0042-6822(74)90301-8. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghendon Y., Blagoveshienskaya O. Polyadenylate sequences of fowl plague virus complementary RNA (cRNA) synthesized in vivo and in vitro. Virology. 1975 Dec;68(2):330–337. doi: 10.1016/0042-6822(75)90276-7. [DOI] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomatos P. J. Reovirus-specific, single-stranded RNA's synthesized in vitro with enzyme purified from reovirus-infected cells. J Mol Biol. 1968 Nov 14;37(3):423–439. doi: 10.1016/0022-2836(68)90112-5. [DOI] [PubMed] [Google Scholar]
- Hoffman D. J., Niyogi S. K. RNA initiation with dinucleoside monophosphates during transcription of bacteriophage T4 DNA with RNA polymerase of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):574–578. doi: 10.1073/pnas.70.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M. Cytoplasmic and nucleoplasmic viral RNPs in influenza virus-infected MDCK cells. Virology. 1972 Oct;50(1):103–113. doi: 10.1016/0042-6822(72)90350-9. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Etkind P. R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973 Nov;56(1):334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Influenza viral RNPs newly synthesized during the latent period of viral growth in MDCK cells. Virology. 1971 Apr;44(1):125–136. doi: 10.1016/0042-6822(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Morgan M. A., Shatkin A. J. Influenza viral mRNA contains internal N6-methyladenosine and 5'-terminal 7-methylguanosine in cap structures. J Virol. 1976 Oct;20(1):45–53. doi: 10.1128/jvi.20.1.45-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski L. J., Content J., Leppla S. H. Characterization of the subunit structure of the ribonucleic acid genome of influenza virus. J Virol. 1971 Nov;8(5):701–707. doi: 10.1128/jvi.8.5.701-707.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Brass L. F., Doty P. A comparison of the binding to polynucleotides of complementary and noncomplementary oligonucleotides. Biochemistry. 1975 Jul 15;14(14):3164–3171. doi: 10.1021/bi00685a020. [DOI] [PubMed] [Google Scholar]
- McGeoch D., Kitron N. Influenza virion RNA-dependent RNA polymerase: stimulation by guanosine and related compounds. J Virol. 1975 Apr;15(4):686–695. doi: 10.1128/jvi.15.4.686-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Grubman M. J., Ehrenfeld E., Banerjee A. K. Studies on the in vivo and in vitro messenger RNA species of vesicular stomatitis virus. Virology. 1975 Oct;67(2):463–473. doi: 10.1016/0042-6822(75)90447-x. [DOI] [PubMed] [Google Scholar]
- Penhoet E., Miller H., Doyle M., Blatti S. RNA-dependent RNA polymerase activity in influenza virions. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1369–1371. doi: 10.1073/pnas.68.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. A reexamination of influenza single-and double-stranded RNAs by gel electrophoresis. Virology. 1976 Feb;69(2):789–792. doi: 10.1016/0042-6822(76)90508-0. [DOI] [PubMed] [Google Scholar]
- Pons M. W. Studies on the replication of influenza virus RNA. Virology. 1972 Mar;47(3):823–832. doi: 10.1016/0042-6822(72)90574-0. [DOI] [PubMed] [Google Scholar]
- Pons M. W. The inhibition of influenza virus RNA synthesis by actinomycin D and cycloheximide. Virology. 1973 Jan;51(1):120–128. doi: 10.1016/0042-6822(73)90372-3. [DOI] [PubMed] [Google Scholar]
- Rott R., Scholtissek C. Specific inhibition of influenza replication by alpha-amanitin. Nature. 1970 Oct 3;228(5266):56–56. doi: 10.1038/228056a0. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Synthesis in vivo of influenza virus plus and minus strand RNA and its preferential inhibition by antibiotics. Virology. 1970 Apr;40(4):989–996. doi: 10.1016/0042-6822(70)90145-5. [DOI] [PubMed] [Google Scholar]
- Schuerch A. R., Mitchell W. R., Joklik W. K. Isolation of intact individual species of single- and double-stranded RNA after fractionation by polyacrylamide gel electrophoresis. Anal Biochem. 1975 May 12;65(1-2):331–345. doi: 10.1016/0003-2697(75)90517-5. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Both G. W. Reovirus mRNA: transcription and translation. Cell. 1976 Mar;7(3):305–313. doi: 10.1016/0092-8674(76)90159-8. [DOI] [PubMed] [Google Scholar]
- Skehel J. J. RNA-dependent RNA polymerase activity of the influenza virus. Virology. 1971 Sep;45(3):793–796. doi: 10.1016/0042-6822(71)90197-8. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylated nucleotides block 5'-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



