Abstract
Aims/hypothesis
Type 1 diabetes results from a chronic autoimmune process continuing for years after presentation. We tested whether treatment with teplizumab (a Fc receptor non-binding anti-CD3 monoclonal antibody), after the new-onset period, affects the decline in C-peptide production in individuals with type 1 diabetes.
Methods
In a randomised placebo-controlled trial we treated 58 participants with type 1 diabetes for 4–12 months with teplizumab or placebo at four academic centres in the USA. A central randomisation centre used computer generated tables to allocate treatments. Investigators, patients, and caregivers were blinded to group assignment. The primary outcome was a comparison of C-peptide responses to a mixed meal after 1 year. We explored modification of treatment effects in subgroups of patients.
Results
Thirty-four and 29 subjects were randomized to the drug and placebo treated groups, respectively. Thirty-one and 27, respectively, were analysed. Although the primary outcome analysis showed a 21.7% higher C-peptide response in the teplizumab-treated group (0.45 vs 0.371; difference, 0.059 [95% CI 0.006, 0.115] nmol/l) (p=0.03), when corrected for baseline imbalances in HbA1c levels, the C-peptide levels in the teplizumab-treated group were 17.7% higher (0.44 vs 0.378; difference, 0.049 [95% CI 0, 0.108] nmol/l, p=0.09). A greater proportion of placebo-treated participants lost detectable C-peptide responses at 12 months (p=0.03). The teplizumab group required less exogenous insulin (p<0.001) but treatment differences in HbA1c levels were not observed. Teplizumab was well tolerated. A subgroup analysis showed that treatment benefits were larger in younger individuals and those with HgbA1c <6.5% at entry. Clinical responders to teplizumab had an increase in circulating CD8 central memory cells 2 months after enrolment compared with non-responders.
Conclusions/interpretations
This study suggests that deterioration in insulin secretion may be affected by immune therapy with teplizumab after the new-onset period but the magnitude of the effect is less than during the new-onset period. Our studies identify characteristics of patients most likely to respond to this immune therapy.
Trial registration
ClinicalTrials.gov NCT00378508
Funding
This work was supported by grants 2007-502, 2007-1059 and 2006-351 from the JDRF and grants R01 DK057846, P30 DK20495, UL1 RR024139, UL1RR025780, UL1 RR024131 and UL1 RR024134 from the NIH.
Keywords: Autoimmunity, Immune therapy, Type 1 diabetes
Introduction
Studies from the past 25 years have described type 1 diabetes as a chronic immune-mediated disease involving a progressive destruction of insulin-producing beta cells often beginning years before clinical presentation and continuing for years after diagnosis [1]. Clinical experience is consistent with a chronic rather than acute failure of beta cell function after the onset of disease. Many newly diagnosed patients show marked improvement in glucose tolerance and reduced insulin requirements [2, 3]. Indeed, experience from the DCCT suggests that for at least 2–3 years after clinical onset, many patients still retain significant levels of insulin production reflected by a stimulated C-peptide level of at least 0.2 nmol/l [4]. Retention of insulin production has been associated with improved prognosis including reduced rates of retinopathy, nephropathy, neuropathy and even hypoglycaemia [5, 6]. This suggests that treatments that are able to arrest the decline in insulin production, even after the new-onset period, may have clinical benefit [7, 8]. However, with time the majority of patients lose the ability to make insulin: from the DCCT, only 3% of participants diagnosed before the age of 18 years and 8% of participants diagnosed as adults retained clinically significant insulin production after 5 years.
Several questions remain about the optimal patients for immune intervention. Most studies have been performed in participants with new-onset type 1 diabetes, generally defined as within 100 days of diagnosis [9–14]. There are at least two reasons for this. First, since C-peptide levels decline with time the proportion of participants with clinically significant levels, who could potentially benefit from a therapy that arrests beta cell destruction, becomes smaller in participants with longer duration of type 1 diabetes [4]. Second, experimental evidence suggests that immune interventions are most effective when there are greater levels of residual insulin production or in the period closer to onset. Anti-CD3 monoclonal antibody (mAb) and ciclosporin treatment were shown to be most effective in those with higher levels of insulin production, and GAD65 immunisation was also most effective in participants treated within 6 months of diagnosis [10, 15, 16]. These observations imply that there may be a window of treatment opportunity in the new onset period related to the immunological events in the peridiagnosis period or to beta cell intrinsic factors. In addition, a previous study suggested that younger participants may show greater responses to immune therapy–a surprising finding since the rate of decline in younger participants is more rapid than in adults [14]. Data from the DCCT showed that glucose levels may affect beta cell function, yet HbA1c levels have largely been considered as an outcome rather than a modulator of immune response [17]. The relationship between glucose control and responses to immune therapy has not been evaluated.
We and others have previously shown that humanised FcR non-binding anti-CD3 mAb can arrest the decline in beta cell function when it is given to patients with type 1 diabetes in the new onset period [9, 10, 18]. We postulated that it may be possible to stop the decline in beta cell function in those with significant levels of C-peptide production, even after the first 100 days following diagnosis. We therefore conducted a randomised double-blind placebo-controlled study to determine whether teplizumab attenuates the decline in C-peptide response in patients with type 1 diabetes of 4–12 months duration.
Methods
Participants and study design
The study was designed as a randomised double-blind placebo-controlled trial with 1:1 randomisation to teplizumab or placebo infusions (NCT00378508). Patients recruited from four clinical sites (Yale University, University of California San Francisco, University of Colorado and Children’s Hospital of Philadelphia) were eligible if they were between the ages of 8 and 30 years and diagnosed with type 1 diabetes at least 4 but not more than 12 months before enrolment. The participants in the study all gave written informed consent and the investigations were approved by each institution’s institutional review board.
The diagnosis of type 1 diabetes was confirmed by clinical history and the presence of at least 1+ autoantibody (islet cell antibody [ICA], anti-GAD65 or anti-ICA512). To qualify for enrolment, individuals needed to have a single stimulated C-peptide level during a 4-h mixed meal tolerance test (MMTT) of at least 0.2 nmol/l, which has been deemed ‘clinically significant’ [4]. Randomisation was stratified by two categories for time from diagnosis: 4–8 months and 9–12 months. Participants and all study personnel were blinded to the treatment assignment.
All participants followed identical study procedures throughout the trial. They received a 14-day course of either intravenous teplizumab (day 1, 51μg/m2; day 2, 103 μg/m2; day 3, 206 μg/m2; day 4, 413 μg/m2; days 5–14, 826 μg/m2) or saline (154 mmol/l NaCl) as described previously [14]. Ibuprofen, diphenhydramine (Benadryl) or paracetamol (known as acetaminophen in the USA and Canada) were given for any infusion related reactions. The participants were instructed to continue their standard diabetes management and were seen in follow-up at intervals of 1–3 months during the year. To assess insulin secretion, 4 h MMTTs, with 11 sampling time points were performed at 6 and 12 months of follow-up as previously described [9, 10, 18]. The mean total daily insulin doses taken for 3 days before study visits was determined at each follow-up visit.
Laboratory tests
C-peptide and HbA1c levels were measured at the Northwest Research Laboratory (Seattle, WA, USA). Chemistries and complete blood counts (CBCs) and differentials were performed in local laboratories. Anti-insulin, anti-GAD65 and anti-IA-2 antibodies were measured at the Barbara Davis Diabetes Center (Aurora, CO, USA), ICA was measured at the University of Florida (Gainesville, FL, USA). Flow cytometry analysis of peripheral blood mononuclear cells (PBMCs) was performed in the Immune Monitoring Core at Yale University on samples shipped overnight and stained using methods described below.
Flow analysis
PBMCs were separated by Ficoll–Hypaque gradient centrifugation. The cells were stained with mAbs to CD4, CD8, CD45RA, CD45RO, CD69, CD127, FoxP3, CD25, CCR3, CCR4, Vα24 and dendritic and myeloid cell markers, and analysed on an LSRII cytometer (Becton Dickinson, San Jose, CA, USA). Subsets of CD4 and CD8 T cells were analysed with the following definitions: CD4 or CD8 central memory (CM) cells; CD45RO+CD62L+, CD4 or CD8 effector memory (EM) cells; CD45RO+CD62L−, naive cells; CD45RA+ [19]. For the analysis, electronic gates were placed on subpopulations of cells and the percentage of the indicated subset was analysed. The absolute number of cells was determined by multiplying the percentage of the subset × %CD4 or CD8 × absolute lymphocyte count that was obtained from the CBC performed in the clinical laboratory.
Statistical design
The pre-designated primary endpoint was a comparison of the area under the C-peptide secretory response curve (C-peptide AUC) from the MMTT at the 12-month follow-up visit. Secondary endpoints were percentage change in C-peptide response, insulin dose and HbA1c. The study was designed to identify a 40% difference in C-peptide AUC. A sample size of 30 participants per group was required for 80% power to detect this difference with a two-sided 0.05 significance level. The primary and secondary endpoints were compared in the modified intent to treat population, which included all participants with at least one post-randomisation MMTT.
A repeated measures linear mixed model was used to test for the effects of teplizumab on outcome variables following randomisation (i.e. at 6 and 12 months). The models included a random effect for participant and fixed effects for treatment, time and time from diagnosis (by stratum) as well as their interactions. Covariate adjustment was also made for the baseline outcome level. Linear contrasts were used to estimate treatment differences and perform hypothesis testing at individual time points. Because of the chance imbalance between the treatment arms at baseline, analyses were further corrected for the baseline HbA1c level. Exploratory subgroup analyses were done post-hoc and included the effects of duration strata, age (i.e. <15 years and ≥15 years, which represents the upper third of age distribution) and HbA1c (i.e. below diagnostic threshold of <6.5% [47.5 mmol/mol] and >6.5% [47.5 mmol/mol]) categories on the C-peptide endpoint. For statistical analyses, the mean C-peptide secretory response over 4 hrs (as nmol/l) and was calculated as: loge ([AUC of the C-peptide from the 240 min MMTT/240] +1) [12, 20]. For presentation, the C-peptide data were converted to the AUC as nmol/l. Comparisons between baseline characteristics were done by ANOVA and the Mantel–Haenszel χ2 test. Mean±SD, SEM, or 95% CI are reported as indicated. A p value of <0.05 was considered statistically significant.
Results
Participants
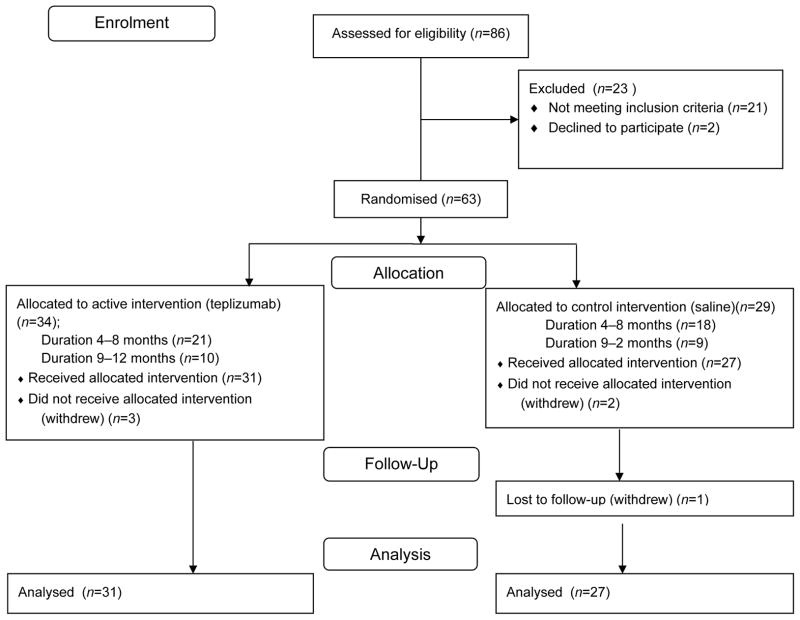
The baseline characteristics and enrolment of participants are shown in Table 1 and Fig. 1. Of 86 screened patients, 63 met eligibility criteria, 43 in the 4- to 8-month stratum 1 and 20 in the 9- to 12-month stratum 2. Five participants withdrew before the first dose of study drug. Two participants withdrew from the 4- to 8-month stratum after receiving the first dose of study drug: one following transient grade 3 neutropoenia and the other voluntarily withdrew before the MMTT at month 6. These two participants are included in the reporting of adverse events.
Table 1.
Patient characteristics
| Characteristic | All participants
|
Stratum 1 (4–8 months) (n=39)
|
Stratum 2 (9–12 months) (n=19)
|
|||
|---|---|---|---|---|---|---|
| Drug (n=31) | Placebo (n=27) | Drug (n=21) | Placebo (n=18) | Drug (n=10) | Placebo (n=9) | |
| Age (years)(±SD) | 12.9±4.18 | 12.0±5.2 | 12.4±3.76 | 10.9±4.24 | 13.4±5.09 | 14.0±5.43 |
| Age category (n) | ||||||
| < 15 yrs | 18 | 20 | 13 | 15 | 5 | 5 |
| ≥ 15 yrs | 13 | 7 | 8 | 3 | 5 | 4 |
| Sex (% male) | 52 | 63 | 62 | 56 | 40 | 44% |
| Baseline C-peptide AUC (nmol/l) (±SD) | 0.618±0.302 | 0.6±0.43 | 0.611±0.289 | 0.557±0.464 | 0.632±0.342 | 0.686±0.365 |
| Baseline insulin use (U kg−1 day−1) (±SD) | 0.405±0.141 | 0.386±0.197 | 0.415±0.111 | 0.423±0.178 | 0.384±0.197 | 0.312±0.222 |
| Duration of diabetes (months) | 7.09±2.45 | 7.14±2.44 | 5.61±1.01 | 5.83±1.27 | 10.4±1.13 | 9.76±2.1 |
| Baseline | 6.37±0.787*** | 7.14±1.21 | 6.28±0.709 | 7.34±1.17 | 6.55±0.94 | 6.62±1.22 |
| HbA1c (%)(mmol/m ol) (±SD) | (46.1±8.6) | (54.1±13.1) | (45.2±7.45)*** | (56.7±12.8) | (48.1±10.32) | (48.9±13.3) |
| Positive for anti-GAD65 antibody (%) | 74 | 55 | 70 | 90 | 80 | 63 |
| Positive for anti-IA-2 antibody (%) | 87 | 73 | 86 | 83 | 80 | 50 |
p<0.01
Fig. 1.
Enrolment and treatment of study patients. The Figure depicts the flow of patients through the protocol. The reasons for screen failures included insufficient stimulated C-peptide levels, absence of detectable autoantibodies, laboratory abnormalities meeting exclusion criteria, and others. Of the eligible participants, five withdrew before the first dose of study drug. Therefore, 58 participants were included in the endpoint analysis
The teplizumab and placebo groups had similar demographics at baseline. The average age was 12.4 (95% CI 11.3, 13.4) years and ranged from 8 to 26 years. Thirty-eight participants were between the ages of 8 and 14 years; the difference in age between the teplizumab and placebo groups was not statistically significant, either overall or in each duration stratum. Despite randomisation, the HbA1c levels were higher in the placebo group (p=0.003) (Table 1). Overall, 27 had HbA1c levels <6.5% (47.5mmol/mol) and 30 had HgbA1c levels >6.5% (47.5 mmol/mol).
Efficacy
In the a priori specified analysis of the pre-designated primary endpoint, the teplizumab group had C-peptide levels that were 21.7% higher than placebo (C-peptide AUC: 0.45 [95% CI 0.40, 0.51] nmol/l vs 0.37 [95% CI 0.32, 0.42] nmol/l, p=0.03). The teplizumab group also lost significantly less C-peptide at 12 months as a percentage of the C-peptide at baseline (18% [95% CI 7.43, 28.5] vs 39.0% [95% CI 27.8%, 50.2%], p=0.006). At 12 months, 5 of the 58 participants did not have detectable C-peptide (i.e. values during the MMTT below 0.03 nmol/l). All of these participants were in the placebo group (Fisher’s Exact test, p=0.02).
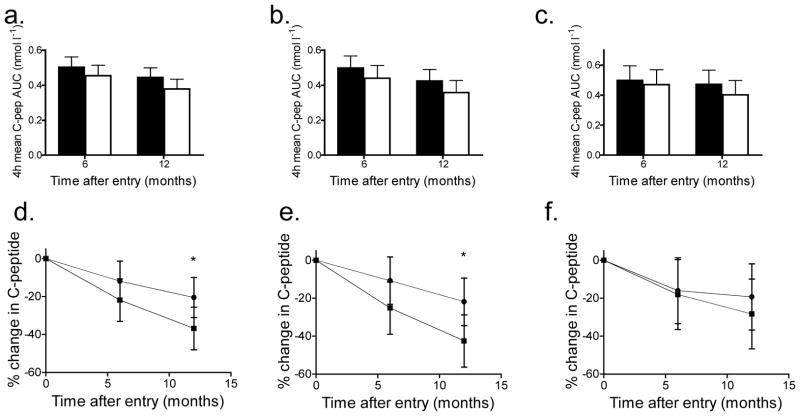
Since the teplizumab group had lower HbA1c levels at baseline we performed additional analyses adjusting for the baseline HbA1c. After adjustment, the teplizumab group had a C-peptide response that was 17.7% higher than that of the placebo group at 12 months (p=0.09) (Fig. 2a). Compared with baseline, the teplizumab group lost, on average, 20.6% (95% CI 10.0, 31.1) of the C-peptide responses whereas the placebo group lost 36.8% (95% CI 25.6, 48.0, p=0.04) when corrected for the baseline HbA1c and C-peptide levels (Fig. 2b).
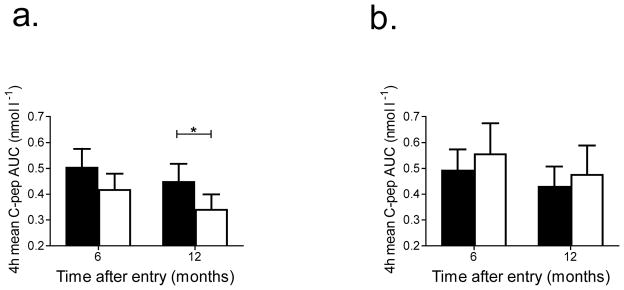
Fig. 2.
C-peptide responses to a MMTT in teplizumab- and placebo-treated participants. (a–c) The mean C-peptide AUC (±95% CI) in participants treated with teplizumab (black bars) and placebo (white bars). The C-peptide AUC at 6 and 12 months adjusting for baseline C-peptide, HbA1c levels and stratum are shown for all participants (a, teplizumab-treated, n=31; placebo-treated, n=27) and for those of diabetes duration 4–8 months (b, teplizumab-treated n=21, placebo-treated n=18) and 9–12 months (c, teplizumab-treated n=10, placebo-treated n=9). (d–f) The percentage change in C-peptide AUC from baseline (±95% CI) in participants treated with teplizumab (circles) and placebo (squares). The percentage change from baseline at months 6 and 12, adjusted for baseline C-peptide, baseline HbA1c level and stratum is shown for all participants (d) and those of diabetes duration 4–8 months (e) and 9–12 months (f) (*p<0.05 teplizumab vs placebo)
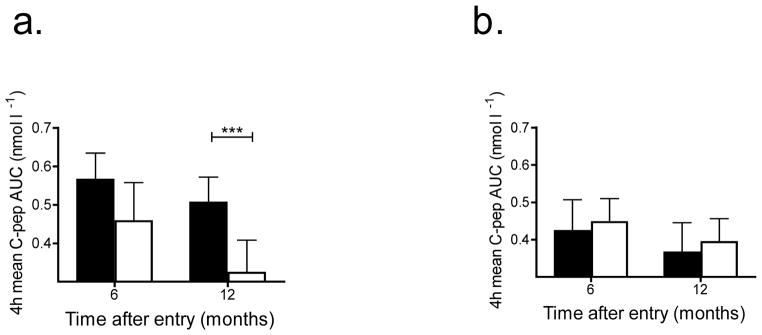
These results suggested that the baseline HbA1c may have affected the responses to teplizumab. Indeed, we found that the magnitude of the difference between teplizumab and placebo on C-peptide response was significantly greater in the lower HbA1c category (<6.5% [47.5 mmol/mol]) compared with the higher HbA1c category (>6.5% [47.5 mmol/mol], p=0.007) (Fig. 3a,b). In participants with an HbA1c <6.5% [47.5 mmol/mol] n=27), the C-peptide levels were 56.3% higher in the teplizumab group compared with the placebo group (p=0.001) at 12 months compared with no improvement in C-peptide responses in the teplizumab group with an HbA1c >6.5% (47.5 mmol/mol) when compared with the placebo group (n=31) (p=0.56). Subjects with lower HbA1c levels at baseline had higher C-peptide levels at baseline (0.725±0.039 [SEM] vs 0.455±0.033 nmol/l, p=0.001). Time from diagnosis did not significantly modify the treatment effect on C-peptide response in the mixed model (p for interaction=0.42). When adjusted for baseline HbA1c, the teplizumab group in the 4- to 8-month stratum had an 18.5% higher C-peptide level compared with the placebo group whereas the teplizumab-treated participants in the 9- to 12-month stratum showed a 17.5% higher level compared with the placebo-treated participants (both p=NS). After adjustment for baseline HbA1c, the C-peptide levels declined by 42.5% in the placebo group in the 4- to 8-month stratum (42.5%; 95% CI 28.8, 56.3) compared with a decline of 28.3% in the placebo group in the 9- to 12-month stratum (95% CI 9.94, 45.73, p=0.22) (Fig. 2b).
Fig. 3.
Effect of baseline HbA1c level on response to teplizumab. The AUC from the MMTT (mean±95% CI) at 6 and 12 months after enrolment, adjusted for baseline C-peptide, are shown for participants treated with teplizumab (black bar) and placebo (white bar). (a) HgbA1c <6.5% (teplizumab-treated n=20, placebo-treated n=7) and (b) >6.5% (teplizumab-treated n=10, placebo–treated n=19) (***p<0.01, teplizumab vs placebo)
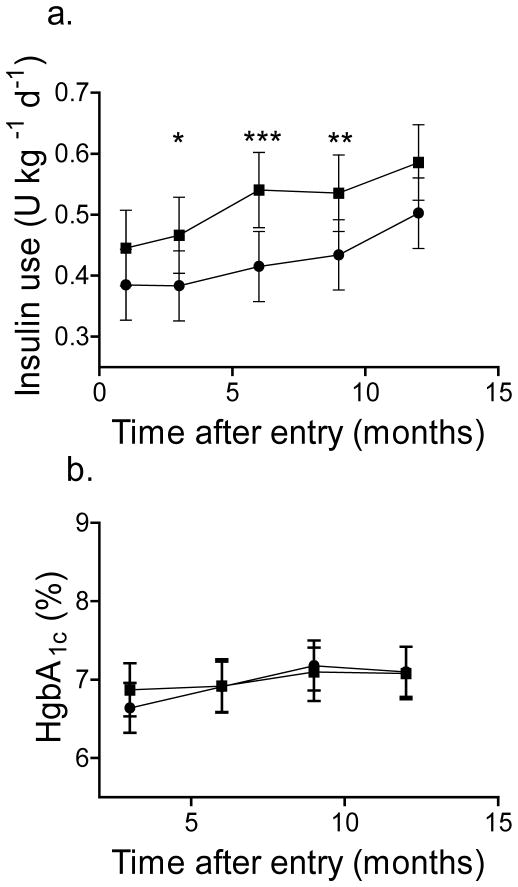
The total insulin requirements were significantly less in the teplizumab group (p=0.01) (Fig. 4a). Teplizumab treatment had no significant effect on the HbA1c levels or on change in the HbA1c levels over the 12-month study period (Fig. 4b, p=0.67).
Fig. 4.
Insulin use and HbA1c levels in participants treated with teplizumab and placebo. (a) The total daily insulin use (mean±95% CI), adjusted for baseline and stratum was compared in all participants treated with teplizumab (n=31, circles) and placebo (n=27, squares). There was a significant effect of drug treatment on insulin usage (p=0.014) (*p<0.05, **p<0.02, ***p<0.01 teplizumab vs placebo). (b) The HbA1c levels, adjusted for HbA1c at study entry (mean±95% CI) in all participants are shown. The drug treatment did not have a significant effect on the HbA1c levels (p=0.67, teplizumab vs placebo). To convert values for HgbA1c in % into mmol/mol, subtract 2.15 and multiply by 10.929
Effect of age on teplizumab response at 12 months
There was a significant difference in the responses of younger (age <15 years) and older participants (≥15 years) (p=0.047). When corrected for baseline C-peptide and HbA1c levels, teplizumab-treated younger participants had C-peptide responses that were 31.7% higher than placebo (p=0.02) whereas the teplizumab-treated older participants showed no difference (p=0.56) (Fig. 5). Younger participants had a lower C-peptide at baseline than older participants (0.492±0.03 [SEM] vs 0.745±0.042 nmol/l, p=0.004). The effect on C-peptide was primarily due to a greater decline in the C-peptide responses in the younger placebo-treated participants who lost 50.2% of the baseline C-peptide response (95% CI 36.5, 63.9, n=20) vs 11.2% (95% CI 11.9, 34.2, n=7) in the placebo-treated older participants (p=0.004); teplizumab-treated participants lost approximately 19% of the response at baseline in both age cohorts.
Fig. 5.
Effect of teplizumab on C-peptide responses and insulin use in young (age <15 years) and older (>15 years) participants. The C-peptide levels at 6 and 12 months after enrolment, adjusted for baseline C-peptide and HbA1c levels, are shown (mean± 95% CI) for participants treated with teplizumab (black bar) and placebo (white bar). (a) Age 8–14 (teplizumab-treated n=18, placebo–treated n=20) and (b) >15 years (teplizumab-treated n=13, placebo-treated n=7) (*p<0.05, teplizumab vs placebo)
Adverse events and safety
In general, teplizumab was well tolerated (Table 2). Five participants experienced serious adverse events during the study, one treated with teplizumab (an allergic reaction—related to injection not drug) and four treated with placebo. One participant discontinued teplizumab because of a transient grade 3 neutropoenia and two placebo-treated participants discontinued treatment (Table 2). The most common adverse events, occurring in at least 40% of participants and more frequent in teplizumab-treated participants, were rash, lymphopoenia and nausea. One participant in the teplizumab group had Epstein Barr virus infection, which resolved without intervention. Two participants treated with teplizumab vs one participant treated with placebo experienced cytokine release syndrome. All adverse events resolved and were expected based on past experiences with teplizumab in type 1 diabetes [18].
Table 2.
Adverse events
| Reported event | Treatment | |
|---|---|---|
| Placebo | Teplizumab | |
| Participants with adverse eventsa | 27 (100) | 33 (100) |
| Treatment-related adverse eventa,b | 25 (92.6) | 30 (90.9) |
| Serious adverse eventa | 4 (14.8) | 1 (3.0) |
| Event resulting in discontinuation of study medicationa | 2 (7.4) | 1 (3.0) |
| Event resulting in withdrawal from the studya | 0 (0.0) | 0 (0.0) |
| Grade 3 or higher adverse eventa | 10 (37.0) | 15 (45.5) |
| Fatal adverse eventa | 0 | 0 |
| Adverse eventsc | 644 (100) | 711 (100) |
| Grade 1 adverse event | 533 (82.8) 589 (82.8) | |
| Grade 2 adverse event | 78 (12.1) | 85 (12.0) |
| Grade 3 adverse event | 25 (3.9) | 21 (3.0) |
| Grade 4 adverse event | 0 (0.0) | 1 (0.1) |
| Grade 5 adverse event | 0 (0.0) | 0 (0.0) |
Data are shown as n (%)
Participants reporting at least one event
Includes events with causality ratings of ‘Possible’, ‘Probable’ or ‘Definite’
Graded according to the Common Terminology Criteria for Adverse Events (version 4.0; http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5x7.pdf)
Mechanistic outcomes and immunological characteristics of clinical response
We did not find a statistically significant change in the titres of anti-GAD65 or anti-ICA512 antibodies or differences between the treatment groups (electronic supplementary material [ESM] Fig. 1), although we did find a modest but statistically significant increase in the titre of anti-insulin antibodies in the drug-treated vs placebo-treated group (p=0.04). Similar to previous experience, the drug caused transient lymphopoenia (ESM Fig. 2) [14, 18]. We did not find consistent changes in immunological markers between drug-treated younger and older participants or between those with non-diabetic and elevated HbA1c levels but the CD4:CD8 T cell ratio trended lower at month 2 in younger participants (p=0.05), a finding we have previously associated with clinical responses to drug treatment [9, 18].
We compared T and B cell subsets among participants who had been treated with teplizumab to identify features that distinguish clinical responders. We designated this subgroup using a previous definition, as those who lost <7.5% of the baseline C-peptide response at 12 months [18]. There was a greater proportion of responders in the teplizumab (13/31, 42%) vs placebo groups (2/27, 7.4%, Fisher’s Exact test, p=0.003). The clinical responders had a higher C-peptide level at baseline (0.55±0.048 [SEM] vs 0.41±0.037 nmol/l, p=0.02) but the insulin use (0.38±0.06 [SEM] vs 0.43±0.03 U kg−1 day−1, p=0.42) and HbA1c levels (6.29±0.24% [45.3±2.57 mmol/mol] [SEM] vs 6.42±0.18% [46.7±1.96 mmol/mol], p=0.66) were not significantly different. The numbers of cells in lymphocyte subsets were not significantly different at baseline and there was similar depletion and repletion of lymphocytes in both subgroups (ESM Fig. 2).
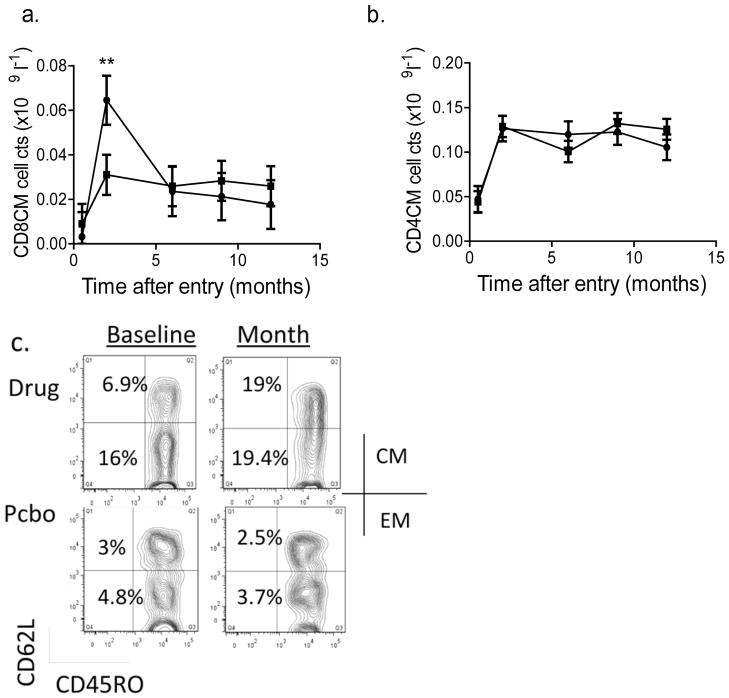
We found a significant increase in CD8CM T cells at month 2 in teplizumab clinical responders compared with teplizumab non-responders (p=0.018) (Fig. 6a,c). We did not identify significant differences in CD8EM, naive, or in CD4 T cell or CD4+Treg subsets between responders and non-responders (not shown and Fig. 6c).
Fig. 6.
Changes in CD8+ T cells in teplizumab-treated participants. (a) The number of circulating CD8CM (CD8+CD45RO+CD62L−) T cells (mean±SEM) is shown in teplizumab-treated responders (circles, n=13) and non-responders (squares, n=18) after drug treatment, corrected for the baseline counts. The baseline count of CD8CM cells was (mean±SEM) 0.011±0.002×109 cells/l in non-responders and 0.014±0.003×109 cells/l in responders. The number of cells was increased in responders at month 2 compared with the non-responders (**p=0.018, drug treated responders vs non-responders). (b) The corresponding CD4CM T cell counts (circles, responders; squares, non-responders) are shown. Significant changes in this subpopulation were not detected. (c) FACS plots showing CD8CM T cells before and at month 2 in representative drug- and placebo (Pcbo)-treated participants. The baseline count of CD4CM T cells was 0.12±0.013×109cells/l in non-responders and 0.12±0.017×109 cells/l in responders. The staining for CD45RO and CD62L is shown on gated CD8+ lymphocytes. In the data from a representative drug-treated participant shown, the percentage of CD8CM and EM cells (in the corresponding L quadrants) increased from 6.9% and 16% of CD8+ T cells to 19.1% and 19.4% of CD8+ T cells, respectively, whereas in the placebo-treated patient, the CD8CM and EM cells were 3% and 4.8% before and 2.5% and 3.7% after treatment
Discussion
In patients treated with a single 14-course regimen of teplizumab, administered up to 1 year following diagnosis of type 1 diabetes, we observed a significant preservation of C-peptide loss after 1 year but this effect was not statistically significant after adjustment for chance imbalances in HbA1c at baseline. However, percentage change in C-peptide from baseline, as well as insulin requirements, were improved in those receiving teplizumab and a greater proportion of placebo-treated participants lost all detectable C-peptide responses. Our exploratory subgroup analyses suggest that younger age and near normal control of blood glucose improves responses to teplizumab.
The effect of the prior glucose control on responses to the drug was unexpected. The participants with lower HbA1c levels had higher baseline C-peptide responses, which may have been a factor in responsiveness to treatment, but younger participants also showed better response to the drug even though their baseline C-peptide levels were lower than in older participants.
Since teplizumab had no direct effect on HbA1c, these findings suggest either an effect of prior glucose levels on beta, immune or other cells such as vascular cells during drug treatment [21, 22]. Glucose may stimulate increased levels of IL-1β by beta cells, which could affect responses to anti-CD3 mAb, as we have recently shown, and thus directly or indirectly cause beta cell toxicity [23–25]. We have found a similar effect of baseline HbA1c in a trial of teplizumab in patients with new-onset type 1 diabetes (K. Herold, unpublished data). Interestingly, in a recent trial of abatacept in type 1 diabetes that showed an effect of that drug on the decline of C-peptide, the drug-treated group had a significantly lower level of HbA1c at randomisation (6.31±0.09% vs 6.74±0.16% [45.5 vs 50.2 mmol/mol], p=0.01). These observations suggest that good glucose control prior to immune therapy is needed for the responses to immune therapies in patients with established disease but this hypothesis will need further evaluation in larger studies.
Our analysis did not identify differences in the efficacy of teplizumab between the duration strata. The differences that were seen reflected the decline in C-peptide in the two placebo groups and not the response to teplizumab. This may be explained by a slower rate of decline of C-peptide during the second year of disease, a finding recently reported [26]. Therefore those in the longer duration stratum may lose C-peptide more slowly during the period on study. A longer follow-up time may be needed to detect differences in participants whose endpoint occurred after the first year of disease. In addition, participants who were recruited after 8 months may represent a ‘survivor’ group—by requiring the same level of C-peptide for enrolment in the 4-month as in the 9- to 12-month strata, we may have selected for individuals who have less aggressive disease: we do not know whether the same degree of preservation would be seen in those with lower stimulated levels at entry.
Based on data from other trials, it appears that the magnitude of the effects of the drug in this population who enrolled after 4 months following diagnosis, is less than in those studied within the first 100 days after diagnosis. In a previous trial recruiting participants of similar age with new-onset disease (i.e. within 100 days from diagnosis), there was an 84% increase in C-peptide in drug-treated vs control participants [18] at 12 mos. In US participants in the Protégé trial, which also enrolled participants within 100 days from diagnosis (n=95), there was a 33% increase in C-peptide at month 12. There are several potential reasons for the difference in the magnitude of response in patients with new-onset vs longer-duration diabetes, such as evolution of the immune response, inflammatory cells that are no longer active following presentation or the effects of an extended duration of damage to beta cells that may render them unrecoverable. The C-peptide responses at entry into new-onset trials has been higher than this study (e.g. 0.722±0.04 vs 0.575±0.030 nmol/l) despite our requirement for a stimulated level of at least 0.2 nmol/l. This might affect the responses to drug since we found that the baseline C-peptide responses were significantly higher in clinical responders, consistent with findings reported by Keymeulen et al [10]. However, the response to drug treatment in our study was not restricted to those within the upper half of C-peptide responses at entry—individuals in the upper half of baseline C-peptide responses lost 19.9±8.8% (SEM) of baseline responses at month 12 whereas those in the lower half of baseline responses lost 18.2±5.2% (p=0.87).
The effect of age in determining responses was previously identified in the Protégé trial [14] and is confirmed by our findings. Although the baseline C-peptide responses were lower in younger participants, their baseline HbA1c levels were not (6.82±0.17% [51±1.88 mmol/mol] [SEM] vs 6.51±0.24% [47.6±11.7 mmol/mol], p=0.29). Since entry into the study required that participants meet a specific stimulated C-peptide level, there may have been a bias in the enrolment of younger participants with less aggressive disease since younger participants in general have lower levels of C-peptide than older participants [27]. Caution is therefore needed in drawing conclusions from these findings.
In previous studies, we had identified an increase in the relative number of CD8+ T cells among clinical responders to anti-CD3 mAb and had shown that the CD8+ T cells from drug-treated participants had suppressor function ex vivo [18, 28–30]. In this trial we found that drug-treated responders could be distinguished from drug-treated non-responders by an increase in the number of CD8CM (CD45RO+CD62L+) T cells 3 months after treatment. A limitation of our finding is that it was restricted to a single time point and we did not perform corrections for multiple comparisons. We hypothesise that these CD8 T cells may be those associated with the functional responses we have previously identified but further studies will be needed to directly analyse the function of these cells at later time points, and to determine whether CD8CM cells respond differently in drug-treated responders and non-responders when they encounter antigen, analogous to adaptive Tr1 cells [31]. We did not identify consistent changes in T cell subsets in young and older participants or in those with non-diabetic vs elevated HbA1c levels although the differences in the CD4:CD8 T cell ratio and other immunological markers warrant further investigation.
In conclusion, our findings for the primary outcome did not conclusively demonstrate a significant benefit for teplizumab. Nevertheless, our secondary outcomes suggest that treatment with teplizumab may modulate the course and reduce the decline of C-peptide in patients with established disease of up to 1 year duration. The analyses we performed identified clinical (age) and laboratory (HbA1c) characteristics that may identify those participants most likely to respond to drug treatment and may serve as a guide to future studies with this or other immunological characteristics tested in this population. These characteristics of ‘responders’ may also provide insights into the mechanisms underlying drug efficacy. Specifically, the HbA1c findings emphasise the need to conduct studies to explore how glucose levels modulate responses to immunological treatments. We also identified immunological determinants of efficacy that may be useful in future trials in tracking responses or selecting those most likely to respond to therapy.
Supplementary Material
Abbreviations
- CBC
Complete blood count
- CM
Central memory
- EM
Effector memory
- FcR
Fc receptor
- mAb
Monoclonal antibody
- MMTT
Mixed meal tolerance test
- PBMC
Peripheral blood mononuclear cell
- ICA
Islet cell antibody
Footnotes
Duality of interest
K. C. Herold and J. A. Bluestone have a patent pending concerning the use of anti-CD3 mAb in combination therapies. J. A. Bluestone has a patent for teplizumab.
Contribution statement
KCH, SEG, JD and JAB designed the trial. KCH, SEG, SMW, PAG, FW-L, LD, JS, SMR, SA, MYJ, AWM and JD acquired and analysed the data. FW-L and LD performed additional mechanistic studies. All of the authors contributed to writing the manuscript. KCH was the trial sponsor. KCH is the guarantor of this work, had full access to all the data and takes full responsibility for the integrity of data and the accuracy of data analysis. All of the authors approved the final manuscript.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57:2883–2888. doi: 10.2337/db07-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madsbad S, Krarup T, Regeur L, Faber OK, Binder C. Insulin secretory reserve in insulin dependent patients at time of diagnosis and the first 180 days of insulin treatment. Acta Endocrinol (Copenh) 1980;95:359–363. doi: 10.1530/acta.0.0950359. [DOI] [PubMed] [Google Scholar]
- 4.Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 5.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Steffes MW, Sibley S, Jackson M, Thomas W. beta-Cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 7.Daneman D, Clarson C. Residual beta-cell function in children with type 1 diabetes: measurement and impact on glycemic control. Clin Invest Med. 1987;10:484–487. [PubMed] [Google Scholar]
- 8.Schiffrin A, Suissa S, Weitzner G, Poussier P, Lalla D. Factors predicting course of beta-cell function in IDDM. Diabetes Care. 1992;15:997–1001. doi: 10.2337/diacare.15.8.997. [DOI] [PubMed] [Google Scholar]
- 9.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 10.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 11.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–327. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–97. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bougneres PF, Carel JC, Castano L, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med. 1988;318:663–670. doi: 10.1056/NEJM198803173181103. [DOI] [PubMed] [Google Scholar]
- 16.Ludvigsson J, Faresjo M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 17.Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31:1966–1971. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinstein R, Genaro AM, Motta A, Cremaschi G, Wald MR. Impaired immune responses in streptozotocin-induced type I diabetes in mice. Involvement of high glucose. Clin Exp Immunol. 2008;154:235–246. doi: 10.1111/j.1365-2249.2008.03742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerman MA, Flores SC. Autoimmune-mediated oxidative stress and endothelial dysfunction: implications of accelerated vascular injury in type I diabetes. J Surg Res. 2009;155:173–178. doi: 10.1016/j.jss.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Mandrup-Poulsen T, Spinas GA, Prowse SJ, et al. Islet cytotoxicity of interleukin 1. Influence of culture conditions and islet donor characteristics. Diabetes. 1987;36:641–647. doi: 10.2337/diab.36.5.641. [DOI] [PubMed] [Google Scholar]
- 24.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ablamunits V, Henegariu O, Hansen JB, et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes. 2012;61:145–154. doi: 10.2337/db11-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenbaum CJ, Beam CA, Boulware D, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from Composite Type 1 Diabetes TrialNet Data. Diabetes. 2012;61:2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes. 2004;53:426–433. doi: 10.2337/diabetes.53.2.426. [DOI] [PubMed] [Google Scholar]
- 28.Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala) J Clin Invest. 2003;111:409–418. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ablamunits V, Bisikirska B, Herold KC. Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF. Eur J Immunol. 2010;40:2891–2901. doi: 10.1002/eji.201040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8 T cell population and induces CD8CD25 Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.