Abstract
In Chuvash polycythemia, a homozygous 598C>T mutation in the von Hippel-Lindau gene (VHL) leads to an R200W substitution in VHL protein, impaired degradation of α-subunits of hypoxia inducible factor (HIF)-1 and HIF-2, and augmented hypoxic responses during normoxia. Chronic hypoxia of high altitude is associated with decreased serum glucose and insulin concentrations. Other investigators reported that HIF-1 promotes cellular glucose uptake by increased expression of GLUT1 and increased glycolysis by increased expression of enzymes such as PDK. On the other hand, inactivation of Vhl in murine liver leads to hypoglycemia associated with a HIF-2-related decrease in the expression of the gluconeogenic enzymes genes Pepck, G6pc, and Glut2. We therefore hypothesized that glucose concentrations are decreased in individuals with Chuvash polycythemia. We found that 88 Chuvash VHLR200W homozygotes had lower random glucose and glycosylated hemoglobin A1c levels than 52 Chuvash subjects with wildtype VHL alleles. Serum metabolomics revealed higher glycerol and citrate levels in the VHLR200W homozygotes. We expanded these observations in VHLR200W homozygote mice and found that they had lower fasting glucose values and lower glucose excursions than wild-type control mice but no change in fasting insulin concentrations. Hepatic expression of Glut2 and G6pc but not Pdk2 was decreased and skeletal muscle expression of Glut1, Pdk1 and Pdk4 was increased. These results suggest that both decreased hepatic gluconeogenesis and increased skeletal uptake and glycolysis contribute to the decreased glucose concentrations. Further study is needed to determine whether pharmacologically manipulating HIF expression might be beneficial for treatment of diabetic patients.
Keywords: VHL, hypoxia inducible factors, glucose, insulin, glycolysis, gluconeogenesis
Introduction
Hypoxia inducible factor (HIF)-1 and HIF-2 are transcription factors that play a major role in cellular responses to hypoxia. Levels of the α subunits of HIFs are tightly controlled. These transcription factors are constantly degraded under normoxia by the proteosome through an iron-requiring interaction with their negative regulator, pVHL [1]. Proline hydroxylation of HIF-α by prolyl hydroxylase domain (PHD) enzymes, a process that requires oxygen and iron, is required for the interaction of HIF-α with VHL protein [2]. During hypoxia this process is inhibited, leading to accumulation of HIFs as HIF-α subunits translocate to the nucleus and interact with HIF-1β to form heterodimers. Subsequently, these heterodimeric transcription factors bind to key hypoxia response elements, thereby governing the transcription of genes that regulate adaptation to hypoxia [3]. Chuvash polycythemia is an autosomal recessive congenital disorder characterized by a homozygous 598C>T mutation in the VHL gene resulting in an R200W amino acid change in the VHL protein [4]. It is characterized by augmented HIF-1α and HIF-2α levels during normoxia and altered expression of erythropoietin, glucose transporter-1 and a number of other genes [4-6].
Chronic hypoxia of high altitude is associated with decreased serum glucose and insulin concentrations [7, 8]. A variety of mechanisms might explain this phenomenon, including enhanced cellular glucose uptake, glycolysis and glycogenesis related to increased HIF-1 expression [9-11] and decreased hepatic gluconeogenesis related to increased HIF-2 expression [12, 13]. Because of the upregulation of HIFs levels during normoxia that characterizes homozygosity for the germline VHLR200W mutation, we sought to determine changes in glucose metabolism in Chuvash polycythemia patients. We measured random blood glucose concentrations, determined glycosylated hemoglobin (HbA1c) levels and assessed the patients’ body mass index (BMI), serum cholesterol, triglyceride levels and several other metabolic intermediates. Finally, we explored potential mechanisms of altered glucose metabolism in VHLR200W mice.
Materials and Methods
Study subjects
The Institutional Review Board of Howard University approved the study and the participants provided written informed consent. Individuals >20 years of age with a diagnosis of familial polycythemia or controls without such a diagnosis were studied in Chuvashia, Russia. Genotyping for the VHLR200W mutation was performed as previously described.[5] The clinical characteristics for the study subjects and the controls including their gender, age, and BMI were recorded.
Laboratory studies
The complete blood count was performed by an automated analyzer (Sysmex XT 2000i, Sysmex Corporation, Kobe, Hyogo, Japan). Serum ferritin concentration was determined by enzyme immunoassay (Ramco Laboratories Inc., Stafford, TX). Serum glucose, bilirubin, creatinine, BUN, triglyceride and cholesterol concentrations were determined by Quest Diagnostics (Auburn Hills, MI) in an Olympus 2700 analyzer (Olympus Optical Co. Ltd, Shizuoka-ken, Japan).
HbA1c level was determined by high performance liquid chromatography (HPLC) using the Primus Diagnostics ultra2 with Resolution™ Software Analytical System for Hemoglobinopathies, commercialized by Trinity Biotech, New Jersey, USA. Samples were processed using the high-resolution method, which requires close to 11 minutes of run time per sample, allowing for higher sensitivity and specificity in detection. The results from samples where the HbA1c peak was not resolved were excluded from the analysis. The HPLC column used in this study is designed for the assessment of hemoglobinopathies and is not the column recommended by the manufacturer for the optimal evaluation of HbA1c.
Metabolomics Analysis
To ensure metabolite volatility for gas chromatographic analysis each sample underwent a two-step derivatization process. The extracted metabolites were suspended in 30 μL of pyridine containing O-methylhydroxylamine (20 mg/mL) and transferred to a 1 mL Recti-Vial (Pierce) fitted with a Teflon cap. The mixture was subjected to microwave irradiation for 30 seconds followed by the addition of 30 μL of N-methyl-N-trimethylsilyltrifluoroacdtamide + 1% trimethylchlorosilane (Pierce) and incubated at 37°C for thirty minutes then transferred to an auto sampler vial containing 70 μL of heptane and analyzed by GC-MS. An Agilent 7683 auto injector was employed to inject 1 μL of each sample into an injector. The injector was set to a 1:1 split ratio and the temperature was held at 250°C. An Agilent 6890 gas chromatograph was used for analysis. A Restek 30 m Rtx-5MS with a 10 m guard column was employed for separation. Helium was used as the carrier gas at a 1 mL/min flow rate. The transfer line into the mass spectrometer was held at 250°C. A MicroMass GCT Premier (Waters, Milford, MA) was used for mass spectrometric analysis. Normal 70 eV electron impact conditions were used for fragmentation. Data were recorded using MassLynx (Waters, Milford, MA). Sample data were divided into two analysis groups, the first was used to build a model of metabolic differences and the second used to test for model robustness. Chromatographic peak detection and height analysis for each chromatogram was performed using MarkerLynx (Waters, Milford, MA). To find possible variation in each sample group the data were transferred to SIMCA-P+ ver.11.5 (Umetrics, Kinnelon, NJ) where principal component analysis (PCA) and partial least squares-discriminate analysis (PLS-DA) were performed. This produced a possible list of metabolites that differed between the groups. Each metabolite was investigated to determine if these changes were real. The chromatographic area for each metabolite was integrated and recorded. Student’s t-test was used to test for significance. This was performed twice using two separate characteristic mass peaks for analysis.
Animal Studies
VHLR200W homozygous mice on a C57BL6 background and wild-type littermates were generated and maintained as previously described [6]. The mice were back-crossed onto the C57BL6 background for more than six generations. The animal experimentation conformed to protocols approved by the animal care committees of the University of Utah. VHLR200W homozygous mice and age-matched wild-type littermates between 4 and 8 months of age were euthanized, then dissected to remove the liver. Total RNA was extracted from livers and skeletal muscle using Trizol (Molecular Research Center, Cincinnati, OH). To quantify G6pc, Glut1, Glut2, Glut4, Pepck, Pdk2 and Pdk4 mRNA levels, hepatic and skeletal muscle RNA (500 ng) was reverse-transcribed into cDNA using SuperScript II with oligo dT primer according to the manufacturer’s instructions (Invitrogen,Carlsbad, CA) and 2μl of cDNA was used for real-time PCR using SYBR Green dye with specific primers (G-6-Pase: 5′-CTTGTACCTGAAAGCCTTGG-3′ and 5′-GTCCCATGAACTTGCTGA TG-3′; Glut4: 5′-CACCAACTCCAGCCAAACTC-3′ and 5′-GAGGTAAAGGGAAAGGGGAAA-3′; Glut1: 5′-GCTGGGAATCGTCGTTGG-3′ and 5′-GATGGGCTGGCGGTAGG-3′; Pdk1: 5′-TTACTCAGTGGAACACCGCC-3′ and 5′-CATGAGAGCGACCATGGAG-3′; Pdk2: 5′-TGACAGGGCTTTCTGGTCTT-3′ and 5′-GGAGATTGACATCCTGCCTG-3′; 18S: 5′-TTGACGGAAGGGCACCACCAG-3′ and 5′-GCACCACCACCCACGGAATCG-3′) and the reaction was carried out at 50°C for 2 min,95°C for 10 min, followed by 50 cycles of 92°C for 15 s and 58°C for 1 min. ΔCt was expressed after normalization against 18S. Fold change was calculated by the ΔΔCt method [14]. Pdk4 and Glut4 are not expressed in liver, while Glut2 and Pdk2 are not expressed in muscle.
Glucose tolerance testing was performed after a 6 hr fast. Mice were injected intraperitoneally (IP) with 1 mg/g body weight of glucose in 0.9% saline. Glucose levels were measured from tail vein blood with a Glucometer (Elite, Bayer Corp., Tarrytown, NY) at 0, 5, 15, 30, 60, and 120 min. Extra tail blood (30 μl) was collected at 0 and 30 min for insulin measurement.
Statistical analysis
Continuous variables that followed a skewed distribution were transformed to normal distribution by natural log or square root transformation. Distribution of clinical and laboratory measurements were tested between VHLR200W homozygotes and controls by students’ t-test or Chi-square. Laboratory measurements that were continuous variables were examined with linear regression; Pearson and Spearman correlation were used to assess the correlation between blood glucose, hemoglobin A1c and other variables in two groups. Multiple linear regression analysis was applied to test the independent relationship of clinical variables with blood glucose and hemoglobin A1c concentration. Models were checked for assumptions. Analyses were done in Stata 10.1 (StataCorp, College Station, TX).
Results
Serum glucose and hemoglobin A1c concentrations in Chuvash polycythemia patients and Chuvash controls
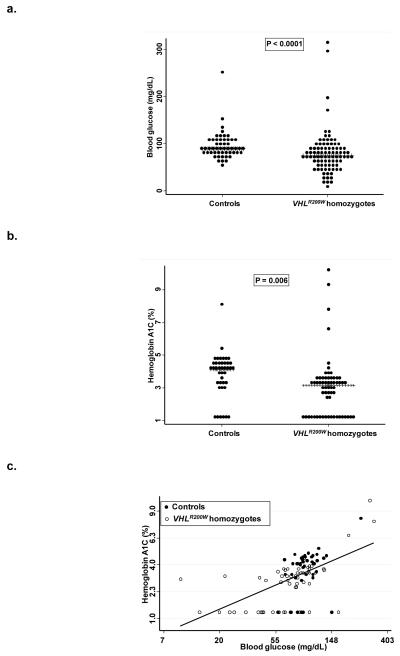
The study population included 88 VHLR200W homozygotes and 52 VHL wildtype subjects. The median (interquartile range) age was 44 (34-54) years. Eighty-four (60%) of the subjects were females. Random serum glucose concentrations and hemoglobin A1c levels were lower in VHLR200W homozygotes (Figure 1). Age, BMI, WBC count, and serum concentrations of cholesterol and ferritin were also lower in VHLR200W homozygotes (Table 1). Hemoglobin concentration, and serum concentrations of bilirubin and triglyceride tended to be greater in the VHLR200W homozygotes. Hemoglobin A1c correlated significantly with the blood glucose concentration in both VHLR200W homozygotes and controls (Figure 1).
Figure 1.
a. Distribution of random serum glucose concentration in VHL wildtype subjects and VHLR200W homozygotes. The median and interquartile ranges were 89 (81-106) mg/dL in VHL wildtype subjects compared to 75 (56-91) mg/dL inVHLR200W homozygotes. b. Distribution of hemoglobin A1c in VHL wildtype subjects and VHLR200W homozygotes. The median and interquartile ranges were 4.1 (3.3-4.6) % in VHL wildtype subjects compared to 3.2 (1.3-3.6) % in VHLR200W homozygotes. c. Relationship of hemoglobin A1c level (vertical axis) to random serum glucose concentration. A significant positive correlation was observed: r = 0.53, P <0.0001.
Table 1. Distribution of variables in controls and Chuvash polycythemia patients. Results are in median (interquartile range) unless otherwise indicated.
| Controls N=52 |
VHLR200W homozygotes, N=88 |
P value | |
|---|---|---|---|
| Age (year) | 50 (39-60) | 42 (33-52) | 0.005 |
| Female gender, no (%) | 34 (65%) | 50 (57%) | 0.3 |
| Phlebotomy in last year, no (%) | 0 | 45 (51%) | <0.0001* |
| Smoking, no (%) | 6 (12%) | 25 (28%) | 0.020 |
| Alcohol drinking, no (%) | 9 (17%) | 16 (18%) | 0.9 |
| Systolic blood pressure (mm Hg) | 126 (118-145) | 118 (109-127) | 0.005 |
| Diastolic blood pressure (mm Hg) | 84 (78-90) | 80 (75-85) | 0.045 |
| BMI (kg/m2) | 24.5 (22.3-27.5) | 22.6 (20.2-25.2) | 0.004 |
| Hemoglobin (mg/dL) | 12.8 (11.8-14.0) | 17.8 (15.7-19.7) | <0.0001* |
| WBC (×1000/uL) | 6.5 (5.6-7.2) | 5.5 (4.4-7.2) | 0.0008* |
| Platelets (×1000/uL) | 250 (213-292) | 213 (163-267) | 0.001* |
| Creatinine (mg/dL) | 0.7 (0.6-0.7) | 0.6 (0.6-0.8) | 0.14 |
| Phosphate (mg/dL) | 3.6 (3.0-4.1) | 3.4 (3.0-3.8) | 0.2 |
| Triglyceride (mg/dL) | 109 (76-174) | 137 (101-201) | 0.030 |
| Cholesterol (mg/dL) | 190 (170-211) | 170 (145-199) | 0.006 |
| Ferritin (ng/mL) | 54 (25-115)1 | 11 (6-22)2 | <0.0001* |
n=50
n=75
Significant after adjustment for Bonferroni correction.
In univariate analyses among subjects stratified by VHL genotype, the most significant positive correlations were those of systemic blood pressure and serum ferritin concentration with hemoglobin A1c levels among the VHL wildtype subjects and BMI with serum glucose concentration among VHLR200W homozygotes (Table 2).
Table 2. Pearson correlation of serum glucose concentration and hemoglobin A1c percent with selected clinical variables.
| Controls | VHLR200W homozygotes | |||
|---|---|---|---|---|
| Glucose r (P) (N = 52) |
HbA1c r (P) (N = 41) |
Glucose r (P) (N = 88) |
HbA1c r (P) (N = 62) |
|
| Age (year) | 0.25 (0.07) | 0.39 (0.012) | 0.22 (0.036) | 0.22 (0.08) |
| Female gender, no (%) | 0.01 (0.9)* | −0.31 (0.051)* | 0.10 (0.4)* | 0.0* |
| Smoking, no (%) | −0.07 (0.6)* | 0.20 (0.2)* | −0.19 (0.07)* | −0.24 (0.06)* |
| Alcohol drinking, no (%) | −0.10 (0.5)* | 0.14 (0.4)* | −0.07 (0.5)* | −0.11 (0.4)* |
| Systolic blood pressure (mm Hg) |
0.24 (0.08) | 0.45 (0.003)** | 0.27 (0.010) | 0.12 (0.3) |
| Diastolic blood pressure (mm Hg) |
0.23 (0.10) | 0.46 (0.003)** | 0.11 (0.3) | 0.01 (0.9) |
| BMI (kg/m2) | 0.24 (0.09) | 0.29 (0.07) | 0.35 (<0.001)** | 0.20 (0.11) |
| Hemoglobin (mg/dL) (natural log) |
0.03 (0.8) | 0.21 (0.18) | −0.05 (0.6) | 0.17 (0.19) |
| Creatinine (mg/dL) (square root) |
0.17 (0.2) | 0.12 (0.5) | 0.06 (0.6) | 0.15 (0.3) |
| Triglyceride (mg/dL) (natural log) |
0.21 (0.13) | 0.15 (0.3) | 0.26 (0.016) | 0.06 (0.6) |
| Cholesterol (mg/dL) (square root) |
0.30 (0.029) | −0.02 (0.9) | 0.02 (0.8) | −0.01 (0.9) |
| Ferritin (ng/mL) (natural log) |
0.18 (0.2)1 | 0.52 (<0.001)2** | −0.09 (0.5)3 | 0.11 (0.4)4 |
Spearman rho
n=50
n=40
n=75
n=57
Significant after adjustment for Bonferroni correction.
In multiple linear regression models that adjusted for age, gender, BMI, and history of smoking, serum glucose concentration and hemoglobin A1c level continued to be lower in VHLR200W homozygotes than controls (Table 3). After adjustment for these covariates and based on the median values in the control group, VHLR200W homozygosity was associated with an average 15 mg/dL decrease in the random glucose concentration (95% CI = 2-26) and with an average 0.7 % decrease in hemoglobin A1c (95%CI = 0.2-1.2).
Table 3. Predictors of serum glucose concentration and hemoglobin A1c percent by multiple linear regression.
| Beta (95% CI) | P value | |
|---|---|---|
|
A. Serum glucose concentration (mg/dL, natural log)
(N = 140) * |
||
| Homozygous VHLR200W mutation | −0.18 (−0.34- −0.02) | 0.031 |
| Female gender | 0.003 (−0.16- 0.17) | 0.9 |
| Age (10 years) | 0.05 (−0.01-0.11) | 0.094 |
| BMI (kg/M2) | 0.02 (0.01- 0.04) | 0.009 |
| History of smoking | −0.08 (−0.29-0.13) | 0.5 |
| B. Hemoglobin A1c (%, square root) (N = 101) ** | ||
| Homozygous VHLR200W mutation | −0.19 (−0.36- −0.02) | 0.028 |
| Female gender | −0.10 (−0.27-0.07) | 0.3 |
| Age (10 years) | 0.06 (−0.01- 0.12) | 0.072 |
| BMI (kg/M2) | 0.02 (0.003- 0.04) | 0.046 |
| History of smoking | −0.04 (−0.25-0.17) | 0.7 |
Variables entered into the model were age, gender, VHL mutation, phlebotomy, BMI, smoking, hemoglobin concentration. R-square = 0.18.
Variables entered into the model were age, gender, VHL mutation, phlebotomy, BMI, smoking, hemoglobin concentration; 2 outliers were removed. R-square = 0.21
Metabolomic studies
To further investigate the metabolic changes in the VHLR200W homozygotes we performed unbiased metabolomics analysis. Glycerol, phosphate, urea, and three unknowns were found to be elevated in VHLR200W homozygote samples (Table 4). Unknown metabolites do not correspond to our in-house developed metabolite library or the commercially available National Institute of Standards and Technologies (NIST) 2.0 database and were excluded from analysis. Citric acid had two very strong outliers (> 20 times the median and >2 standard deviations from the mean); however, we could not rule out the possibility that this was caused by an interfering metabolite. If those are removed from the analysis, citric acid is also elevated in VHLR200W homozygotes.
Table 4. Metabolomic analysis of sera*.
| Metabolite | Controls (N = 8) |
VHLR200W homozygotes (N = 13) |
P value |
|---|---|---|---|
| Glycerol | 189 (141-208) | 298 (245-459) | 0.012 |
| Phosphate | 437 (332-518) | 576 (500-663) | 0.026 |
| Urea | 554 (499-732) | 1073 (847-1337) | 0.004 |
| Citrate | 166 (81-204) | 355 (194-437) | 0.008 |
Values reflect median ± interquartile range and are presented as the area under the chromatographic curve.
Chuvash polycythemia mice
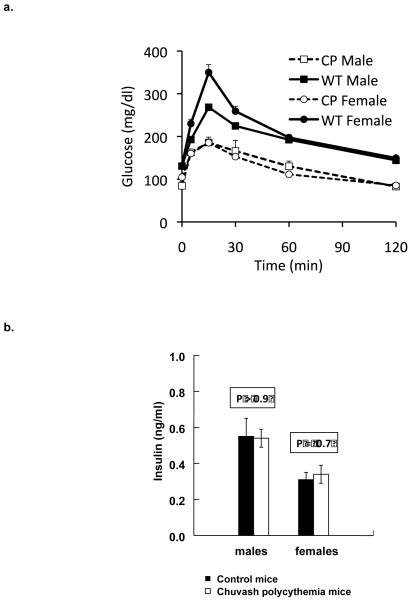
Glucose tolerance testing was performed on male and female Chuvash polycythemia mice and age matched wild-type C57BL6 controls. Both sexes of Chuvash polycythemia mice had significantly lower fasting glucose values and glucose excursions than wild type mice (Figure 2a). Despite the lower fasting glucose values, insulin levels did not differ between the Chuvash polycythemia and wildtype mice (Figure 2b; P >0.6).
Figure 2.
a. Glucose tolerance testing in Chuvash polycythemia (CP) mice and C57BL6 wild type (WT) controls. Mice (4-10 per group, aged 3-8 months and age-matched across groups) had fasting glucose tolerance tests performed. Shown are the mean values ±SEM, with the error bars in most cases hidden within the data points. By panel data analysis, the values for CP were significantly different compared to WT (P <0.0001). b. Despite the lower fasting glucose values, insulin levels did not differ between the Chuvash polycythemia and wildtype mice (Chuvash polycythemia males, 0.54 ± 0.05 ng/ml, wildtype males 0.55 ±0.10 ng/ml, P =0.96; Chuvash polycythemia females 0.34 ± 0.05, wildtype females 0.31 ± 0.04 ng/ml, P = 0.66).
Realtime RT-PCR analysis of mRNAs from the livers of the Chuvash polycythemia mice (Table 5) showed significant decreases at transcript levels of G6pc, encoding glucose-6-phosphatase, decrease of Pepck, encoding phosphoenolpyrvate carboxykinase, enzymes that are important in gluconeogenesis. There was no significant change in the transcript levels of Pdk2, encoding pyruvate dehydrogenase kinase isoenzyme 2, or Glut1, two important regulators of glucose transport and glycolysis, although the HIF-regulated Glut1 trended toward an increase in the Chuvash polycythemia mice. In skeletal muscle, the tissue responsible for most glucose uptake after glucose challenge, Glut1, Pdk1 and Pdk4 were upregulated and there was a trend toward increased Glut4 expression. These data are consistent with the lower glycemia in Chuvash mice related to decreased hepatic gluconeogenesis and increased skeletal muscle glucose uptake and glycolysis.
Table 5. Relative expression measured by realtime RT-PCR of genes involved in glucose metabolism in Chuvash polycythemia and control mice.
| Tissue | Gene | Control mice (n = 8, mean ± SEM) |
Chuvash polycythemia mice (n = 8, mean ± SEM) |
P value |
|---|---|---|---|---|
| Liver | G6pc | 4.32 ± 1.26 | 0.97 ± 0.21 | 0.02 |
| Liver | Pepck | 1.32 ± 0.13 | 0.99 ± 0.14 | 0.10 |
| Liver | Glutl | 1.03 ± 0.11 | 1.39 ± 0.14 | 0.07 |
| Liver | Glut2 | 1.30 ± 0.08 | 1.07 ± 0.04 | 0.04 |
| Liver | Pdk2 | 0.34 ± 0.04 | 0.43 ± 0.06 | 0.36 |
| Muscle | Glutl | 0.81 ± 0.29 | 1.18 ± 0.14 | 0.03 |
| Muscle | Glut4 | 1.27 ± 0.08 | 1.43 ± 0.18 | 0.14 |
| Muscle | Pdk1 | 0.86 ± 0.18 | 1.67 ± 0.43 | 0.003 |
| Muscle | Pdk4 | 0.50 ± 0.09 | 1.86 ± 0.24 | <0.001 |
Discussion
In this study we found that, similar to chronic hypoxia of high altitude, the homozygous VHLR200W mutation is associated with a lower serum glucose concentration. These effects were seen in both humans and a mouse model. We have probed the principal mechanisms including hepatic glucose production, insulin levels, and peripheral glucose uptake mechanisms, all of which are known to be affected by HIF pathway signaling. It should be emphasized, however, that the regulation of plasma glucose concentrations is highly complex, resulting from the interplay of several tissues (brain, muscle, fat, liver, gut, pancreatic islets, and others) and several discrete pathways (insulin secretion, insulin signaling, insulin-dependent and –independent glucose uptake, and multiple metabolic fates of glucose). Without the analysis of multiple tissue-specific deletions of VHL and its downstream targets that include the different HIF isoforms, it is impossible from the current data to ascribe the changes in glucose to specific pathways in specific tissues. Furthermore, all of the pathways enumerated above feed back upon one another, so any changes observed in a tissue may be intrinsic to altered HIF pathway signaling but also to endocrine, nutrient, or neural feedback based on HIF pathway signaling in other tissues. Nevertheless, the data do offer suggestions as to the mechanisms by which VHLR200W affects metabolism. It should be noted that a previous study found no significant difference in glucose tolerance between humans with Chuvash polycythemia and controls, but the subjects in this relatively small study (5 subjects and 5 controls) varied over a large range of ages and weights that might have obscured any intergroup differences [15].
Among the several potential mechanisms whereby the upregulation of HIF-1 or HIF-2 in VHLR200W homozygotes might lead to the reduction in serum glucose concentration are changes in hepatic metabolism, as the liver is known to play a central role in maintaining serum glucose concentrations by balancing the uptake of glucose from the circulation with the release of glucose to the circulation via gluconeogenesis [16]. We observed decreased hepatic expression of the glucose transporter Glut2 and the gluconeogenic gene glucose-6-phosphatase (G6pc) and a trend to decreased expression of the rate-limiting enzyme in gluconeogenesis phosphoenolpyruvate carboxykinase (Pepck), but not significantly increased expression of Glut1 or Pdk2. These findings suggest that impairment of hepatic gluconeogenesis contributes to the overall decline in glycemia in the Chuvash polycythemia subjects. The results are consistent with the previous observation that HIF-2 decreases hepatic gluconeogenesis [12, 13]. Namely, mice with Vhl-inactivated hepatocytes had down-regulation of Glut2 and G6pc, leading to impaired release of glucose from the liver, elevated hepatic glycogen storage and hypoglycemia [12]. The elevated plasma pyruvate and lactate concentrations observed after a standard meal in five VHLR200W homozygotes by other investigators [15] are consistent with such a scenario; pyruvate and lactate are gluconeogenic precursors and their increased concentrations may result from a block in gluconeogenesis. It should be noted that HIF-1 enhances hepatic glucose production during hypoxia by activating PEPCK through direct binding of HIF-1 to the promoter region of the PEPCK gene [17]. Thus, the observed effects in Chuvash polycythemia are more consistent with an effect mediated by HIF-2.
In addition to effects on gluconeogenesis, enhanced HIF signaling also affects glycolysis and glycogenesis in ways that would be expected to lower serum glucose and glycosylated hemoglobin levels [9-11]. Specifically, HIF-1 enhances expression of the glucose transporter, GLUT1 [9, 10], and the glycolytic enzymes, PDK [9] and LDHA [18]. PDK inactivates pyruvate dehydrogenase, thus pushing pyruvate to lactate through the action of LDHA rather than to mitochondrial oxidation. In turn, lactate is necessary to regenerate NAD+ to re-feed the earlier steps in glycolysis. HIF-1 also promotes glycogenesis, or increased production of glycogen from glucose in the liver and other tissues, by inducing glycogen synthase-1 (GYS1) [11, 19] and protein phosphatase 1 regulatory subunit 3 C (PPP1RsC) [20], which can lead to lowered glucose concentration [21].
In the current study, Chuvash polycythemia transgenic mice exhibited both decreased fasting glycemia and decreased glucose excursions after glucose tolerance testing. These results suggest that VHLR200W homozygotes experience lower glucose levels not only because of changes in hepatic glucose production (a major determinant of fasting glucose level) but also because of increased peripheral glucose utilization, which in the fed or glucose-challenged state is primarily in skeletal muscle. Consistent with this hypothesis, in skeletal muscle of the Chuvash polycythemia mouse model we observed up regulation of Glut1, the basal (non-insulin-stimulated) glucose transporter, and a trend toward an increase in Glut4, the insulin-stimulated transporter. Increased glucose utilization would also be expected to contribute to the observed high lactate levels by increased production, adding to the decreased utilization of lactate predicted by the changes in gluconeogenesis. Indeed, both Pdk1 and Pdk4 were highly up regulated in muscle of the Chuvash polycythemia transgenic mice, which would contribute to decreased glucose oxidation and increased lactate production through the inhibition of pyruvate dehydrogenase. Similar up regulation of PDK1 and PDK4 has also been reported in humans with Chuvash polycythemia [15]. All of these changes are consistent with the improved glucose tolerance in Chuvash polycythemia mice being related both to decreased hepatic glucose production and increased glucose utilization by skeletal muscle.
Altered regulation of the beta islet cells of the pancreas is another potential mechanism for reduced glucose concentrations in Chuvash polycythemia but it was not explored in humans in this study. In the Chuvash polycythemia mice model, fasting glucose levels were significantly lower than in wild types. Normally, lower fasting glucose levels would trigger lower insulin levels. Fasting insulin levels, however, did not differ between the groups. This suggests that the Chuvash polycythemia model exhibits augmented insulin secretion (i.e. enhanced stimulus-secretion coupling), enhanced insulin sensitivity, or a combination of the two. Consistent with augmented fasting insulin secretion in the Chuvash polycythemia mice, enhanced HIF-1 activity in mice with homozygous deletion of VHL results in stimulation of basal rates of glycolysis and therefore basal ATP production in pancreatic β-cells, in turn resulting in higher fasting insulin levels.[22] Conversely, mice engineered to lack HIF-1β in pancreatic β-cells exhibited impaired insulin secretion and changes in the islet gene expression resembling that of human diabetic islets [23, 24]. When glucose-challenged, however, the mice with homozygous deletion of VHL exhibit impaired glucose-stimulated insulin secretion because of decreased rates of glucose oxidation [19, 22, 25]. This effect by itself would be expected to lead to higher glucose excursions after glucose challenge in the Chuvash polycythemia mice, and instead the opposite was observed, suggesting that any effects of the VHL mutation on insulin secretion are balanced by the effects of HIF-1 to decrease glucose production and enhance glucose utilization, as described above. The situation may be even more complex, in that decreased levels of HIF-1 have also been associated with impaired β-cell function, and increasing HIF-1 levels by inhibiting degradation of HIF-1α through iron chelation improves insulin secretion and glucose tolerance in mice fed a high-fat diet [26]. Thus, it has been proposed that there may be a dose-response curve for the effect of HIF-1 on β-islet cell function, with deletion of HIF-1α being deleterious, with mild increases of HIF-1 being beneficial, and with very high levels also being deleterious [26].
The increased glycerol level observed in metabolomic analysis may reflect increased lipolysis and is consistent with the trend toward increased serum triglycerides. Hypoxia is known to increase lipolysis [27] but fat anabolic pathways are also up-regulated in hypoxia, mediated at least in part by up regulation of the transcription factors PPARγ and SREBP-1 [28-30]. Thus, the effects of the VHLR200W mutation on lipid metabolism may be pleiotropic and require further study. The increases in citrate, phosphate, and urea levels in metabolomics analysis are at present of unknown significance or mechanism. A speculation that is consistent with known effects of hypoxic signaling is that higher citrate in the VHLR200W population could reflect increased glycolysis and delivery of pyruvate to the TCA cycle [9]. We did observe increased expression of Glut1, although the net effect of increased production of pyruvate on increased TCA cycle activity would be dampened by the observed increases in Pdk1 and Pdk4 that were seen in the skeletal muscle of Chuvash polycythemia mice.
Our study has some limitations. First, the blood glucose, cholesterol, triglyceride and metabolomics levels obtained from the VHLR200W homozygotes and controls were random rather than fasting. Random levels can be altered by consumption of food, especially high fat and or carbohydrate content, and therefore may not be the best indicators for glucose or lipid metabolism. Second, the type of diet, the degree of physical activity and having a first-degree family member with diabetes mellitus are factors that might affect glucose tolerance in both VHLR200W homozygotes as well as in controls. We have not adjusted our results for these variables. Third, a number of factors not specifically addressed by this study might affect hemoglobin A1c levels, such as blood transfusions [31], hemolysis [32], chronic renal insufficiency [33], severe hypertriglyceridemia [34], chronic alcohol consumption [35]. In our cohort, none of the participants had received blood transfusion and there was no difference in alcohol consumption between the two groups. There is no evidence that Chuvash polycythemia subjects have lower red cell survival. There were trends to lower rather than higher BUN and creatinine concentrations in VHLR200W homozygotes. The serum triglyceride level tended to be higher in the VHLR200W homozygotes compared to controls, but nevertheless the glycosylated hemoglobin levels were lower in the VHLR200W homozygotes.
In conclusion, in this study we report that VHL 598C>T homozygosity is associated with lower glucose concentrations and lower glycosylated hemoglobin (HbA1c) levels indicating tighter glucose control in individuals with Chuvash polycythemia compared to controls and suggesting a possible role for manipulation of HIF expression as a means to treat diabetic patients.
Acknowledgements
Supported in part by 2 R25-HL03679-08 (VRG) from the National Heart, Lung and Blood Institute (NHLBI) and the Office of Research on Minority Health, by Howard University General Clinical Research Grant No. MO1-PR10284, by R01HL079912-04 (VRG) from NHLBI, by 1R01 DK081842 (DAM) from NIDDK, by UL1 RR025764 (DAM) from NCRR, by the Research Service of the Veterans Administration (DAM), by the Marilyn Jane Robinson Foundation (DAM), NIDDK P30 DK072437 (JEC) and by 1P01CA108671-O1A2 (National Cancer Institute, Bethesda, MD) awarded to the Myeloproliferative Disorders Consortium (project 1, PI: J.T.P.)
Footnotes
Disclosure Statement
Disclosure summary: No conflicts of interest
References
- 1.Safran M, Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 4.Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 5.Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, Butman JA, Jedlickova K, Prchal JT, Polyakova LA. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103:3924–3932. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]
- 6.Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis. J Clin Invest. 2007;117:3879–3889. doi: 10.1172/JCI32614. DOI 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindgarde F, Ercilla MB, Correa LR, Ahren B. Body adiposity, insulin, and leptin in subgroups of Peruvian Amerindians. High Alt Med Biol. 2004;5:27–31. doi: 10.1089/152702904322963663. [DOI] [PubMed] [Google Scholar]
- 8.Baracco R, Mohanna S, Seclen S. A comparison of the prevalence of metabolic syndrome and its components in high and low altitude populations in peru. Metabolic syndrome and related disorders. 2007;5:55–62. doi: 10.1089/met.2006.0019. [DOI] [PubMed] [Google Scholar]
- 9.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol. 2008;172:738–747. doi: 10.2353/ajpath.2008.070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pescador N, Villar D, Cifuentes D, Garcia-Rocha M, Ortiz-Barahona A, Vazquez S, Ordonez A, Cuevas Y, Saez-Morales D, Garcia-Bermejo ML, Landazuri MO, Guinovart J, del Peso L. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS One. 2010;5:e9644. doi: 10.1371/journal.pone.0009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SK, Haase VH, Johnson RS. von Hippel Lindau tumor suppressor regulates hepatic glucose metabolism by controlling expression of glucose transporter 2 and glucose 6-phosphatase. International journal of oncology. 2007;30:341–348. [PubMed] [Google Scholar]
- 13.Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. DOI 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–25711. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- 15.Formenti F, Constantin-Teodosiu D, Emmanuel Y, Cheeseman J, Dorrington KL, Edwards LM, Humphreys SM, Lappin TR, McMullin MF, McNamara CJ, Mills W, Murphy JA, O’Connor DF, Percy MJ, Ratcliffe PJ, Smith TG, Treacy M, Frayn KN, Greenhaff PL, Karpe F, Clarke K, Robbins PA. Regulation of human metabolism by hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2010;107:12722–12727. doi: 10.1073/pnas.1002339107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annual review of nutrition. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- 17.Choi JH, Park MJ, Kim KW, Choi YH, Park SH, An WG, Yang US, Cheong J. Molecular mechanism of hypoxia-mediated hepatic gluconeogenesis by transcriptional regulation. FEBS Lett. 2005;579:2795–2801. doi: 10.1016/j.febslet.2005.03.097. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Cantley J, Selman C, Shukla D, Abramov AY, Forstreuter F, Esteban MA, Claret M, Lingard SJ, Clements M, Harten SK, Asare-Anane H, Batterham RL, Herrera PL, Persaud SJ, Duchen MR, Maxwell PH, Withers DJ. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest. 2009;119:125–135. doi: 10.1172/JCI26934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen GM, Zhang FL, Liu XL, Zhang JW. Hypoxia-inducible factor 1-mediated regulation of PPP1R3C promotes glycogen accumulation in human MCF-7 cells under hypoxia. FEBS Lett. 2010;584:4366–4372. doi: 10.1016/j.febslet.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Luo X, Zhang Y, Ruan X, Jiang X, Zhu L, Wang X, Ding Q, Liu W, Pan Y, Wang Z, Chen Y. Fasting-induced protein phosphatase 1 regulatory subunit contributes to postprandial blood glucose homeostasis via regulation of hepatic glycogenesis. Diabetes. 2011;60:1435–1445. doi: 10.2337/db10-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zehetner J, Danzer C, Collins S, Eckhardt K, Gerber PA, Ballschmieter P, Galvanovskis J, Shimomura K, Ashcroft FM, Thorens B, Rorsman P, Krek W. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta cells. Genes Dev. 2008;22:3135–3146. doi: 10.1101/gad.496908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O’Connell PJ, Gonzalez FJ, Kahn CR. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Pillai R, Huypens P, Huang M, Schaefer S, Sheinin T, Wettig SD, Joseph JW. Aryl hydrocarbon receptor nuclear translocator/hypoxia-inducible factor-1{beta} plays a critical role in maintaining glucose-stimulated anaplerosis and insulin release from pancreatic {beta}-cells. J Biol Chem. 2011;286:1014–1024. doi: 10.1074/jbc.M110.149062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puri S, Cano DA, Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes. 2009;58:433–441. doi: 10.2337/db08-0749. DOI db08-0749 [pii] 10.2337/db08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O’Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, Okada T, Wang XL, Yim SH, Shah Y, Grey ST, Biankin AV, Kench JG, Laybutt DR, Gonzalez FJ, Kahn CR, Gunton JE. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120:2171–2183. doi: 10.1172/JCI35846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen TS, Myrmel T, Skulberg A, Severson DL, Mjos OD. Effects of hypoxia on lipolysis in isolated rat myocardial cells. Molecular and cellular biochemistry. 1989;88:139–144. doi: 10.1007/BF00223435. [DOI] [PubMed] [Google Scholar]
- 28.Piguet AC, Stroka D, Zimmermann A, Dufour JF. Hypoxia aggravates non-alcoholic steatohepatitis in mice lacking hepatocellular PTEN. Clin Sci (Lond) 2010;118:401–410. doi: 10.1042/CS20090313. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan A, Karnad DR, Limaye U, Siddharth W. Cerebral venous and dural sinus thrombosis in severe falciparum malaria. J Infect. 2004;48:86–90. doi: 10.1016/s0163-4453(03)00130-0. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiological genomics. 2006;25:450–457. doi: 10.1152/physiolgenomics.00293.2005. [DOI] [PubMed] [Google Scholar]
- 31.Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem. 2011;57:344–346. doi: 10.1373/clinchem.2010.157321. DOI 10.1373/clinchem.2010.157321. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 1995;18:896–909. doi: 10.2337/diacare.18.6.896. [DOI] [PubMed] [Google Scholar]
- 33.de Boer MJ, Miedema K, Casparie AF. Glycosylated haemoglobin in renal failure. Diabetologia. 1980;18:437–440. doi: 10.1007/BF00261697. [DOI] [PubMed] [Google Scholar]
- 34.Falko JM, O’Dorisio TM, Cataland S. Spurious elevations in glycosylated hemoglobin (HbA1) secondary to hypertriglyceridemia. Arch Intern Med. 1982;142:1370–1371. [PubMed] [Google Scholar]
- 35.Stevens VJ, Fantl WJ, Newman CB, Sims RV, Cerami A, Peterson CM. Acetaldehyde adducts with hemoglobin. J Clin Invest. 1981;67:361–369. doi: 10.1172/JCI110043. [DOI] [PMC free article] [PubMed] [Google Scholar]