Abstract
Myoblasts are precursor muscle cells that lie nascent to mature skeletal muscle. Once muscle is damaged, these cells migrate, fuse, and regenerate the muscle tissue. It is known that skeletal muscle can partially regenerate in vivo after muscle tissue damage. However, this regeneration does not always occur, especially in more severe injuries. Cellular therapy using tissue-engineering approaches has been shown to improve organ repair and function. To exploit potential benefits of using cell therapy as an avenue for skeletal muscle repair, it is important to understand the cellular dynamics underlying skeletal myocyte formation and growth. Cardiac fibroblasts have been shown to have a major influence on cardiomyocyte function, repair, and overall spatial distribution. However, little is known regarding fibroblasts’ role on skeletal myocyte function. In this study, we utilized a reconfigurable co-culture device to understand the contact and paracrine effects of fibroblasts on skeletal myocyte alignment and differentiation using murine myoblast and fibroblast cell lines. We demonstrate that myotube alignment is increased by direct contact with fibroblasts, while myotube differentiation is reduced both in the gap and contact configurations with fibroblasts after 6 days of co-culture. Furthermore, neutralizing antibodies to FGF-2 can block these effects of fibroblasts on myotube differentiation and alignment. Finally, bi-directional signaling is critical to the observed myoblast-fibroblast interactions, since conditioned media could not reproduce the same effects observed in the gap configuration. These findings could have direct implications on cell therapies for repairing skeletal muscle, which have only utilized skeletal myoblasts or stem cell populations alone.
Keywords: Co-culture, myogenesis, reconfigurable, myoblast, fibroblast, FGF-2
INTRODUCTION
Myocytes are the primary cell type found in skeletal muscle. They are arranged in an organized fashion, developing into muscle fibers that align with each other to create a functional tissue. In vivo, myoblast precursor cells develop from quiescent satellite cells nascent to muscle that proliferate and migrate to the site of injury (Schultz and Lipton 1978). These mononucleated cells are responsible for the regenerative or repair mechanisms of skeletal muscle (Hawke and Garry 2001). These cells fuse with one another when in close proximity and become terminally differentiated. This fusion creates multinucleated cells called myotubes, the building blocks in muscle fiber formation (Sharples, Al-Shanti et al.). It is well established that most skeletal muscle has a large regenerative capacity due to satellite cell infiltration in response to injury (Grounds and Yablonka-Reuveni 1993). However, in larger and more severe injuries, skeletal muscle may not regenerate completely, and fibrosis and scar tissue can cause decreased functionality of the tissue (McKeon-Fischer, Flagg et al. 2011). Additionally, in regions that are not associated with limbs, there is heterogeneity in the proliferation efficiency of these satellite cells due to injury response, and simultaneously there may be a lack of these cells to cause an appreciable difference in repair at the site of injury (Pavlath, Thaloor et al. 1998). There is therefore a need to develop strategies for skeletal muscle repair.
A number of other cell types may be potentially important in this repair and satellite cell differentiation process. In vivo, myogenic cells are also influenced by fibroblasts at the site of injury, particularly in establishing a stabilizing extracellular matrix (Mann, Perdiguero et al. 2011). These fibroblasts sit in close proximity to muscle tissue and develop the basement membrane of this tissue. This relationship between fibroblasts and myocytes is well characterized in the heart. Cardiomyocytes undergo phenotypic and morphological changes when in the presence of cardiac fibroblasts in vitro, including increased contractility (LaFramboise, Scalise et al. 2007). It has also been shown that merely the fibroblast paracrine signals are enough to attribute to these differences (LaFramboise, Scalise et al. 2007). Other studies have shown that cardiomyocytes align, contract, and avoid apoptosis pathways when co-cultured with cardiac fibroblasts (Nichol, Engelmayr et al. 2008). Extensive in vitro data has suggested that cardiac fibroblasts are necessary in repair, function, matrix synthesis/degradation, and important cytokine secretion (Pirskanen, Kiefer et al. 2000; Porter and Turner 2009). Moreover, producing functional cardiac tissue in vivo improves with the implantation of both fibroblast and cardiomyocyte cell types in organized cell sheets (Kobayashi, Shimizu et al. 2008). The requirement for these two cell types to be present and synergistically create a functional tissue could analogously have similar implications for skeletal muscle; however, the effect of fibroblasts on myoblast differentiation and alignment has not been well documented or studied.
Interestingly, in diseased models such as arrhythmia and myocardial infarction, myofibroblasts actually have a negative effect on cardiac remodeling. They can undergo “fibrotic remodeling” which causes an overabundant secretion of ECM proteins that separates cardiomyocytes and inhibits electrical conduction (Rohr 2009). These cells are unable to go through normal apoptosis in this state, and persist to create scar tissue (Peterson, Ju et al. 1999; Sun, Kiani et al. 2002; Rohr 2009). From this data, there is a potential for skeletal fibroblasts to also have a negative effect on skeletal muscle formation in characteristic phenotypic states.
Few groups have studied how fibroblasts influence skeletal myoblasts. The most extensive study to date involved plating C2C12 myoblasts on a fibroblast feeder layer, which resulted in myotubes that were highly adherent and contractile with functional electrical propagation as evidenced by calcium transients between cell types (Cooper, Maxwell et al. 2004). This study showed that fibroblasts play a beneficial role in maintaining mature myotube functionality. However, it did not examine how fibroblasts influence differentiation of myoblasts into mature myotubes. Another more recent study used a transwell assay to co-culture myoblasts and fibroblasts, and demonstrated that fibroblast paracrine factors protected myoblasts from apoptosis when undergoing differentiation (Zhang, Li et al. 2010). Understanding fibroblast influence on these muscle progenitor cells, as in the case with cardiac muscle cells, could have direct implications on cell therapies for repairing skeletal muscle, which have to date only utilized skeletal myoblasts or stem cell populations alone (Otto, Collins-Hooper et al. 2009). Those that have used myoblast or satellite cell therapies alone have shown some improvement, but demonstrate the need for more efficient delivery, inhibited apoptosis, and increased therapeutic response (Rando and Blau 1994; Fan, Maley et al. 1996; Beauchamp, Morgan et al. 1999; Peault, Rudnicki et al. 2007; Otto, Collins-Hooper et al. 2009).
In this study, we investigated the role of fibroblasts on skeletal muscle progenitor differentiation and alignment using a microfabricated, reconfigurable co-culture device (Hui and Bhatia 2007). This setup allows for both contact and paracrine signals to be monitored independently and in tandem. It also provides the user with the ability to separate two cell populations and dynamically study and manipulate the co-culture configuration (Hui and Bhatia 2007). Furthermore, this technology can: 1) determine whether a cell type has an influence on another’s differentiation, proliferation, etc., 2) conclude whether a paracrine signal or a contact dependency is needed, and 3) isolate only the cell type of interest for analysis. These advantages allow one to better understand the mechanisms of cross talk between two cell types of interest.
Herein, we analyzed C2C12 murine myoblasts co-cultured with 3T3 murine fibroblasts on the reconfigurable co-culture device. We demonstrate that fibroblasts inhibited myoblast differentiation independent of contact, while cell-cell contact between the two cell types yielded more organized alignment of myotubes upon differentiation. Secretion of basic fibroblasts growth factor (FGF-2) is an important player in both regulating differentiation and, in conjunction with contact signals, improving myotube alignment. Lastly, these two cell types are required to be in the presence of one another to elicit such responses. It has been shown with multiple cell types, most commonly with tumor or cancer cell communication, that protein signaling is only turned on when two or more cells “cross-talk” with one another (Buchanan, Szot et al. 2011). In this study we show that fibroblasts and myoblasts must be in close proximity with one another (as opposed to using conditioned media of one on the other) to show these differences.
MATERIALS AND METHODS
Cell Culture
Murine C2C12 myoblasts and 3T3 fibroblast cells (ATCC, Manassas, VA) were each cultured in growth media (GM) consisting of high glucose Dulbecco’s Modified Eagle Medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (Hyclone), 100 units/mL penicillin, and 100 µg/mL streptomycin (Gibco, Grand Island, NY). 3T3 and C2C12 cells were cultured at 37°C and 5% CO2 and split at 1:10 when 80% confluence was reached; media was changed every two days. For myoblast culture, tissue culture flasks were coated with 1 mg/mL collagen in 0.1 M acetic acid for 1 hour at 37°C and rinsed with 1X Dulbecco’s Phosphate Buffer Saline (PBS) prior to seeding. Fibroblasts were cultured on uncoated tissue culture flasks.
Reconfigurable Co-Culture Device
The silicon reconfigurable co-culture devices were fabricated according to previously established protocols (Hui and Bhatia 2007; Hui EE 2009). Each half of the device, called “combs” were individually coated with 1 mg/mL collagen prior to seeding for 1 hour. The collagen was removed and the combs were rinsed with sterile PBS. For each comb pair, myoblasts were seeded on the male half; the female half was seeded with either fibroblasts or myoblasts. Each collagen coated comb half was placed in a 12-well plate. Approximately 1,000,000 C2C12 and 750,000 3T3 cells were added to each half to create complete confluence upon seeding. The plate was then placed in the incubator at 37°C. In order to achieve a complete monolayer on the devices, the plate was lightly shaken for 5 seconds by hand after 30 minutes and 1 hour after seeding. After six hours, the combs were each individually rinsed with 1X PBS and the parts were fit together with sterile tweezers (SPI, West Chester, PA). The combs were connected in the proper configuration and maintained in a new 12-well plate containing 2 mL of cell culture media. The combs were visualized via an upright microscope to ensure that the fingers fit properly (in either gap or contact) and no cellular debris was floating in the media. Media was changed every two days.
The following groups were examined: myoblasts cultured with myoblasts in gap, myoblasts cultured with myoblasts in contact, myoblasts cultured with fibroblasts in gap, and myoblasts cultured with fibroblasts in contact (4 groups). Each group contained two sets of comb pairs and every finger of the male combs (9 fingers per comb), containing the cells of interest, was individually analyzed (n = 18 per group). After six days the four groups were stained, mounted and analyzed by immunofluorescence as described below. For the FGF-2 neutralization experiments, 2 comb pairs (n=18) per group were cultured with anti-FGF-2 neutralizing antibody (Millipore, Temecula, CA) at 10 µg/uL for six days. This was well mixed in pre-warmed media and replenished every 2 days. 2 mL of this media was added to each comb in the same manner as stated previously.
Conditioned Media Assays
3T3 fibroblasts were cultured independently of C2C12 myoblasts prior to the experiment. Once the fibroblasts had reached confluence, myoblasts were seeded at 500,000 cells/well in eight wells of a collagen coated (1 mg/mL) 48-well plate. The wells were filled with a total volume of 1mL of GM. Once the myoblasts were confluent, media was removed from these wells and 500 µL of new GM was added with 500 µL of media from cultured fibroblasts. In the other four wells, this 50:50 ratio was composed of new GM and myoblast cultured media. 500 µL of media was removed from these wells and 500 µL of fresh GM was added. These media changes were carried out from day 1 and continued every subsequent day until day 6. On day 6, the media was removed and cells were rinsed with PBS. They were fixed with 4% paraformaldehyde, stained, and imaged as described below. Three 200X images were taken for each well (n=12).
Transwell Assays
Eight wells of a 24-well transwell plate (Corning, Union City, CA) were coated with 1 mg/mL collagen. Four of the wells contained 3T3 fibroblasts on a membrane over C2C12 myoblasts. The other four were myoblasts co-cultured over myoblasts. All wells were seeded with 500,000 C2C12s and the membrane was seeded with either 100,000 myoblasts or fibroblasts. The cells on membranes were cultured separately until adherent, at which point they were placed on top of the myoblast wells. A total of 700 µL of GM covered each transwell. This media was changed every two days and on day 6, the membranes were removed and wells were fixed and stained according to the immunofluorescence procedures outlined below. Three 200X images were taken per well for analysis (n=12).
Immunofluorescence Quantification
After 6 days, cells were fixed with 4% paraformaldehyde (Wako Chemicals, Richmond, VA) for ten minutes at room temperature. Once fixed, the cells were rinsed with PBS to remove any excess paraformaldehyde. The fixed cells were permeabilized, and blocked in “blocking buffer,” containing .03% Triton-X 100 and 1% Bovine Serum Albumin in 1X PBS. Primary antibody, mouse anti-mouse myosin heavy chain (skeletal, fast, 1:200, SIGMA, St. Louis, MO), was incubated in the blocking buffer for 1 hour at room temperature. Secondary antibodies (Alex Fluor 588, 1:200, Invitrogen, Carlsbad, CA) were incubated for 30 minutes at room temperature in blocking buffer. Cells were stained with Phalloidin 468 (1:200, Millipore, Temecula, CA) in buffer for 30 additional minutes. Hoescht 33342 (0.1µg/ml in DI water, Invitrogen, Carlsbad, CA) staining was used to visualize the nuclei. Once stained, the combs were taken out of wells and mounted on slides with Fluoromount-G (Southern Biosciences, Birmingham, AL).
A Zeiss Observer.D1 fluorescent Axio Observer scope was used for image acquisition, and Axiovision software was employed for image analysis. 100X images were taken at the free end of the finger of each male comb. Three 200X images were analyzed from each well of the conditioned media and transwell assays. Percent differentiation was calculated by manually counting the total number of nuclei within myotubes and dividing by total nuclei within the image. The total number of nuclei was quantified automatically via AxioVision software. Each myotube was identified and the angle deviation relative to the parallel of the comb finger was measured by AxioVision angle measurement tool.
RESULTS
To study the effect of the presence of fibroblasts on myoblast differentiation and subsequent alignment, we employed a previously described microfabricated co-culture device (Hui and Bhatia 2007; Hui EE 2009). This device has interlocking “combs” that can be positioned either to allow direct cell-cell contact or to separate the cell populations by a gap of 80 microns (Figure 1). In this study, the male end contained the analyzed cell type (myoblasts only) and the female end contained either fibroblasts or myoblasts.
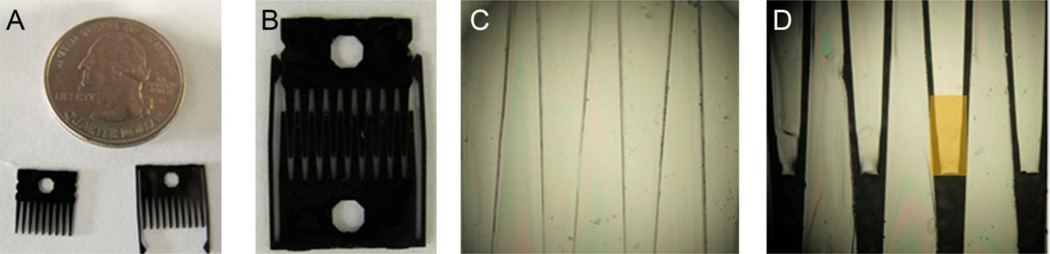
Fig. 1.
Visual representation of the reconfigurable co-culture device. (A) Both male and female pairs of “combs.” The holes in the comb allow the user to move and manipulate the device with the use of forceps. (B) Photo of a comb pair in gap mode. (C, D) 40X brightfield images in both contact and gap mode respectively. Orange box represents area of interest for analysis in which we refer to as the “finger” of the comb. An 80 µm gap separates the fingers in gap mode. Cells are unable to traverse this gap, only secreted factors.
Each finger of every configuration (myoblasts co-cultured with myoblasts or myoblasts co-cultured with fibroblasts, in gap or contact) was imaged by the methods described above. Figure 2 demonstrates examples of comb images with staining for F-actin, myosin heavy chain, and nuclei, as shown in green, red, and blue respectively. Each of the four images represented are one of 18 fingers analyzed for each of the four groups. The 80 µm gap was clearly visible in these cultures and showed no positive staining. Myosin heavy chain, a marker for myotube formation, was used to determine percent differentiation of myoblasts. Fibroblast comb halves did not stain positive for myosin heavy chain as expected. This demonstrates that no cross-contamination had occurred in either gap or contact configurations.
Fig. 2.
Immunofluorescent images of a given myoblast finger in all culture configurations after 6 days in culture. Nuclei, f-actin, and myosin heavy chain are stained in blue, green, and red respectively (scale bar = 200 µm.) (A) Myoblasts co-cultured with myoblasts in contact configuration. (B) Myoblasts co-cultured with myoblasts in gap mode. (C, D) Fibroblasts co-cultured with myoblasts in contact and gap mode respectively. Note: No myosin heavy chain staining is found on fibroblast fingers.
Percent differentiation, as reflected by myosin heavy chain positive cells and highly aligned myotubes are two phenotypic characteristics reflective of a mature muscle phenotype. To assess the effects of fibroblasts on myoblast maturation, we quantified both the percent differentiation and degree of alignment of myoblasts in myoblast-myoblast and myoblast-fibroblast co-cultures, when in contact and in gap.
Myotube alignment is increased only when in contact with fibroblasts and percent differentiation is decreased in the presence of fibroblasts
Myotube alignment, as well as percent differentiation, is a desirable phenotype for myoblast differentiation into mature myotubes. More highly aligned myotubes recapitulates the more favorable mature muscle phenotype in vitro. We quantified the alignment of the myotubes on the fingers of each of the male combs (n=18). The alignment was calculated by averaging all of the angles between each myotube and that of the parallel of the finger of the comb. After six days in culture, the co-cultures containing fibroblasts in contact with myoblasts showed an increase in alignment with the finger parallel by approximately 10 degrees (Figure 3A), which is more evident when representing the data in a histogram format (Figure 3B). Myoblast-fibroblast cultures in contact show a skewed distribution towards lower degree angles, suggesting that a larger number of myotubes are more highly aligned to the parallel in this culture type. All other co-culture settings showed no significant difference in alignment between each other.
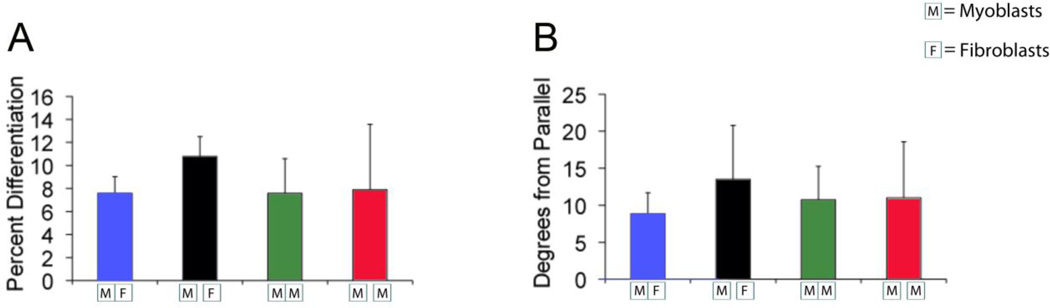
Fig. 3.
Quantification of myotube alignment in the reconfigurable co-culture device after 6 days in culture. “M” represents myoblasts; “F” represents fibroblasts in each figure. Contact mode is represented by adjacent boxes touching one another and gap is represented by a space between boxes. (A) Graphical representation of degrees from parallel versus culture type. Lower values indicate more aligned myotubes. The average degree from parallel of the finger of the comb in the myoblast-fibroblast contact co-culture was significantly less (*p< 0.05) compared to all other groups, indicating that the myoblasts were more aligned. (B) Histogram representation of myotube alignment. The data for degrees from parallel is grouped into 6 bins. Lower bin numbers represent more aligned myotubes with the comb finger. Graph shows a skewed distribution of myoblasts and fibroblasts in contact toward lower degree numbers
We show that regardless of configuration, contact or gap, fibroblasts caused a significant inhibition of myoblast differentiation (Figure 4). Since percent differentiation between gap and contact in myoblast-fibroblast co-cultures showed no differences, this further suggested that the inhibition of differentiation seen in fibroblast cultures is due to a paracrine signal and not a contact dependent signal. In myoblast-myoblast co-cultures, a higher percentage of nuclei were contained within myotubes than in co-cultures with fibroblasts. There were up to 50% more nuclei that co-stained positive with myosin heavy chain for these myoblast only cultures. No significant differences were observed between myoblasts in either contact or gap. This finding suggested that fibroblasts inhibited this myotube formation in culture on the male half of the comb. Unlike these differentiation studies, myotube alignment was only increased in contact with fibroblast cultures, suggesting contact dependent (alignment) and contact independent (differentiation) pathways underlying the effects of fibroblasts on myoblast differentiation.
Fig. 4.
Quantification of percent differentiation in the reconfigurable co-culture device. Co-culture with fibroblasts reduced the percent differentiation on the myoblast comb compared to myoblast only cultures regardless of configuration after 6 days (*p <0.01), suggesting a paracrine factor is responsible.
Myotube differentiation is reduced and alignment is increased in fibroblast co-culture through an FGF-2-dependent mechanism
It is well documented that FGF-2 has a major influence on increasing skeletal muscle cell proliferation and prevents their terminal differentiation (Cook, Doumit et al. 1993; Wiedlocha and Sorensen 2004; Li, Wei et al. 2010; Stratos, Madry et al. 2011). To determine whether FGF-2 could influence differentiation of myoblasts in contact with fibroblasts, we sought to assess percent myoblast differentiation in these co-cultures containing media, which includes or excludes neutralizing antibodies to FGF-2. Neutralizing antibodies to FGF-2 have been shown to bind and neutralize the effects of soluble FGF-2 to its receptors, and thus eliminate its functionality (Sheikh, Fandrich et al. 1999). Unlike our previous observations, we show that fibroblasts do not significantly influence myoblast differentiation and alignment in myoblast-fibroblast co-cultures in the presence of FGF-2-neutralizing antibodies, when compared to other co-culture conditions (Figure 5). Interestingly, our results reveal that the percent of differentiated myoblasts in myoblast-fibroblast co-cultures increased to the level endogenously observed in myoblast-myoblast cultures. However, myotube alignment in these studies remained similar to what was previously observed in all other cell culture types.
Fig. 5.
FGF-2 blocking by anti-FGF-2 neutralizing antibody. No significance is found between any groups for (A) percent differentiation or (B) quantification of alignment of myoblasts after 6 days in co-culture with an FGF-2 neutralizing antibody added into the media. Percent differentiation of both myoblast-fibroblast contact and gap cultures and alignment in the fibroblast-myoblast contact culture increased as a result of adding the neutralizing FGF-2 antibody
Bi-directional signaling is critical in myoblast-fibroblast interactions
In this study we show that FGF-2 plays a major influential role in myoblast differentiation and alignment in myoblast-fibroblast co-cultures. We wanted to further explore whether fibroblast-secreted factors, independent of a myoblast signal, resulted in the same effect. We tested this scenario by growing myoblasts in media transferred from fibroblast cultures. By utilizing this conditioned media, we were able to determine whether fibroblast secreted factors were enough to maintain the phenotypic changes seen when the cells were in direct co-culture. We reproducibly show that there are no statistical differences in percent myoblast differentiation when myoblasts are cultured with fibroblast-conditioned media (Figure 6). Percent differentiation in both groups lied between 13–15% after six days. Conditioned media experiments, therefore, did not produce the same result as the gap configuration from our previous co-culture experiments.
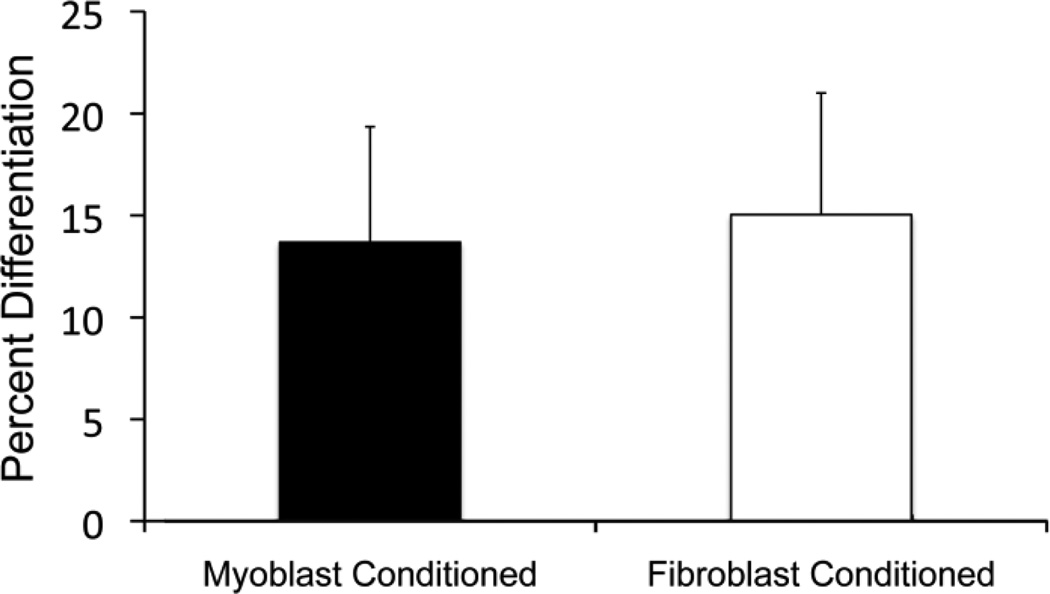
Fig. 6.
Quantification of percent differentiation of conditioned media experiments. Conditioned media from either fibroblast only cultures or myoblast only cultures was added to additional myoblasts cultures for 6 days. No significance difference in terms of differentiation was observed between cultures with fibroblast conditioned and myoblast conditioned media when staining with myosin heavy chain after 6 days.
One possible explanation of this phenomenon could be due to the secreted factors having only a short-range paracrine effect, which has previously been observed in gap co-culture (Hui and Bhatia 2007). To test the hypothesis whether paracrine factors were responsible in eliciting differentiation changes between co-culture types, we mimicked gap conditions of the comb by utilizing a transwell assay where fibroblasts were separated from myoblasts by a transwell membrane. The major differences between these assays and the gap conformation is 1) a larger distance separates the two cell types (1.35 mm rather than 80 µm), and 2) spatially the transwell has cells on top of one another (z-direction) rather than adjacent to one another on the same plane. The transwell assays recapitulated our findings from the reconfigurable co-culture device. We show that myoblast differentiation was inhibited by up to 10% in the presence of fibroblasts that were separated from myoblast-myoblast wells (Figure 7). The difference in inhibition between culture types could be readily visualized by comparing the number of myotubes in 100X images of each culture type. The behaviors of Transwell and gap co-cultures were similar, which is not consistent with short-range paracrine signaling.(Sheikh, Fandrich et al. 1999) Instead, the data support a critical role for bi-directional signaling, or cross talk. Constitutively expressed fibroblast paracrine factors captured in conditioned media do not produce the same behavior that occurs when feedback signaling between the two cell types is made possible. As mentioned previously many cells only secrete factors in the presence of another cell signal (Decker, Abdelmoneim et al. 2008; Buchanan, Szot et al. 2011). For example, drug therapies for cancer are targeted to block the cross-talk between cancer cells and stromal cells to reverse detrimental effects (Said, Smith et al. 2011). These data strongly suggest that there is a need for the two cell types to be in close proximity to communicate with one another and allow for signaling between them to cause these phenotypic changes seen by the comb and transwell assays.
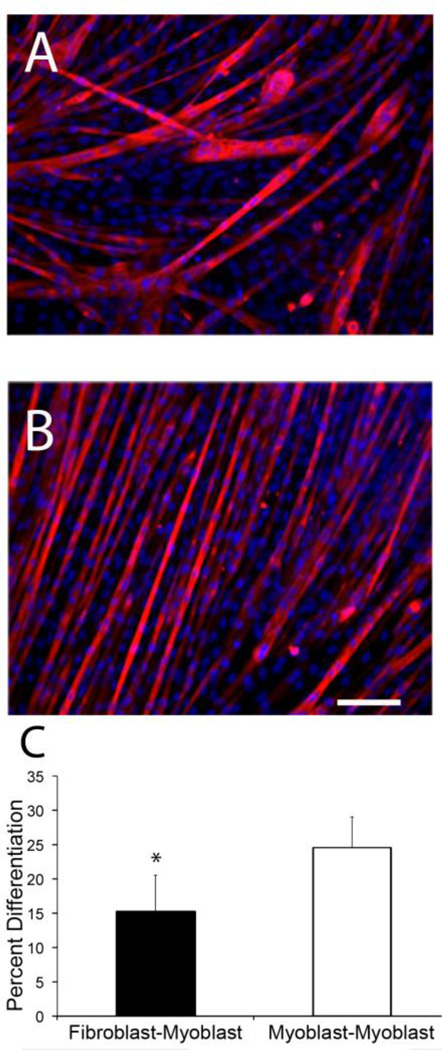
Fig. 7.
Confirmation of inhibition of differentiation by use of a transwell assay. (A) Immunofluorescence image of myoblasts when co-cultured with fibroblasts using a transwell setup. (B) Immunofluorescence image of myoblasts when co-cultured with myoblasts. The decrease in density of myotubes stained with myosin heavy chain in A shows the decrease in percent differentiation (C) Graphical representation of difference in percent differentiation between both groups after 6 days. Percent differentiation of the fibroblast co-culture is significantly decreased compared to myoblast (*p <0.01, scale bar = 100 µm)
DISCUSSION
From the results of this study, we have reproducibly shown that myoblasts differentiate into myotubes at a slower rate when fibroblasts are present in culture. Whether the fibroblasts were in contact or in gap mode in the microfabricated co-culture devices, myoblast differentiation into myotubes was inhibited. This suggests that a paracrine signal from the fibroblasts caused these phenotypic changes. These cell types are needed to be in close proximity, in co-culture, to cause such changes. Fibroblast-secreted factors alone are not enough to show any significant changes in myoblast differentiation compared to standard myoblast culture. With the reconfigurable co-culture device we were also able to show that there were changes in myotube alignment when the cultures are in physical contact with one another. Myotubes aligned parallel to the comb finger direction when fibroblasts flanked the cells in contact. This implies that cell-cell signaling via junctional (gap, connexin, or cadherin) proteins between fibroblasts and myoblasts may be necessary in order to drive myotube directionality.
We have also shown that without the reconfigurable co-culture device, these findings would not be easily observed. By modulating between gap and contact modes we are able to witness phenotypic changes. In addition, the desired readouts are more manageable on the fingers of these combs because of the spatial patterning. The ability to distinguish one cell type from another by direction and placement on each comb half was an advantage in immunofluorescence quantification. Every comb half also contains a great deal of information due to the individual “microenvironments” that every finger occupies, providing a larger sample size.
Previous literature and standard primary myoblast cell-culture, has shown that FGF-2 is needed for in vitro control over myoblast cell cycle, proliferation, and differentiation (Hannon, Kudla et al. 1996; Flanagan-Steet, Hannon et al. 2000; Neuhaus, Oustanina et al. 2003; Wiedlocha and Sorensen 2004). FGF-2 has also been used to engineer muscle cells in vitro to promote increased proliferation and regeneration in skeletal muscle (Stratos, Madry et al. 2011). By adding FGF-2, cells are stalled from differentiating in culture (Hannon, Kudla et al. 1996). By inactivating this factor with large concentrations of neutralizing antibody as done previously by several groups (Santiago, Ma et al.; Savage, Hart et al. 1993; Seghezzi, Patel et al. 1998), we observed an offset of this inhibition. Myoblasts were able to differentiate more readily even when fibroblasts were in culture with the anti-FGF-2 antibody present.
We show that FGF-2 is ultimately responsible for both the inhibition of differentiation and a player in myotube alignment. The FGF-2 pathway is well characterized and extensively studied in the context of myogenesis. FGF-2 activates RhoA thus blocking muscle differentiation (Lim, Choi et al. 2007). When RhoA is inhibited, RhoE levels increase and muscle terminally differentiates. RhoE also increases myotube alignment by an m-cadherin dependent pathway (Fortier, Comunale et al. 2008). Thus far, there was no evidence that solely fibroblast cells cause alignment of myoblast fusion when organized in contact with myoblasts. However, it has been demonstrated that myoblast alignment is dependent on a number of factors, including substrate geometries as shown by micropatterning (Bajaj, Reddy et al. 2011). Specific growth factors (some of which are secreted by fibroblasts) have been shown to play a regulatory role in both myogenesis and alignment (Peng, Wen et al. 1997; Pirskanen, Kiefer et al. 2000; Li, Wei et al. 2010; Ogawa, Firth et al. 2011; Stratos, Madry et al. 2011). As mentioned, none of these studies attribute fibroblasts as causing such changes, only growth factors that can be secreted by a host of cell types and may be abundantly found in the extracellular matrix. The necessary cell contact of fibroblasts to myoblasts to show such changes in alignment is potentially due to the cell-cell contact signals that are created between cell types in addition to the FGF-2 secreted signal.
The inhibition due to FGF-2 is only noticed when myoblasts are in communication with fibroblasts, as seen from the results of conditioned media and transwell assays. We hypothesize that this could be due to “bilateral signaling,” meaning that the myoblasts are eliciting a signal(s) that in turn causes fibroblasts to signal back causing this change in differentiation. Alternatively, multiple paracrine signals could be responsible for this change but require FGF-2 to be present. This has potential implications for skeletal muscle engineering. First, it supports the case that cellular therapy may be a necessary option in order to elicit alignment and differentiation responses. It may require a number of cells in particular ratios to cause the highest percent differentiation and alignment to form a functional tissue. As shown with cardiomyocytes, cardiac fibroblasts are required for functional electrical conduction through the heart (Baudino, Carver et al. 2006). Likewise, adding fibroblasts or even other cell types found in vivo may be necessary in regulating skeletal muscle repair or regeneration. FGF-2 has been shown to increase proliferation in myoblasts that over express the growth factor in muscle regeneration and reduces apoptosis (Stratos, Madry et al. 2011). Consequently, our results suggest that adding fibroblasts in tandem could elicit a similar effect; however, a balance between proliferation and differentiation will likely be necessary for proper regeneration.
CONCLUSION
We demonstrate that the reconfigurable co-culture device is a useful tool in understanding changes in cell phenotype as a result of another cell type. We show that myotube formation is regulated and inhibited by FGF-2 secretion by fibroblast cells. Alignment of these myotubes is governed by a contact dependent mechanism between these two cell types. However, if FGF-2 is neutralized, improvement in alignment via contact signaling is eliminated. Lastly, these two cells must be in proximity with one another, implying that there is a more than one signal between the two causing such changes. These findings are important in understanding how these cells interact together and could provide potential avenues for skeletal muscle repair in vivo.
Acknowledgements
The authors would like to thank Monica Kim for helping expedite the process of receiving the combs from UC Irvine and providing the preparation protocols. Funding for this project was provided in part by the NIH Director’s New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant no. 1-DP2-OD004309-01, and by 1R21HL104493-01. N.R. would like to thank the Jacobs Graduate Fellowship and the NIH T32 EB009380-02 Interfaces training grant. K.S. would like to thank the NSF GRFP fellowship and NIH T32-HD060555 training grant in Systems Biology of Development. E.H. would like to thank the UC Irvine ACS/IRG grant (98-279-07).
References
- Bajaj P, Reddy B, Jr, et al. Integr Biol (Camb) 2011;3:9. doi: 10.1039/c1ib00058f. [DOI] [PubMed] [Google Scholar]
- Baudino TA, Carver W, et al. Am J Physiol Heart Circ Physiol. 2006;291:3. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, et al. Journal of Cell Biology. 1999;144:6. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CF, Szot CS, et al. J Cell Biochem. 2011 [Google Scholar]
- Cook DR, Doumit ME, et al. J Cell Physiol. 1993;157:2. doi: 10.1002/jcp.1041570216. [DOI] [PubMed] [Google Scholar]
- Cooper ST, Maxwell AL, et al. Cell Motil Cytoskeleton. 2004;58:3. doi: 10.1002/cm.20010. [DOI] [PubMed] [Google Scholar]
- Decker NK, Abdelmoneim SS, et al. Am J Pathol. 2008;173:4. doi: 10.2353/ajpath.2008.080158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Maley M, et al. Muscle Nerve. 1996;19:7. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Flanagan-Steet H, Hannon K, et al. Dev Biol. 2000;218:1. doi: 10.1006/dbio.1999.9535. [DOI] [PubMed] [Google Scholar]
- Fortier M, Comunale F, et al. Cell Death Differ. 2008;15:8. doi: 10.1038/cdd.2008.34. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. Mol Cell Biol Hum Dis Ser. 1993;3 doi: 10.1007/978-94-011-1528-5_9. [DOI] [PubMed] [Google Scholar]
- Hannon K, Kudla AJ, et al. Journal of Cell Biology. 1996;132:6. doi: 10.1083/jcb.132.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon K, Kudla AJ, et al. Journal of Cell Biology. 1996;132:6. doi: 10.1083/jcb.132.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. J Appl Physiol. 2001;91:2. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Hui EE, Bhatia SN. Proc Natl Acad Sci U S A. 2007;104:14. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui EE, K S, Bhatia SN. In: Methods in Bioengineering: Microdevices in Biology and Medicine. Yarmush ML, Langer RS, editors. Artech House; 2009. [Google Scholar]
- Kobayashi H, Shimizu T, et al. J Artif Organs. 2008;11:3. doi: 10.1007/s10047-008-0421-8. [DOI] [PubMed] [Google Scholar]
- LaFramboise WA, Scalise D, et al. Am J Physiol Cell Physiol. 2007;292:5. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- Li J, Wei Y, et al. Microvasc Res. 2010;80:1. doi: 10.1016/j.mvr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Lim MJ, Choi KJ, et al. Mol Endocrinol. 2007;21:9. doi: 10.1210/me.2007-0114. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, et al. Skelet Muscle. 2011;1:1. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon-Fischer KD, Flagg DH, et al. Journal of Biomedical Materials Research Part A. 2011;99:3. doi: 10.1002/jbm.a.33116. [DOI] [PubMed] [Google Scholar]
- Neuhaus P, Oustanina S, et al. Molecular and Cellular Biology. 2003;23:17. doi: 10.1128/MCB.23.17.6037-6048.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol JW, Engelmayr GC, Jr, et al. Biochem Biophys Res Commun. 2008;373:3. doi: 10.1016/j.bbrc.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Firth AL, et al. Am J Physiol Cell Physiol. 2011 [Google Scholar]
- Otto A, Collins-Hooper H, et al. J Anat. 2009;215:5. doi: 10.1111/j.1469-7580.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlath GK, Thaloor D, et al. Developmental Dynamics. 1998;212:4. doi: 10.1002/(SICI)1097-0177(199808)212:4<495::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Peault B, Rudnicki M, et al. Mol Ther. 2007;15:5. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Peng H, Wen TC, et al. Arch Histol Cytol. 1997;60:2. doi: 10.1679/aohc.60.163. [DOI] [PubMed] [Google Scholar]
- Peterson DJ, Ju H, et al. Cardiovasc Res. 1999;41:3. doi: 10.1016/s0008-6363(98)00264-8. [DOI] [PubMed] [Google Scholar]
- Pirskanen A, Kiefer JC, et al. Dev Biol. 2000;224:2. doi: 10.1006/dbio.2000.9784. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA. Pharmacology & Therapeutics. 2009;123:2. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Journal of Cell Biology. 1994;125:6. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S. Heart Rhythm. 2009;6:6. doi: 10.1016/j.hrthm.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Said N, Smith S, et al. J Clin Invest. 2011;121:1. doi: 10.1172/JCI42912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago JJ, Ma X, et al. Cardiovasc Res. 89:1. doi: 10.1093/cvr/cvq261. [DOI] [PubMed] [Google Scholar]
- Savage MP, Hart CE, et al. Dev Dyn. 1993;198:3. doi: 10.1002/aja.1001980302. [DOI] [PubMed] [Google Scholar]
- Schultz E, Lipton BH. Anat Rec. 1978;191:3. doi: 10.1002/ar.1091910308. [DOI] [PubMed] [Google Scholar]
- Seghezzi G, Patel S, et al. J Cell Biol. 1998;141:7. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples AP, Al-Shanti N, et al. J Cell Biochem. doi: 10.1002/jcb.23308. [DOI] [PubMed] [Google Scholar]
- Sheikh F, Fandrich RR, et al. Cardiovasc Res. 1999;42:3. doi: 10.1016/s0008-6363(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Stratos I, Madry H, et al. Tissue Eng Part A. 2011 doi: 10.1089/ten.tea.2011.0239. [DOI] [PubMed] [Google Scholar]
- Sun Y, Kiani MF, et al. Basic Research in Cardiology. 2002;97:5. doi: 10.1007/s00395-002-0365-8. [DOI] [PubMed] [Google Scholar]
- Wiedlocha A, Sorensen V. Curr Top Microbiol Immunol. 2004;286 doi: 10.1007/978-3-540-69494-6_3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li H, et al. Dev Growth Differ. 2010;52:8. doi: 10.1111/j.1440-169X.2010.01209.x. [DOI] [PubMed] [Google Scholar]