Abstract
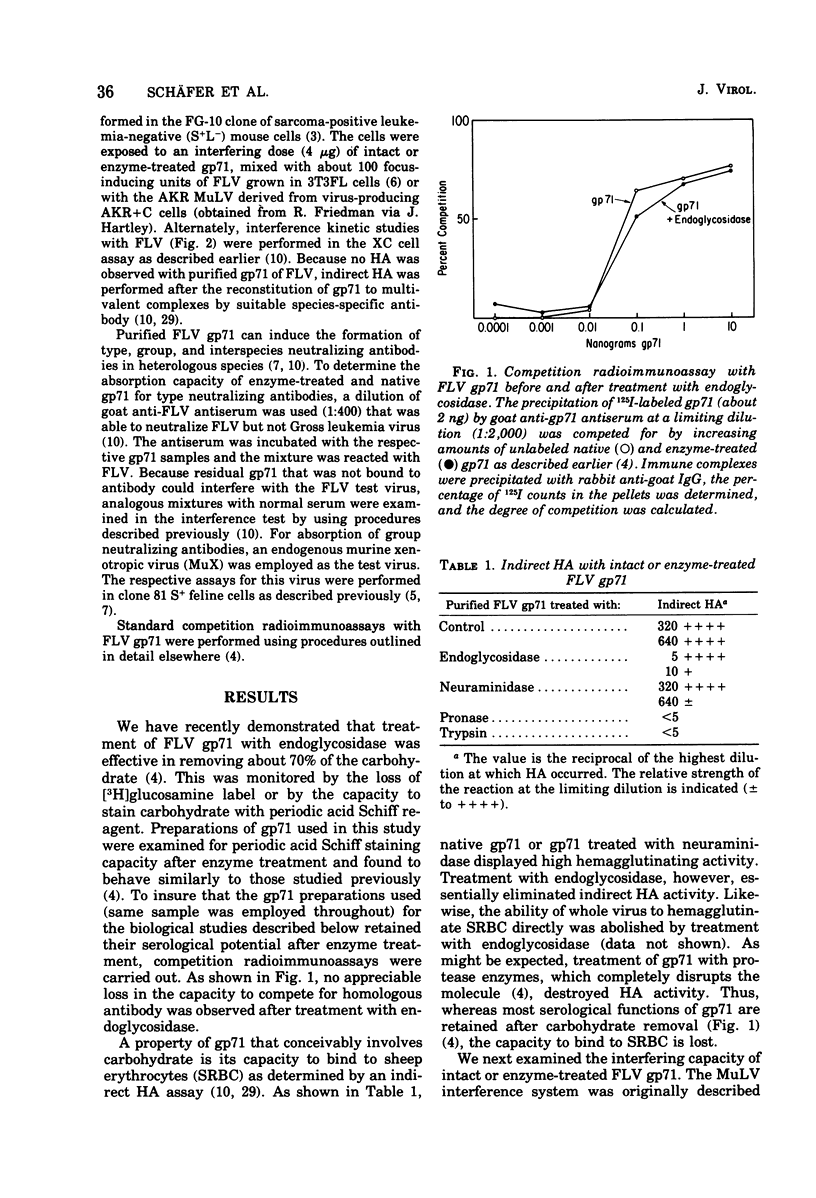
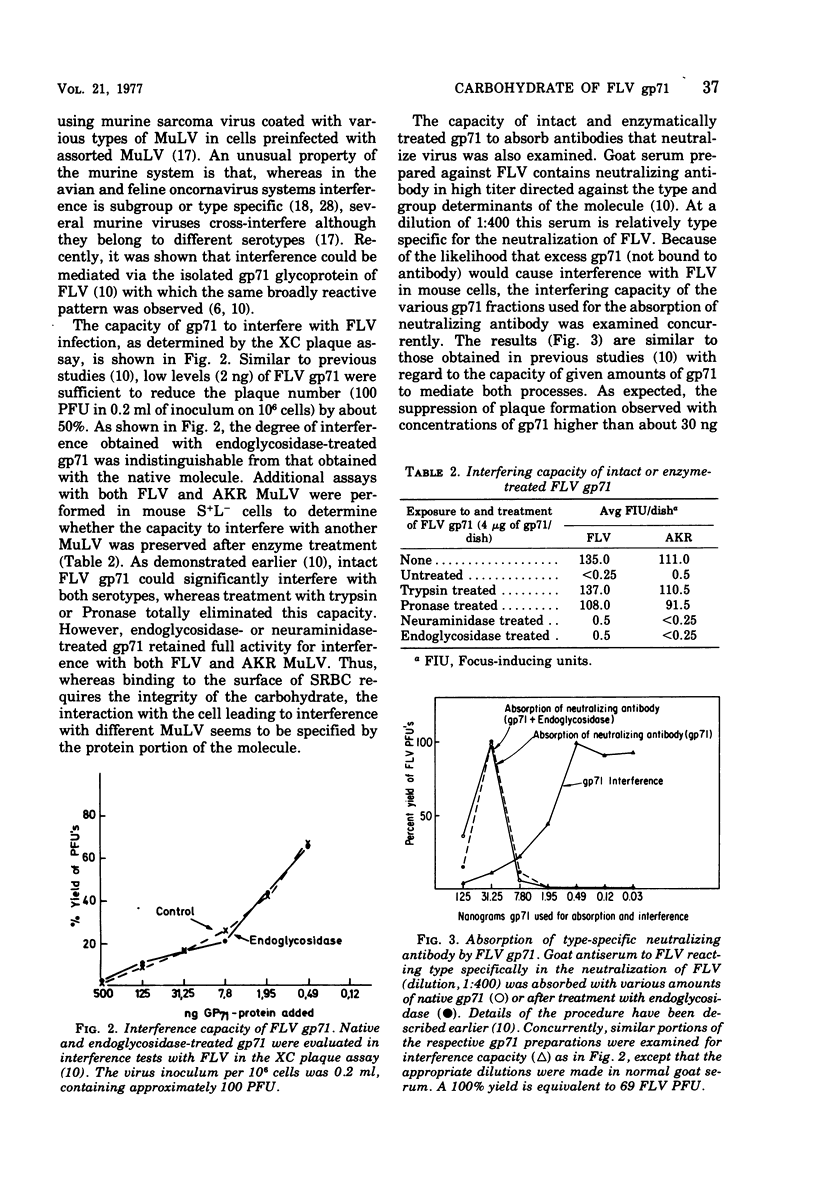
Purified gp71 of Friend murine leukemia virus (FLV) can interfere with virus infection, absorb neutralizing antibody, and in the presence of group-specific anti-gp71 antibody, hemagglutinate sheep erythrocytes. Interference by FLV gp71 with several murine leukemia viruses (MuLV) was tested in the XC and S + L- assay systems. Treatment of gp71 with trypsin or Pronase eliminated its interfering capacity. However, treatment with neuraminidase or a mixture of glycosidase enzymes, which left the major serological properties of gp71 intact, did not reduce the interference potential of gp71 for FLV or AKR MuLV. The capacity of gp71 to absorb type- or group-specific virus-neutralizing antibodies was similarly affected by the various enzyme treatments. In contrast, indirect hemagglutination by gp71 was abolished not only by proteases but also by treatment with glycosidase enzymes, although neuraminidase had no effect. Preliminary data indicate that infectivity of FLV or xenotropic MuLV was not affected by short treatment with glycosidase enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Collins J. J., Leis J. P., Moennig V., Schäfer W., Atkinson P. H. Role of carbohydrate in determining the immunochemical properties of the major glycoprotein (gp71) of Friend murine leukemia virus. J Virol. 1975 Dec;16(6):1453–1463. doi: 10.1128/jvi.16.6.1453-1463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. J., Bolio L. D., Denny T. P., Bolognesi D. P. Interspecies determinants of Friend leukemia virus antigens involved in cytolysis of virus-pfoducing cells. J Virol. 1977 Jan;21(1):113–118. doi: 10.1128/jvi.21.1.113-118.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Sch5AAFER W., Bolognesi D. P. Neutralization of homologous and heterologous oncornaviruses by antisera against the p15(E) and gp71 polypeptides of Friend murine leukemia virus. Virology. 1976 May;71(1):169–184. doi: 10.1016/0042-6822(76)90103-3. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Lewandowski L. J. Isolation of the major viral glycoprotein and a putative precursor from cells transformed by avian sarcoma viruses. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2342–2346. doi: 10.1073/pnas.71.6.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Radiommunoassays for the 70,000-molecular-weight glycoproteins of endogenous mouse type C viruses: viral antigen expression in normal mouse tissues and sera. J Virol. 1976 Jun;18(3):933–941. doi: 10.1128/jvi.18.3.933-941.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Pister L., Seifert E., Schäfer W. Properties of mouse leukemia viruses. VIII. The major viral glycoprotein of Friend leukemia virus. Seroimmunological, interfering and hemagglutinating capacities. Virology. 1974 Dec;62(2):307–318. doi: 10.1016/0042-6822(74)90394-8. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Denny T. P., Bolognesi D. P. Purification and serological characterization of the major envelope glycoprotein from AKR murine leukemia virus and its reactivity with autogenous immune sera from mice. J Virol. 1976 Mar;17(3):727–736. doi: 10.1128/jvi.17.3.727-736.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D. Viral envelopes and their relationship to cellular membranes. Curr Top Microbiol Immunol. 1974;(68):29–58. doi: 10.1007/978-3-642-66044-3_2. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Wöllert W., Rott R., Scholtissek C. Association of influenza virus proteins with cytoplasmic fractions. Virology. 1974 Jan;57(1):28–41. doi: 10.1016/0042-6822(74)90105-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Moennig V., Frank H., Hunsmann G., Schneider I., Schafer W. Properties of mouse leukemia viruses. VII. The major viral glycoprotein of friend leukemia virus. Isolation and physicochemical properties. Virology. 1974 Sep;61(1):100–111. doi: 10.1016/0042-6822(74)90245-1. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Tsang J. M., Atkinson P. H., Summers D. F. Oligosaccharide moieties of the glycoprotein of vesicular stomatitis virus. J Virol. 1976 Apr;18(1):167–175. doi: 10.1128/jvi.18.1.167-175.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Cheong M. P., Hartley J. W., Huebner R. J. A viral interference test for mouse leukemia viruses. Virology. 1967 Sep;33(1):180–184. doi: 10.1016/0042-6822(67)90111-0. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Schafer W., Hunsmann G., Moennig V., Noranha F., Bolognesi D. P., Green R. W., Hüper G. Polypeptides of mammalian oncornaviruses. II Characterization of murine leukemia virus polypeptide (p 15) bearing interspecies reactivity. Virology. 1975 Jan;63(1):48–59. doi: 10.1016/0042-6822(75)90369-4. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Protease activation mutants of sendai virus. Activation of biological properties by specific proteases. Virology. 1976 Jan;69(1):265–277. doi: 10.1016/0042-6822(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Schlom J., Schochetman G., Kimball P., Wagner R. R. Sialylatin of glycoproteins of murine mammary tumor virus, murine leukemia virus, and Mason-Pfizer monkey virus. J Virol. 1976 May;18(2):804–808. doi: 10.1128/jvi.18.2.804-808.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Sialoglycoprotein of vesicular stomatitis virus: role of the neuraminic acid in infection. J Virol. 1974 Aug;14(2):270–281. doi: 10.1128/jvi.14.2.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer W., Lange J., Fischinger P. J., Frank H., Bolognesi D. P., Pister L. Properties of mouse leukemia viruses. II. Isolation of viral components. Virology. 1972 Jan;47(1):210–228. doi: 10.1016/0042-6822(72)90253-x. [DOI] [PubMed] [Google Scholar]
- Stollar V., Stollar B. D., Koo R., Harrap K. A., Schlesinger R. W. Sialic acid contents of sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology. 1976 Jan;69(1):104–115. doi: 10.1016/0042-6822(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- Witter R., Hunsmann G., Lange J., Schäfer W. Properties of mouse leukemia viruses. V. Hemagglutination-inhibition and indirect hemagglutination tests. Virology. 1973 Aug;54(2):346–358. doi: 10.1016/0042-6822(73)90148-7. [DOI] [PubMed] [Google Scholar]


