Abstract
In the present study, we investigated whether the essential nutrient choline may protect against schizophrenic-like cognitive deficits in a rat model. Theories regarding the etiology of schizophrenia suggest that early life events render an individual more vulnerable to adult challenges, and the combination may precipitate disease onset. To model this, the adult male offspring of dams who either experienced stress during late gestation or did not were given a 5 mg/kg dose of the NMDA antagonist, MK-801. The presence of both the prenatal challenge of stress and the adult challenge of MK-801 was expected to impair memory in these offspring. Memory was not expected to be impaired in rats that did not experience prenatal stress, but did receive MK-801 as adults. To study whether choline levels altered outcomes in these groups, rats were fed a choline-supplemented, -deficient, or standard diet during the period between the two challenges: beginning at weaning and continuing for 25 days. All rats consumed regular rat chow thereafter. The efficacy of the model was confirmed in the standard fed rats in that only those that were prenatally stressed and received MK-801 as adults displayed impaired memory on a novelty preference test of object recognition. Contrary to this finding and consistent with our hypothesis, choline-supplemented rats that were also both prenatally stressed and given MK-801 as adults showed intact memory. Choline deficiency impaired memory in rats that were just prenatally stressed, just given MK-801 as adults, and subjected to both. Thus, a choline deficient diet may render rats vulnerable to either challenge. Taken together, we offer evidence that developmental choline levels modulate the effects of prenatal stress and/or MK-801 and thereby alter the cognitive outcome in a rat model of schizophrenia.
Keywords: prenatal stress, nutrition, mk-801, hippocampus, novelty preference, object recognition
1. Introduction
Schizophrenia affects approximately 1% of the global population and though it manifests in many ways, there are characteristic positive symptoms which include paranoia, delusions, hallucinations, and negative symptoms which include flattened affect and social deficits that appear in some combination across patients (American Psychiatric Association, 2000). Schizophrenia is also commonly characterized by marked deficits in cognition, including impairments in memory and attention (e.g. Assaf et al., 2006; Schwartz et al., 2009; van Erp et al., 2008). There is increasing awareness of the neurological abnormalities that occur in this disorder and noted among them are diminished excitatory neurotransmission in the frontal cortex leading to less inhibitory neurotransmission in the ventral tegmental area in the midbrain (Volk and Lewis, 2010; see also Carr and Sesack, 2000; Harte and O’Connor, 2005). These effects are thought to contribute to negative symptoms in schizophrenia (Lynch, 1992; Sesack and Carr, 2002). They also lead to over-excitation of neurons which release dopamine into the nucleus accumbens, which is linked to the positive symptoms of schizophrenia (Gray, 1995; 1998). Another neurological feature of schizophrenia is pathology in the hippocampus, including disorganized cell layers (Harrison, 2004) and reductions in overall volume (Heckers and Konradi, 2010). This, along with pathology in the frontal cortex (Gruber et al., 2010), is likely to be the source of the cognitive deficits in the disorder.
The present work was designed to contribute to an emerging body of work on the neuroprotective capacity of dietary factors in mental health disorders, particularly in schizophrenia. Choline, an essential dietary nutrient, is known to profoundly impact the development of cholinergic-rich brain areas, such as the hippocampus, basal forebrain, and cortical regions (for reviews see Blusztajn, 1998; Meck and Williams, 2003; Zeisel and da Costa, 2009). Life-long enhancements in neural function and plasticity produced in these areas by supplemental choline levels during pre- and postnatal sensitive periods could mediate the risk for, and severity of, a host of neuropsychiatric conditions—particularly those for which hippocampal pathology and loss of neural plasticity are contributing factors. Consistent with this hypothesis, there is mounting evidence of choline’s neuroprotective capacity against a variety of neural insults (Holmes et al., 2002; Ryan et al., 2008; Thomas et al., 2000; Wong-Goodrich et al., 2008; Yang et al., 2000). Thus, the specific goal of this study was to examine whether levels of the nutrient choline during the postnatal periadolescent developmental period would affect adult cognitive performance in a rat model of schizophrenia.
A number of key parallels between the neuropathological features of schizophrenia and the neural and behavioral effects of choline led to the rationale for this study. Neonatal choline supplementation enhances cognition, particularly spatial memory, in adult rats (Meck et al., 1988, 1989; Meck and Williams, 1997; Schenk and Brandner, 1995; Tees, 1999; Tees and Mohammadi, 1999) and prevents age-associated declines (Meck et al., 2007; Meck and Williams, 1997; 2003). These effects are thought to occur through changes in hippocampal and cortical function, including acetylcholine neurotransmission (Blusztajn et al., 1998; Montoya and Swartzwelder, 2000), neuron morphology (Loy et al., 1991; Williams et al., 1998), and excitability (Pyapali et al., 1998). Second, failure of neural plasticity in the hippocampus is currently linked to a host of neuropsychiatric disorders such as schizophrenia, depression, anxiety, and addiction (reviewed in Eisch et al., 2008). Prenatal choline supplementation significantly enhances adult hippocampal plasticity (Glenn et al., 2007; 2008; Mellott et al., 2004; Pyapali et al., 1998) and accompanying this are marked increases in growth factor expression in the hippocampus (Glenn et al., 2007, 2008; Napoli et al., 2008), frontal cortex (Glenn et al., 2008) and striatum (Nag and Berger-Sweeney, 2007). Thus, choline supplementation, at least when given during development, may be neuroprotective in adulthood because the microenvironment of vulnerable areas, like the hippocampus, are enriched and mechanisms of support and repair may be triggered more readily in the face of potentially harmful factors.
In stark contrast to the benefits of choline supplementation, prenatal choline deficiency compromises the cognitive abilities of rats (Meck and Williams, 1997; reviewed in Meck and Williams, 2003), reduces neuron excitability (Pyapali et al., 1998) and prevents a normal enrichment-induced increase in adult neurogenesis (Glenn et al., 2007). Choline deficiency also accelerates normal rates of apoptosis and slows rates of cell division in the fetal hippocampus during development (Albright et al., 1999; Craciunescu et al., 2003). Thus, in addition to assessing the attenuation of cognitive symptoms by periadolescent choline supplementation, we also explored the hypothesis that periadolescent choline deficiency may worsen the cognitive deficits in the schizophrenia model used in the present study.
Though the exact cause of schizophrenia is still under investigation, several etiological models exist (Young et al., 2010). The procedures used in this study followed a ‘two-hit’ model of schizophrenia by combining prenatal stress and adult administration of the noncompetitive NMDA antagonist, dizocilpine (MK-801) in rats. In this model, two distinct events are required to produce impairments typical of those seen in schizophrenic patients (e.g. cognitive impairments). These can be manifested in animal models during young adulthood with drug-administration which compounds an initial neuronal insult, thereby creating an epidemiologically relevant model of the disorder (Meyer & Feldon, 2010). The stress during gestation was hypothesized to induce a neurodevelopmental abnormality, to which hippocampal and cortical areas would be particularly vulnerable (Markham et al., 2010; Martinez-Tellez et al., 2009). The adult MK-801 exposure was further hypothesized to induce a marked reduction in excitatory neurotransmission akin to that seen in schizophrenia (Javitt, 2007). Similar rodent models using either or both of these factors are known to induce cognitive deficits in rats (Markham et al., 2010; see also Powell and Miyakawa, 2006) comparable to those documented in schizophrenic humans. However, the aim of the present model, in keeping with the two-hit premise of schizophrenia, was to use these factors in ways that would minimize their individual impact so as to focus on their combined impact. To do this, male rats were used since they are less impacted by prenatal stress than females (e.g. McCormick et al., 1995). In addition, a relatively easy memory task was used, and we lengthened the interval between MK-801 treatment and memory assessment considerably compared to other studies (e.g. de Lima et al., 2005). To our knowledge, there are no other reports that have combined these two factors—prenatal stress and acute MK-801 exposure in adulthood—in this way.
Based on the two-hit model, it was hypothesized that the most substantial cognitive deficit would be observed in prenatally stressed rats that received MK-801 as adults when compared to rats that were not prenatally stressed, but also received MK-801. Dietary choline levels were manipulated during the postnatal periadolescent period—the time intervening between our two factors (prenatal stress and adult MK-801 treatment). While much of the previous work has been conducted on the effects of prenatal choline availability, there is emerging evidence that postnatal choline intake may also modulate behavior (Glenn et al., 2012; Meck et al., 2007) and is neuroprotective against prenatal trauma (Ryan et al., 2008). Memory was assessed using a novelty preference task which takes advantage of rats’ natural propensity to explore novel objects and features of this task are cortically- and hippocampal-dependent (Mumby et al., 2002; Gaskin et al., 2010, respectively). It was hypothesized that rats fed choline-supplemented diets following prenatal stress would not show a memory deficit following acute MK-801 treatment and that all rats fed choline-deficient diets, whether they were prenatally stressed or not, would exhibit impaired memory when given MK-801 as adults.
2. Materials and methods
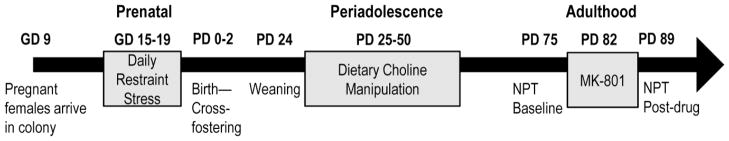
The experimental timeline is depicted in Fig. 1 and the different stages of the experiment are described in the sections below.
Fig. 1.
Experimental timeline showing the overall study design.
2.1 Colony conditions
All rats, except pregnant dams and dams with litters, were pair-housed in individually ventilated cages (Thoren Caging Systems, Inc., Hazleton, PA) in a colony room maintained at 21 ± 2°C with 40–60% humidity. Cages were clear polycarbonate and contained a thin layer of corncob bedding. Rats had free access to food and water throughout the experiment. A 12 hour light/12 hour dark cycle was used, with lights turning on daily at 08:00 h. All procedures were carried out during the light phase of the cycle and were approved by the Colby College Institutional Animal Care and Use Committee and in compliance with federally regulated standards.
2.2 Prenatal stress manipulations, cross-fostering, and rearing conditions
Twelve timed-pregnant female Long-Evans rats were obtained from Charles River Laboratories (Wilmington, MA) on gestational day (GD) 9. Pregnant dams were individually housed in standard laboratory conditions as described above. During the third week of pregnancy (gestational days 15–19; inclusive), half of the pregnant dams (n=6) were subjected to chronic restraint stress. Rats were removed from their home cages and put into clear restraint tubes (16cm in length; 7.5cm in diameter) for one hour, twice per day on each of the 5 consecutive days. The exact time of day and location during the restraint procedures were randomly altered to prevent habituation or time-of-day effects. The other half of the pregnant dams (n=6) were assigned to the control condition (no restraint stress) and were exposed only to normal daily handling through feeding, weighing, and cage cleaning regimens equal to those experienced by all rats throughout the study.
Following birth, pups were taken from their mothers and toes marked to indicate their prenatal stress condition. Pups were then cross-fostered among all of the dams in mixed litters of 10 male and female pups from stressed and non-stressed conditions. At this time pup weights were taken and no differences were found between those from stressed and non-stressed mothers. We also observed patterns of mothering between stressed and non-stressed dams and did not detect any differences in nursing postures, incidence of licking and grooming, nest building, or pup contact. Pups were weaned on postnatal day (PD) 24 to same sex pairs. The male offspring of the pregnant dams (N=47) were the subjects of the present study; 24 were prenatally stressed (Prenatal-Stress) and 23 were not stressed (No-stress). All dams and the male rats that served as subjects in the study were fed commercially available rat chow (Harlan Teklad, Madison, WI) except during the choline manipulation described in the next section.
2.3 Periadolescent choline manipulation
Custom prepared synthetic diets based on formulations by the American Institute of Nutrition were used to manipulate post-weaning choline levels (AIN76A; Dyets Inc., Bethlehem, PA). Rats from each group, Prenatal-Stress and No-stress, were randomly assigned to one of three dietary conditions exclusively during PDs 25–50: choline-deficient (DEF n = 16; 0 g/kg choline), standard choline (STD n = 15; 1.1 g/kg choline chloride in the place of choline bitartrate), or choline-supplemented (SUP n = 16; 5 g/kg choline chloride). As stated above, rats were returned to regular rat chow following the diet manipulation. Combined, the prenatal conditions (stress or no stress) and the adolescent diet conditions (DEF, STD, or SUP) yield 6 experimental groups (n’s=8, except for n=7 in the No-stress/STD condition).
2.4 Apparatus and materials used in the novelty preference task
Novelty preference testing was conducted in a 70 × 70 cm open field with 60 cm high walls. The field was constructed of painted wood with a removable bottom that was covered with bedding throughout habituation and testing. At approximately 20 cm from each inside corner were baby food jar lids that were bolted to the removable bottom of the field with ½ inch screws. The lids were used to secure baby food jars which were glued to the bottoms of the objects used to assess object recognition memory in this study. The lids and bottles prevented the objects from being displaced during both the study and test phases of the task. Eight objects (four sets of two identical objects) were used to assess memory. The objects were ordinary items purchased locally and constructed from hard plastic or ceramic. They differed in texture and color and ranged in size from approximately 4–6 inches high and 2–3 inches wide. They were pretested before being used in the present study to ensure adequate interest by rats in the absence of chewing or excessive interaction.
2.5 Procedure
The following sections describe the experimental procedures that started once rats reached adulthood (approximately PD 75).
2.5.1 Novelty preference task: Pre-MK-801
Baseline cognition was measured using the novelty preference task. Rats were habituated to the testing environment in the absence of objects over three consecutive days. On the first day, cage-mate pairs were placed into the empty field for 10 minutes, and then on each of the next two days, rats were placed in the field individually for 5 minutes. On the day following the third habituation session, the baseline test was conducted.
The novelty preference task consisted of a study and a test phase. In the study phase, two identical objects were positioned in opposite corners of the field. Rats were individually placed in a corner of the field, equidistant from both objects, and were allowed to freely explore for 5 minutes. A 20-minute retention delay separated the study and test phases during which rats were placed individually in small opaque cages. After the delay, rats were returned to the field for a 3-minute test phase in which one of the previously studied objects was replaced with a different, novel object. Object positions, as well as which of the object pairs were designated as study or novel, were counterbalanced across all conditions. Study and test phases were recorded and digital videos were archived for later analysis. All scoring of archived footage was done by an experimenter blind to the conditions of each rat. Object exploration was defined as the time spent by the rat, in seconds, with its snout oriented towards the object and within 2 cm of it (Mumby et al., 2002).
2.5.2 MK-801 administration
After baseline memory testing, a major subset of each group received a single injection of MK-801 (5 mg/kg i.p. in a volume of 1 ml/kg; Sigma-Aldrich, Saint Louis, MO). This dose was selected based on past work showing that its effects on rats resembled an acute psychotic episode with led to lasting disturbances in hippocampal LTP and spatial memory (Manahan-Vaughan et al., 2008. It was therefore expected that the impact of this amount of MK-801 on a non-spatial test of memory would have less impact, unless, as we hypothesized, it is given to rats made vulnerable by either prenatal stress or periadolescent choline deficiency. In further support of this, de Lima et al. (2005) reported deficits in object-recognition with a smaller dose of MK-801 (1 mg/kg) but drug-training/testing intervals where all within 1 day. Thus, we used the larger dose, a longer post-drug interval, and the object recognition test to achieve the goals of our “two-hit” model. To confirm the effectiveness of this dose of MK-801 on the locomotor measures described below and as reported in Manahan-Vaughan et al. (2008), a smaller subset of rats from each group was randomly selected to receive an injection of 0.9% physiological saline in matched volumes (1 ml/kg) instead of MK-801. Thus, there were a total of 29 rats that received MK-801 (n’s=5, except n=4 in the No-Stress/STD) and 18 that received saline (n=3 from each of the 6 experimental conditions).
Immediately following MK-801 or saline administration, rats were placed individually in a clear plastic bin with corncob bedding and a metal cage top for observation. Rats were observed via a closed-circuit camera connected to a computer in an adjacent room and all digital video footage was archived. The observation period lasted 30 minutes, and each rat’s behavior was sampled and assessed every 5 minutes for a total of 6 assessments per rat. Based on published methods (Andiné et al., 1999; Manahan-Vaughan et al., 2008), the behavior of the rat at each assessment was coded for three specific characteristics: locomotion (0 = stationary; 1 = movements within a localized area forelimbs only; 2 = intermittent movements within half of the area of the cage; 3 = continuous movement within half of the cage; 4 = intermittent movements within the whole area of the cage; 5 = continuous movements within the whole area of the cage); stereotypy (0 = no sniffing; 1 = discontinuous sniffing; 2 = continuous sniffing); and ataxia (0 = normal body control; 1 = falling tendency upon movement; 2 = falling upon movement; 3 = almost unable to move). These scales are specifically designed to reveal the motor effects of drugs like MK-801. Following the observation period, rats were returned to their home cages and placed back in the colony for recovery. After 24 hours, there were no further observable drug effects on motor function and all rats in the study behaved normally upon handling and displayed normal food and water consumption, activity, and grooming.
2.5.3 Novelty preference task: Post-MK-801
Memory was re-assessed with the novel object preference task one week after the administration of the large dose of MK-801 or a vehicle saline injection. The procedures were the same as those used for baseline memory testing, except that two new pairs of objects were used. As with the previous testing, all digital video of study and test phase trials were recorded and archived and object placement and assignment were counterbalanced across all conditions.
2.6 Statistical Analyses
The primary factors in this study were Prenatal Condition (Prenatal-stress, No-stress) and Periadolescent Diet (DEF, STD, SUP). Daily food intake in grams (g) consumed by each cage of two rats was recorded and these values divided by 2 to obtain an approximate measure of g of food consumed by each rat while dietary choline levels were being manipulated. Each cage contained rats from the same experimental conditions and in the two instances where this was not the case the food intake data were excluded. Food intake at 4 points over the 25-day periadolescent choline manipulation (Days 1, 7, 14, and 21) as a function of Prenatal condition and Periadolescent Diet was analyzed using a 2×3×4 mixed factorial analysis of variance (ANOVA).
Two dependent measures were collected from the test phases of the two novelty preference tests: total object exploration time and a ratio reflecting the proportion of total object exploration time that was directed at the novel object. Object exploration times were evaluated using 2×3 ANOVAs. Ratio values were analyzed using one-sample t-tests to compare each group’s data to the value of 0.5, which reflects no preference for the novel object and thus impaired memory: ratio values that approach 1 indicate that the rat was spending more time exploring the novel object, from which memory of the previously studied object was inferred (Dix & Aggleton, 1999; e.g. Gaskin et al., 2003; Mumby et al., 2002; 2007). The data were analyzed in this way to maximize the detection of memory for novel objects in each experimental condition.
To assess the acute effects of MK-801 during the 30-minute observation period, mixed factorial ANOVAs were used. The ANOVAs were 2×3×2×6 mixed factorials with the between factors of Prenatal Condition, Periadolescent Diet, and Drug (MK-801, Saline), and the repeated measure of Time since injection (5, 10, 15, 20, 25, 30 minutes). The dependent measures of motor function assessed were the scores on the locomotion, stereotypy, and ataxia scales. Because no rats given saline displayed ataxia at any time, only scores on this scale from those rats given MK-801 were assessed with a 2×3×6 (Prenatal condition, Periadolescent Diet, and Time) mixed factorial ANOVA. To follow up significant main effects or interactions, simple effects analyses and post hoc Tukey tests were conducted where appropriate. All statistical tests were assessed with a significance level of p=0.05 and without correction for multiple one-sample t-tests.
3. Results
3.1 Food intake during the periadolescent choline manipulation
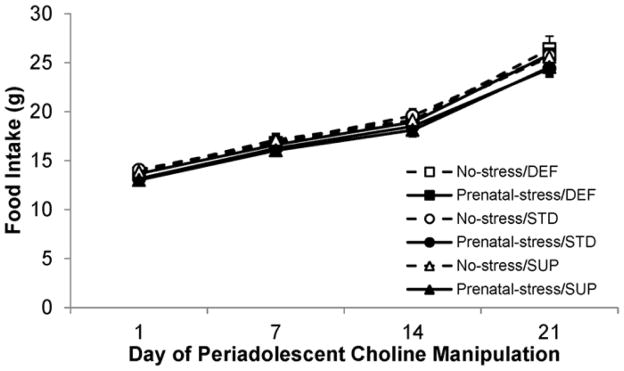
As is evident in Fig. 2 there were no statistically significant main effects of Prenatal condition or Diet and no interactions between any of the factors in the analysis (all p’s>0.05). These data indicate that all rats started off consuming similar amounts of the diet and this was still the case throughout and at the end of the diet manipulation. There was a significant main effect of Time (F[3,114]=1354.226, p<0.001) indicating that all rats increased their consumption over the 25-day period, consistent with their growth.
Fig. 2.
Food consumption (in grams) for the six experimental groups over the course of the postnatal choline diet manipulation. All rats show comparable food intake throughout the course of the dietary manipulation regardless of experimental condition, as well as a steady increase in consumption commiserate with growth of the animals. Bars represent ±SEM.
3.2 Pre-MK-801 novelty preference task
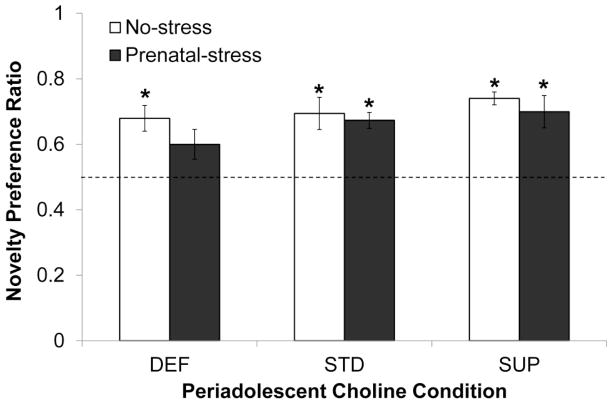
Fig. 3 shows the ratios reflecting the preference for the novel object in each group. Only the Prenatal-stress/DEF rats did not display a statistically significant preference for the novel object (t[7]=2.198, p<0.05); all other groups had ratio values that were significantly higher than 0.5, or no preference (all p’s < 0.01). These effects were not accounted for by total object exploration times as there were no statistically significant results of the ANOVA conducted on this variable.
Fig. 3.
Memory performance in the baseline novelty preference task conducted prior to MK-801 administration as a function of prenatal stress conditions and periadolescent dietary choline conditions. The dashed line represents a novelty preference ratio value of 0.5 indicating no preference for either object. Bars represent ±SEM. * p<0.05, one-sample t-test comparing group performance to no-preference value of 0.5
3.3 Acute motor responses to MK-801 administration
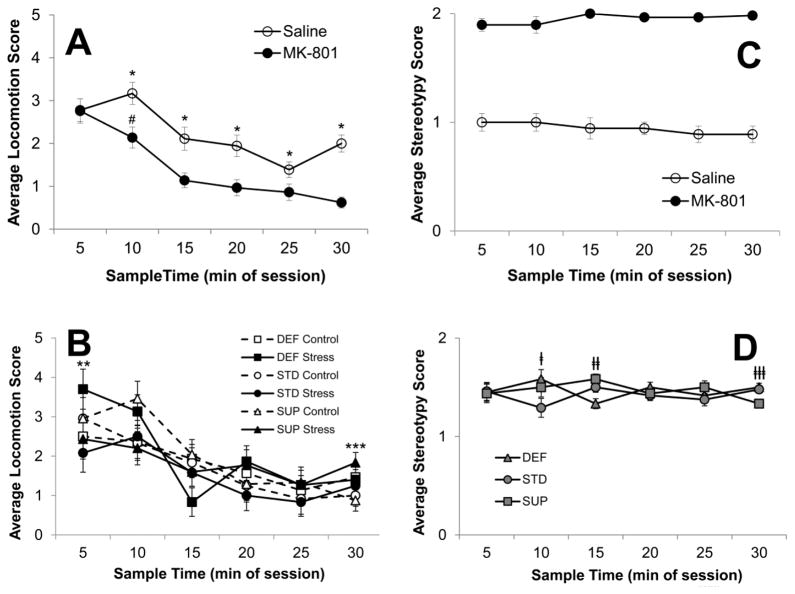
The saline-treated rats were used to confirm the effects of the drug on these behavioral scales and, as expected, the large dose of MK-801 used in this study produced robust effects on motor behaviors. ANOVA results on the locomotion scores revealed significant main effects of Drug (F[1,35]=17.373, p<0.001) and Time since injection (F[5,175]=27.931, p<0.001) and significant interactions between Drug and Time since injection (F[5,175]=3.142, p<0.01) and Time since injection, Prenatal condition, and Periadolescent Diet (F[10.175]=2.655, p<0.01). As can be seen in Fig. 4A, both saline and MK-801-treated rats displayed declining locomotion over the course of the 30-minute observation period, but the decline in locomotion in rats given MK-801 began early in the observation period with a significant decline from the 5- to the 10-min observation in MK-801-treated rats (p = 0.043) whereas saline-treated rats showed an increase over the same period, though it was not statistically significant (p = 0.10). MK-801-treated rats continued to display significantly lower locomotion scores over the observation period (all p’s<0.05) and reached a significantly lower plateau than that shown by the saline treated rats (p < 0.001 at 30 min time point). Interestingly, the 3-way interaction (see Fig. 4B) resulted from a differential impact of Prenatal stress on DEF and SUP rats: Prenatal-stress/DEF rats were more active at the beginning of the observation period compared to Prenatal-no stress/DEF rats, while the opposite was true among SUP rats (p’s<0.05).
Fig. 4.
Behavioral response of rats to injections of MK-801 (5 mg/kg) or saline assessed every 5 minutes over the first 30 minutes of drug exposure. Average scores for locomotion as a function of treatment (MK-801 vs. saline) and collapsed over prenatal and diet conditions are displayed in A, and average locomotion scores as a function of prenatal and diet conditions at each sample time, collapsed over drug, are shown in B. Average stereotypy scores are displayed in the right panel: C shows the comparison between rats receiving MK-801 or saline; D shows stereotypy scores as a function of periadolescent dietary choline condition at each sample time. Bars represent ±SEM. *p<0.05 Saline v. MK-801; # p<0.05 5-min v. 10-min in MK-801 rats; **p<0.05 DEF Control v. Stress; ***p<0.05 SUP Control v. Stress; †p<0.05 STD v. SUP and DEF; ††p<0.05 DEF v. STD and SUP; †††p<0.05 SUP v. STD and DEF
ANOVA results on the stereotypy scores also revealed a significant main effect of Drug (F[1,35]=802.433, p<0.001), but in this case neither the main effect of Time since injection or the interaction were significant. Stereotypy in rats given MK-801 was evident at the first observation and remained high throughout the observation period; whereas normal amounts of sniffing were evident in saline treated rats and these levels did not change over the 30-minute period (see Fig. 4C). There was a significant interaction between Periadolescent Diet and Time since injection (F[1,175]=2.099, p<0.05), however this did not reflect a meaningful pattern in the data (see Fig. 4D). For example, STD rats displayed significantly less stereotypy at Time 2, DEF rats displayed significantly less at Time 3, and SUP rats displayed significantly less at Time 6 (p’s<0.05).
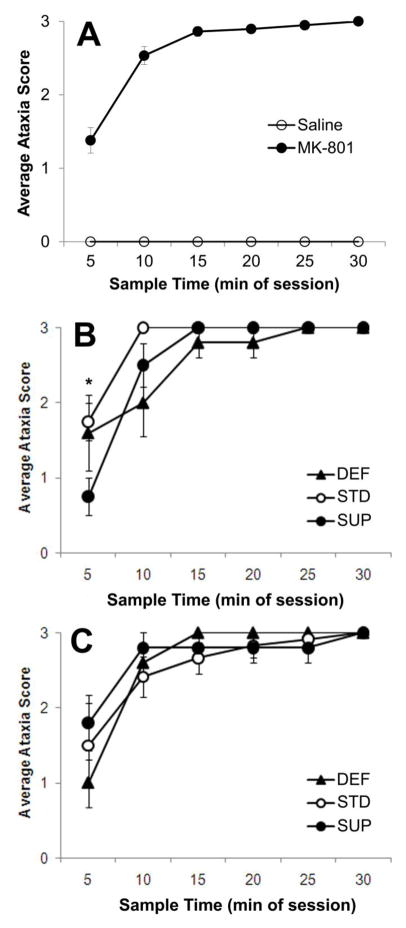
The drug effects on ataxia scores were very clear such that no saline injected rats displayed ataxia at any time during the observation period (see Fig. 5A). Thus an ANOVA was conducted on the ataxia scores based on Prenatal Condition, Periadolescent Diet, and Time since MK-801 injection and excluded the saline-treated rats. There was a statistically significant effect of Time (F[5,115]=62.074, p<0.001), with ataxia worsening in all groups over the observation period. There was also a significant 3-way interaction between Prenatal condition, Periadolescent diet, and Time since MK-801 injection. A simple effects analysis of the interaction between Periadolescent diet and Time at each level of Prenatal condition revealed a significant interaction only in No-Stress rats (F[10,55]=2.014, p<0.05). As can be seen in Fig. 5B, No-stress/SUP rats displayed significantly less ataxia than No-Stress/STD rats 5 minutes after the MK-801 injection (p<0.05). No diet effects were seen thereafter and none were observed among the Prenatal-stress rats (see Fig. 5C).
Fig. 5.
Average ataxia score as a function of treatment (MK-801 or saline) and collapsed over prenatal and diet conditions (A) show that only rats given MK-801 registered any form of ataxia. Rats given MK-801 that were not stressed prenatally and received postnatal choline supplementation (SUP) showed attenuated ataxia scores within the first 5 minutes of observation (B). However, this difference was not evident in rats that were prenatally stressed (C). Bars represent ±SEM. *p<0.05 SUP vs. STD
3.4 Post MK-801 novelty preference task
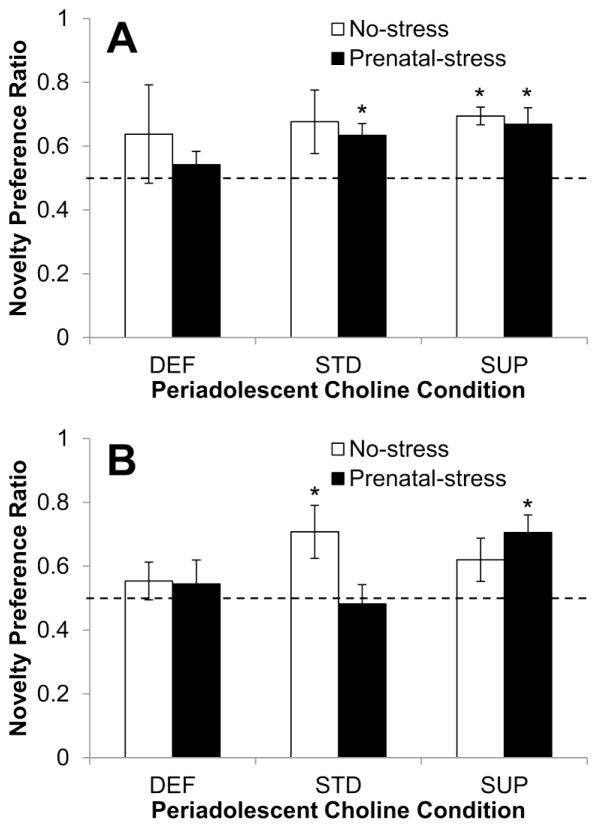
Fig. 6A shows the memory performance of saline-treated rats from each Prenatal Condition and Periadolescent Diet group one week after they received the injection. In contrast to the findings from the pre-test, neither deficient group (No-stress and Prenatal-stress) showed a preference for the novel object on the second test (p = 0.233 and p = 0.212, respectively). No-stress/STD rats also did not show a significant preference for the novel object (p = 0.110); though, as can be seen in Fig. 6A, this group did, on average, prefer the novel object (M=0.676, SEM=0.099). The remaining groups all showed a statistically significant preference for the novel object: Prenatal-stress/STD (p = .035), No-stress/SUP (p = 0.010), and Prenatal-stress/SUP (p = 0.042).
Fig. 6.
Memory performance in the post-injection novelty preference task as a function of prenatal stress conditions and periadolescent dietary choline conditions. (A) shows the performance of rats given a saline injection and (B) shows the performance of rats given an acute dose of MK-801. The dashed line represents a novelty preference ratio value of 0.5 indicating no preference for either object. Bars represent ±SEM. * p<0.05, one-sample t-test comparing group performance to no-preference value of 0.5
Fig. 6B shows the memory performance one week after the large dose of MK-801 as a function of Prenatal Condition and Periadolescent Diet. Analyses of these data revealed that: 1) neither Prenatal-stress/DEF (p = 0.418) or No-stress/DEF (p = 0.587) rats displayed a significant preference for the novel object; 2) No-stress/STD rats displayed a significant preference for the novel object (p = 0.044) but Prenatal-stress/STD rats did not (p = 0.774); and 3) Prenatal-stress and No-stress SUP rats exhibited a preference for the novel object, though the preference was only statistically significant for the Prenatal-stress/SUP rats (p = 0.011) with No-stress/SUP rats showing a non-significant preference for the novel object (p = 0.09). A 2×3 ANOVA conducted on time spent exploring the objects during the 3-minute test revealed no statistically significant main effects or interactions and thus did not account for any of the patterns of novel object preference.
4. Discussion
The results of the present study provided compelling support for the hypotheses that supplemental dietary choline during periadolescent development could mitigate the adverse interaction of prenatal stress and adult administration of an acute large dose of the NMDA antagonist, MK-801. Evidence is also shown indicating that periadolescent choline deficiency may worsen the effects of either or both of these factors. An integral finding was that rats fed a standard amount of choline throughout life that were both stressed prenatally during the last week of gestation and given MK-801 as adults displayed a memory deficit on the novelty preference task of object recognition. Thus, exposing rats to factors that produce effects like those seen in schizophrenia led to the characteristic cognitive deficits (Corcoran et al., 2003; Walker and Diforio, 1997). By contrast, prenatally stressed rats treated during periadolescence with choline supplementation did not display cognitive deficits in the novelty preference task after MK-801 administration. This finding fits well with other work on choline in a rodent model of the sensory-gating deficits in neural function which is also considered to be a hallmark feature of schizophrenia (Stevens et al., 2008a, 2008b). Stevens and colleagues (2008a) demonstrated that choline supplementation shortly after birth improved the marked deficiencies in sensory inhibition characteristically seen in the inbred DBA/2 mouse that models the deficits of schizophrenic humans. Accompanying this improvement, Stevens et al. observed increases in the numbers of α-7 nicotinic receptors in the hippocampus, for which choline is a direct agonist. In addition, emerging ideas about the neuropathological features of psychological disorders, including schizophrenia, point to dysfunctions in adult neural plasticity, especially hippocampal neurogenesis, as important mediators of prognosis and treatment (DeCarolis and Eisch, 2010; Eisch et al., 2008). Supplemental choline, at least when administered prenatally, produces a marked increase in several markers for neural plasticity, including LTP (Pyapali et al., 1998), dendritic branching patterns (Li et al., 2004; Loy et al., 1991), growth factor content (Glenn et al., 2007; Glenn et al., 2008; Mellot et al., 2004; Napoli et al., 2008), and, of particular note, hippocampal neurogenesis (Glenn et al., 2007; Mellot et al., 2004). Thus, there are several candidate mechanisms through which choline could be acting to exert the neuroprotective effects observed in the present study. Importantly, however, this is the first demonstration of a complete prevention of the memory deficits that were successfully induced in the model.
Also as predicted, combining choline deficiency with either element of the model—the prenatal stress or the adult MK-801 administration—resulted in cognitive deficits. Thus, choline deficiency may exacerbate other existing vulnerabilities and thereby trigger or accelerate onset of pathological conditions. These findings are in agreement with many others in the literature: In regard to bodily health, choline deficiency worsens the effects of coronary heart disease and colorectal cancer risk factors (Bidulescu et al., 2009; Lee et al., 2010), and, in regard to brain health, prenatal choline deficiency reduces adult neural plasticity (Glenn et al., 2007; Pyapali et al., 1998), and severely disrupts rats’ abilities to adapt to cognitively demanding tasks and enriching conditions (Glenn et al., 2007; Tees, 1999). These findings are also consistent with those of Stevens et al. (2008a), showing that choline deficiency significantly worsened the sensory processing deficits in their mouse model. Taken together, these findings suggest that the impact of other known factors that increase the risk for, and outcome of, schizophrenia may be compounded by choline deficiency.
Another intriguing result from the present study was that choline supplementation mildly attenuated the motor effects of the high dose of MK-801 given to rats in adulthood. Previously published and well-documented methods (e.g. Manahan-Vaughan et al., 2008) were used to assess and quantify the response of rats to the drug over the course of the first 30 minutes of exposure. Based on this work, there is a reliable progression of symptoms during this period that is characteristic of the dose we used and the rats in this study progressed through these symptoms as expected: locomotor behavior declined over the first 5–10 minutes as stereotypy and ataxia increased. Rats began to display stereotyped head-weaving and repetitive sniffing within the first 5 minutes and continued to display these behaviors for the remainder of the session. There were some small but statistically significant differences between the diet groups when the data were collapsed over the drug and prenatal conditions. However, the lack of a consistent pattern in these effects makes them difficult to interpret: STD rats showed less stereotypy at 10 min; DEF rats showed less stereotypy at 15 min; and SUP rats showed less stereotypy at 30 min. It is possible that the diet was affecting the adaptation of the rats to novel conditions of the observation period but the lack of any diet effects in each of the treatment conditions, particularly the saline-treated/No-stress rats, suggests that these effects are subtle and inconsistent.
Compared to stereotypy, ataxia progressed more slowly but was still evident to some degree in the first 5 minutes. After 10–15 minutes all rats had some to full loss of their righting reflex. Interestingly, at the 5-minute observation, choline-supplemented rats were maintaining the lowest ataxia scores compared to standard-fed and choline-deficient rats, which was only true in rats that were not prenatally stressed. This suggests that, in addition to a protection from the MK-801-induced sequelae that emerges the week after exposure, choline supplementation may also confer protection by reducing the acute actions of the drug. These findings fit well with other evidence that prenatal choline supplementation can prevent the neurotoxic properties of MK-801 delivery directly into the brain (Guo-Ross et al., 2002; 2003). Further research is clearly warranted to investigate this provocative finding in a more direct and comprehensive manner.
In contrast to the results discussed so far, there were aspects of the data that were unexpected and difficult to interpret. In particular, the finding that the No-stress/MK-801 choline-supplemented rats did not display a clear preference for the novel object during the post-drug test was surprising. The bias of this group toward the novel object was above chance, but not statistically significant. It is possible that this reflects an impairment in that group but such an interpretation would be in conflict with a host of other findings on choline’s neuroprotective capacity (Glenn et al., 2008; Nag and Berger-Sweeney, 2007; Ryan et al., 2008; Thomas et al., 2011; Wong-Goodrich et al., 2008; 2010), particularly those showing that MK-801 neurotoxicity is markedly reduced with choline supplementation (Guo-Ross et al., 2002; 2003). It is possible that the non-significant preference in the No-stress/MK-801 supplemented rats was an artifact of the small sample size of the group and the fact there was only one post-drug test. Consistent with this interpretation, these rats showed the slowed progression of ataxia during post-injection observations. In addition, unlike this groups’ tendency to investigate the novel object more, the deficits seen in the Prenatal-stress/MK-801 standard-fed rats and the No-stress/MK-801 and Prenatal-stress/MK-801 deficient-fed rats showed little discernible differences from the line of no object preference during the same test (see Fig. 6).
It should also be noted that, in the present study, neither prenatal stress nor MK-801 alone was sufficient to induce deficits on this task, except in the choline-deficient rats. These results are not surprising in light of several important points. Prenatal stress of this sort has a much more potent effect in females than in males (McCormick et al., 1995; see also Markham et al., 2010) and our task was relatively easy considering the short retention delay used (Markham et al., 2010). We expect that adverse effects of prenatal stress on memory would have been more likely had we used different stress procedures, like chronic variable stress rather than chronic restraint stress (Markham et al., 2010), and/or a longer retention delay or a different test of memory, particularly one with a more heavy reliance on the integrity of the hippocampus (Hosseini-Sharifabad & Hadinedoushan, 2007). Similarly, the dose of MK-801 was large but administered only once. Again, we expect we would have observed deficiencies had rats been tested closer to the time of injection (de Lima et al., 2005), or if a more difficult or hippocampally-dependent procedure been used (Manahan-Vaughan et al., 2008). The present aim was to use procedures that were unlikely to yield deficits with either factor alone to ensure that the two-hit aspect of the model would be critical. Also note that all of our manipulations were systemic and thus many brain regions may have been impacted by the procedures. It is possible that pathological changes in hippocampus and cortical regions are critically involved in the reported effects and investigations into them are ongoing.
5. Conclusion
In summary, we provide compelling new evidence that choline supplementation administered over the adolescent period may diminish the combined negative impact of prenatal stress and adult MK-801 exposure on memory. Chronically stressing rat dams during the last week of gestation leads to significant elevations in circulating glucocorticoid hormones which can be detrimental to the development of the hippocampus (reviewed in McEwen, 2005; also see Corcoran et al., 2003). Abnormal neural development, particularly centered on the hippocampus, then yields a vulnerability to the acutely challenging experience of a large dose of an NMDA-antagonist. MK-801 significantly reduces the overall excitatory tone of the frontal cortical projections to the ventral tegmental area, thereby resulting in a disinhibition and overexpression of dopaminergic signals in the nucleus accumbens (Mathé et al., 1999). This is the contributing pathology of schizophrenia. It is not clear from the present work whether supplemental choline levels corrected the vulnerability brought on by prenatal stress or whether it reduced the neurotoxic capabilities of MK-801 in adulthood; ample previous research point convincingly to the latter, but the possibility of both remains. We also provide compelling evidence that choline deficiency over the same period can lead to memory deficits when combined with either component of our model. This provides further support for the notion that choline may be generally neuroprotective and thus mitigates the effects of both of our factors. When considered together, the results of the present study are convincing evidence for the use of dietary choline as a preventative measure for the mediation of cognitive deficits seen in schizophrenia. This could be especially beneficial to individuals who may be considered ‘at-risk’ for schizophrenia, such as those with schizophrenic relatives (e.g., Chubb et al., 2008; Whyte et al., 2006), or who may have experienced significant stressors during the second trimester of gestation (e.g., Brown et al., 2004; Pallast et al., 1994). Furthermore, these results suggest that supplemented levels of important dietary nutrients, such as choline, should be considered and implemented in postnatal and periadolescent nutrition (Zeisel & da Costa, 2009).
Research Highlights.
In this study we employed a rat model of schizophrenia to study how dietary choline modulates outcomes in it.
Early-life stress was compounded by a large dose of the NMDA antagonist, MK-801, to induce cognitive deficits in rats.
When rats were supplemented with choline during the intervening periadolescent period they showed no memory deficits.
Choline deficiency during the intervening periadolescent period in combination with either or both components of the model impaired memory.
Dietary choline may be a novel target for therapeutic interventions aimed at lessening or slowing the cognitive symptoms of schizophrenia.
Acknowledgments
The authors would like to thank Marie Hayes and Amanda Kimball for their helpful comments on an earlier version of this manuscript and Olivia Bordiuk for her assistance with the breeding. This project was supported by grants from the National Center for Research Resources (5P20RR016463) and the National Institute of General Medical Sciences (8 P20 GM103423) from the National Institutes of Health to MJG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Institute of General Medical Sciences, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Research Developmental Brain Research. 1999;115:123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Press, Inc; 2000. (DSM-IV-TR) [Google Scholar]
- Andiné P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Mårtensson E, Sandberg M. Characterization of MK-801-induced behavior as a putative rat model of psychosis. The Journal of Pharmacology and Experimental Therapeutics. 1999;290:1393–1408. [PubMed] [Google Scholar]
- Assaf M, Rivkin PR, Kuzu CH, Calhoun VD, Kraut MA, Groth KM, Yassa MA, Hart J, Jr, Pearlson GD. Abnormal object recall and anterior cingulate overactivation correlate with formal thought disorder in schizophrenia. Biological Psychiatry. 2006;59:452–459. doi: 10.1016/j.biopsych.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Repeatability and measurement error in the assessment of choline and betaine dietary intake: The atherosclerosis risk in communities (ARIC) study. Nutrition Journal. 2009;8:14. doi: 10.1186/1475-2891-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Cermak JM, Holler T, Jackson DA. Imprinting of hippocampal metabolism of choline by its availability during gestation: Implications for cholinergic neurotransmission. Journal of Physiology, Paris. 1998;92:199–203. doi: 10.1016/s0928-4257(98)80010-7. [DOI] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. The Journal of Neuroscience. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Molecular Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: Etiology and onset. Schizophrenia Bulletin. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. The Journal of Nutrition. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: A critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima MNM, Laranja DC, Bromberg E, Roesler R, Schröder N. Pre- or post-training administration of the NMDA receptor blocker MK-801 impairs object recognition memory in rats. Behavioural Brain Research. 2005;156:139–143. doi: 10.1016/j.bbr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: Hope or hype? Journal of Neuroscience. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde objection recognition in rats with hippocampal lesions. Hippocampus. 2003;13:962–969. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- Gaskin S, Tardif M, Cole E, Piterkin P, Kayello L, Mumby DG. Object familiarization and novel-object preference in rats. Behavioural Processes. 2010;83:61–71. doi: 10.1016/j.beproc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulated hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. European Journal of Neuroscience. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Research. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Adams RS, McClurg L. Supplemental dietary choline during development exerts anti-depressant like effects in adult female rats. Brain Research. 2012;1443:52–63. doi: 10.1016/j.brainres.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. Integrating schizophrenia. Schizophrenia Bulletin. 1998;24:249–266. doi: 10.1093/oxfordjournals.schbul.a033324. [DOI] [PubMed] [Google Scholar]
- Gray JA. Dopamine release in the nucleus accumbens: The perspective from aberrations of consciousness in schizophrenia. Neuropsychologia. 1995;33:1143–1153. doi: 10.1016/0028-3932(95)00054-7. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: A disinhibited prefrontal cortex impairs cognitive flexibility. Journal of Neuroscience. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Clark S, Montoya DA, Jones KH, Obernier J, Shetty AK, White AM, Blusztajn JK, Wilson WA, Swartzwelder HS. Prenatal choline supplementation protects against postnatal neurotoxicity. Journal of Neuroscience. 2002;22:RC195. doi: 10.1523/JNEUROSCI.22-01-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Jones KH, Shetty AK, Wilson WA, Swartzwelder HS. Prenatal dietary choline availability alters postnatal neurotoxic vulnerability in the adult rat. Neuroscience Letters. 2003;341:161–163. doi: 10.1016/s0304-3940(03)00119-8. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Harte M, O’Connor WT. Evidence for a selective prefrontal cortical GABAb receptor-mediated inhibition of glutamate release in the ventral tegmental area: A duel probe microdialysis study in the awake rat. Neuroscience. 2005;130:215–222. doi: 10.1016/j.neuroscience.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Current Topics in Behavioral Neurosciences. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Research. 2002;48:3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Hosseini-Sharifabad M, Hadinedoushan H. Prenatal stress induces learning deficits and is associated with a decrease in granules and CA3 cell dendritic tree size in rat hippocampus. Anatomical Science International. 2007;82:211–217. doi: 10.1111/j.1447-073X.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. International Review of Neurobiology. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Lee JE, Giovannucci E, Fuchs CS, Willett WC, Zeisel SH, Cho E. Choline and betaine intake and the risk of colorectal cancer in men. Cancer Epidemiology, Biomarkers & Prevention. 2010;19:884–887. doi: 10.1158/1055-9965.EPI-09-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo-Ross S, Lewis DV, Turner D, White AM, Wilson WA, Swartzwelder HS. Dietary prenatal choline supplementation alters postnatal hippocampal structure and function. Journal of Neurophysiology. 2004;91:1545–1555. doi: 10.1152/jn.00785.2003. [DOI] [PubMed] [Google Scholar]
- Loy R, Heyer DD, Williams CL, Meck WH. Choline-induced spatial memory facilitation correlated with altered distribution and morphology of septal neurons. 1991. [DOI] [PubMed] [Google Scholar]
- Lynch MR. Schizophrenia and the D1 receptor: Focus on negative symptoms. Progress in Neuropsychopharmacology & Biological Psychiatry. 1992;16:797–832. doi: 10.1016/0278-5846(92)90102-k. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Frontiers in Behavioral Neuroscience. 2010;4:1–15. doi: 10.3389/fnbeh.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- Mathé JM, Nomikos GG, Blakeman KH, Svensson TH. Differential actions of dizocilpine (MK-801) on the mesolimbic and mesocortical dopamine systems: Role of neuronal activity. Neuropharmacology. 1999;38:121–128. doi: 10.1016/s0028-3908(98)00163-4. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Developmental Brain Research. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism: Clinical and Experimental. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Frontiers in Integrative Neuroscience. 2007;1:7, 1–11. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neuroscience and Biobehavioral Reviews. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behavioral Neuroscience. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Developmental Psychobiology. 1988;21:339–359. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Perinatal choline supplementation increased the threshold for chunking in spatial memory. Neuroreport. 1997b;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MARK and CREB activation. The FASEB Journal. 2004;18:545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Progress in Neurobiology. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Montoya D, Swartzwelder HS. Prenatal choline supplementation alters hippocampal N-methyl-D-aspartate receptor-mediated neurotransmission in adult rats. Neuroscience Letters. 2000;296:85–88. doi: 10.1016/s0304-3940(00)01660-8. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learning & Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Glenn MJ, Nesbitt C, Kyriazis DA. Dissociation in retrograde memory for object discriminations and object recognition in rats with perirhinal cortex damage. Behavioural Brain Research. 2002;132:215–226. doi: 10.1016/s0166-4328(01)00444-2. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Piterkin P, Lecluse V, Lehmann H. Perirhinal cortex damage and anterograde object-recognition in rats after long retention intervals. Behavioural Brain Research. 2007;185:82–87. doi: 10.1016/j.bbr.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Napoli I, Blusztajn JK, Mellott TJ. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Research. 2008;1237:124–135. doi: 10.1016/j.brainres.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiology of Disease. 2007;26:473–480. doi: 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Pallast EG, Jongbloet PH, Straatman HM, Zielhuis GA. Excess seasonality of births among patients with schizophrenia and seasonal ovopathy. Schizophrenia Bulletin. 1994;20:269–276. doi: 10.1093/schbul/20.2.269. [DOI] [PubMed] [Google Scholar]
- Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: A uniquely human disorder? Biological Psychiatry. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. Journal of Neurophysiology. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Research. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk F, Brandner C. Indirect effects of peri- and postnatal choline treatment on place-learning abilities in the rat. Psychobiology. 1995;23:302–313. [Google Scholar]
- Schwartz BL, Parker ES, Rosse RB, Deutsch SI. Recognition memory probes affect what is remembered in schizophrenia. Psychiatry Research. 2009;167:21–27. doi: 10.1016/j.psychres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: Implications for schizophrenia. Physiology & Behavior. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Adams CE, Yonchek J, Hickel C, Danielson J, Kisley MA. Permanent improvement in deficient sensory inhibition in DBA/2 mice with increased perinatal choline. Psychopharmacology. 2008a;198:413–420. doi: 10.1007/s00213-008-1170-3. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Adams CE, Mellott TJ, Robbins E, Kisley MA. Perinatal choline deficiency produces abnormal sensory inhibition in sprague-dawley rats. Brain Research. 2008b;1237:84–90. doi: 10.1016/j.brainres.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behavioral Brain Research. 1999;105:173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Developmental Psychobiology. 1999;35:226–240. [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicology and Teratology. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Therman S, Pirkola T, Tuulio-Henriksson A, Glahn DC, Bachman P, Huttunen MO, Lönnqvist J, Hietanen M, Kaprio J, Koskenvuo M, Cannon TD. Verbal recall and recognition in twins discordant for schizophrenia. Psychiatry Research. 2008;159:271–280. doi: 10.1016/j.psychres.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Current Topics in Behavioral Neuroscience. 2010;4:485–508. doi: 10.1007/7854_2010_44. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: A neural diathesis-stress model. Psychological Review. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Whyte MC, Brett C, Harrison LK, Byrne M, Miller P, Lawrie SM, Johnstone EC. Neuropsychological performance over time in people at high risk of developing schizophrenia and controls. Biological Psychiatry. 2006;59:730–739. doi: 10.1016/j.biopsych.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Research. 1998;794:225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiology of Disease. 2008;30:255–269. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu Z, Tandon P, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK, Holmes GL. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. The Journal of Neuroscience. 2000;20:RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Zhou X, Geyer MA. Animal models of schizophrenia. Current Topics in Behavioral Neurosciences. 2010;4:391–433. doi: 10.1007/7854_2010_62. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, da Costa KA. Choline: An essential nutrient for public health. Nutrition Reviews. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. The fetal origins of memory: The role of dietary choline in optimal brain development. The Journal of Pediatrics. 2006;149:131–136. doi: 10.1016/j.jpeds.2006.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]