Abstract
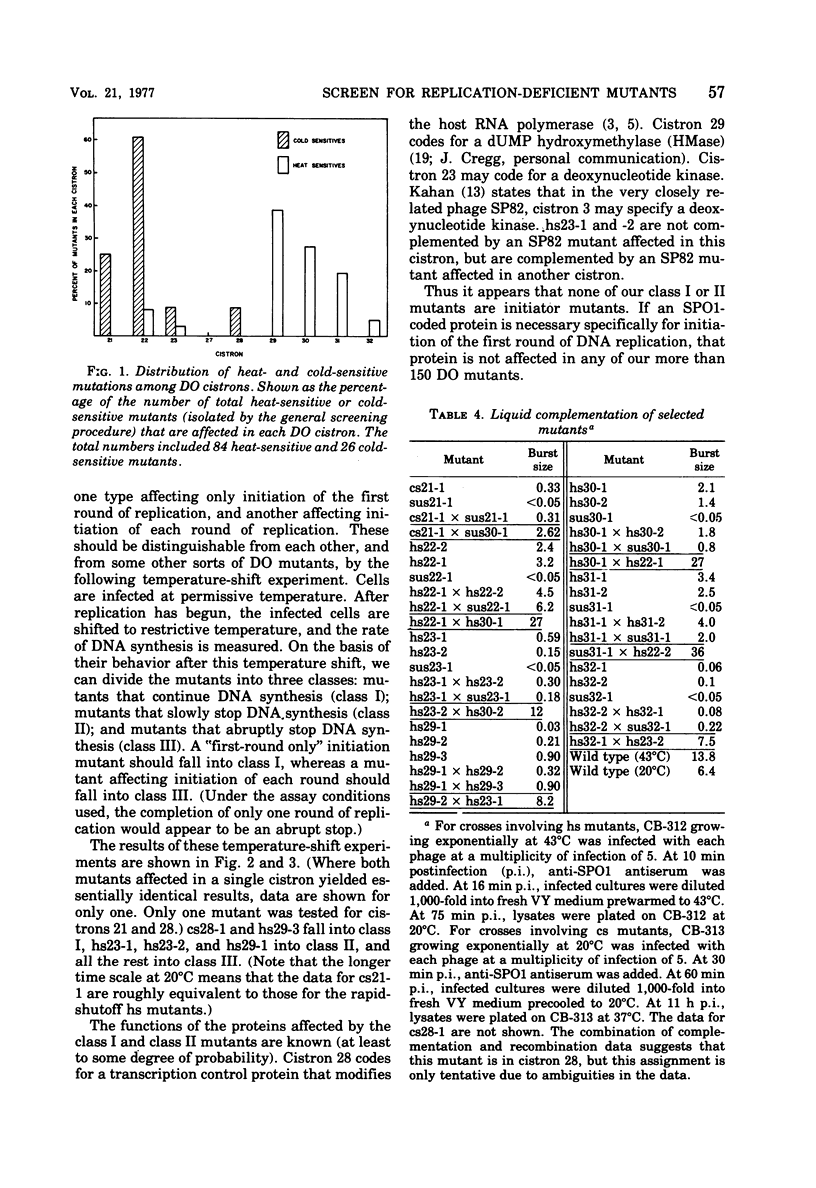
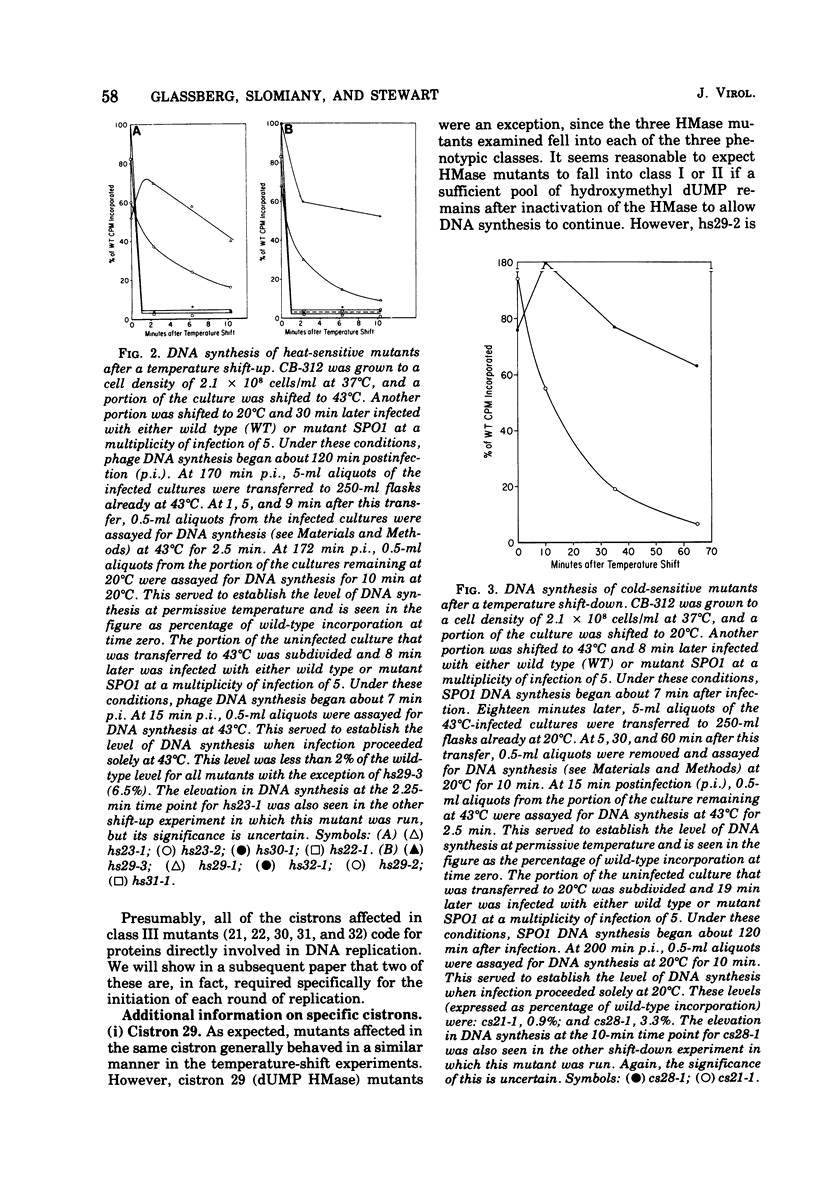
A procedure is described for the selective isolation of temperature-sensitive replication-deficient mutants of Bacillus subtilis phage SPO1. A modification of the procedure permits the isolation of temperature-sensitive mutants in specific cistrons of interest. The applicability of these procedures to other viral systems is discussed. The mutations isolated were assigned to eight replication-deficient cistrons, with the cold-sensitive mutations showing a distribution strikingly different from that of the heat-sensitive mutations. As a preliminary to the identification of initiation-deficient mutants, the mutants were divided into three classes on the basis of their ability to synthesize DNA after a shift to nonpermissive temperature. We also report two incidental results: (i) the SPO1 dUMP hydroxymethylase, like the T4 dCMP hydroxymethylase, may be part of a multifunctional complex; and (ii) mutants were isolated that were replication positive but lysis deficient and failed to complement one of the replication-deficient mutants.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiu C. S., Tomich P. K., Greenberg G. R. Simultaneous initiation of synthesis of bacteriophage T4 DNA and of deoxyribonucleotides. Proc Natl Acad Sci U S A. 1976 Mar;73(3):757–761. doi: 10.1073/pnas.73.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Strack H. B. Cold-sensitive mutants of bacteriophage lambda. Genetics. 1971 Jan;67(1):5–17. doi: 10.1093/genetics/67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J. J., Petrusek R. L., Geiduschek E. P. Conversion of Bacillus subtilis RNA polymerase activity in vitro by a protein induced by phage SP01. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2366–2370. doi: 10.1073/pnas.72.6.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR R. S., LIELAUSIS I. TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4D: THEIR ISOLATION AND GENETIC CHARACTERIZATION. Genetics. 1964 Apr;49:649–662. doi: 10.1093/genetics/49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Losick R., Pero J. Regulatory gene 28 of bacteriophage SPO1 codes for a phage-induced subunit of RNA polymerase. J Mol Biol. 1976 Mar 5;101(3):427–433. doi: 10.1016/0022-2836(76)90157-1. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriphage SPO1 development. II. Some modulations and prerequisites of the transcription program. Virology. 1971 Apr;44(1):200–210. doi: 10.1016/0042-6822(71)90165-6. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Nashimoto H., Nomura M. Structure and function of E. coli ribosomes. 8. Cold-sensitive mutants defective in ribosome assembly. Proc Natl Acad Sci U S A. 1969 Jun;63(2):384–391. doi: 10.1073/pnas.63.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik J., Botstein D. A genetic method for determining the order of events in a biological pathway. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2046–2050. doi: 10.1073/pnas.70.7.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan E. A genetic study of temperature-sensitive mutants of the subtilis phage SP82. Virology. 1966 Dec;30(4):650–660. doi: 10.1016/0042-6822(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Kahan E. Early and late gene function in bacteriophage SP82. Virology. 1971 Dec;46(3):634–637. doi: 10.1016/0042-6822(71)90066-3. [DOI] [PubMed] [Google Scholar]
- Klotz G., Spatz H. C. A biological assay for intracellular SPP1 DNA. Mol Gen Genet. 1971;110(4):367–373. doi: 10.1007/BF00438279. [DOI] [PubMed] [Google Scholar]
- Levner M. H., Cozzarelli N. R. Replication of viral DNA in SPO1-infected Bacillus subtilis. I. Replicative intermediates. Virology. 1972 May;48(2):402–416. doi: 10.1016/0042-6822(72)90051-7. [DOI] [PubMed] [Google Scholar]
- Levner M. H. Eclipse of viral DNA infectivity in SPO1-infected Bacillus subtilis. Virology. 1972 Oct;50(1):267–272. doi: 10.1016/0042-6822(72)90369-8. [DOI] [PubMed] [Google Scholar]
- Levner M. H. Replication of viral DNA in SPO1-infected Bacillus subtilis. II. DNA maturation during abortive infection. Virology. 1972 May;48(2):417–429. doi: 10.1016/0042-6822(72)90052-9. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Green D. M. Effects of the decay of incorporated radioactive phosphorus on the transfer of the bacteriophage SP82G genome. J Virol. 1973 Aug;12(2):300–309. doi: 10.1128/jvi.12.2.300-309.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Yanagida T., Fujita D. J., Olsson-Wilhelm B. M. The genetics of bacteriophage SPO1. Biken J. 1972 Jun;15(2):81–97. [PubMed] [Google Scholar]
- Scotti P. D. A new class of temperature conditional lethal mutants of bacteriophage T4D. Mutat Res. 1968 Jul-Aug;6(1):1–14. doi: 10.1016/0027-5107(68)90098-5. [DOI] [PubMed] [Google Scholar]
- Shub D. A., Johnson G. G. Bacteriophage SPO1 DNA- and RNA-directed protein synthesis in vitro: the effect of TF1, a template-selective transcription inhibitor. Mol Gen Genet. 1975;137(2):161–169. doi: 10.1007/BF00341682. [DOI] [PubMed] [Google Scholar]
- Stewart C. R., Click B., Tole M. F. DNA replication and late protein synthesis during SP82 infection of Bacillus subtilis. Virology. 1972 Dec;50(3):653–663. doi: 10.1016/0042-6822(72)90419-9. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Kessler D. P., Ingraham J. Cold-sensitive mutations in Salmonella typhimurium which affect ribosome synthesis. J Bacteriol. 1969 Mar;97(3):1298–1304. doi: 10.1128/jb.97.3.1298-1304.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera A., Jimenez F., Salas M., Viñuela E. Temperature-sensitive mutants of bacteriophage phi-29. Virology. 1971 Dec;46(3):586–595. doi: 10.1016/0042-6822(71)90062-6. [DOI] [PubMed] [Google Scholar]
- Tessman I. Mutagenic treatment of double- and single-stranded DNA phages T4 ans S13 with hydroxylamine. Virology. 1968 Jun;35(2):330–333. doi: 10.1016/0042-6822(68)90275-4. [DOI] [PubMed] [Google Scholar]
- Tevethia M. J., Baptist J. N., Mandel M. Pleiotropic effects of suppressor mutations in Bacillus subtilis. J Bacteriol. 1974 Sep;119(3):961–975. doi: 10.1128/jb.119.3.961-975.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Weintraub S., Dales S. Biogenesis of poxviruses: genetically controlled modifications of structural and functional components of the plasma membrane. Virology. 1974 Jul;60(1):96–127. doi: 10.1016/0042-6822(74)90369-9. [DOI] [PubMed] [Google Scholar]
- Yehle C. O., Ganesan A. T. Deoxyribonucleic acid synthesis in bacteriophage SP01-infected Bacillus subtilis. II. Purification and catalytic properties of a deoxyribonucleic acid polymerase induced after infection. J Biol Chem. 1973 Nov 10;248(21):7456–7463. [PubMed] [Google Scholar]
- Yehle C. O., Ganesan A. T. Deoxyribonucleic acid synthesis in bacteriophage SPO1-infected Bacillus subtilis. I. Bacteriophage deoxyribonucleic acid synthesis and fate of host deoxyribonucleic acid in normal and polymerase-deficient strains. J Virol. 1972 Feb;9(2):263–272. doi: 10.1128/jvi.9.2.263-272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



