Abstract
Rationale
Ischemic heart disease is characterized by contractile dysfunction and increased cardiomyocyte death, induced by necrosis and apoptosis. Increased cell survival after an ischemic insult is critical and depends on several cellular pathways, which have not been fully elucidated.
Objective
To test the hypothesis that the anti-apoptotic haematopoietic-lineage-substrate-1-associated protein-X-1 (HAX-1), recently identified as regulator of cardiac Ca-cycling, may also ameliorate cellular injury in the face of an ischemic insult.
Methods and Results
We report that cardiac ischemia/reperfusion injury is associated with significant decreases in HAX-1 levels ex vivo and in vivo. Accordingly, over-expression of HAX-1 improved contractile recovery, coupled with reduced infarct size, plasma troponin I level and apoptosis. The beneficial effects were associated with decreased ER stress response through specific inhibition of the inositol-requiring-enzyme (IRE-1) signaling pathway, including its downstream effectors caspase-12 and the transcription factor C/EBP homologous protein. Conversely, HAX-1 heterozygous deficient hearts exhibited increases in infarct size and IRE-1 activity. The inhibitory effects of HAX-1 were mediated by its binding to the N-terminal fragment of the heat shock protein 90 (Hsp90). Moreover, HAX-1 sequestered Hsp90 from IRE-1 to the phospholamban/SERCA calcium transport complex. The HAX-1 regulation was further supported by loss of IRE-1 inhibition in presence of the Hsp90 inhibitor, 17-N-Allylamino-17-Demethoxygeldanamycin.
Conclusions
Cardiac ischemia/reperfusion injury is associated with decreases in HAX-1 levels. Consequently, over-expression of HAX-1 promotes cardiomyocyte survival, mediated by its interaction with Hsp90 and specific inhibition of IRE-1 signaling at the ER/SR.
Keywords: Apoptosis, ER stress, ischemia/reperfusion injury, ischemic heart disease
INTRODUCTION
Coronary heart disease is the leading cause of morbidity and mortality worldwide, with over 7 million deaths per year1. Myocardial infarction, caused by ischemia/reperfusion injury, results in massive death of cardiomyocytes and plays a key role in the development of coronary heart disease2. Cell death during ischemia/reperfusion is an active, multifactor process mediated by apoptosis, necrosis, or both3. Several studies in human and animal models have indicated that there is an association between apoptotic loss of cardiomyocytes and progression of heart failure4, 5 and suggested that inhibition by appropriate interventions may hold therapeutic promise3, 6.
Recently, the role of endoplasmic/sarcoplasmic reticulum (ER/SR) in ischemia/reperfusion injury has gained significant interest, as dysfunction of this membrane-associated organelle can lead to activation of cell death responses7, 8. ATP depletion, abnormal oxidative status and disrupted calcium homeostasis during cardiac ischemic/reperfusion injury can cause the accumulation of misfolded proteins in the ER/SR lumen, which is known to trigger ER stress8. ER stress induces two major protecting responses: attenuation of protein synthesis and increase in the expression of genes encoding chaperones to facilitate the protein folding in the ER. Three major signaling pathways are involved in the pro-survival ER stress response7, 8: a) the RNA-dependent protein kinase-like ER kinase (PERK), which regulates cellular protein synthesis and limits additional influx of proteins into the lumen of the stressed ER; b) the ER transmembrane kinase or the inositol-requiring enzyme-1 (IRE-1), a Ser/Thr kinase with an endonuclease domain that can remove 26-nucleotides from the mRNA of x-box binding protein 1 (XBP-1), resulting in the translation of stable XBP-1 transcription factor to promote ER stress gene program; and c) the type II transmembrane protein or the activating transcription factor-6 (ATF-6), which can facilitate the ER folding capacity through induction of chaperone expression. Although the importance of these pathways has been recognized, there are only a few studies on their functional significance in the intact heart. Specifically, the role of ATF6 has been addressed in cardiac ischemic injury9, 10, but the roles of PERK or IRE-1 have not been delineated. Although the ER stress response is initially directed towards cellular adaptation to alleviate the unfolded protein load, prolonged ER stress is associated with activation of apoptosis. In this regard, the kinase domain of IRE-1 was reported to activate c-Jun N-terminal kinase (JNK) by interacting with TNF receptor-associated factor 2 (TRAF2) and apoptosis signal-regulating kinase 1 (ASK1) in neuronal and pancreatic tumor cell lines11, 12. The IRE-1/TRAF2 complex also contributes to apoptosis through caspase 12 released from the ER and the ensuing cell death13.
The anti-apoptotic HS-1-associated protein X-1 (HAX-1) may be a new regulator of ER-mediated cell survival. HAX-1 is a ~35 kDa protein, which was originally found to form a complex with HS-1 (hematopoietic lineage cell-specific protein-1) in lymphocytes, and mediate lymphocyte differentiation14. In vitro studies provided the first experimental evidence that HAX-1 protects against cell death15, such as promotion of cardiomyocyte survival through caspase-9 inhibition upon hydrogen peroxide treatment16. Its protective role against cell death in vivo was subsequently demonstrated in a global genetic deletion mouse, which had a short life-span due to progressive loss of neuronal cells17. More importantly, human mutations were found in the HAX-1 gene, which can result in loss of this protein, and the affected individuals present with severe neutropenia, a rare immunodeficiency disease with scarce neutrophil counts15. However, although HAX-1 has been reported to regulate cell survival in multiple tissues15, its potential protective role in cardiac muscle is virtually unknown. HAX-1 has been shown to be present in both the mitochondria and the sarcoplasmic reticulum (SR) and to associate with phospholamban (PLN), regulating cardiac calcium homeostasis18. Given the regulatory role of HAX-1 in calcium cycling and its anti-apoptotic properties in other tissues, it becomes important to delineate the functional role of HAX-1 in the stressed heart as this may reveal novel insights for potential therapeutic interventions.
Here we demonstrate for the first time, that HAX-1 reduces cardiac infarct size and improves contractile recovery after ischemia/reperfusion ex vivo and in vivo. The protective effects of HAX-1 are mediated by formation of a regulatory complex between HAX-1 and heat shock protein 90 (Hsp90), resulting in inhibition of ER stress-induced cell death response through the IRE-1 signaling pathway. Moreover, the HAX-1/Hsp90 complex is recruited to PLN/SERCA2a, suggesting a “functional coupling” between ER stress signaling elements and calcium homeostasis in cardiac myocytes. Thus, HAX-1 protection may be partially mediated at the ER/SR level, promoting cell survival against noxious conditions such as ischemia/reperfusion injury.
METHODS
A detailed Methods section is available in the Online Supplement at http://circres.ahajournals.org, which includes the description of animal models, ex vivo global ischemia/reperfusion, in vivo regional ischemia, rat myocytes isolation and virus infection, mouse cardiomyocyte isolation and calcium kinetics measurement, cardiomyocyte apoptosis treatment and Annexin V staining, western blot analysis, caspase-3 and calpain activities measurement, DNA fragmentation measurement, terminal dUTP nick end labeling assays, plasma troponin I measurement, quantitative real-time PCR assay, generation of recombinant protein and blot overlay assay, GST-pull down assay, competitive protein binding ELISA assay, co-immunoprecipitation, immuno-fluorescence staining and statistical analysis.
RESULTS
HAX-1 protects hearts from ischemia/reperfusion injury
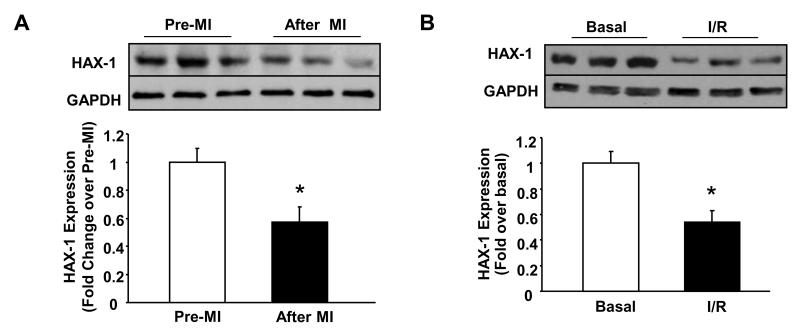
Ischemia/reperfusion (I/R) injury can tilt the balance between anti-apoptotic and pro-apoptotic protein expression, inducing cell death19, 20. Although HAX-1 has been suggested to be an anti-apoptotic protein, its participation in controlling the above balance is virtually unknown in the heart. To address this question, we assessed the expression levels of HAX-1 after 30 minutes of coronary artery ligation, followed by 24 hours of reperfusion. There was a decrease in HAX-1 expression (Figure 1A), which was also confirmed in isolated hearts, subjected to 40 minutes of global no-flow ischemia followed by 60 minutes of reperfusion (Figure 1B). The reduction was not due to changes in HAX-1 mRNA (Online Figure II), suggesting a post-translational regulation of HAX-1 levels during I/R. These findings suggest that decreases in HAX-1 expression may contribute to tissue death after ischemia/reperfusion injury.
Figure 1. HAX-1 levels are decreased in WT hearts after ischemia-reperfusion injury.
A: Representative immunoblot and quantification of cardiac HAX-1 protein expression after in vivo myocardial infarction (MI), performed by 30 minutes of left coronary artery occlusion followed by 24 hours reperfusion (n=3 hearts/group). * P<0.05, compared to sham. B: Representative immunoblot and quantification of HAX-1 protein expression in isolated hearts after 40 min of no flow global ischemia, followed by 60 min of reperfusion (I/R) (n=4 hearts/group). *P<0.05, compared to basal. Data are presented as mean±SEM.
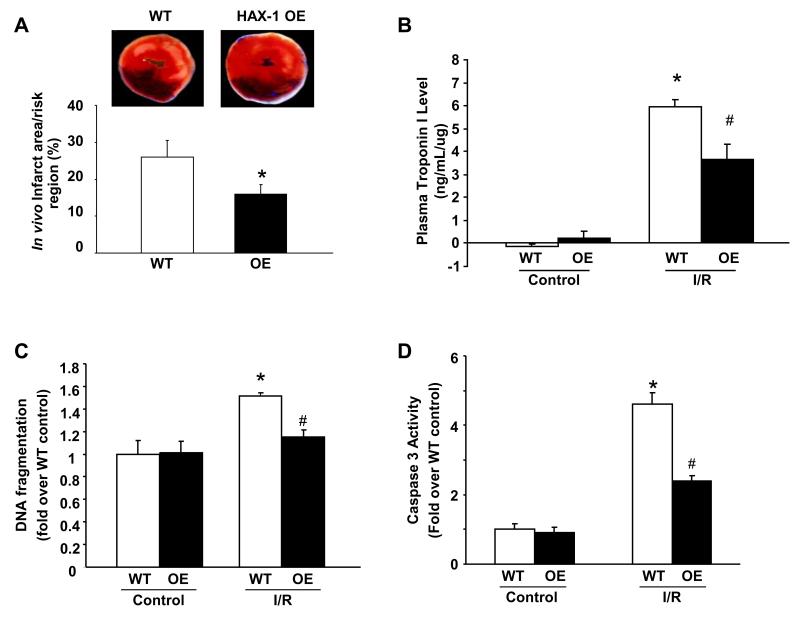
To examine whether restoring HAX-1 levels can confer protection after ischemia/reperfusion, mice with cardiac specific HAX-1 over-expression (HAX-1 OE: 2.5-fold) 18 along with their isogenic WTs were subjected to in vivo coronary artery ligation. The ratio of infarct to risk region was significantly reduced from 23.5± 7% in WT hearts, typical for FVBN strain21, 22 , to 16±3% in HAX-1 OEs (Figure 2A), while the region at risk was not significantly different between these two groups (HAX-1 OE, 58±4.3%; WT, 59±3.6%; P>0.05). Protection elicited by HAX-1 over-expression was also confirmed by a reduction in plasma troponin I (TnI) levels (Figure 2B). To examine whether the reduction in infarct size by increased HAX-1 expression was related to inhibition of apoptotic events, we measured the extent of DNA fragmentation. HAX-1 OE hearts showed significantly attenuated DNA fragmentation compared to WTs (Figure 2C). This finding was further supported by reduced caspase-3 activity in HAX-1 OE (Figure 2D), demonstrating the anti-apoptotic role of cardiac HAX-1 in vivo.
Figure 2. Over-expression of HAX-1 decreases myocardial infarction in vivo by inhibiting apoptosis.
A: Representative images of TTC staining and quantification of in vivo infarct area/risk region in WT and HAX-1 OE hearts after MI. n=6 for WT and 8 for HAX-1 OE. *P<0.05, compared to WT. B: Quantification of plasma troponin I level, normalized to protein loaded, in WT and HAX-1 OE mice at 24 hours after surgery (n=3 for sham, 4 for WT and 5 for HAX-1 OE I/R hearts). C and D: DNA fragmentation and caspase 3 activity expressed as percentage of WT basal levels (n=3 for both WT and OE controls, and n=4 for both WT and OE ischemia/reperfusion samples). *P<0.05, compared to WT control. #P<0.05, compared to WT ischemia/reperfusion samples.
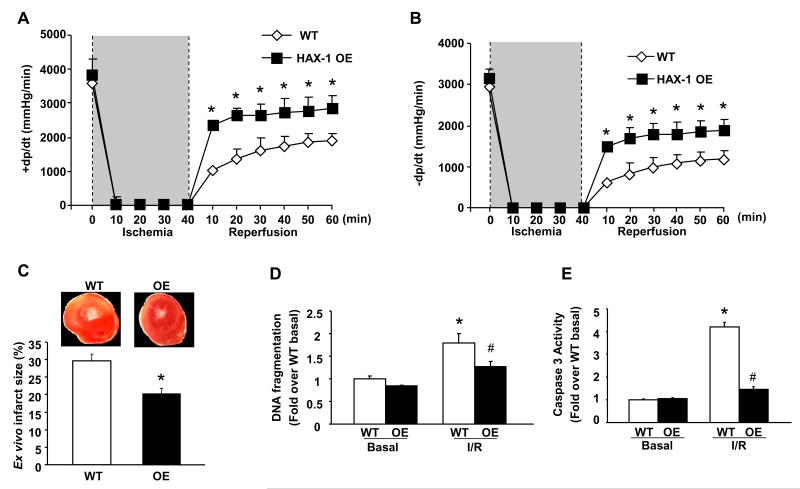
To further delineate the cardioprotective mechanisms associated with HAX-1 in the absence of neuro-hormonal factors, isolated hearts were subjected to ex vivo ischemia/reperfusion injury, using the Langendorff perfusion system. Interestingly, the decreased contractility previously observed in isolated cardiomyocytes by HAX-1 overexpression18, did not translate to depressed function in the intact heart (Figures 3A and B). This apparent discrepancy was also previously noted with studies on Bcl-223-26 and other mouse models27, 28, indicating that cardiomyocyte contractility is not the sole determinant but a number of additional factors, such as sheer force on the sarcolemma, cell-cell/matrix interactions and the endothelium, influence whole heart performance. Upon ischemia/reperfusion, functional recovery, measured by rates of contraction (+dP/dt) and relaxation (−dP/dt), was significantly increased in HAX-1 OE hearts (Figures 3A and 3B), leading to enhanced left ventricular developed pressure (LVDP) and lower left ventricular diastolic pressure (LVEDP, Online Figure III). This improvement in contractility was accompanied with a 30% reduction in myocardial infarct size, compared with WTs (Figure 3C). Similar to in vivo myocardial infarction studies, the protective effect was related to inhibition of apoptosis, where over-expression of HAX-1 significantly attenuated the increases in DNA fragmentation, TUNEL positive nuclei and caspase-3 activity to 70%, 77% and 35% of WT values, respectively (Figures 3D and E, Online Figure IV). Thus, both in vivo and ex vivo studies suggest that restoring HAX-1 levels after ischemia/reperfusion injury may prevent cell death and improve heart contractile recovery.
Figure 3. Over-expression of HAX-1 improves cardiac contractile recovery after ischemia/reperfusion by decreasing apoptosis.
Isolated WT and HAX-1 OE hearts were subjected to either 100 min of perfusion (basal) or 40 minutes of no-flow ischemia, followed by 60 minutes of reperfusion (I/R). A and B: Rates of contraction and relaxation (±dP/dt, mmHg/min) (n=6 for WT and 8 for HAX-1 OE); C: Representative images of TTC staining and quantification of infarct size in WT and HAX-1 OE hearts after I/R injury (n=6 hearts for each group); D: DNA fragmentation expressed as fold over WT basal (n=4 hearts for each group); E: Caspase 3 activity expressed as fold increase over WT basal (n=6 hearts for each group). *P<0.05, compared to WT basal. #P<0.05, compared to WT I/R. Data are presented as mean±SEM.
HAX-1 attenuates ER stress response through specific inhibition of IRE-1
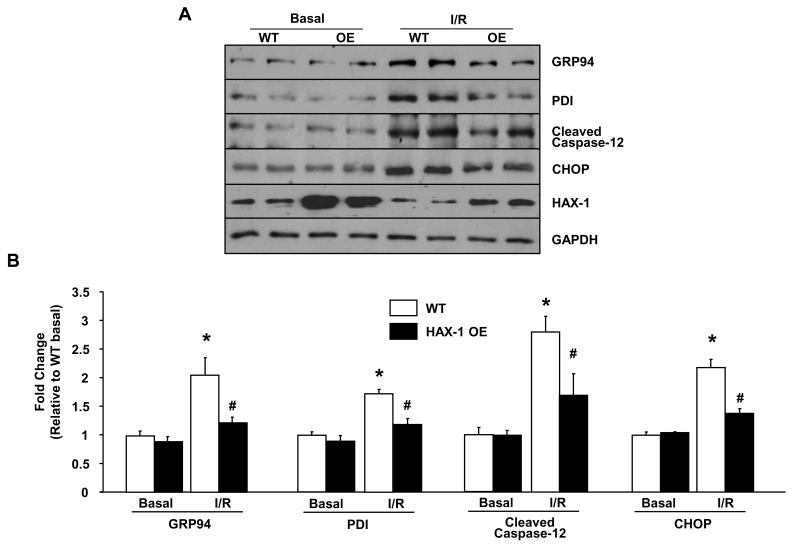
During ischemia/reperfusion injury, proper protein folding in the ER/SR compartment is disturbed. The accumulation of misfolded peptides can elicit ER stress signaling response (IRE-1, PERK and ATF6), that is capable of inducing apoptosis through caspase activation and increased pro-apoptotic protein expression7, 8. Although HAX-1 shows ER/SR localization and regulates SR calcium cycling in cardiomyocytes18, its involvement in ER stress response is unknown. To address this, we examined the ER stress response in our HAX-1 OE model after ischemia/reperfusion injury. Under basal conditions, there was no significant difference in the expression levels of the ER stress-induced chaperones, glucose-regulated protein 94 (GRP94) and protein disulfide isomerase (PDI), as well as the pro-apoptotic transcription factor C/EBP homologous protein (CHOP) between WT and HAX-1 OE hearts (Figure 4). After ischemia/reperfusion injury, the expression levels of these players were increased in both groups, but the increases in HAX-1 OE hearts were significantly lower compared to WTs (Figure 4). Furthermore, activation of caspase-12, an ER stress associated caspase, was significantly attenuated by HAX-1. These findings suggest that the anti-cell death effect of HAX-1 is associated with reduction of pro-apoptotic gene expression and caspase activation that are coupled to ER stress response (Online Figure I).
Figure 4. Over-expression of HAX-1 attenuates ER stress after ex vivo ischemia reperfusion.
Isolated WT and HAX-1 OE hearts were subjected to either 100 minutes of perfusion (Basal) or 40 minutes of no-flow ischemia, followed by 60 minutes of reperfusion (I/R). A: Representative immunoblot for GRP94, PDI, cleaved caspase-12 and CHOP expression levels. GAPDH was used as control. B: Quantification of GRP94, PDI, cleaved caspase-12 and CHOP expression levels (n = 4-6 hearts). *P<0.05, compared to WT basal. #P<0.05, compared to WT I/R. Data are presented as mean±SEM.
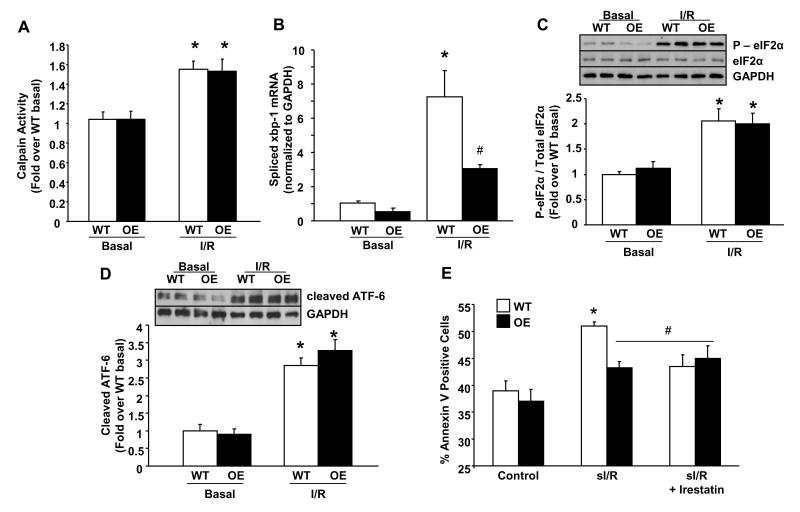
Since caspase-12 is a known substrate for calpain and inhibition of calpain has been shown to attenuate ER stress mediated apoptosis29, we examined the calpain activity. There were no differences between HAX-1 OE and WT hearts after ischemic injury (Figure 5A). This suggests that the reduced caspase-12 activity is regulated by a calpain-independent mechanism. Indeed, processing of caspase-12 and initiation of the caspase cascade may be facilitated by auto-phosphorylation and activation of IRE-1, one of the three canonical ER stress sensors13, 30. To test this hypothesis, we examined the IRE-1 phosphorylation status and found a 50% decrease in HAX-1 OE hearts after ischemia/reperfusion injury (Online Figure V). The inhibition of IRE-1 function by HAX-1 was also confirmed by its decreased endoribonuclease activity, measured by splicing of the full-length xbp-1 transcript (Figure 5B). Processing of the xbp-1 transcript allows translation of stable XBP-1 protein, a transcription factor that regulates the expression of ER stress related genes. Indeed, assessment of XBP-1 protein levels indicated parallel alterations as the spliced xbp-1 transcript (Data not shown). Therefore, reduction of IRE-1 activity and the associated XBP-1 levels appear consistent with the attenuated expression of GRP94, PDI and CHOP in HAX-1 OE hearts after reperfusion injury (Online Figure I).
Figure 5. HAX-1 specifically inhibits the IRE-1 pathway.
WT and HAX-1 OE hearts, subjected to no-flow ischemia followed by reperfusion (I/R) in figure 4, were processed for the examination of ER stress signaling pathways. A: Calpain activity expressed as fold over WT basal (n = 4-6 hearts); B: Quantification of spliced xbp1 mRNA levels using real-time PCR. GAPDH was used as control (n = 3 for basal, n=4-6 for I/R); C and D: Quantification of p-eIf2α/total-eIf2α and cleaved ATF-6 expression. GAPDH was used as control (n = 4-6 hearts). E: Apoptosis, measured by annexin V staining, of isolated cardiomyocytes after simulated ischemia/reperfusion procedure (sI/R), with or without Irestatin (n=16 hearts for WT and 13 for HAX-1 OE in each treatment group).*P<0.05, compared to WT basal or control. #P<0.05, compared to WT I/R or sI/R. Data are presented as mean±SEM.
Surprisingly, there was no significant attenuation of the other two ER stress signaling pathways, PERK and ATF-6, by HAX-1. Activation of PERK, measured by phosphorylation of the eukaryotic translation initiation factor 2alpha (eIF-2α), was elevated to similar levels in both models after ischemia/reperfusion (Figure 5C). In addition, the ER transmembrane transcriptional factor ATF-6, which requires proteolytic cleavage for activation to translocate to the nucleus and up-regulate ER stress-induced genes, including the primary full-length xbp-1 transcript31, was increased to similar levels in both groups, as indicated by ATF-6 cleavage (Figure 5D). This finding was also supported by the lack of attenuated expression of total (full-length + spliced) xbp-1 transcript in the HAX-1 OE group (Online Figure VI). Thus, HAX-1 appears to specifically regulate the IRE-1 pathway without affecting the other two ER stress pathways (Online Figure I).
To examine whether IRE-1 inhibition can confer protection during ER stress, isolated WT cardiomyocytes were treated with brefeldin A (BA) to induce ER stress, in the absence or presence of the IRE-1 inhibitor, irestatin. Indeed, induction of apoptosis, associated with BA and measured by annexin V staining (Online Figure VII), was blocked by irestatin treatment (Online Figures VIII and IX). These findings indicate that IRE-1 inhibition promotes myocyte survival under ER stress conditions. Over-expression of HAX-1 completely abrogated apoptosis, similar to IRE-1 inhibition (Online Figure IX), further supporting the role of HAX-1 in prevention of cell death induced by ER stress. Additionally, inhibition of IRE-1 in isolated WT cardiomyocytes, subjected to simulated ischemia/reperfusion32 (sI/R), attenuated apoptosis (Figure 5E), demonstrating that IRE-1 inhibition is protective in cardiomyocytes under this condition. More importantly, HAX-1 OE cardiomyocytes, which exhibited better survival than WTs, did not respond to irestatin treatment (Figure 5E). These findings on BA and sI/R studies suggest that IRE-1 inhibition is one of the mechanisms underlying cardioprotection by HAX-1.
Reduction of HAX-1 level exacerbates cardiac I/R injury
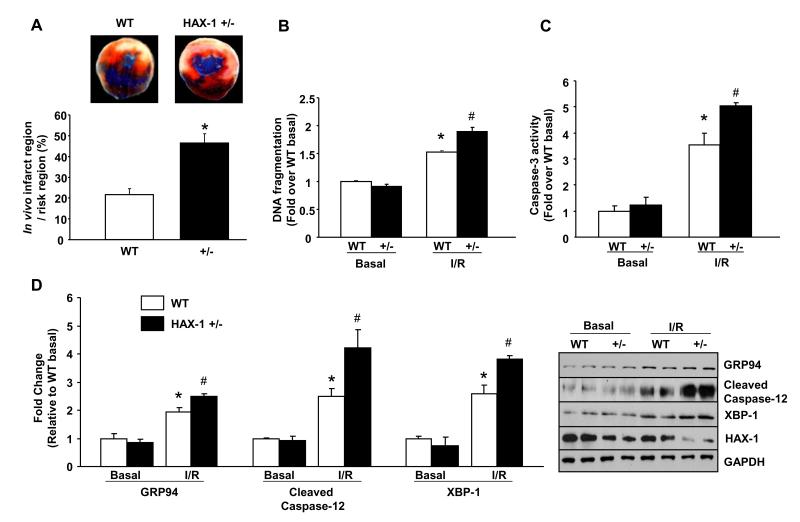
To test whether loss of HAX-1 contributes to cardiac injury, we subjected the HAX-1 heterozygous deficient mice (HAX-1 +/−) to ischemia/reperfusion. Reduced HAX-1 expression (by 64%)18 was associated with increased infarct size (Figure 6A) and plasma Tnl level (Online Figure X) after in vivo ischemia/reperfusion injury. Furthermore, exacerbated cardiac contractile dysfunction was observed in HAX-1 +/− hearts with 60% and 47% recovery of +dP/dt and −dP/dt parameters, respectively, when compared to WTs, after ex vivo ischemia/reperfusion (Online Figure XI). These findings indicate the importance of HAX-1 in preserving contractile function upon ischemia/reperfusion injury.
Figure 6. HAX-1 heterozygous deficient (HAX +/−) hearts exhibit exacerbated apoptotic response after ischemia/reperfusion.
A. Infarct size/risk area in WT and HAX-1 +/− hearts after 30 minutes of left coronary artery ligation followed by 24 hours of reperfusion (n=8 for WT and 5 for HAX-1+/− hearts). B, C and D: DNA fragmentation, caspase 3 activity and quantification of GRP94, cleaved caspase-12 and XBP-1 expression in isolated WT and HAX-1+/− hearts after 40 minutes of ischemia followed by 60 minutes of reperfusion (n = 3 hearts for either WT or HAX+/− basal, and 4 for either WT or HAX-1+/− I/R). *P<0.05, compared to WT basal. #P<0.05, compared to WT I/R. Data are presented as mean±SEM.
Consistent with compromised cardiac function, the extent of apoptosis was elevated after ex vivo ischemia/reperfusion in HAX-1 heterozygous hearts, as indicated by significant increases in DNA fragmentation, TUNEL positive nuclei and caspase-3 activity, compared to WTs (Figures 6B and C, Online Figure XII). The elevated apoptosis was related to exacerbated ER stress response, associated with increased expression of ER stress markers (GRP94 and cleaved caspase-12) (Figure 6D). The IRE-1 activity was also enhanced as demonstrated by significant increases in XBP-1 protein expression (Figure 6D). Together with the HAX-1 over-expression studies, our findings suggest that HAX-1 prevents ER stress induced apoptotic responses through inhibition of IRE-1 activity.
HAX-1 regulates IRE-1 activity through Hsp90
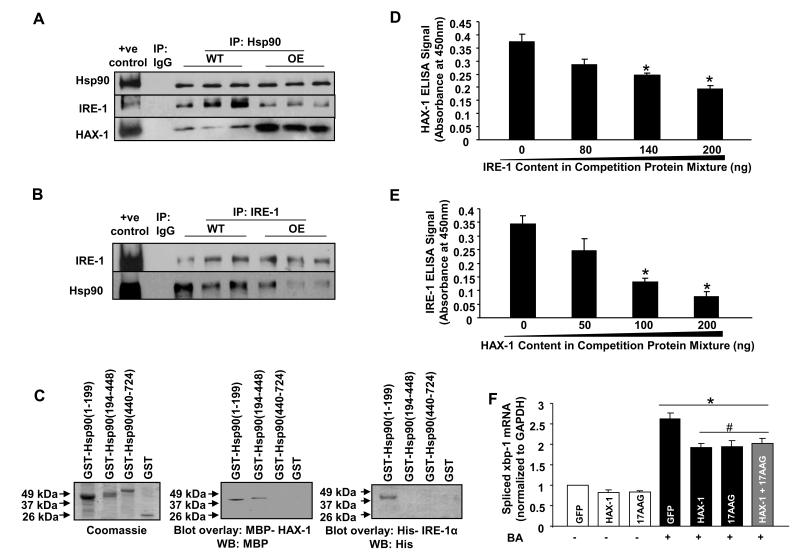
Previous studies in COS-7 and pancreatic tumor cell lines reported that IRE-1 binds Hsp90 and this promotes its stable conformation or activity33. Thus, we examined the expression levels of Hsp90 in WT and HAX-1 OE hearts before and after I/R. However, there were no differences among these groups (Online Figures XIII and XIV). It was then determined whether HAX-1 may disturb the IRE-1/Hsp90 interaction, resulting in decreased IRE-1 activity. Immuno-precipitations, using the HAX-1 antibody as bait, revealed that Hsp90 was pulled down from both WT and HAX-1 OE hearts (Online Figure XV). This association was also observed in reciprocal experiments, using the Hsp90 antibody as bait (Figure 7A). Importantly, over-expression of HAX-1 enhanced the HAX-1/Hsp90 association (Figure 7A), while the Hsp90/IRE-1 interaction was reduced, compared to WTs (Figures 7A and B). To determine the binding domains of HAX-1 or IRE-1 on Hsp90, recombinant proteins were used in in vitro binding assays. Both HAX-1 and the cytosolic domain of IRE-133, directly interacted with the N-terminal fragment (amino acid 1-199) of Hsp90 (Figure 7C). The binding of these proteins on Hsp90 appeared to be competitive, as demonstrated by recombinant protein binding ELISA assays. The interaction of HAX-1 with Hsp90 gradually decreased with increasing amounts of the IRE-1 cytosolic fragment. The same trend was also observed for IRE-1/Hsp90 interaction, when the levels of HAX-1 were increased (Figures 7D and E). These findings suggest that over-expression of HAX-1 in the heart destabilizes IRE-1 activity by displacing it from Hsp90.
Figure 7. HAX-1 over-expression reduces Hsp90/IRE-1 interaction and inhibits IRE-1 activity.
Centrifuged homogenates from WT and HAX-1 OE hearts were subjected to co-immunoprecipitation, using Hsp90 (A) or IRE-1 (B) antibody. Eight independent experiments were performed. C: SDS-gel stained with Coomassie blue showing various purified GST-Hsp90 fragments. Blot overlay assay using either MBP-HAX-1 or His-IRE-1α, followed by immunodetection with the MBP or His antibody to determine the binding region of HAX-1 and IRE-1 on Hsp90. D: HAX-1/Hsp90 (200ng/200ng) interaction, in the presence of a competition protein mixture (200ng of total protein), containing various compositions of GST and the IRE-1α cytosolic fragment. ELISA signals were calculated after subtraction of the mean value of controls, represented by GST coated wells (n=4 experiments; *P<0.05, compared to IRE-1 at 0ng). E: Same procedure was performed for IRE-1/Hsp90 interaction, using GST/HAX-1 in the competition mixture (n=7 experiments; *P<0.05, compared to HAX-1 at 0ng). F: Rat cardiomyocytes were infected with adenoviruses encoding either GFP or HAX-1. 17-AAG was used as Hsp90 inhibitor and brefeldin A (BA) was used to induce ER stress (n=6 hearts; *P<0.05, compared to GFP control; #P<0.05, compared to GFP + BA). Data are presented as mean±SEM.
To further examine whether HAX-1 regulates IRE-1 activity through Hsp90, we used isolated rat cardiomyocytes and induced ER stress pharmacologically, while HAX-1 expression or Hsp90 activity was manipulated. Brefeldin A administration (Online Figure XVI) in GFP infected cardiomyocytes resulted in a ~2.7-fold up-regulation of spliced xbp-1 transcript levels, reflecting increased IRE-1 activity. This increase was attenuated to a similar extent by either over-expression of HAX-1 or Hsp90 inhibition by 17-N-Allylamino-17-Demethoxygeldanamycin (17-AAG; 0.1μmol/L). When HAX-1 over-expressing cardiomyocytes were treated with Hsp90 inhibitors, there was no additional attenuation, compared to either treatment alone (Figure 7F), indicating that HAX-1 can no longer inhibit IRE-1 function, in the face of Hsp90 inhibition. This finding was further supported by studies in isolated mouse cardiomyocytes, where Hsp90 inhibition abolished the difference between WT and HAX-1 OE cells in ER stress induced apoptosis. Furthermore, Hsp90 inhibition also abolished the enhancement of BA induced apoptosis in HAX-1 +/− cardiomyocytes (Online Figure XVII).
Since HAX-1 expression was reduced after I/R (Figures 1A and 1B), accompanied with enhanced IRE-1 activity (Figure 5B), co-immunoprecipitation experiments were performed to further address the role of HAX-1 in the formation of the Hsp90/IRE-1 complex. Using the Hsp90 antibody as bait, there was a decrease in the HAX-1 levels associated with Hsp90, which was accompanied with an enhanced Hsp90/IRE-1 association in WT hearts after ischemia/reperfusion. This increase was also observed in reciprocal experiments, using the IRE-1 antibody (Online Figure XVIII). Thus, the loss of HAX-1 during ischemia/reperfusion may allow Hsp90 to stabilize IRE-1 activity and promote the ER stress response.
PLN facilitates recruitment of HAX-1/Hsp90 to SERCA
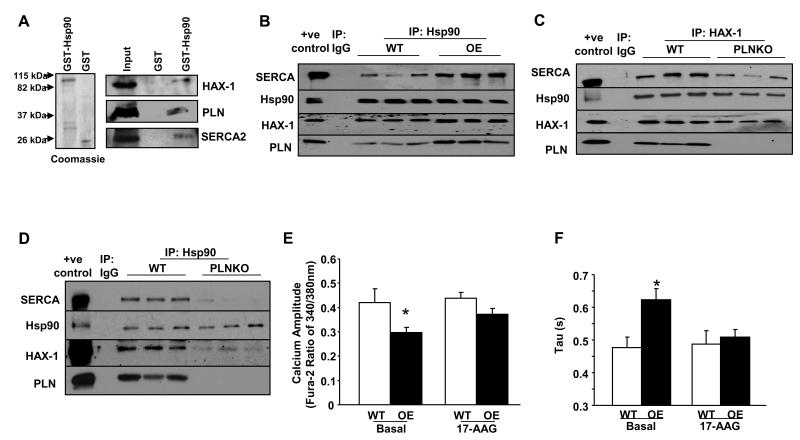
The studies above indicate that Hsp90 may get recruited away from the IRE-1 signaling complex in the presence of HAX-1. Since HAX-1 has been reported to interact with the PLN/SERCA complex18, we examined the potential association of Hsp90 with PLN/SERCA, using a GST-recombinant protein pull-down assay. GST-Hsp90 pulled-down HAX-1, PLN and SERCA, as revealed by western blot analysis (Figure 8A). This SERCA/PLN/Hsp90 association was also observed in GST-PLN pull-down samples (Online Figure XIX). Furthermore, immuno-precipitations from WT or HAX-1 OE hearts, using the Hsp90 as bait, confirmed the presence of HAX-1, PLN and SERCA in the precipitated protein complex (Figure 8B). Accordingly, immuno-fluorescence studies showed that Hsp90 co-localizes with SERCA in the HAX-1 OE cardiomyocytes (Online Figure XX). Control experiments, using HAX-1 as bait in PLN knockout (PLNKO) cardiac homogenates, indicated reduced Hsp90 levels in co-immunoprecipitates (Figure 8C). These findings were also confirmed using the Hsp90 antibody as bait in PLNKO hearts (Figure 8D). Thus, the protective effects of HAX-1 in the stressed heart may involve the formation of a multimeric protein complex of Hsp90/HAX-1/PLN/SERCA.
Figure 8. Hsp90 associates with PLN/SERCA.
A: SDS-gel stained with Coomassie blue showing purified GST and GST-Hsp90 recombinant proteins. GST-pull down assays were performed using mouse cardiac homogenates and GST-Hsp90 or GST recombinant proteins. The association with SERCA2, HAX-1 and PLN was determined by western blot analysis with appropriate antibodies. B: Centrifuged homogenates from WT and HAX-1 OE hearts were subjected to co-immunoprecipitation using the Hsp90 antibody. Five independent experiments were performed. C and D: Same procedure (as B above) was performed with WT and PLNKO homogenates, using HAX-1 and Hsp90 antibodies. Five independent experiments were performed. E and F: Calcium transient peak (n= 47-73 cells from 9 WT hearts and 8 HAX-1 OE hearts) and time constant for calcium decay (Tau, n=16-30 cells from 4 hearts in each group) in isolated WT and HAX-1 OE myocytes with or without the administration of 17-AAG. *P<0.05, compared to WT basal. Data are presented as mean±SEM.
Hsp90 and HAX-1 inhibition of SR Ca-cycling
We previously reported that HAX-1 over-expression augments PLN inhibition of SERCA and attenuates calcium cycling18. Since HAX-1 interacts with Hsp90, we sought to determine whether this chaperone protein participates in HAX-1 inhibition. Thus, we examined the calcium transient kinetics in isolated WT and HAX-1OE cardiomyocytes in the absence and presence of the Hsp90 inhibitor 17-AAG. The administration of 17-AAG (0.1μmol/L) had no significant effect in WT cardiomyocytes. However, it reversed the HAX-1 inhibitory effects by enhancing the calcium transient amplitude (Figure 8E) and abbreviating the time constant of calcium decay (Figure 8F) in HAX-1 OE cardiomyocytes. The stimulated parameters in treated HAX-1 OE cells were similar to those in WTs. These findings suggest a potential regulatory role of Hsp90 in calcium cycling.
DISCUSSION
In the current study, we demonstrate for the first time that HAX-1 interacts with Hsp90, mediating cardioprotection through specific attenuation of IRE-1 activity in the ER/SR. A previous study in pancreatic tumor cell lines showed that Hsp90 is a binding partner of IRE-1 and promotes its activity33. A similar mechanism appears to also operate in the heart and over-expression of HAX-1 recruits Hsp90 to PLN/SERCA, resulting in depressed IRE-1 activity and attenuation of apoptosis (Online Figure I).
Since its discovery in 1997, HAX-1 has been suggested to be anti-apoptotic14. Indeed, ablation of HAX-1 led to excessive neuronal cell death, which was caused by accumulation of Bax resulting from inadequate maturation of HtrA serine peptidase 2 (HtrA2), a mitochondrial serine protease that is released to cytosol upon maturation and promotes the activity of the inhibitor of apoptosis proteins (IAPs)17. However, as the cellular distribution of HAX-1 differs in various cell-types34, it is important to note that survival mechanisms mediated by HAX-1 may be different in the heart, where it shows significant ER/SR localization18. This notion is supported by the findings that re-distributing HAX-1 to ER through PLN co-expression enhances survival in HEK293 cells35, 36. In this regard, the present study provides the first evidence that the ER/SR localized HAX-1 can modulate apoptotic signaling through the ER stress response pathways.
Growing evidence supports the significance of ER stress signaling pathways in ischemia/reperfusion injury, as they impact both survival and death decisions. HAX-1 plays a critical role in fine-tuning the ER stress response through indirect but specific inhibition of IRE-1 pathway, which has been suggested to serve as the switch to death signaling37, due to its ability to activate caspase-1213, JNK signaling11 and pro-apoptotic gene expression38. This beneficial effect of IRE-1 inhibition is also demonstrated by improved cardiomyocyte survival in simulated ischemia/reperfusion studies. Notably, HAX-1 does not inhibit the other two ER response-elicited pathways, PERK and ATF-6. This is particularly important since inhibition of the ATF-6 ER stress response pathway was shown to be detrimental to the heart in ischemic injury9. Altogether, HAX-1 is able to ameliorate cell-death signaling by limiting caspase-12 initiation and pro-apoptotic protein expression, without complete abrogation of the chaperone protein induction (ATF-6) and global protein synthesis suppression (PERK). Thus, HAX-1 may be beneficial in ischemic injury by preventing cell death, as it widens the window for cardiomyocytes to rectify contractile dysfunction.
The inhibitory effects of HAX-1 on IRE-1 appear to be mediated through its interaction with Hsp90, an abundant cytosolic chaperone protein that can be recruited to virtually every cellular compartment and facilitate proper folding, post-translational modification, trafficking and degradation of a great array of client proteins39. Cardiac Hsp90 has been generally considered to be protective, since the Hsp90 inhibitor increases cardiomyocyte apoptosis through reducing pro-survival Akt signaling40 and enhancing the activity of pro-apoptotic p38α41, a member of mitogen-activated protein kinases (MAPKs). In isolated hearts, Hsp90 has also been shown to guide the transclocation of protein kinase Cε (PKCε) to the mitochondria and reduce injury after ischemia/reperfusion insults42. In endothelium, Hsp90 plays an integral role in eNOS signaling43, a pro-survival mechanism upon activation in cardiomyocytes3. The present study shows for the first time that Hsp90 may also mediate some of its cardioprotective effects at the ER/SR level. This regulation is facilitated by HAX-1, which sequesters Hsp90 to the PLN/SERCA calcium transport complex.
Substrate specificity of Hsp90 under various cellular conditions is facilitated by its co-chaperone proteins44. When a particular cellular pathway is activated, a co-chaperone protein in the signaling complex can recruit or alter the activity of Hsp90, affecting its substrate regulation39, 44. The current study suggests that HAX-1 may be a novel co-chaperone protein for Hsp90. Increased HAX-1 expression recruits Hsp90 to the PLN/SERCA, shifting the “apparent substrate specificity” of Hsp90 and reducing apoptosis induced from the ER/SR. This is especially important under ischemia/reperfusion, when the HAX-1 levels get reduced and favor increased association of Hsp90 with IRE-1 that promotes the stress response. Thus, the HAX-1/Hsp90 complex may serve as a brake on cell-death signaling. However, the regulatory effects of HAX-1 on Hsp90 may go beyond the level of ER/SR, as HAX-1 has been shown to interact with an array of proteins with different subcellular localizations, including mitochondria in various tissues34, 45.
Furthermore, Hsp90 appears to facilitate the inhibitory effect of HAX-1 on SR calcium uptake18, 35, which may provide another mechanism of cardioprotection. Pretreatment with a SERCA inhibitor to reduce SR calcium content was previously shown to be protective in ischemia/reperfusion injury46, 47, possibly by preventing mitochondria calcium overload3, 48. The current study indicates that Hsp90 may modulate the inhibitory function of HAX-1 on calcium cycling and suggests that regulation of SR calcium transport may occur through a putative quaternary protein complex, composed of SERCA/PLN/HAX-1/Hsp90. Moreover, given the role of PLN in maintaining HAX-1 at the ER/SR36, it is interesting to propose that the detrimental effects of PLN deficiency in ischemia/reperfusion injury49 may not be simply mediated by calcium dysregulation, since enhancing SR calcium uptake by either over-expressing SERCA1a50 or active protein phosphatase 1 (PP1) inhibitor-1, a repressor of PLN activity21, showed improved function and reduced injury after ischemic insults. The removal of PLN may disrupt the compartmentalization of the HAX-1/Hsp90 complex and disinhibit the apoptotic signaling through the ER pathways, affecting life or death decisions in ischemia/reperfusion injury.
In summary, the current study is the first to demonstrate the interaction of HAX-1 with Hsp90, and the role of this complex in protection against IRE-1 activation during cardiac ischemia/reperfusion injury. Our findings not only suggest an important gate-keeping role of HAX-1 in apoptosis, but also have wide-ranging implications in multiple tissues and cellular pathways, given the ubiquitous and abundant nature of Hsp90.
Supplementary Material
Novelty and Significance.
What Is Known?
Neutropenia, a disease with scarce number of neutrophils, is associated with loss of hematopoietic substrate-1 associated protein X-1 (HAX-1) protein due to mutations in this gene.
HAX-1 is expressed in the heart and it regulates contractility by controlling sarcoplasmic reticulum (SR) calcium cycling.
Acute over-expression of HAX-1 in cardiomyocytes limits cell death after hydrogen peroxide treatment, partly through caspase-9 inhibition.
What New Information Does This Article Contribute?
HAX-1 protects heart from ischemia/reperfusion injury by reducing the infarct area and improving cardiac contractile recovery.
The protective effects of HAX-1 are ascribed to attenuation of apoptosis induced by endoplasmic reticulum (ER) stress, due to specific inhibition of the inositol-requiring enzyme (IRE1) activity.
Inhibition of IRE-1 activity results from the direct physical interaction of HAX-1 with the heat shock protein 90 (Hsp90), displacing it from IRE-1.
Human mutations in HAX-1, associated with loss of this protein, result in severe loss of neutrophils, suggesting its role in maintaining cell survival in the immune system. In this study, we identified a novel regulatory role of HAX-1 in cardiomyocyte survival at the ER/SR level involving Hsp90, a chaperone protein that promotes IRE-1 activity. HAX-1 attenuates cell death responses initiated by IRE-1, through its interaction with Hsp90 and displacement of Hsp90 from IRE-1. Thus, the observed reduction of HAX-1 during ischemia/reperfusion injury may remove this protection and facilitate cell death pathways. Indeed, over-expression of HAX-1 reduces cardiac injury and improves contractile recovery, suggesting a beneficial effect by restoring HAX-1 levels. These findings reveal a novel protein complex that can be potentially targeted to limit cardiac injury in ischemic disease.
Acknowledgments
SOURCES OF FUNDING This work was supported by NIH grants: HL64018, HL26057, and HL77101 and the Leducq Foundation (to E.G.K), American Heart Association predoctoral fellowship (to C.K.L) and the European Community’s Seventh Framework Programme FP72007-2013 under grant agreement HEALTH-F2-2009-241526, EUTrigTreat.
Non-Standard Abbreviations
- 17-AAG
17-N-Allylamino-17-Demethoxygeldanamycin
- Ad
Adenovirus
- ATF-6
Activating transcription factor 6
- BA
Brefeldin A
- CHOP
C/EBP homologous protein
- GRP94
Glucose-regulated protein 94
- GST
Glutathione-S-transferase
- HAX-1
HS-1-associated-protein-X-1
- Hsp90
Heat shock protein 90
- I/R
Ischemia/reperfusion
- IRE-1
Inositol-requiring-enzyme 1
- KO
Knockout
- OE
Over-expression
- PERK
RNA-dependent protein kinase-like ER kinase
- PLN
Phospholamban
- SERCA
Sarcoplasmic/endoplasmic reticulum calcium ATPase
- TnI
Troponin I
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
Wild-type
- XBP-1
X-box binding protein 1
Footnotes
DISCLOSURES E.G.K. is a scientific founder of Nanocor.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, Dorn GW., 2nd Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 3.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuss M, Crow MT, Chesley A, Lakatta EG. Apoptosis in cardiac disease--what is it--how does it occur. Cardiovasc Drugs Ther. 2001;15:507–523. doi: 10.1023/a:1013715704835. [DOI] [PubMed] [Google Scholar]
- 6.Buja LM, Vela D. Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovasc Pathol. 2008;17:349–374. doi: 10.1016/j.carpath.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res. 2007;101:975–984. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- 8.Groenendyk J, Sreenivasaiah PK, Kim do H, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–1197. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 9.Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 10.Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284:29735–29745. doi: 10.1074/jbc.M109.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 12.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Demoliere C, Kitamura D, Takeshita H, Deuschle U, Watanabe T. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol. 1997;158:2736–2744. [PubMed] [Google Scholar]
- 15.Fadeel B, Grzybowska E. HAX-1: a multifunctional protein with emerging roles in human disease. Biochim Biophys Acta. 2009;1790:1139–1148. doi: 10.1016/j.bbagen.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Han Y, Chen YS, Liu Z, Bodyak N, Rigor D, Bisping E, Pu WT, Kang PM. Overexpression of HAX-1 protects cardiac myocytes from apoptosis through caspase-9 inhibition. Circ Res. 2006;99:415–423. doi: 10.1161/01.RES.0000237387.05259.a5. [DOI] [PubMed] [Google Scholar]
- 17.Chao JR, Parganas E, Boyd K, Hong CY, Opferman JT, Ihle JN. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 2008;452:98–102. doi: 10.1038/nature06604. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W, Waggoner JR, Zhang ZG, Lam CK, Han P, Qian J, Schroder PM, Mitton B, Kontrogianni-Konstantopoulos A, Robia SL, Kranias EG. The anti-apoptotic protein HAX-1 is a regulator of cardiac function. Proc Natl Acad Sci U S A. 2009;106:20776–20781. doi: 10.1073/pnas.0906998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafsson AB, Gottlieb RA. Mechanisms of apoptosis in the heart. J Clin Immunol. 2003;23:447–459. doi: 10.1023/b:joci.0000010421.56035.60. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 21.Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, Mitton B, Pathak A, Robbins J, Hajjar RJ, Jones K, Kranias EG. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, Fan GC, Kranias EG. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105:1223–1231. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 24.Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) Biochem J. 2004;383:361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium. 2005;38:291–302. doi: 10.1016/j.ceca.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Dremina ES, Sharov VS, Schoneich C. Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation. Biochemistry. 2006;45:175–184. doi: 10.1021/bi050800s. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt AG, Gerst M, Zhai J, Carr AN, Pater L, Kranias EG, Hoit BD. Evaluation of left ventricular diastolic function from spectral and color M-mode Doppler in genetically altered mice. J Am Soc Echocardiogr. 2002;15:1065–1073. doi: 10.1067/mje.2002.121863. [DOI] [PubMed] [Google Scholar]
- 28.Song Q, Schmidt AG, Hahn HS, Carr AN, Frank B, Pater L, Gerst M, Young K, Hoit BD, McConnell BK, Haghighi K, Seidman CE, Seidman JG, Dorn GW, 2nd, Kranias EG. Rescue of cardiomyocyte dysfunction by phospholamban ablation does not prevent ventricular failure in genetic hypertrophy. J Clin Invest. 2003;111:859–867. doi: 10.1172/JCI16738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 31.Belmont PJ, Chen WJ, San Pedro MN, Thuerauf DJ, Gellings Lowe N, Gude N, Hilton B, Wolkowicz R, Sussman MA, Glembotski CC. Roles for endoplasmic reticulum-associated degradation and the novel endoplasmic reticulum stress response gene Derlin-3 in the ischemic heart. Circ Res. 2010;106:307–316. doi: 10.1161/CIRCRESAHA.109.203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolaou P, Knoll R, Haghighi K, Fan GC, Dorn GW, 2nd, Hasenfub G, Kranias EG. Human mutation in the anti-apoptotic heat shock protein 20 abrogates its cardioprotective effects. J Biol Chem. 2008;283:33465–33471. doi: 10.1074/jbc.M802307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcu MG, Doyle M, Bertolotti A, Ron D, Hendershot L, Neckers L. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol Cell Biol. 2002;22:8506–8513. doi: 10.1128/MCB.22.24.8506-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yap SV, Koontz JM, Kontrogianni-Konstantopoulos A. HAX-1: a family of apoptotic regulators in health and disease. J Cell Physiol. 2011;226:2752–2761. doi: 10.1002/jcp.22638. [DOI] [PubMed] [Google Scholar]
- 35.Vafiadaki E, Sanoudou D, Arvanitis DA, Catino DH, Kranias EG, Kontrogianni-Konstantopoulos A. Phospholamban interacts with HAX-1, a mitochondrial protein with anti-apoptotic function. J Mol Biol. 2007;367:65–79. doi: 10.1016/j.jmb.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 36.Yap SV, Vafiadaki E, Strong J, Kontrogianni-Konstantopoulos A. HAX-1: a multifaceted antiapoptotic protein localizing in the mitochondria and the sarcoplasmic reticulum of striated muscle cells. J Mol Cell Cardiol. 2010;48:1266–1279. doi: 10.1016/j.yjmcc.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab. 2011;22:266–274. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. Embo J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Peng Y, Wang Y, Zhao X, Yuan Z. The Anti-Apoptotic Effect of Heat Shock Protein 90 on Hypoxia-Mediated Cardiomyocyte Damage through the Pi3k/Akt Pathway. Clin Exp Pharmacol Physiol. 2009 doi: 10.1111/j.1440-1681.2009.05167.x. [DOI] [PubMed] [Google Scholar]
- 41.Ota A, Zhang J, Ping P, Han J, Wang Y. Specific regulation of noncanonical p38alpha activation by Hsp90-Cdc37 chaperone complex in cardiomyocyte. Circ Res. 2010;106:1404–1412. doi: 10.1161/CIRCRESAHA.109.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Budas GR, Churchill EN, Disatnik MH, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88:83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 44.Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93:211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vafiadaki E, Papalouka V, Arvanitis DA, Kranias EG, Sanoudou D. The role of SERCA2a/PLN complex, Ca(2+) homeostasis, and anti-apoptotic proteins in determining cell fate. Pflugers Arch. 2009;457:687–700. doi: 10.1007/s00424-008-0506-5. [DOI] [PubMed] [Google Scholar]
- 46.Kumada Y, Yamamoto F, Yamamoto H, Ishikawa T, Kagisaki K, Hirose H. Decreasing sarcoplasmic reticular calcium gives rise to myocardial protection--the effect of thapsigargin for myocardial protection under conditions of normothermia. Jpn J Thorac Cardiovasc Surg. 1998;46:368–374. doi: 10.1007/BF03217757. [DOI] [PubMed] [Google Scholar]
- 47.Avellanal M, Rodriguez P, Barrigon S. Protective effects of cyclopiazonic acid on ischemia-reperfusion injury in rabbit hearts. J Cardiovasc Pharmacol. 1998;32:845–851. doi: 10.1097/00005344-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 48.Zucchi R, Ronca F, Ronca-Testoni S. Modulation of sarcoplasmic reticulum function: a new strategy in cardioprotection? Pharmacol Ther. 2001;89:47–65. doi: 10.1016/s0163-7258(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 49.Cross HR, Kranias EG, Murphy E, Steenbergen C. Ablation of PLB exacerbates ischemic injury to a lesser extent in female than male mice: protective role of NO. Am J Physiol Heart Circ Physiol. 2003;284:H683–690. doi: 10.1152/ajpheart.00567.2002. [DOI] [PubMed] [Google Scholar]
- 50.Talukder MA, Kalyanasundaram A, Zhao X, Zuo L, Bhupathy P, Babu GJ, Cardounel AJ, Periasamy M, Zweier JL. Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H2418–2428. doi: 10.1152/ajpheart.00663.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.