Abstract
Humans are especially tuned to the movements of other people. Neural correlates of this social attunement have been proposed to lie in and around the right posterior superior temporal sulcus (STS) region, which robustly responds to biological motion in contrast to a variety of non-biological motions. This response persists even when no form information is provided, as in point-light displays (PLDs). The aim of the current study was to assess the ability of functional near-infrared spectroscopy (fNIRS) to reliably measure brain responses to PLDs of biological motion, and determine the sensitivity of these responses to interpersonal contextual factors. To establish reliability, we measured brain activation to biological motion with fNIRS and functional magnetic resonance imaging (fMRI) during two separate sessions in an identical group of 12 participants. To establish sensitivity, brain responses to biological motion measured with fNIRS were subjected to an additional social manipulation where participants were either socially included or excluded before viewing PLDs of biological motion. Results revealed comparable brain responses to biological motion using fMRI and fNIRS in the right supramarginal gyrus. Further, social inclusion increased brain responses to biological motion in right supramarginal gyrus and posterior STS. Thus, fNIRS can reliably measure brain responses to biological motion and can detect social experience-dependent modulations of these brain responses.
Keywords: social exclusion, magnetic resonance imaging, optical topography, cognition, biological motion
Introduction
The human visual system is tuned to the movement of other humans. We are skilled in perceiving biological motion even when limited form information is provided, as in the case of point-light displays (PLDs), stimuli composed of dots following the movements of major joints on the body (Johansson 1973; for review see Blake and Shiffrar 2007). Perceptual sensitivity to biological motion can be seen in infants, suggesting that sensitivity to biological motion arises early in life and supports social development across the lifespan (Ichikawa et al. 2010; Simion et al. 2008). A distinct neural network supports the visual perception of biological motion. Notably, the superior temporal sulcus (STS) region has been repeatedly implicated in biological motion processing in adults (Allison et al. 2000; Saygin 2007).
The current study investigated temporal lobe responses to biological motion stimuli by taking advantage of a neuroimaging technique well-suited to measuring cortical brain activation: functional near-infrared spectroscopy (fNIRS). This method uses near-infrared light absorption as a proxy for indexing changes in cortical blood oxygenation (oxy-Hb) and deoxygenation (deoxy-Hb). Measurements are performed with emitting and recording optodes placed in a lattice on the scalp of the participant. Like fMRI, fNIRS can measure changes in blood oxygenation in response to visual stimuli such as PLDs of biological motion.
The study's primary aim was to use fNIRS to detect socially-dependent experimental manipulations of right temporal lobe brain responses to biological motion. Past research has suggested that perception of biological motion is affected in individuals with neurodevelopmental disorders including autism (Kaiser et al. 2010a), obsessive-compulsive disorder (Kim et al. 2008) and schizophrenia (Kim et al. 2005). Further, individual differences in physical motor abilities have also been shown to influence action perception (Bidet-Ildei et al. 2010; Casile and Giese 2006; Serino et al. 2010), suggesting that while responses to biological motion are robust, they are also subject to modulation by experimental factors.
Previous research also suggests that factors affecting social behavior can impact basic biological motion processing. For example, administration of oxytocin, an endogenous neuropeptide associated with a variety of prosocial behaviors (Guastella et al. 2008a; Guastella et al. 2008b; Israel et al. 2009; Zak et al. 2005; for review see Heinrichs et al. 2009), has been shown to facilitate both perception (Keri and Benedek 2009) and neural processing of biological motion cues (Perry et al. 2010). In addition, research suggests that social interaction in the form of physical touch increases oxytocin levels (Grewen et al. 2005; Holt-Lunstad et al. 2008). Thus, we hypothesize that a manipulation of social interaction may influence biological motion processing through a neurobiological mechanism such as endogenous oxytocin release.
We used social inclusion and exclusion in a computer ball-toss game to assess whether different types of social interactions can manipulate temporal lobe cortical responses to biological motion PLDs. Research has found that compared to social inclusion, social exclusion affects subsequent interpersonal interactions, both increasing (Lakin and Chartrand 2003; Maner et al. 2007) and decreasing (Twenge et al. 2007) prosocial behavior. Mediating these equivocal results, a recent fMRI investigation demonstrated that brain responses to social exclusion related to neural processing of subsequent social stimuli dependent on the valence, positive or negative (Powers et al. in press). Thus, since evidence suggests that social interactions influence subsequent high-level social processing such as viewing complex social scenes, we tested the hypothesis that social inclusion and exclusion would also modulate brain responses to basic, low-level social stimuli in the form of PLDs of biological motion.
Social inclusion and exclusion were elicited using an interactive ball-toss game (Cyberball; Williams et al. 2000). During an fNIRS session, participants viewed PLDs of biological and scrambled motion at three time points: baseline, post-social inclusion, and post-social exclusion. In addition, all participants underwent a separate fMRI session where they viewed identical PLDs of biological and scrambled motion in the absence of the social manipulation. Thus, a secondary goal of the current study was to validate the ability of fNIRS to precisely measure cortical responses to PLDs of biological motion in the right posterior temporal cortex in a group of healthy adults by comparing brain responses to biological and scrambled motion PLDs measured in an identical participant group during separate sessions of fNIRS and fMRI, as many studies have demonstrated that neural responses to a variety of other tasks can be co-localized with fMRI and fNIRS (Cui et al. 2011; Ferrani and Quaresima 2012; Steinbrink et al. 2006).
While fMRI and fNIRS both measure changes in blood oxygenation in the brain, fNIRS measurements incur limitations that do not exist in fMRI. Unlike fMRI, fNIRS data is subject to a loss of spatial resolution resulting from inevitably variable cap placement on participants’ heads. The current study implemented two important methods to account for this variability. First, digital localization of optode placement was used to normalize the site of recording channels to standard space in each participant. Second, digital localization was used to visualize the non-normalized placement of each recording site on a participant's structural MRI, allowing for the identification of channels placed over each participant's STS region. To help account for a decreased signal to noise ratio in fNIRS compared to fMRI, a hemodynamic response function was used to model the blood oxygen level dependent (BOLD) response. In this way, we took advantage of the a priori knowledge of the shape of hemodynamic response and used a general linear model framework to analyze the fNIRS data. These analysis strategies are essential to enhance spatial resolution in the identification of hemodynamic responses recorded with fNIRS.
With the described fNIRS analysis strategies, the current study aimed to replicate and extend previous work on biological motion processing in the right temporal lobe. First, we verified the ability of fNIRS to measure brain responses to biological motion by comparing brain activation to PLD stimuli measured with fNIRS and fMRI. Next, we compared neural activation to biological versus scrambled motion PLDs at baseline, after social inclusion, and after social exclusion in order to examine whether social interactions modulate brain responses to basic social stimuli, in order to better understand how interpersonal interactions can affect subsequent social processing.
Experimental Procedures
Participants
The participants included 17 typical adults (5 male, 24.2 years ± 2.51). Two of these participants were excluded for having no data collected from one or more of the 52 recording channels thus preventing spatial interpolation of the results. Three additional participants were excluded because they played with non-race matched players in the ball-toss sections of the task, as past research has suggested that the race of the excluding players in relation to the participant affects brain responses to exclusion (Krill and Platek 2009; Masten et al. 2011). Consequently, 12 participants were included in analyses (3 male, 24.6 years ± 2.73). All 12 of these participants also partook in an fMRI scan where structural data was collected, as well as a functional imaging data during a similar task involving alternating videos of the same scrambled and biological motion PLDs as were used in the fNIRS paradigm.
Design
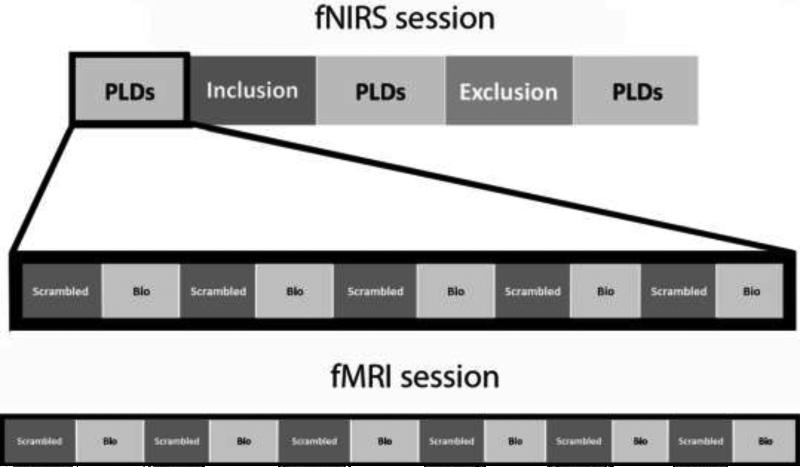
The present study used fNIRS to investigate the effects of social inclusion and exclusion on neural responses to biological (compared to scrambled) motion in PLDs. These stimuli depicted either coherent point-light motion of an adult male actor performing social movements (such as waving) or scrambled motion created by random selection of 16 points from the biological motion displays. Our group (Kaiser et al. 2010b) and others (Klin et al. 2009) have used these PLDs in previous work. Here, during the fNIRS session we presented three runs of PLD stimuli, each run alternating between videos of biological and scrambled motion. Runs were presented at baseline, post-social inclusion, and post-social exclusion (Figure 1). A PLD run consisted of 10 unique videos (24 seconds each) which alternated between biological and scrambled motion, separated by a jittered fixation of 12, 14, or 16 seconds. Within the alternating design, the order of the 10 videos differed between iterations of PLD runs within each subject. Half of the participants saw biological motion first in each PLD run, while the other half saw scrambled motion first. While the order of social inclusion and exclusion was not counterbalanced for concern that the psychological effects of social exclusion might persist into a subsequent inclusion condition, the use of a scrambled motion condition allowed us to assess the possibility of repetition priming or order effects on neural responses, as either of these confounds would affect both biological and scrambled motion processing. In addition, participants also underwent an fMRI session where they viewed identical biological and scrambled motion videos (6 of each type, 12 videos in total; Figure 1) in an alternating design.
Figure 1.
Visual depiction of the experimental paradigm for the fNIRS session (top) and fMRI session (bottom). Each block of biological (bio) and scrambled motion consisted of a 24-second point-light video clip. The fNIRS session consisted of three runs of 10 PLD videos, presented at baseline, post-inclusion, and post-exclusion. The fMRI session consisted of one run of 12 PLD videos.
For the fNIRS session where we applied the social manipulation, we used an interactive computer ball-toss game called Cyberball (Williams et al. 2000) to induce social inclusion and exclusion. Participants were initially prompted to choose a playing glove for the game, and they were given instructions to use buttons labeled with arrows to throw the ball to either of the two online players. After the baseline run of PLDs, the participants viewed their own playing glove, as well as images of two other players matched to the participant on gender and ethnicity. Pictures were of adults with neutral facial expressions taken from the collection of face stimuli known as NimStim (Tottenham et al. 2009). In social inclusion, the participant received the ball on one-third of the throws (10 out of 30). In social exclusion, the participant initially received the ball on 3 (of 6) throws, and then was subsequently excluded from the game for the remaining 24 throws.
As a manipulation check, following the post-exclusion PLD presentation and the completion on of the fNIRS session participants were asked to respond to ten questions assessing their exclusion-related distress. Items were rated on a 1 to 5 Likert scale from “not at all” to “extremely”. Items were a subset of questions from the Need Threat Scale (Wirth and Williams 2009), previously used to assess Cyberball-induced distress in healthy adults (Bolling et al. 2011).
Data acquisition
NIRS
Participants’ head dimensions were assessed as per the international 10-20 system. The average measured distances from nasion to inion, left ear to right ear, and nasion to right ear were 29.54 cm (± 2.60), 35.67 cm (± 1.71), and 16.04 cm (± 0.84), respectively. Subsequently, measurements of neural activation were taken using a fifty-two-channel near-infrared spectroscopy machine (ETG-4000, Hitachi Medical) with thirty-three optodes separated by 3 cm configured in a 3 × 11 lattice. Changes in oxygenated (oxy-Hb) and deoxygenated (deoxy-Hb) hemoglobin were measured using two wavelengths of infrared light (695 nm and 830 nm). Previous work investigating the correlation between fMRI BOLD signal and blood oxygenation measured with fNIRS has come to mixed conclusions (Steinbrink et al. 2006), with some studies showing preferential coupling between BOLD and deoxy-Hb (e.g. Toronov et al. 2001) and others showing a stronger relationships between BOLD and oxy-Hb (e.g. Strangman et al. 2002). In the present study we visually inspected both oxy-Hb and deoxy-Hb responses to biological motion to determine the relative task-related modulation of the two measures. The magnitude of signal change was greater for oxy-Hb than deoxy-Hb, in response to both biological and scrambled motion stimuli (Online Resource 1), and thus subsequent analyses focused on oxy-Hb. All data were collected at a frequency of 10 Hz. To standardize the placement of the optode lattice, source probe 25 was placed directly above the right ear in all participants. Following the acquisition of functional data, all optodes were removed from the lattice, and a 3D digitizer system (Polhemus, Vermont, USA) was used to localize the placement of each optode in relation to reference points on the participant's head (nasion, left and right ears, top and back of the head).
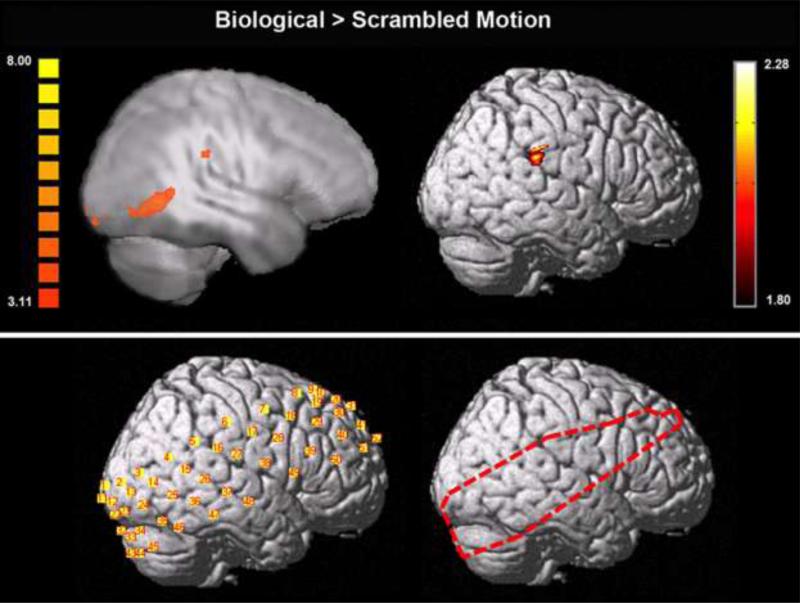
Whole-brain T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1900 ms; TE = 2.96 ms; flip angle = 9°; FOV = 256 mm; image matrix 256 mm2; voxel size =1 × 1 × 1 mm; 160 slices; NEX = 1). Brain and head polygons were constructed from these anatomical images using the Hitachi 3D topographic image display system (TopoGraph 3D 2.4.1). Optode and channel placements were coregistered to each participant's structural MRI image using reference points for each optode as well as the location of the nasion and the left and right ears, as measured by the Polhemus 3D digitizer system. The four channels along each participant's right STS were identified for subsequent region of interest analyses. In addition, the coordinate placements of each participant's channels as well as 5 reference points (nasion, left and right ears, top and back of the head) were used to normalize the location of each recording channel into MNI space for subsequent general linear model group-level analyses (see Figure 2 for an example of one participant's recording channel placement in MNI space).
Figure 2.
Activation to biological > scrambled motion measured with functional MRI (top left) and functional NIRS (collapsed across conditions; top right) in an identical group of 12 participants. Functional MRI activation is displayed on a Talairach-transformed template brain depicting functional activation in the plane x = 54 projected onto a smoothed surface map. Functional NIRS activation is displayed on a template brain normalized to MNI space. Functional MRI results were assessed at a corrected threshold of α < .05. Functional NIRS results were assessed at a threshold of p < .05. An example of normalized recording channel placement in MNI space for one participant is shown on the bottom left, while the outline of pixels used in the group analysis (where 11 or more participants had functional data) is shown on the bottom right.
Functional MRI
Each participant in the current study also participated in a functional MRI scan where he or she viewed 24-second PLDs identical to the biological and scrambled motion stimuli used in the fNIRS session. Images were collected on a Siemens 3T Tim Trio scanner located in the Yale Magnetic Resonance Research Center. Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 64 mm2; voxel size = 3.4 × 3.4 × 4 mm; 34 slices) sensitive to BOLD contrast.
Data analysis
fNIRS
GLM-based analyses
For data collected in the fNIRS session, 24-second blocks of scrambled and coherent biological motion were modeled separately for each PLD run. Additionally, the periods of social inclusion and social exclusion were modeled separately. Continuous data were integrated over each of the 8 block types (baseline-biological, baseline-scrambled, post-inclusion-biological, post-inclusion-scrambled, post-exclusion-biological, post-exclusion-scrambled, social inclusion, and social exclusion). Data were low pass filtered at a frequency of 0.2 Hz. For region of interest analyses only, a moving average of 1 second was applied and integrated data was baseline corrected using a pre-block period of 3 seconds and a post-block period of 3 seconds (after a recovery time of 6 seconds following the block completion). Using NIRS SPM version 3.2 (Jang et al. 2009; Tak et al. 2010a, 2010b; Ye et al. 2009), global trends were removed from each single participant's measurement data using a wavelet-minimum description length (MDL) detrending algorithm. A single-participant General Linear Model (GLM) was then performed using a double-gamma hemodynamic response curve to model the oxy-Hb response during each experimental condition in the task. On a single-participant level, channel placement was normalized to MNI space, and the t-value in each channel was interpolated to increase spatial resolution (Ye et al. 2009). These activation maps were subsequently combined in group-level mixed-effect GLM-based analyses, analyzing any pixel where 11 of the 12 participants had overlapping functional data (see Figure 2 for the extent of analyzed pixels). All analyses were assessed at an uncorrected statistical threshold of p < .05.
ROI analyses
For fNIRS data, region of interest (ROI) analyses were performed on a single-participant level by integrating oxy-Hb signals over each 24 second video of biological motion (15 videos) and scrambled motion (15 videos). These integrated signals in each of the 4 channels identified as corresponding to a participant's right STS were then averaged for each time point of data acquisition in the 24 second stimulus block, as well as for the 3 seconds pre-stimulus onset and 9 seconds post-stimulus offset. These signals were subsequently averaged on a group-level to visualize the oxy-Hb response to biological and scrambled motion in the STS. An identical ROI analysis was performed for integrated data from biological and scrambled motion blocks in each condition (baseline, post-inclusion, post-exclusion) separately. To quantify the amplitude of activation in the described waveforms, average activation during the 10 second time window from 6 seconds to 16 seconds post stimulus onset (corresponding to a hypothesized peak of the hemodynamic response) was calculated for biological motion and scrambled motion in each of the three study conditions.
Functional MRI
GLM-based analyses
The functional MRI scan consisted of 6 alternating blocks of biological and scrambled motion videos. Data from these scans were preprocessed and analyzed using the BrainVoyager QX 2.0.8 software package (Brain Innovation, Maastricht, The Netherlands). Preprocessing of the functional data included slice time correction (using sinc interpolation), 3-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a FWHM 4-mm Gaussian kernel, and temporal high-pass filtering (GLM with Fourier basis set, using 2 cycles/time course). Functional datasets were coregistered to within-session anatomical images, which were in turn normalized to Talairach space. Boxcar functions with values of “1” during each motion type (biological and scrambled) and “0” otherwise were included in the general linear model, as well as predictors for each dimension of head motion (included as predictors of no interest). Analyses were restricted to all voxels within the extent of the right hemisphere of the MNI template brain normalized to Talairach space. We limited analyses to only the right hemisphere to make these results most comparable to the NIRS data collected only from the right side of the head. Results were combined in group-level random-effects GLM-based analyses, and assessed in the contrast of biological motion > scrambled motion at a p < .01 with a cluster threshold of 9 functional voxels, calculated by the BrainVoyager cluster threshold estimator plug-in to correspond to α < .05 (Xiong et al. 1995). The more stringent threshold used with the fMRI data (compared to the group-level NIRS analysis assessed at p < .05) was used to discern distinct regions of activation to biological motion.
Results
Self-report measures
The average total score on our 10-item exclusion-related distress questionnaire was 24 (± 4.60, maximum score of 50). This score corresponds to an average item score of 2.4, on a Likert scale of 1 to 5 (“1” indicating no distress, “5” indicating extreme distress). The average total score of 24 is significantly greater than a baseline score of 10 indicating no distress (t = 13.61, p < .001).
NIRS
GLM-based analyses
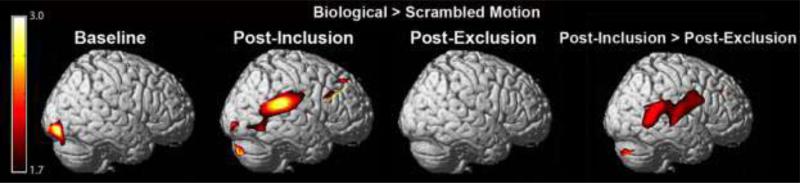
Group-level analyses on fNIRS data were performed using a mixed-effect GLM. Group-results were first assessed in the contrast of biological motion > scrambled motion collapsed across all experimental conditions (Figure 2). The comparison of biological motion > scrambled motion was then performed in each experimental condition separately (baseline, post-inclusion, post-exclusion). All activations reported are in the right hemisphere of the brain. Collapsed across experimental conditions, a region of supramarginal gyrus showed greater activation to biological (versus scrambled) motion. The only region showing greater activation to biological versus scrambled motion at baseline was posterior inferior temporal gyrus. Following social inclusion, several regions showed greater activation to biological versus scrambled motion including an extensive region of supramarginal gyrus extending into STS and inferior temporal gyrus, lateral occipital cortex and dorsolateral prefrontal cortex. No regions showed increased activation to biological (versus scrambled) motion after social exclusion (Figure 3).
Figure 3.
Activation to biological > scrambled motion in each experimental condition and in the contrast of post-inclusion > post-exclusion, measured with functional NIRS. Activation is displayed on a template brain normalized to MNI space, and assessed at a threshold of p < .05.
To directly investigate how social inclusion and exclusion modulated brain responses to biological (versus scrambled) motion, we compared the contrast of biological > scrambled motion among the three conditions (post-inclusion > baseline, post-exclusion < baseline, and post-inclusion versus post-exclusion). Brain responses to biological (versus scrambled) motion increased post-inclusion as compared to baseline in regions of dorsolateral prefrontal cortex, cerebellum, and supramarginal gyrus extending into posterior STS and inferior temporal gyrus. Only a small region of precentral gyrus showed decreased activation to biological (versus scrambled) motion post-exclusion compared to baseline. No regions showed increased activation to biological motion post-exclusion compared to baseline. In comparing brain responses to biological (versus scrambled) motion post-inclusion and post-exclusion, regions of inferior temporal gyrus and posterior STS extending into supramarginal gyrus showed decreased activation to biological motion after participants were socially excluded (Figure 3). No regions showed increased activation to biological versus scrambled motion post-exclusion compared to post-inclusion.
ROI analyses
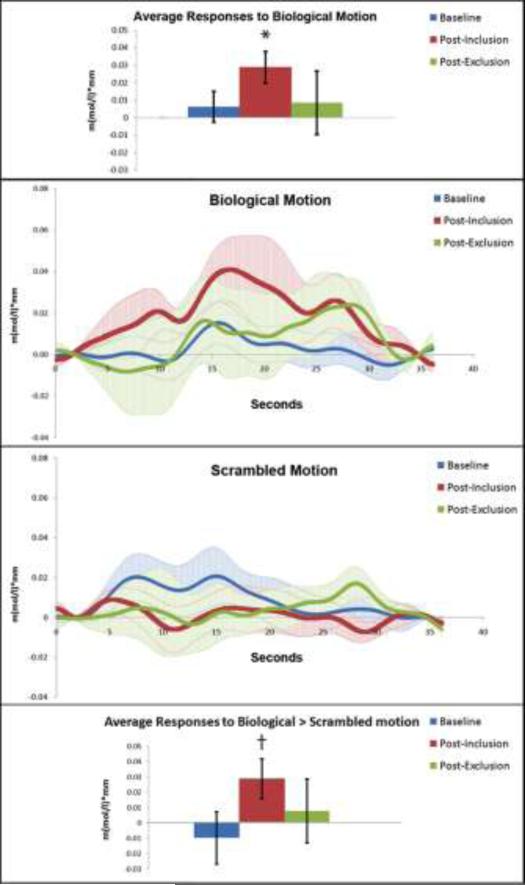
Activation to biological and scrambled motion in each experimental condition was assessed for each participant in 4 optodes lying most closely along his or her right STS. This activation was averaged over the time period from 6 to 16 seconds post-stimulus onset (Figure 4). Average activation over this time period for biological motion was significantly greater than zero post-inclusion (t = 3.17, p = .009) but not post-exclusion or at baseline (p's > .05). This result precludes the possibility of a repetition priming effect, as that would predict the greatest response to biological motion at baseline, decreasing with time. Activation to scrambled motion in this region was not significantly greater than zero in any condition (p's > .05). Activation to biological versus scrambled motion assessed in each condition with paired-sample t-tests revealed a significant difference post-inclusion (t = 2.20, p = .05) but not at baseline or post-exclusion (p > .05). Repeated measures ANOVAs did not reveal a significant effect of condition on responses to biological motion (F(10, 2) = 1.31, p = .313) or scrambled motion (F(10, 2) = .39, p = .69). In addition, there was no main effect of condition on the contrast of activation to biological and scrambled motion (F(10, 2) = 1.13, p = .36). To address our main question of interest, we contrasted the neural responses to biological > scrambled motion post-inclusion versus post-exclusion in a repeated measures ANOVA, which did not yield a significant effect of time point (F(11, 1) = 0.653, p = 0.44). The same repeated measures analysis restricted only to brain responses to biological motion revealed a slightly larger but still non-significant effect (F(11, 1) = 1.39, p = 0.26).
Figure 4.
Activation in participant-specific structurally defined regions of superior temporal sulcus measured with functional NIRS. Average responses to biological motion in each condition 6-16 seconds post-stimulus onset are depicted in the top bar graph. Waveforms depict brain responses to biological motion (middle panel, top) and scrambled motion (middle panel, bottom) in each condition. Average responses to biological > scrambled motion (calculated for each participant, then averaged across the group) in each condition 6-16 seconds post-stimulus onset are depicted in the bottom bar graph. All bars depict standard error, stimulus onset and offset are at 3 and 27 seconds, respectively. * denotes significance at p < .01, while † denotes significance of p = .05.
Functional MRI analyses
Results from the functional MRI analysis identified regions in the right hemisphere of the brain that showed greater activation to biological versus scrambled motion (Table 1, Figure 2). These regions included supramarginal gyrus, posterior STS, fusiform gyrus, precentral gyrus, middle temporal gyrus, temporal pole, intraparietal lobule, posterior insula, amygdala, thalamus, medial frontal gyrus, and cerebellum.
Table 1.
Activation in the right hemisphere to biological > scrambled point-light display motion measured with fMRI. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Region sizes are reported in structural voxels (1 × 1 × 1 mm).
| Brain Region | X | Y | Z | Size (mm3) | t | p |
|---|---|---|---|---|---|---|
| Biological > Scrambled | ||||||
| Precentral gyrus | 42 | -7 | 46 | 855 | 5.27 | 0.000266 |
| MTG | 36 | -4 | -17 | 753 | 5.84 | 0.000113 |
| Temporal pole | 27 | 8 | -38 | 557 | 6.95 | 0.000024 |
| IPL | 30 | -40 | 52 | 416 | 4.72 | 0.00063 |
| Posterior insula | 30 | -16 | -2 | 674 | 8.32 | 0.000004 |
| Amygdala | 0 | -10 | -8 | 631 | 7.39 | 0.000014 |
| Thalamus | 12 | -28 | 1 | 509 | 5.39 | 0.000221 |
| Thalamus | 6 | -13 | 13 | 307 | 4.8 | 0.000556 |
| Medial frontal gyrus | 3 | -4 | 64 | 816 | 5.97 | 0.000093 |
| Cerebellum | 0 | -58 | -38 | 562 | 5.81 | 0.000118 |
| Supramarginal gyrus | 36 | -19 | 28 | 1167 | 8.51 | 0.000004 |
| Posterior STS | 45 | -34 | 10 | 296 | 5.25 | 0.000273 |
| Fusiform gyrus | 39 | -58 | -5 | 24804 | 9.51 | 0.000001 |
Abbreviations: middle temporal gyrus (MTG), inferior parietal lobule (IPL), superior temporal sulcus (STS).
Discussion
The results of the present study confirm that brain responses to PLDs of biological motion in healthy adults can be reliably measured using fNIRS. Further, we demonstrate that the experience of social inclusion modulates subsequent neural responses to PLDs of biological motion in the posterior STS, a key node of the social brain (Brothers 1990).
To our knowledge, the present study is the first to co-localize neural responses to PLDs of biological motion using fMRI and fNIRS. Previous work has demonstrated corresponding results of hemispheric lateralization using these two imaging methods (Kennan et al. 2002). Comparisons of fMRI and fNIRS measurements during multiple types of tasks corroborate this result (for reviews see Cui et al. 2011; Ferrani & Quaresima 2012; Steinbrink et al. 2006). The present study found that a region of supramarginal gyrus just adjacent to STS showed significant activation to PLDs of biological versus scrambled motion measured during separate fMRI and fNIRS sessions in an identical sample of 12 healthy adults. This replication is essential in our subsequent investigation of the social experience-driven modulation of this brain activation.
In addition to identifying comparable neural responses to biological motion in fMRI and fNIRS, the present investigation tested the hypothesis that the experiences of social inclusion and exclusion would modulate brain responses to biological motion in a key node of the social brain, namely the right STS. With interpolated general linear model-based analyses, we found decreased activation to biological motion (versus scrambled motion) following exclusion compared to inclusion in posterior STS extending into supramarginal gyrus. Thus, we showed an interaction between condition and time-point such that the magnitude of difference in brain activation to biological versus scrambled motion was significantly different post-inclusion compared to post-exclusion. To elucidate the nature of these results, we subsequently identified the separate effects of inclusion and exclusion on biological motion processing. The majority of research on social inclusion and exclusion simply contrasts these two conditions, and thus cannot determine the main effects of each condition independently. In the current study, the comparison of each social condition to baseline (pre-social inclusion and exclusion) revealed unique results. One region of precentral gyrus showed decreased activation to PLDs of biological (versus scrambled) motion following exclusion. In comparison, brain responses to biological (versus scrambled) motion were increased following social inclusion in dorsolateral prefrontal cortex, and supramarginal gyrus extending into posterior STS and inferior temporal gyrus. Structural ROI results corroborate these findings, with activation to biological motion in the STS reaching statistical significance only following social inclusion. Taken together, these results support the conclusion that modulation of activation to biological motion in posterior STS and supramarginal gyrus is driven primarily by the effects of being socially included.
Using traditional analyses with social inclusion as a “control” condition for social exclusion, our results would appear to support the conclusion that social exclusion decreases brain responses to biological motion. However, the addition of a non-social baseline condition of biological motion processing (biological motion processing before experiencing social inclusion or exclusion) revealed that a main effect of social inclusion was driving this difference. The current study highlights the importance of considering the individual effects of both social inclusion and exclusion on brain and behavior in an experimental context.
Our results are consistent with findings from an investigation of the emotional effects of Cyberball in adolescents with and without autism spectrum disorder. Sebastian et al. (2009) reported increased anxiety following social exclusion compared to social inclusion, with this effect being driven by a significant decrease in anxiety following social inclusion as compared to anxiety at baseline and following exclusion. This effect did not show modulation by autism diagnosis and suggests that the effects of Cyberball on anxiety are driven specifically by the anxiolytic experience of social inclusion. Additional work has suggested that social inclusion also increases trust behavior (Hillebrandt et al. in press). Further, merely the suggestion of social connection through priming with words implying interpersonal relatedness increased prosocial behaviors and intentions in adults (Pavey et al. 2011). This evidence gives credence to the findings in the current study that brain responses to biological motion were increased following social inclusion. Further, this enhancement did not persist following social exclusion. Finally, these results existed while controlling for modulation of brain responses to scrambled motion displays. We posit that the experience of social inclusion enhances prosocial tendencies resulting in an increased sensitivity to social cues, seen in the significant effects on brain responses to biological but not scrambled motion stimuli.
Previous research has shown that the motor system is involved in action perception, as physical learning (Casile and Giese 2006) and performance ability (Serino et al. 2010) of the observed motion enhance perception of such biological motion cues. Thus, while speculative, the inclusive engagement in a hypothetical ball-toss game may cause participants to visualize physical ball tossing, subsequently enhancing neural responses to biological motion PLD stimuli. We would expect that this effect would be significantly decreased during social exclusion where participants are no longer throwing the ball in the computer game.
A final explanation for our results comes from the observation that oxytocin plays a role in both social cognition and biological motion processing. Specifically, oxytocin is involved in social interactions, such as acts of interpersonal trust (Zak et al. 2005), the sounds of a mother's voice (Seltzer et al. 2010), and warm contact with supportive partners (Grewen et al. 2005; Holt-Lunstad et al. 2008), all of which result in increased levels of oxytocin. The engagement of participants in a ball-toss game during social inclusion might have resulted in a release of endogenous oxytocin, thus facilitating neural processing of biological motion stimuli (Perry et al. 2010). The lack of social engagement in social exclusion may have truncated the duration of this oxytocin effect. Indeed, previous research on the effects of social inclusion has hypothesized that an endogenous release of oxytocin in response to social inclusion may underlie subsequent prosocial behaviors in a trust game (Hillebrandt et al. in press).
Although several theories lend themselves to explaining the effects of social inclusion on biological motion processing, we originally hypothesized that the experience of social exclusion would also significantly affect brain responses to biological motion. Previous research illuminating a range of social, behavioral, and psychological effects of social exclusion supports the notion that humans have an innate, driving need to belong (Baumeister and Leary 1995). Thus, one would expect that activation in regions of the social brain especially tuned to the actions of others (i.e. STS; Allison et al. 2000) would be modulated by the interpersonal threat elicited by peer rejection. When we compared activation to biological (versus scrambled) motion post-exclusion versus at baseline, we found only a small region of precentral gyrus that showed decreased activation following social exclusion. In addition, our STS ROI analysis revealed no differential activation to biological versus scrambled motion post-exclusion, and no difference in this condition from baseline.
While previous behavioral work has shown that social exclusion affects subsequent social behavior, it has also been shown that specific characteristics of these social interactions modulate said effects. Maner et al. (2007) found that exclusion did not elicit prosocial behaviors when subsequent interactions were with individuals with whom no face-to-face interaction was eminent. Thus, the lack of potential interpersonal interaction with the PLD stimuli of the present study may have diminished effects of exclusion on social processing. In addition, DeWall et al. (2009) noted an effect of ostracism such that social exclusion increased perceptual sensitivity to faces depicting positive but not negative emotion. Similarly, Powers et al. (in press) found that social exclusion affected subsequent brain responses to social stimuli in medial prefrontal cortex differentially depending on valence. The current study utilized PLDs of moderately positive valence, and at a more lenient threshold of p < 0.1, distinct regions of supramarginal gyrus and posterior STS showed increased activation to biological motion following exclusion compared to baseline. Thus, while the modulation of brain responses to biological motion following social exclusion were only trending towards significance in the current study, future investigations using different variations of social assays (other than the current PLDs) may yield stronger results. In addition, the current study is limited by its modest sample size. Thus, a larger participant group may yield more robust results.
While theoretically and methodologically novel, the current study incurred several limitations. First, adults in the current study may have suspected that the players they were interacting with were not real people. While for ecological validity it is advantageous to have participants believe the players are real, past research has demonstrated that social exclusion has negative psychological effects even when participants know they are being excluded by a computer (Zadro et al. 2004). In line with this conclusion, we did find that participants in the current study were significantly distressed by the exclusion experience. Second, the comparison of activation to biological versus scrambled motion in fNIRS and fMRI did not utilize equal numbers of stimulus trials in the two sessions. The fMRI session consisted of 12 videos of biological and scrambled motion, while the fNIRS session consisted of 30 videos (disregarding the social manipulation). The resulting replication of activation at similar thresholds with both methods is likely due to the decreased signal to noise ratio in fNIRS, and suggests that fNIRS requires a greater number of stimulus trials to reveal significant patterns of activation. Finally, while GLM-based analyses revealed significant differences in neural activation to biological > scrambled motion between social exclusion and inclusion, ROI analyses only demonstrated a main effect of biological motion processing post-inclusion, with no modulation by condition. This could be due to the greater sensitivity of a model-based analysis to accurately identify a veritable HRF in the data, or alternatively this could be due to low power related to the limited participant number.
The current study is the first report of comparable neural responses to PLDs of biological motion using both fMRI and fNIRS in an identical set of healthy adults. In addition, we were able to demonstrate modulation of this brain response by the experience of social inclusion using fNIRS. The results of this study suggest that basic, low-level social processing such as brain responses to biological motion PLDs can be significantly affected by brief social interactions. Future studies may investigate endogenous neurological factors such as oxytocin release in an attempt to elucidate the mechanisms by which social inclusion affects social processing.
Supplementary Material
Online Resource 1. Activation in participant-specific structurally defined regions of superior temporal sulcus for Oxy-Hb and Deoxy-Hb fNIRS measurements. Waveforms depict brain responses to biological (top) and scrambled motion (bottom) collapsed across all 3 runs (baseline, post-inclusion, post-exclusion). Stimulus onset and offset are at 3 and 27 seconds, respectively.
Acknowledgements
The research presented herein was supported by grants from the National Institute of Mental Health to Kevin Pelphrey.
References
- Allison T, Puce A, McCarthy G. Social Perception from visual cues: Role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. doi:10.1016/S1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117:497–529. doi: 10.1037/0033-2909.117.3.497. [PubMed] [Google Scholar]
- Bidet-Ildei C, Chauvin A, Coello Y. Observing or producing a motor action improves later perception of biological motion: Evidence for a gender effect. Acta Psychol. 2010;134:215–224. doi: 10.1016/j.actpsy.2010.02.002. doi:10.1016/j.actpsy.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annu Rev Psychol. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. doi:10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McParland JC, Mayes LC, Pelphrey KA. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. doi:10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L. The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Casile A, Giese MA. Nonvisual motor training influences biological motion perception. Curr Biol. 2006;16:69–74. doi: 10.1016/j.cub.2005.10.071. doi:10.1016/j.cub.2005.10.071. [DOI] [PubMed] [Google Scholar]
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. doi:10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, Maner JK, Rouby DA. Social exclusion and early-stage interpersonal perception: Selective attention to signs of acceptance. J Pers Soc Psychol. 2009;96:729–741. doi: 10.1037/a0014634. doi:10.1037/a0014634. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.03.049. doi:10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. doi:10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008a;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. doi:10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008b;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. doi:10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. doi:10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hillebrandt H, Sebastian C, Blakemore S-J. Experimentally induced social exclusion influences behavior on trust games. Cogn Neurosci. 2:27–33. doi: 10.1080/17588928.2010.515020. in press. doi:10.1080/17588928.2010.515020. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. doi:10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Kanazawa S, Yamaguchi MK, Kakigi R. Infant brain activity while viewing facial movement of point-light displays as measured by near-infrared spectroscopy (NIRS). Neurosci Lett. 2010;482:90–94. doi: 10.1016/j.neulet.2010.06.086. doi:10.1016/j.neulet.2010.06.086. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, Uzefovsky F, Riebold M, Laiba E, Bachner-Melman R, Maril A, Bornstein G, Knafo A, Ebstein RP. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS ONE. 2009;4:e5535. doi: 10.1371/journal.pone.0005535. doi:10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KE, Tak S, Jung J, Jang J, Jeong Y, Ye JC. Wavelet-MDL detrending for near-infrared spectroscopy (NIRS). J Biomed Opt. 2009;14:1–13. doi: 10.1117/1.3127204. doi:10.1117/1.3127204. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Percept Psychophys. 1973;14:201–211. doi:10.3758/BF03212378. [Google Scholar]
- Kaiser MD, Delmolino L, Tanaka JW, Shiffrar M. Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Res. 2010a;3:191–195. doi: 10.1002/aur.137. doi:10.1002/aur.137. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, Deen B, Pitskel NB, Sugrue DR, Voos AC, Saulnier CA, Ventola P, Wolf JM, Klin A, Vander Wyk BC, Pelphrey KA. Neural signatures of autism. Proc Nat Acad Sci USA. 2010b;49:21223–21228. doi: 10.1073/pnas.1010412107. doi:10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennan RP, Kim D, Maki A, Koizumi H, Constable RT. Non-invasive assessment of language lateralization by transcranial near infrared optical tomography and functional MRI. Hum Brain Mapp. 2002;16:183–189. doi: 10.1002/hbm.10039. doi:10.1002/hbm.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Benedek G. Oxytocin enhances the perception of biological motion in humans. Cogn Affective Behav Neurosci. 2009;9:237–241. doi: 10.3758/CABN.9.3.237. doi: 10.3758/CABN.9.3.237. [DOI] [PubMed] [Google Scholar]
- Kim J, Blake R, Park S, Shin Y-W, Kang D-H, Kwon JS. Selective impairment in visual perception of biological motion in obsessive-compulsive disorder. Depress Anxiety. 2008;25:E15–E25. doi: 10.1002/da.20402. doi:10.1002/da.20402. [DOI] [PubMed] [Google Scholar]
- Kim J, Doop ML, Blake R, Park S. Impaired visual recognition of biological motion in schizophrenia. Schizophr Res. 2005;77:299–307. doi: 10.1016/j.schres.2005.04.006. doi:10.1016/j.schres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. doi:10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill A, Platek SM. In-group and out-group membership mediates anterior cingulate activation to social exclusion. Front Evol Neurosci. 2009;1:1–7. doi: 10.3389/neuro.18.001.2009. doi:10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin JL, Chartrand TL. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol Sci. 2003;14:334–339. doi: 10.1111/1467-9280.14481. doi:10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- Maner JK, DeWall CN, Baumeister RF, Schaller M. Does social exclusion motivate interpersonal reconnection? Resolving the “porcupine problem”. J Pers Soc Psychol. 2007;92:42–55. doi: 10.1037/0022-3514.92.1.42. doi:10.1037/0022-3514.92.1.42. [DOI] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Eisenberger NI. An fMRI investigation of attributing negative social treatment to racial discrimination. J Cogn Neurosci. 2011;23:1042–1051. doi: 10.1162/jocn.2010.21520. doi:10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- Pavey L, Greitemeyer T, Sparks P. Highlighting relatedness promotes prosocial motives and behavior. Pers Soc Psychol Bull. 2011;37:905–917. doi: 10.1177/0146167211405994. doi:10.1177/0146167211405994. [DOI] [PubMed] [Google Scholar]
- Perry A, Bentin S, Shalev I, Israel S, Uzefovsky F, Bar-On D, Ebstein RP. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35:1446–1453. doi: 10.1016/j.psyneuen.2010.04.011. doi:10.1016/j.psyneuen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Powers KA, Wagner DD, Norris CJ, Heatherton TF. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Soc Cog Affect Neurosci. doi: 10.1093/scan/nsr079. in press. doi:10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin AP. Superior temporal and premotor brain areas necessary for biological motion perception. Brain. 2007;130:2452–2461. doi: 10.1093/brain/awm162. doi:10.1093/brain/awml62. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Blakemore S-J, Charman T. Reactions to ostracism in adolescents with autism spectrum conditions. J Autism Dev Disord. 2009;39:1122–1130. doi: 10.1007/s10803-009-0725-4. doi:10.1007/s10803-009-0725-4. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social Vocalizations can release oxytocin in humans. Proc Biol Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. doi:10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino A, De Filippo L, Casavecchia C, Coccia M, Shiffrar M, Ladavas E. Lesions to the motor system affect action perception. J Cogn Neurosci. 2010;22:413–426. doi: 10.1162/jocn.2009.21206. doi:10.1162/jocn.2009.21206. [DOI] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proc Natl Acad Sci USA. 2008;105:809–813. doi: 10.1073/pnas.0707021105. doi:10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink J, Villringer A, Kempf F, Haux D, Boden S, Obrig H. Illuminating the BOLD signal: Combined fMRI-fNIRS studies. Magn Reson Imaging. 2006;24:495–505. doi: 10.1016/j.mri.2005.12.034. doi:10.1016/j.mri.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. NeuroImage. 2002;17:719–731. doi:10.1006/nimg.2002.1227. [PubMed] [Google Scholar]
- Tak S, Jang J, Lee K, Ye JC. Quantification of CMRO2 without hypercapnia using simultaneous near-infrared spectroscopy and fMRI measurements. Phys Med Biol. 2010a;55:3249–3269. doi: 10.1088/0031-9155/55/11/017. doi:10.1088/0031-9155/55/11/017. [DOI] [PubMed] [Google Scholar]
- Tak S, Yoon SJ, Jang J, Yoo K, Jeong Y, Ye JC. Quantitative analysis of hemodynamic and metabolic changes in subcortical vascular dementia using simultaneous near-infrared spectroscopy and fMRI measurements. NeuroImage. 2010b;55:176–184. doi: 10.1016/j.neuroimage.2010.11.046. doi:10.1016/j.neuroimage.2010.11.046. [DOI] [PubMed] [Google Scholar]
- Toronov V, Webb A, Choi JH, Wolf M, Michalos A, Gratton E, Hueber D. Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Med Phys. 2001;28:521–527. doi: 10.1118/1.1354627. doi:10.1118/1.1354627. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. doi:10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, DeWall CN, Ciarocco NJ, Bartels JM. Social exclusion decreases prosocial behavior r. J Pers Soc Psychol. 2007;92:56–66. doi: 10.1037/0022-3514.92.1.56. doi:10.1037/0022-3514.92.1.56. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: Effects of being ignored over the internet. J Pers Soc Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. doi:10.1037/0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Wirth JH, Williams KD. ‘They don't like our kind’: Consequences of being ostracized while possessing a group membership. Group Process Intergroup Relat. 2009;12:111–127. doi:10.1177/1368430208098780. [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. doi:10.1002/hbm.460030404. [Google Scholar]
- Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. NeuroImage. 2009;44:428–447. doi: 10.1016/j.neuroimage.2008.08.036. doi:10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. J Exp Soc Psychol. 2004;40:560–567. doi:10.1016/j.jesp.2003.11.006. [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. doi:10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1. Activation in participant-specific structurally defined regions of superior temporal sulcus for Oxy-Hb and Deoxy-Hb fNIRS measurements. Waveforms depict brain responses to biological (top) and scrambled motion (bottom) collapsed across all 3 runs (baseline, post-inclusion, post-exclusion). Stimulus onset and offset are at 3 and 27 seconds, respectively.