Abstract
Rationale
Limited pharmacological data are available to guide methadone treatment during pregnancy and postpartum.
Objectives
Study goals were to: 1) Characterize changes in methadone dose across childbearing, 2) Determine enantiomer-specific methadone withdrawal kinetics from steady-state during late pregnancy, 3) Assess enantiomer-specific changes in methadone level/dose (L/D) ratios across childbearing, and 4) Explore relationships between CYP2B6, CYP2C19 and CYP3A4 single nucleotide polymorphisms and maternal dose, plasma concentration and L/D.
Methods
Methadone dose changes and timed plasma samples were obtained for women on methadone (n=25) followed prospectively from third trimester of pregnancy to three months postpartum.
Results
Participants were primarily white, Medicaid insured and multiparous. All women increased their dose from first to end of second trimester (mean peak increase=23 mg/day); 71% of women increased from second trimester to delivery (mean peak increase=19 mg/day). Half took a higher dose 3 months postpartum than at delivery despite significantly larger clearance during late pregnancy. Third trimester enantiomer-specific methadone half-lives (range R-methadone 14.7-24.9 hours; S-methadone 8.02-18.9 hours) were about half of those reported in non-pregnant populations. In 3 women with weekly 24-hour methadone levels after delivery, L/D increased within 1-2 weeks after delivery. Women with the CYP2B6 Q172 variant GT genotype have consistently higher L/D values for S-methadone across both pregnancy and postpartum.
Conclusions
Most women require increases in methadone dose across pregnancy. Given the shorter half-life and larger clearances during pregnancy, many pregnant women may benefit from split methadone dosing. L/D increases quickly after delivery and doses should be lowered rapidly after delivery.
Keywords: rac-methadone, R-methadone, S-methadone, pregnancy, postpartum, pharmacokinetics, half-life, CYP3A4, CYP2B6, CYP2C19
RATIONALE
Medication dosing across pregnancy is challenging due to the physiological adaptations that occur. Methadone has a complicated pharmacologic profile and significant metabolic heterogeneity among individuals. It has a wide therapeutic dosing range and plasma drug concentrations do not correlate with drug dose(Eap, Bertschy et al. 1998; Foster, Somogyi et al. 2004). Although its use in pregnancy is well established(NIH Consensus Statement 1997), limited data are available to inform methadone dosing through childbearing. Methadone dose during pregnancy is titrated against clinical signs and symptoms of opioid withdrawal and many women report the need to increase dosing during pregnancy. Elucidation of the impact of pregnancy on methadone pharmacokinetics is needed to optimize clinical efficacy.
In the US, methadone is dispensed as racemic (rac) methadone, a mixture of equal components of R and S-stereoisomers. The R enantiomer is ten times more pharmacologically and clinically active(Kristensen, Christensen et al. 1995; Crettol, Déglon et al. 2005), the S enantiomer is responsible for some of the drug’s side effects, and has an augmenting effect on R enantiomer’s actions(Eap, Buclin et al. 2002). The stereoisomers are predominantly metabolized through separate cytochromes and have different half-lives and protein binding properties.
Physiological adaptations of pregnancy provide an explanation for the observed pattern of higher doses across pregnancy. Methadone undergoes hepatic metabolism although controversy remains as to which cytochrome P450 (CYP) isoform(s) are the primary pathways, with CP3A4 and CYP2B6 the main contenders with involvement of CYP2C19 (followed by Phase II glucuronidation)(Gerber, Rhodes et al. 2004; Totah, Sheffels et al. 2008; Kharasch, Hoffer et al. 2009). CYP3A4 activity is increased during pregnancy(Anderson 2005; Tracy, Venkataramanan et al. 2005) which supports the need to increase doses while CYP2C19 activity is decreased(van Heeswijk, Khaliq et al. 2004; Anderson 2005) or unchanged(Hirt, Treluyer et al. 2006), which implies no need to increase the dose. Changes in CYP2B6 in pregnancy have not been described but CYP2B6 is associated more with S- than R-enantiomer metabolism(Gerber, Rhodes et al. 2004; Kharasch, Hoffer et al. 2004; Totah, Sheffels et al. 2008). Increased volume of distribution and renal clearance also support increased dose requirements. Stereospecific differences have been reported in the plasma binding of methadone, with greater free fractions of R- than S-methadone(Eap, Cuendet et al. 1990). During pregnancy, concentrations of key plasma binding proteins (primarily alpha-1-acid glycoprotein and secondarily albumin) decrease, which increase availability of the more biologically active R-enantiomer. This would suggest less need for dose increases during pregnancy. Alternatively, less protein binding could also increase clearance of both enantiomers, resulting in the need for higher doses in pregnancy. The relative importance of each adaptation on overall methadone bioavailability across pregnancy is unknown and additional studies are needed. The postpartum time course of return to pre-pregnancy metabolic characteristics is unknown; therefore, no protocol for adjusting methadone dosage after birth is available.
Our study goal was to define enantiomer-specific methadone pharmacokinetics in late pregnancy and postpartum to provide an evidence-base upon which to make dosing decisions during and after pregnancy. The specific aims of this longitudinal study of pregnant women enrolled in a community-based methadone maintenance treatment program (MMTP) were to: 1. Characterize changes in methadone dose during pregnancy and postpartum based on titration to withdrawal symptoms.
2. Determine enantiomer-specific methadone withdrawal kinetics from steady-state during the third trimester of pregnancy.
3. Assess the enantiomer-specific changes in methadone level/dose (L/D) ratios from third trimester of pregnancy to the postpartum period.
4. Explore the relationships between CYP2B6, CYP2C19 and CYP3A4 single nucleotide polymorphisms (SNPs) and maternal dose, plasma concentration and L/D.
METHODS
This longitudinal study included pregnant women with opioid dependency who were being treated with methadone at a single community-based MMTP (Narcotics Addiction Treatment Program) and its sister behavioral support program (Perinatal Addiction Center). Most women were recruited into the L/D protocol and, if they met additional criteria, into the pharmacokinetics (PK) protocol which included formal studies with timed serial blood sampling during pregnancy. We recruited a minority of women into the PK arm of the study to minimize participant burden and minimize risk to the fetus. The PK arm required mothers to take their second day methadone dose more than 4 hours late and there were little data in the literature to support this during pregnancy. Since, we planned to estimate PK parameters from the L/D data for all women, this approach allowed us to balance the needs of the study with the needs of the participants.
Inclusion Criteria
Women in both study arms were eligible if they were 18-45 years old, were enrolled in the MMTP, planned to deliver at Magee-Women’s Hospital of UPMC (Magee), did not intend to place their baby for adoption and were at least 20 weeks pregnant at enrollment. There were no limitations on length of time since starting on methadone, concurrent medical and psychiatric conditions or use of other medications.
Exclusion Criteria
Women with HIV/AIDS, inability to provide informed consent or to speak English were excluded. Women were excluded from the PK arm, but not L/D arm, if they were prescribed split methadone dosing (dose more than once daily).
Third Trimester Study Visits for L/D Arm
Women in the L/D arm had two visits during the third trimester (27-39 weeks gestation) from one to 15 days apart. For each visit, women went to the MMTP at their usual dosing time and an investigator (DLB) drew 5 ml of blood for 24-hour methadone concentration just prior to taking their usual dose. After being observed taking their methadone, women went to the Magee Clinical and Translational Research Center (CTRC) where they completed questionnaires (health, dietary and mental health), had anthropometric measurements and had blood drawn for pharmacogenetics and nutritional biomarkers. Dietary and nutritional biomarker data were previously published.(Tomedi, Bogen et al. 2012) For the second visit, women again had 24-hour methadone sampling at their MMTP with follow-up at the CTRC for repeat anthropometric measurements and questionnaire completion. To achieve steady state PK, women were required to have been on the same methadone dose for at least one week before each study visit.
Third Trimester Study Visits for PK Arm
Women in the PK study arm had their two third trimester appointments scheduled on consecutive days. For the first visit, women went to their MMTP at their usual dosing time and had 5 ml of blood drawn for 24-hour methadone concentration just prior to their morning dose. After being observed taking their methadone, women went to Magee’s CTRC where blood was taken for methadone concentrations at ½, 1½, 3, 4½, and 6 hours post-dose. Women returned to the CTRC the next morning before obtaining their methadone dose and had blood samples obtained at 24, 26, and 28 hours. Women received their usual methadone dose after the final blood sample. Because of the 4 to 6 hour delay past their usual dosing interval on the second day, women completed the Clinical Opiate Withdrawal Scale (COWS)(Wesson and Ling 2003) on arrival to the CTRC both mornings. One woman had a higher second day score which suggests withdrawal symptoms due to delayed dosing.
Postpartum Visit
Subjects were asked to return 3 to 6 months after delivery to obtain data during a non-gravid state for comparison to pregnancy. They went to their MMTP and the same procedures as described for the third trimester were implemented.
Late in the study, a protocol modification was made (with Institutional Review Board approval) to assess the timing of normalization of post-delivery plasma methadone concentrations. This modification was inspired by the observation that postpartum women appeared sedated. We hypothesized that the accelerated clearance of pregnancy rapidly dissipated and mothers developed sedation due to higher post-birth plasma levels. Three women had 24-hour plasma samples obtained prior to their dose in the first few weeks after delivery.
Methadone Concentration Samples
All methadone samples were collected by venipuncture into EDTA tubes and cold centrifuged within 60 minutes. Plasma was removed using polyethylene Pasteur pipettes into polypropylene vials and frozen at −80C.
Chiral Methadone Analysis
The plasma samples were analyzed for R- and S-methadone by the application of a modified Chiral High Performance Liquid Chromatographic/Ultraviolet Detection Method (HPLC/UV)(Foster, Somogyi et al. 2000). The modifications used 4.0 ml of 3:7 Methyl tertButyl ether : n-hexane as the extraction solvent, rac-norfluoxetine as Internal Standard instead of 3-Methoxymorphinan, a mobile HPLC phase of Methanol/Acetonitrile/pH 5.7 Triethylamine phosphate buffer (9/11/80) and evaporation of the re-extracted 5mM Hydrochloric acid phase to dryness with a Savant Speed-Vac centrifuge evaporator, followed by reconstitution with 100 microliters of the mobile phase from which 80 microliters were injected via an Autoanalyzer Calculation, weighted linear regression of peak height ratio versus concentration for each compound; and Retention times (min), R-Methadone 10.1, S-Methadone 11.8, S-Norfluoxetine 41.3 (Internal standard I), R-Norfluoxetine 45.5 (Internal Standard II ). The limit of detectability was 5 ng/ml, mean % Coefficients of Variation ranged (CV) from 8.6 (high controls) to 12.7 (low controls).
Methadone dosing
Methadone was administered at the licensed MMTP, with multiple safety measures in place to confirm patient identity and verify dose (using Methasoft Version 4) as required under local, state and federal regulations. Study staff were not involved in dosing decisions. Methadone dose adjustments were made per the MMTP’s standard protocol. Dose adjustments were usually patient initiated. Patients complete an opioid withdrawal scale and provide the reason(s) for dose change to staff, who add input before the physician reviews the information and makes a decision. Dose changes ranged from 1 to 10 mg, with the total increase usually split over 2 days. Urine screens for illicit drug were obtained at least monthly according to the MMTP routine and by obstetrical care providers, but not specifically for this study.
Methadone 24-Hour L/D
For all 24-hour methadone concentrations obtained, the ratio of the measured plasma R, S and rac-methadone concentration to dose at time of sampling was calculated. For R and S-methadone, half of the mother’s rac-methadone dose was used in the calculation.
Genotyping samples and analysis
CYP2B6, 2C19 and 3A4 have been implicated in the metabolism of rac-methadone with increasing evidence that CYP2B6 is largely responsible for S-methadone metabolism and less for R-methadone(Crettol, Déglon et al. 2005; Totah, Sheffels et al. 2008). One ml of whole blood was obtained for CYP2B6, CYP2C19 and CYP3A4 genotyping during the third trimester of pregnancy. DNA and whole blood samples were stored at −80 F. Genomic DNA from the whole blood samples was extracted and purified using the manufacturer’s protocol (Gentra Puregene Blood Kit (Qiagen). We selected SNPs for each of the CYPs that were at the time (2005-2006) identified as demonstrating phenotypic differences. For CYP2B6, the following SNPs were evaluated: rs8015382, rs11083595, rs7254579, rs4803419, rs3745274 and rs3211371. Two showed no heterogeneity (rs8015382 and rs3211371) and were not evaluated further. For CYP2C19, two SNPs were evaluated (rs4244285 and rs4986893) but one showed no heterogeneity (rs4986893) and was not evaluated. For CYP3A4, only rs2740574 was evaluated.
All genotyping analyses were performed using TaqMan® allele discrimination based assays (Applied Biosystems, Foster City, CA). Positive and negative PCR controls were included with each amplification reaction. For both genotyping analyses, previously sequenced genomic DNA samples were used as positive controls for the homozygous wild-type, heterozygous and homozygous variant genotypes with every PCR analysis to verify reproducibility of the assay and to confirm accuracy of genotype classifications. Approximately 10% of randomly selected samples were repeated blindly for verification of genotyping assays. All results were interpreted independently by two laboratory personnel and no discordant genotype classifications were identified.
IRB Approval was obtained from the University of Pittsburgh. Study participants provided written, informed consent for themselves and their infants. Consent included permission to review the Magee medical records (mother and infant). Women signed releases of information for the results of urine drug screens, methadone dose changes and psychiatric diagnoses from their MMTP.
Data Analysis by Specific Aim
Subject Characteristics
Women who underwent screening but did not participate or who consented but did not complete the two pregnancy study visits were compared on demographic and health related variables to women who completed the two pregnancy study visits. Chi-square statistics were used for categorical variables and t-tests for continuous variables. For variables with small cell frequencies, Fishers exact tests were used.
Aim 1: Characterize changes in methadone dose during pregnancy
Changes in dosages across pregnancy were compared with a series of paired t-tests because women converted to methadone in different trimesters of pregnancy. Dose comparisons were made between groups for women who were taking methadone in two trimesters (compared 1st and 2nd trimester peak doses and 2nd and 3rd trimester peak doses). For women with postpartum data, peak doses in 3rd trimester and dose at postpartum follow-up visit were compared.
Aim2: Determine maternal enantiomer-specific methadone withdrawal kinetics from steady-state during late pregnancy
For the 5 women in the PK study arm, area under the curve (AUC) was calculated from t=0 to t= using the WinNonlin in Noncompartmental Analysis Program (v0.3,1A), core version October 29, 1999 (Pharsight Corp) and by application of the trapezoidal rule. Withdrawal rates are first-order kinetics and the final three data points serve to accurately establish PK parameters with statistically valid Akai Criteria of Concordances. Comparisons of the R and S parameters were carried out with a random effects regression using the R and S levels of the PK parameters as dependent measures within each subject. This statistical procedure allows each woman to be compared to her own withdrawal kinetics rather than a fixed level established by the mean across all observations.
For women in both the PK and L/D arms, clearance was estimated from the L/D data using the following formula: Cl x f = Dose/(CSS x T). In this calculation, f was assumed to equal to one, CSS is concentration at steady state and T is time of dosing interval (24 hours). The 3 women taking split methadone dosing were not included in this analysis.
Aim3: Assess the enantiomer-specific changes in methadone L/D ratios from third trimester of pregnancy to postpartum period
Two third trimester samples and one postpartum sample of maternal R, S and rac-methadone L/D values were compared using mixed effect regression. The primary effects of interest were the separate comparisons of R, S and rac-methadone levels across assessment times.
Aim 4: Examine the associations between CYP2B6, CYP2C19 and CYP3A4 SNPs and maternal dose, plasma concentration and L/D
The primary interest was main effects for different genetic variants and the interactions of genetic variants and pregnancy state. These significance of effects were tested with the chi-square statistics for changes in log-likelihood when the interaction terms were removed from models with the interactions.
For data presented on individual participants (eg clearance), participant number is internally consistent across all tables and figures, meaning that data with the same identification number represents the same woman.
RESULTS
Of 51 women screened for study enrollment, 40 met eligibility criteria, 31 consented to participate, 25 women came to the two third trimester pregnancy visits (study sample) and 18 completed the postpartum study visit. The 25 women included in the analysis did not differ from the 15 women who were screened as eligible but did not participate with respect to age, race, ethnicity, education, methadone dose or gestational age. The participants (Table 1) were on average 26.8 years (sd 6.06; range 18-40), primarily white, Medicaid insured, multiparous and smokers. At study enrollment, 4 women reported use of other psychotropic medications (sertraline, s-citalopram and clonazepam) and 6 reported taking non-psychiatric medications other than prenatal vitamins. At delivery, according to medical record review, 9 were prescribed psychotropic medications (clonazepam, alprazolam, sertraline, citalopram, escitalopram, bupropion and haloperidol) and 3 of 9 were taking two psychotropic medications.
Table 1.
Study participant characteristics (n=25)
| Participant Characteristic | N | % |
|---|---|---|
| Age | ||
| 18 – 25 | 12 | 48 |
| 26 – 40 | 13 | 52 |
| Race (% Caucasian) | 22 | 88 |
| High School Education or more | 21 | 84 |
| Insurance (% Medicaid) | 25 | 100 |
| Primiparous | 9 | 36 |
| Married or living with partner | 9 | 36 |
| Employed during pregnancy | 5 | 20 |
| Smoked during pregnancy | 19 | 76 |
| Timing of conversion to methadone | ||
| Before Pregnancy | 1 | 4 |
| 1st Trimester | 13 | 52 |
| 2nd Trimester | 7 | 28 |
| 3rd Trimester | 4 | 16 |
| Taking psychotropic medication | ||
| At enrollment (self report) | 4 | 16 |
| At delivery (medical record review; n=22) | 9 | 41 |
| Hepatitis C positive | 14 | 56 |
| Hepatitis B positive | 0 | -- |
| Drug Screen Positive for Illicit Drugs at Delivery (positive for benzodiazepines and opiates) |
1 | 4 |
| Edinburgh Postnatal Depression Scale ≥ 10 (3rd tri) | 20 | 80 |
| aMood Disorder Questionnaire33 | 4 | 16 |
| bPrimary Care PTSD Screen34 | 12 | 48 |
| Methadone Dose (mg/day) | Mean (sd) | Range |
| At Delivery | 102.6 (44.3) | 22-200 |
| Late Postpartum (n=22) | 105.9 (42.1) | 26-190 |
Positive if “yes” to seven or more of the 13 items in question number 1 and yes to question 2 and answers moderate or serious to question 3.
PTSD: post-traumatic stress disorder screen positive screen if patient answered “yes” to any two items or to item three (hyper-arousal).
Aim 1: Methadone dose changes during pregnancy and postpartum
Most women (84%) converted to methadone before the third trimester of pregnancy (Table 2). Three women were prescribed split dosing during pregnancy and two postpartum. Among 14 women who converted to methadone before or during the first trimester, all increased their dose by the end of the second trimester. Their average dose increased by 23 mg/day (range 5-55 mg) or 46% (range to 4-183%). Similarly, among 21 women on methadone during their second trimester, 15 (90%) increased their dose during the third trimester. Their average dose increased by 19 mg/day (range 0-65 mg/day) or 61% (range 0-71%) (paired t (df20)=4.71, p<0.0001). Three months postpartum dose information was available on 22 women. Compared to their dose at delivery, 7 (32%) were taking a lower dose (mean change −32, range −10 to-91), 4 (18%) were taking the same dose and 11 (50%) were taking a higher dose (mean change 21, range 5-70). Although the 3 women who converted to methadone in the 3rd trimester all had increased doses postpartum, the relationship between dose changes postpartum and trimester women converted to methadone was not significant (Fisher exact=0.47).
Table 2.
Methadone dose by trimester of pregnancy and postpartum
| Methadone Dose | N | Mean | Median | Range |
|---|---|---|---|---|
| Women who converted during or before the first trimester a | ||||
| Peak dose 1st trimester | 14 | 70.4 | 57.5 | 30-145 |
| Peak dose 2nd trimester | 14 | 93.4 | 89 | 50-155 |
| Peak dose 3rd trimester/delivery | 14 | 111.7 | 114 | 50-180 |
| Women who converted during the second trimester b | ||||
| Peak dose 2nd trimester | 7 | 90 | 80 | 60-135 |
| Peak dose 3rd trimester/delivery | 7 | 111.4 | 100 | 75-200 |
| Women who converted during the third trimester | ||||
| Peak dose 3rd trimester/delivery | 4 | 46.3 | 45 | 20-75 |
| Dose 3 to 6 months postpartum c | 22 | 106 | 97.5 | 26-190 |
Paired t-test for dose in 1st trimester to 2nd trimester, t(13)=5.45, p=0.0001.
Paired t-test for dose on 2nd trimester to 3rd trimester, t(13)=4.15, p=0.001.
Paired t-test for dose on 2nd trimester to 3rd trimester, t(6)=2.35, p=0.06
Paired t-test of 3rd trimester and postpartum dose levels, t(21)=0.05, p=0.96.
Aim 2: Methadone Pharmacokinetics Data
Non-compartmental PK parameters for the 5 women who completed third trimester PK protocol are provided in Table 3. R-methadone half-life ranged from 14.7-24.9 hours and S-methadone half-life ranged from 8.02-18.9 hours. Although there was large individual variability in half-life and 24-hour clearance rates, for each individual woman the more biologically active R-enantiomer had a longer half-life (3 -7 hours longer) and smaller clearance (.002-.15 L/(hr*kg) than the S-enantiomer and substantially shorter than reported in non-gravid populations(Eap, Buclin et al. 2002). However, the VD/F was not significantly different for R-, S- and rac-methadone. The average number of days between third trimester visits was 4.3 (sd + 5.6, range 1-15). These second study visit occurred a mean of 34 days before delivery (range: 2 to 101 days) and the postpartum visit occurred a mean of 153 days following delivery (range 123-242 days). The stereoisomer 24-hour L/D was very stable between the two third trimester visits for all participants (paired t-test for rac-methadone, t=0.28, p=0.78, R-methadone t=-0.06, p=0.96, S-methadone t=-0.06, p=0.76), which demonstrates the consistency of sampling techniques and assays. Using the formula provide in the methods section, the R, S and rac-methadone clearances were estimated from L/D data on 22 women during the third trimester of pregnancy who were not on split dosing (Table 4). These data demonstrate the inter-individual variability of methadone clearance and are consistent with the PK data that 3rd trimester R-methadone clearance is significantly smaller than for S-methadone. Postpartum, this difference in stereoisomer clearance is not evident.
Table 3.
Methadone Non-compartmental Pharmacokinetic Parameters
| Patient | Mat Weight (kg) |
Dose per isomer (mg) |
t½ (hrs) |
AUC∞ {hr*(ng/mL)} |
VD / F {L/kg} |
Clearance {L/(hr*kg)} |
|
|---|---|---|---|---|---|---|---|
| R-MTD | 11.0 | 24.7 | 2,478 | 1.89 | 0.0530 | ||
| S-MTD | 1 | 82.4 | 11.0 | 18.9 | 2,423 | 1.49 | 0.0548 |
| rac-MTD | 22.0 | 21.2 | 4,848 | 1.67 | 0.0545 | ||
|
| |||||||
| R-MTD | 30.0 | 14.7 | 3,684 | 2.72 | 0.128 | ||
| S-MTD | 2 | 61.5 | 30.0 | 11.3 | 3,656 | 2.13 | 0.130 |
| rac-MTD | 60.0 | 12.8 | 7,286 | 2.40 | 0.130 | ||
|
| |||||||
| R-MTD | 30.0 | 14.8 | 2,196 | 5.38 | 0.252 | ||
| S-MTD | 3 | 53.6 | 30.0 | 8.02 | 1,252 | 5.16 | 0.446 |
| rac-MTD | 60.0 | 11.3 | 3,342 | 5.40 | 0.332 | ||
| R-MTD | 77.5 | 24.9 | 15,941 | 2.28 | 0.0635 | ||
| S-MTD | 4 | 75.0 | 77.5 | 17.2 | 10,944 | 2.32 | 0.0933 |
| rac-MTD | 155.0 | 20.7 | 26,346 | 2.30 | 0.0772 | ||
|
| |||||||
| R-MTD | 80.0 | 20.7 | 4,575 | 6.23 | 0.208 | ||
| S-MTD | 5 | 83.7 | 80.0 | 13.6 | 2,792 | 6.72 | 0.341 |
| rac-MTD | 160.0 | 17.1 | 7,210 | 6.50 | 0.264 | ||
|
| |||||||
| R-MTD | 45.7 | 20.00* | 5,775** | 3.70# | 0.141## | ||
| S-MTD | Means for 1-5 |
71.2 | 45.7 | 13.80 | 4,213 | 3.56 | 0.213 |
| rac-MTD | 91.4 | 16.62 | 9,806 | 3.65 | 0.172 | ||
t½ for R-MTD was significantly higher than t½ for S-MTD (p=0.000)
AUC for R-MTD was marginally higher than AUC for S-MTD (p=0.089)
VD / F was not significantly different for R-MTD and S-MTD (p=0.47)
Clearance for S-MTD was marginally higher than for R-MTD (p=0.064)
Statistics based on random effects regressions with R and S levels treated as a within subject variable
Table 4.
Methadone clearance estimated from L/D for women on single daily dosinga
| 3rd trimester b | Postpartum | |||||
|---|---|---|---|---|---|---|
| ID a | Clearance L/(hr*kg) | Clearance L/(hr*kg) | ||||
| R | S | Total | R | S | Total | |
| 1 | 0.156 | 0.135 | 0.145 | -- | -- | -- |
| 2 | 0.319 | 0.319 | 0.318 | 0.165 | 0.131 | 0.146 |
| 3 | 0.518 | 1.086 | 0.692 | 0.365 | 0.741 | 0.489 |
| 4 | 0.271 | 0.297 | 0.281 | 0.142 | 0.184 | 0.160 |
| 5 | 0.532 | 0.966 | 0.684 | 0.257 | 0.257 | 0.257 |
| 6 | 0.313 | 0.638 | 0.420 | -- | -- | -- |
| 7 | 0.140 | 0.192 | 0.162 | 0.144 | 0.136 | 0.139 |
| 8 | 0.352 | 0.864 | 0.500 | 0.237 | 0.404 | 0.298 |
| 9 | 0.387 | 0.861 | 0.534 | -- | -- | -- |
| 10 | 0.139 | 0.309 | 0.191 | -- | -- | -- |
| 11 | 0.212 | 0.244 | 0.227 | 0.074 | 0.051 | 0.060 |
| 12 | 0.135 | 0.195 | 0.159 | -- | -- | -- |
| 13 | 0.269 | 0.382 | 0.315 | 0.246 | 0.242 | 0.244 |
| 14 | 0.261 | 0.566 | 0.357 | 0.148 | 0.207 | 0.172 |
| 15 | 0.095 | 0.128 | 0.109 | -- | -- | -- |
| 16 | 0.395 | 1.176 | 0.589 | -- | -- | -- |
| 17 | 0.302 | 0.405 | 0.346 | 0.222 | 0.399 | 0.285 |
| 18 | 0.343 | 0.493 | 0.404 | 0.156 | 0.127 | 0.140 |
| 19 | 0.409 | 0.579 | 0.479 | 0.120 | 0.148 | 0.132 |
| 20 | 0.277 | 0.412 | 0.332 | 0.224 | 0.233 | 0.228 |
| 21 | 0.365 | 0.492 | 0.419 | -- | -- | -- |
| 22 | 0.182 | 0.186 | 0.184 | 0.279 | 0.250 | 0.263 |
|
| ||||||
| Mean | 0.290 | 0.497 | 0.377 | 0.199 | 0.251 | 0.215 |
| Range | 0.095-.0532 | 0.125-0.176 | 0.109-0.692 | 0.074-0.365 | 0.051-.741 | 0.060-0.489 |
Three women on split methadone dosing are not included in this table.
Average of 2 third trimester measurements
Aim 3: Comparison of Pregnancy to Postpartum L/D and clearance data
L/D and clearance data were compared for women with both pregnancy and postpartum samples. For 12 of the 14 women not on split dosing with clearance data in pregnancy and postpartum, clearances of R, S, and rac-methadone were significantly larger during pregnancy than postpartum (p<=0.002, for each of the 3 tests). For one woman R-methadone clearance was the same in pregnancy and postpartum but S and rac-methadone clearance was larger in pregnancy. For one woman, R, S and rac-methadone clearance was smaller postpartum than during pregnancy (Table 4 and Figure 1).
Figure 1.
Comparison of L/D between third trimester of pregnancy and late postpartum
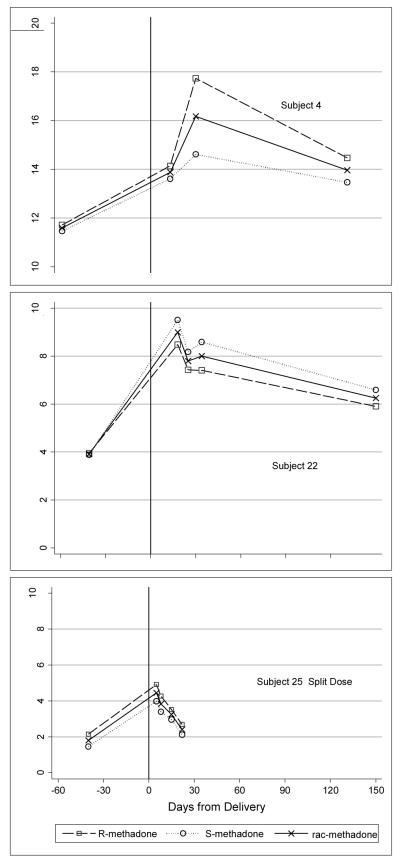
Three women provided multiple 24-hour samples in first month following delivery as well as a late postpartum sample. The findings (Figure 2) demonstrate that R, S and rac-methadone L/D concentrations rise quickly within one to two weeks after delivery and remain above the third trimester level postpartum. These limited data suggest that women may tolerate a significant decline in methadone dose within one to two weeks after delivery. Additional data are needed to determine how rapidly the L/D increases and the optimal manner to individualize post-birth dosing.
Figure 2.
L/D for 3 women with multiple postpartum samples
Aim 4: Explore CYP2B6, 2C19 and 3A4 SNPs activity and both L/D and change in L/D
Main effects data
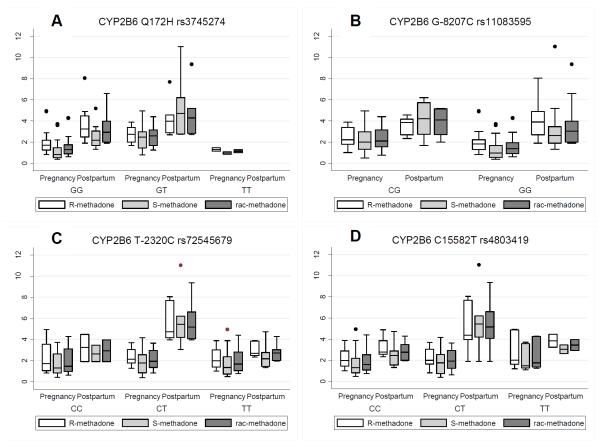
With one exception R, S, and rac-methadone L/D ratios were significantly higher postpartum than in pregnancy. The analyses reported here focus on different patterns observed for women with different genotype variants. Women with the CYP2B6 Q172 variant GT genotype have higher L/D values for S and rac-methadone across both pregnancy and postpartum time points than women with the variant GG genotype (Figure 3A p=0.001, 0.006 respectively). Other notable differences that did not reach significance were: 1) L/D-R-methadone in women with variant GT of CYP2B6 Q172 (Figure 3A, p=0.11) were higher and 2) L/D-S-methadone in women with the CG variant of the CYP2B6 G-8207C SNP were higher than those with the GG variant (Figure 3B, p=0.10). When variants of the CYP2B6 T-2320C (Figure 3C) were included in the modeling, the main effect for pregnancy vs. postpartum L/D ratios were no longer significant (p=0.07, 0.09, 0.06 for R, S, and rac-methadone respectively).
Figure 3.
Variation in L/ D ratios of R-, S- and rac-methadone across pregnancy and postpartum related to CYP2B6 SNPs Boxes represent interquartile range (IQR) of observed values. The median value is represented with a contrasting line within the box. The vertical lines above and below the box (whiskers) are values that are 1.5 times the median but beyond the IQR. Markers (dots) outside the whiskers represent any values outside of these values. (Tukey, J.W. 1977 Exploratory Data Analysis. Reading MA: Addison-Wesley)
Interactions between genetic SNPs and pregnancy/postpartum L/D ratios
More intriguing differences related to the CYP allelic variants were seen in the interactions of the variants and the time of L/D measurement. For women with the GG variant of the CYP2B6 G-8207C, there was a larger increase between pregnancy and postpartum in the L/D R-methadone ratio than for women with the CG variant (Figure 3B, p=0.001). For women with the CT variant of the CYP2B6 T-2320C SNP, L/D R, S and rac-methadone were higher postpartum than women with the CC or TT variants (Figure 3C, p= 0.01, 0.04, 0.004 for R, S and rac-methadone respectively). This SNP did not affect the L/D ratios in pregnancy. For women with the CT variant of CYP2B6 C15582T postpartum L/D R, S and rac-methadone were higher than for women with the CC or TT variant (Figure 3D, p=0.07, 0.009, 0.018 respectively). This SNP did not affect levels in pregnancy.
DISCUSSION
This prospective study of pregnant women prescribed methadone for treatment of opioid dependence confirms that increased doses across pregnancy in response to clinical symptoms are required. The average increase in methadone dose was 23 mg from first to second trimester and 19 mg from second to third trimester of pregnancy. This clinical finding is strongly supported by the steady state PK data. For most women, R-, S- and rac-methadone clearances during pregnancy were significantly larger than late postpartum (non-pregnant state). Compared to previously published data on steady-state stereoselective methadone PK in non-pregnant pain clinic populations, we found the half-life in late pregnancy to be approximately half for both enantiomers(Kristensen, Blemmer et al. 1996). Compared to Eap and colleagues’ summary of published reports on rac-methadone half-life (range 22 to 54 hours(Eap, Buclin et al. 2002)), we found a range in pregnancy of 11 to 21 hours. Given the shorter half-life in late pregnancy, twice daily dosing may be appropriate for more pregnant women than currently are offered this option and could potentially decrease the total dose requirement during pregnancy. Among 3 women with split methadone dosing during pregnancy, two had no dose increase from second to third trimester. The third had a 35 mg increase from early pregnancy until delivery, which was lower than women on once daily dosing. Twice daily dosing during pregnancy may also benefit the fetus. Jansson demonstrated less fetal neurobehavioral changes at peak versus trough levels when women’s methadone dose was given twice daily compared to once daily(Jansson, Dipietro et al. 2009).
Data from 3 women who provided multiple early postpartum trough samples suggests that methadone doses could be reduced within the first one to two weeks postpartum. This finding is consistent with the clinical observation made following delivery that prompted this line of inquiry. This rapid increase in methadone concentration is particularly concerning given the common use of benzodiazepine and SSRIs in this population. Some of these medications inhibit methadone metabolizing enzymes which could increase the risk of opioid toxicity and drug interactions. The time course should be studied in a larger sample and 24-hour concentrations examined at closer intervals to determine the rapidity of postpartum dose reduction. Other investigators have reported methadone dose changes in pregnancy and shortly after delivery(Pond, Kreek et al. 1985),(Jones, Johnson et al. 2008). Jones et al. recommended postpartum dose reductions due to mild signs and symptoms of overmedication in one of eight patients stabilized on methadone three weeks before delivery(Jones, Johnson et al. 2008). Blood concentrations of methadone were not reported. Optimal adjustment of methadone dose postpartum is critical to maternal function. Higher than necessary doses may lead to tolerance, over-sedation, increased risk for overdose and may interact with medications that cause respiratory depression. Maternal sedation may put infants at risk due to inattention or lack of coordination.
Dose changes are common during and after pregnancy for a variety of medications due to changes in plasma volume, protein binding, clearance and CYP activity(Anderson 2005). Pond suggested that the increased methadone doses during pregnancy were due to increased metabolism and clearance(Pond, Kreek et al. 1985). Data as to when maternal blood volume and metabolic changes return to the prepregnancy level are conflicting. Plasma volume returns to the prepregnancy level from 2 to 12 weeks (Robson, Mutch et al. 1990; Frederiksen 2001) while the cytochrome P450 system requires up to 6 weeks to return to prepregnancy activity levels(Wisner, Perel et al. 1997). Even in our small sample, we found that specific CYP2B6 variants relate to methadone L/D during pregnancy and postpartum. Our data are consistent with recent publications that suggest that CYP2B6 plays a role in S-methadone metabolism. Methadone induces the hepatic expression of multiple drug metabolizing enzymes, including CYP2B6 and CYP3A4(Tolson, Li et al. 2009), but the impact during pregnancy is unknown. Although a number of studies implicate polymorphisms in CYP2D6 (Chang, Fang et al. 2011)in methadone metabolism, particularly as it relates to its impact on multidrug metabolism, at the time we designed and implemented the study, it was not considered a significant contributor and the cost of testing was prohibitive.
Our study is limited by the small sample size and should be considered pilot data. However, we were able to follow this pregnancy cohort across time and obtain unique data. We did not measure methadone metabolites which also undergo changes during pregnancy. We focused on the third trimester of pregnancy for our L/D data and additional work is needed to evaluate L/D changes earlier in pregnancy. We are not yet able to characterize changes in methadone concentration at individual sites of action or how changes in individual sites affect the constellation of observed side effects. Like most populations prescribed methadone for treatment of opiate addiction, our subjects usually smoked cigarettes, took other psychoactive medications and some intermittently used illicit drugs. We did not study the impact of these other exposures on methadone pharmacokinetics although others have studied these interactions in non-pregnant populations. Methadone plasma concentration is not affected by nicotine, although it does impact subjective measures of opiate withdrawal(Elkader, Brands et al. 2009). Cytochrome P450 metabolized benzodiazepines (excluding oxazepam and lorazepam), and specifically CYP3A4 metabolized benzodiazepines are associated with higher trough plasma R- and, to a greater extent, S-methadone/dose(Hallinan, Crettol et al. 2009). Women who are adherent to their MMTP should be supported to breastfeed(The Academy of Breastfeeding Medicine Protocol Committee and Jansson 2009) due to very low levels in milk(Jansson, Choo et al. 2008; Bogen, Perel et al. 2011) and increasing evidence that breastfeeding is associated with improved neonatal outcomes(Ballard 2002; Arlettaz, Kashiwagi et al. 2005; Abdel-Latif, Pinner et al. 2006); however, the effect of breastfeeding on maternal methadone plasma concentrations has not been studied. In our study, a few of the women were partially breastfeeding at the postpartum visit.
Our study findings suggest that serious consideration should be given to offering women split methadone dosing in late pregnancy due to its significantly shorter half-life at this time. Regulations related to take-home dosing for patients who only recently were started on methadone make this practice challenging; diversion of methadone remains a serious challenge in the US. Our limited data also suggest methadone doses should be reduced within 1-2 weeks following delivery but this time frame requires confirmation through further study.
Acknowledgements
This study was funded by The Children’s Hospital of Pittsburgh Research Advisory Committee and The Gerber Foundation. Study visits were conducted in the Magee-Womens Hospital Clinical and Translational Research Center which is supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005. Medela, Inc. supported this study by providing the breast pump supplies to collect breastmilk samples and educational materials. Dr. Bogen’s contribution to this work was supported by K12 HD043441 (BIRCWH Award). Support for the time of Dr. Wisner, Mr. Helsel and Dr. Perel was funded by R01 MH 075921 (K. Wisner, PI). This project was also supported in part by the award 5MO1 RR00056 and P30CA047904 for the genotyping analyses by Drs. Romkes and Nukui. We would like to thank the staff of NATP, in particular Millie Hopkins, for their support of this project. .
Abbreviation
- AAP
American Academy of Pediatrics
- AUC
Area under the curve
- CTRC
Clinical and Translational Research Center
- CYP
Cytochrome P450
- HPLC/UV
High performance liquid chromatographic/ultraviolet detection method
- L/D
Level/Dose
- Magee
Magee-Womens Hospital
- MMTP
Methadone maintenance treatment program
- NAS
neonatal abstinence syndrome
- PK
Pharmacokinetic
- PTSD
Post traumatic stress disorder
- Rac
Racemic
- SNPs
single nucleotide polymorphisms
Footnotes
Presentations Study findings were presented at the following meetings: Organization of Teratology Information Specialists Meeting, Louisville, KY, June 2010; North American Society for Psychosocial Obstetrics and Gynecology, Marce’ Symposium, New Haven, CT, Feb 2009; 72nd Annual Meeting of The College of Problems of Drug Dependence, Scottsdale, Arizona. June 2010; and Western Psychiatric Institute and Clinic Tenth Annual Research Day, June 3, 2010.
Conflicts of Interest Debra L. Bogen, MD: Medela, Inc provided breastfeeding supplies for this study
James M Perel, PhD: none
Joseph Helsel, BS: none
Barbara H. Hanusa, PhD: none
Marjorie Romkes, PhD: none
Tomoko Nukui, PhD: none
Catherine Friedman, MD: none
Katherine L. Wisner, MD, MS: none
References
- Abdel-Latif ME, Pinner J, et al. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics. 2006;117(6):e1163–9. doi: 10.1542/peds.2005-1561. [DOI] [PubMed] [Google Scholar]
- Anderson GD. Pregnancy-Induced Changes in Pharmacokinetics: A Mechanistic-Based Approach. Clinical Pharmacokinetics. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- Arlettaz R, Kashiwagi M, et al. Methadone maintenance program in pregnancy in a Swiss perinatal center (II): neonatal outcome and social resources. Acta Obstetricia et Gynecologica Scandinavica. 2005;84(2):145–50. doi: 10.1111/j.0001-6349.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Ballard JL. Treatment of neonatal abstinence syndrome with breast milk containing methadone. Journal of Perinatal & Neonatal Nursing. 2002;15(4):76–85. doi: 10.1097/00005237-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Bogen D, Perel J, et al. Exposure to Enantiomer-specific Methadone Levels in Breastmilk. Breastfeeding Medicine. 2011;6(6):377–384. doi: 10.1089/bfm.2010.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Fang WB, et al. Stereo-Selective Metabolism of Methadone by Human Liver Microsomes and cDNA-Expressed Cytochrome P450s: A Reconciliation. Basic & Clinical Pharmacology & Toxicology. 2011;108(1):55–62. doi: 10.1111/j.1742-7843.2010.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crettol S, Déglon J-J, et al. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78:593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Eap CB, Bertschy G, et al. High interindividual variability of methadone enantiomer blood levels to dose ratios. Archives of General Psychiatry. 1998;55(1):89–90. doi: 10.1001/archpsyc.55.1.89. [DOI] [PubMed] [Google Scholar]
- Eap CB, Buclin T, et al. Interindividual Variability of the Clinical Pharmacokinetics of Methadone: Implications for the Treatment of Opioid Dependence. Clinical Pharmacokinetics. 2002;41(14):1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- Eap CB, Cuendet C, et al. Binding of d-methadone, l-methadone, and dl-methadone to proteins in plasma of healthy volunteers: role of the variants of alpha 1-acid glycoprotein. Clinical Pharmacology & Therapeutics. 1990;47(3):338–46. doi: 10.1038/clpt.1990.37. [DOI] [PubMed] [Google Scholar]
- Elkader AK, Brands B, et al. Methadone-Nicotine Interactions in Methadone Maintenance Treatment Patients. Journal of Clinical Psychopharmacology. 2009;29(3):231–238. doi: 10.1097/JCP.0b013e3181a39113. 10.1097/JCP.0b013e3181a39113. [DOI] [PubMed] [Google Scholar]
- Foster D, Somogyi A, et al. Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. British Journal of Clinical Pharmacology. 2000;50(5):427–440. doi: 10.1046/j.1365-2125.2000.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Somogyi AA, et al. Population pharmacokinetics of (R)-, (S)- and rac-methadone in methadone maintenance patients. British Journal of Clinical Pharmacology. 2004;57(6):742–55. doi: 10.1111/j.1365-2125.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M. Physiologic Changes in Pregnancy and Their Effect on Drug Disposition. Semin Perinatol. 2001;25(3):120–3. doi: 10.1053/sper.2001.24565. [DOI] [PubMed] [Google Scholar]
- Gerber JG, Rhodes RJ, et al. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16(1):36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- Hallinan R, Crettol S, et al. Cannabis and benzodiazepines as determinants of methadone trough plasma concentration variability in maintenance treatment: a transnational study. European Journal of Clinical Pharmacology. 2009;65(11):1113–1120. doi: 10.1007/s00228-009-0706-8. [DOI] [PubMed] [Google Scholar]
- Hirt D, Treluyer J, et al. Pregnancy-related effects on nelfinavir-M8 pharmacokinetics: a population study with 133 women. Antimicrob Agents Chemother. 2006;50(6):2079–86. doi: 10.1128/AAC.01596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson LM, Choo R, et al. Methadone maintenance and long-term lactation. Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine. 2008;3(1):34–7. doi: 10.1089/bfm.2007.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson LM, Dipietro JA, et al. Maternal methadone dosing schedule and fetal neurobehaviour. Journal of Maternal-Fetal & Neonatal Medicine. 2009;22(1):29–35. doi: 10.1080/14767050802452291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H, Johnson R, et al. Dosing adjustments in postpartum patients maintained on buprenorphine or methadone. Journal of Addiction Medicine. 2008;2(2):103–107. doi: 10.1097/ADM.0b013e31815ca2c6. [DOI] [PubMed] [Google Scholar]
- Kharasch E, Hoffer C, et al. Role of hepatic and Intestinal Cytochrome P450 3A and 2B in the metabolism, disposition and miotic effects of methadone. Clin Parmacol Ther. 2004;76:250–269. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Hoffer C, et al. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport. Anesthesiology. 2009;110:660–72. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen K, Blemmer T, et al. Stereoselective pharmacokinetics of methadone in chronic pain patients. Therapeutic Drug Monitoring. 1996;18(3):221–7. doi: 10.1097/00007691-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Kristensen K, Christensen CB, et al. The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sciences. 1995;56(2):PL45–50. doi: 10.1016/0024-3205(94)00426-s. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Statement Effective medical treatment of opiate addiction. 1997;15(6):1–38. [Google Scholar]
- Pond SM, Kreek MJ, et al. Altered methadone pharmacokinetics in methadone-maintained pregnant women. Journal of Pharmacology & Experimental Therapeutics. 1985;233(1):1–6. [PubMed] [Google Scholar]
- Robson S, Mutch E, et al. Apparent liver blood flow during pregnancy: a serial study using indocyanine green clearance. Brit J Obstet Gynaeco. 1990;97:720–4. doi: 10.1111/j.1471-0528.1990.tb16246.x. [DOI] [PubMed] [Google Scholar]
- The Academy of Breastfeeding Medicine Protocol Committee. Jansson LM. ABM Clinical Protocol #21: Guidelines for Breastfeeding and the Drug-Dependent Woman. Breastfeeding Medicine. 2009;4(4):225–8. doi: 10.1089/bfm.2009.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson AH, Li H, et al. Methadone Induces the Expression of Hepatic Drug-Metabolizing Enzymes through the Activation of Pregnane X Receptor and Constitutive Androstane Receptor. Drug Metabolism and Disposition. 2009;37(9):1887–1894. doi: 10.1124/dmd.109.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomedi L, Bogen D, et al. A Pilot Study of the Nutritional Status of Opiate Abusing Pregnant Women on Methadone Maintenance Therapy. Substance Use and Misuse. 2012;47(3):286–295. doi: 10.3109/10826084.2011.635324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah RA, Sheffels P, et al. Role of CYP2B6 in Stereoselective Human Methadone Metabolism. Anesthesiology. 2008;108:363–74. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- Tracy TS, Venkataramanan R, et al. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. American journal of obstetrics and gynecology. 2005;192(2):633–9. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- van Heeswijk R, Khaliq Y, et al. The pharmacokinetics of nelfinavir and M8 during pregnancy and postpartum. Clinical Pharmacology and Therapeutics. 2004;766:588–97. doi: 10.1016/j.clpt.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Wesson D, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003 Apr-Jun;35(2):253–9. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, et al. Effects of the postpartum period on nortriptyline pharmacokinetics. Psychopharmacology Bulletin. 1997;33(2):243–8. [PubMed] [Google Scholar]