Abstract
Rationale
Activin receptor-Like Kinase-1 (ALK1) is an endothelial TGF-β receptor involved in angiogenesis. ALK1 expression is high in the embryo vasculature, becoming less detectable in the quiescent endothelium of adult stages. However, ALK1 expression becomes rapidly increased after angiogenic stimuli such as vascular injury.
Objective
To characterize the molecular mechanisms underlying the regulation of ALK1 upon vascular injury.
Methods and Results
Alk1 becomes strongly upregulated in endothelial (EC) and vascular smooth muscle cells (vSMC) of mouse femoral arteries after wire-induced endothelial denudation. In vitro, denudation of monolayers of Human Umbilical Vein Endothelial Cells (HUVEC) also leads to an increase in ALK1. Interestingly, a key factor in tissue remodeling, Krüppel-like factor 6 (KLF6), translocates to the cell nucleus during wound healing, concomitantly with an increase in the ALK1 gene transcriptional rate. KLF6 knock down in HUVECs promotes ALK1 mRNA downregulation. Moreover, Klf6+/− mice have lower levels of Alk1 in their vasculature compared with their wild type siblings. Chromatin immunoprecipitation assays show that KLF6 interacts with ALK1 promoter in ECs, and this interaction is enhanced during wound healing. We demonstrate that KLF6 is transactivating ALK1 gene, and this transactivation occurs by a synergistic cooperative mechanism with Sp1. Finally, Alk1 levels in vSMCs are not directly upregulated in response to damage, but in response to soluble factors, such as IL-6, released from ECs after injury.
Conclusions
ALK1 is upregulated in ECs during vascular injury by a synergistic cooperative mechanism between KLF6 and Sp1, and in vSMCs by an EC-vSMC paracrine communication during vascular remodeling.
Keywords: ALK1, KLF6, endothelial cell, vascular injury, smooth muscle cell, remodeling, cell culture, vascular biology
INTRODUCTION
Endothelial integrity is essential to regulate many aspects of vascular biology, including angiogenesis, inflammation, vasoconstriction, vasodilation, control of the blood pressure, blood clotting and barrier function. The consequences of endothelial injury have strengthened the concept of endothelium as an organ.1,2 The impairment of the endothelial integrity leads to prothrombotic phenomena, aberrant angiogenesis, the loss of endothelial-selective permeability to leukocytes, and inflammatory processes.3-6 All these consequences of endothelial dysfunction are associated with a range of diseases such as sepsis, haemolytic uremic syndrome, thrombotic thrombocytopenic purpura, diabetes and hypertension. Thus, the study of the regulatory mechanisms involved in vascular remodeling is a crucial step in the search of targets for therapy.
Upon vascular injury, a coordinated gene expression program is triggered among those genes coding for extracellular matrix proteins, growth factors, receptors and proteases.7,8 One of these classes of proteins is the transforming growth factor-β (TGF-β) family, which includes TGF-β, activins and bone morphogenetic proteins (BMPs)9. Several lines of evidence support a pivotal role for TGF-β in response to injury: i) TGF-β expression is upregulated after injury;8,10,11 ii) infusion of TGF-β polypeptide or transfection of TGF-β cDNA into injured arteries increases extracellular matrix production associated with the repair process;12 iii) antibodies against TGF-β1 suppress intimal hyperplasia;13 iv) radiation-mediated vascular injury causes a rapid and persistent increase in expression of TGF-β receptors and mediators;14 v) BMP-9 is involved in postnatal retinal vascular remodeling15; and vi) TGF-β has a role in intimal thickening after vascular injury.16,17 Moreover, increased TGF-β1 activity underlies the wound-healing response in liver,18,19 kidney, lung12 and vascular tissue.20,21
TGF-β family members exert their effects via type I (TβRI) and type II (TβRII) serine/threonine kinase receptors, helped by the co-receptors (TβRIII) and transduce the signal from the membrane to the nucleus through the intracellular Smad factors.9 In endothelial cells (ECs), the TGF-β signaling acquires an important level of regulation due to the coexistence of two different type I receptors, the ubiquitous Activin receptor-like kinase 5 (ALK5), and ALK1.22 ALK1 is highly expressed in the vascular structures of the embryo,23-26 and it is essential during vascular development as demonstrated by the lethality of the ALK1/Activin A receptor type II-like 1 (ACVRL1) gene disruption. The Alk1 knock out embryos die at E10.5, due to the absence of mature blood vessels in the yolk sac, showing aberrant hyperdilated vascular structures and clumps of blood cells.27,28 Moreover, the heterozygous mutation of ALK1 results in a vascular dysplasia called Hereditary Haemorrhagic Telangiectasia type 2 (HHT2), characterized by skin and mucosa telangiectases as well as liver and lung arteriovenous malformations (AVMs).29,30 Despite of the essential role exerted by ALK1 in the vasculogenic process during embryonic development, its expression is diminished in the quiescent endothelium during adult life24. The activation of the endothelial cell ALK1 expression is crucially upregulated in certain locations in response to several angiogenic stimuli.24,31
Krüppel-like factor 6 (KLF6) is a transcriptional regulator which mediates cellular differentiation and tissue development through its roles in growth-related signal transduction pathway, cell proliferation, apoptosis and angiogenesis.32-34 KLF6 is considered as a damage-response factor that promotes tissue remodeling due to its ability of transactivating several target genes by direct binding to their promoters.19,35 These genes comprise several members of the TGF-β signaling pathway such as TGF-β1, its receptors TβRI (ALK5) and TβRII,36 the co-receptor Endoglin (ENG),37uPA (urokinase-type plasminogen activator)38 and Col1A (collagen 1A).35 Furthermore, we have recently described a specific functional relationship between KLF6 and TGF-β pathway by the direct formation of a ternary Smad3-Sp1-KLF6 complex.39 These effects suggest that KLF6 is a common regulatory factor for all the TGF-β functions related to injury, so KLF6 seems to orchestrate the repair mechanisms in order to return the endothelium to its regular state and to avoid the complications derived of its dysfunction.40 In this article, we have explored the regulation of ALK1 expression under vascular injury. Our results demonstrate the transactivation of ALK1 gene by KLF6 and as a consequence the ALK1 upregulation in the migrating ECs. These data provide new insights in the molecular mechanisms mediated by KLF6 for the coordination of the vascular remodeling process and provide additional evidences for a pivotal role of ALK1 in the activated state of the endothelial cell during the angiogenic response after vascular injury.
METHODS
Cell culture37,41, expression vectors,35,42,43 transfection and reporter assays,41 stable infection of primary endothelial cell cultures,44 real time PCR,41in vitro endothelial cell denudation,37 immunofluorescence microscopy37, flow cytometry,37 immunohistochemistry, mechanical injury model in mouse femoral arteries,45,46 laser microdissection (LMD) and chromatin immunoprecipitation41 are described in an expanded manner in material and methods section of the supplementary data.
RESULTS
Alk1 expression is increased in vivo after endothelial injury
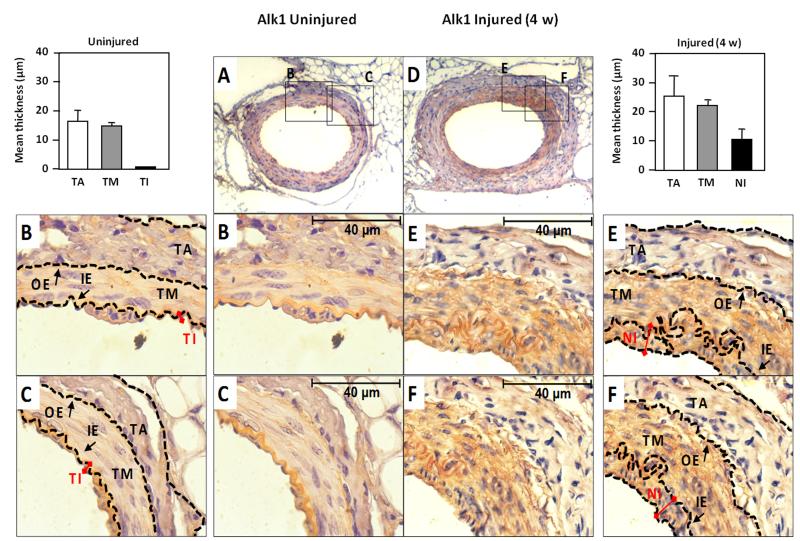
To assess the effect of vascular injury in vivo on Alk1 expression, we used a model of wire-induced endothelial injury in mouse. Mice were subjected to endothelial mechanical injury by using an angioplasty guidewire that removes the tunica intima (TI) of the hindlimb femoral artery. Then, the Alk1 expression levels post-injury were examined by immunohistochemistry after 4 weeks, when the proliferative response to arterial injury was prominent.45,46 At day 28, a clear hyperplasia of the neointima, the new layer formed from inner elastic lamina, was detectable in the wounded area, as shown in Fig. 1. Alk1 expression was restricted to the endothelial single monolayer in uninjured femoral arteries. However, after injury, the hyperplasia was associated with a marked upregulation of Alk1 levels in the neointima (NI), and tunica media (TM), which is composed mainly by vascular smooth muscle cells (vSMC). These results suggest a potential active role for Alk1 during vascular remodeling after an acute injury, in concordance with previous findings of the involvement of TGF-β pathway in the formation of the neointima.16
Figure 1. Alk1 expression is upregulated in vivo in neointima of mouse femoral artery after endothelial injury.
A, D. Immunohistochemical staining of Alk1 in mouse femoral artery in (A) control, and (D) after endothelial injury (28 days). Pictures were taken at 25X magnification. B, C, E and F. Zoom (100X) of different areas of the vessel wall in each case. The outer (OE) and inner (IE) elastica laminae, divide the vessel wall into the three regions: tunica adventitia (TA), tunica media (TM) and tunica intima (TI) as indicated. In the injured vessel wall, the TI has been replaced for a hyperplasic neointima (NI), where Alk1 expression is highly increased. The thickness of both intima layers are indicated with red connectors. The mean thickness of each layer in both control and injured arteries was measured and the data are represented in the histograms.
KLF6 translocates to the nucleus prior to ALK1 upregulation in wounded endothelial cells
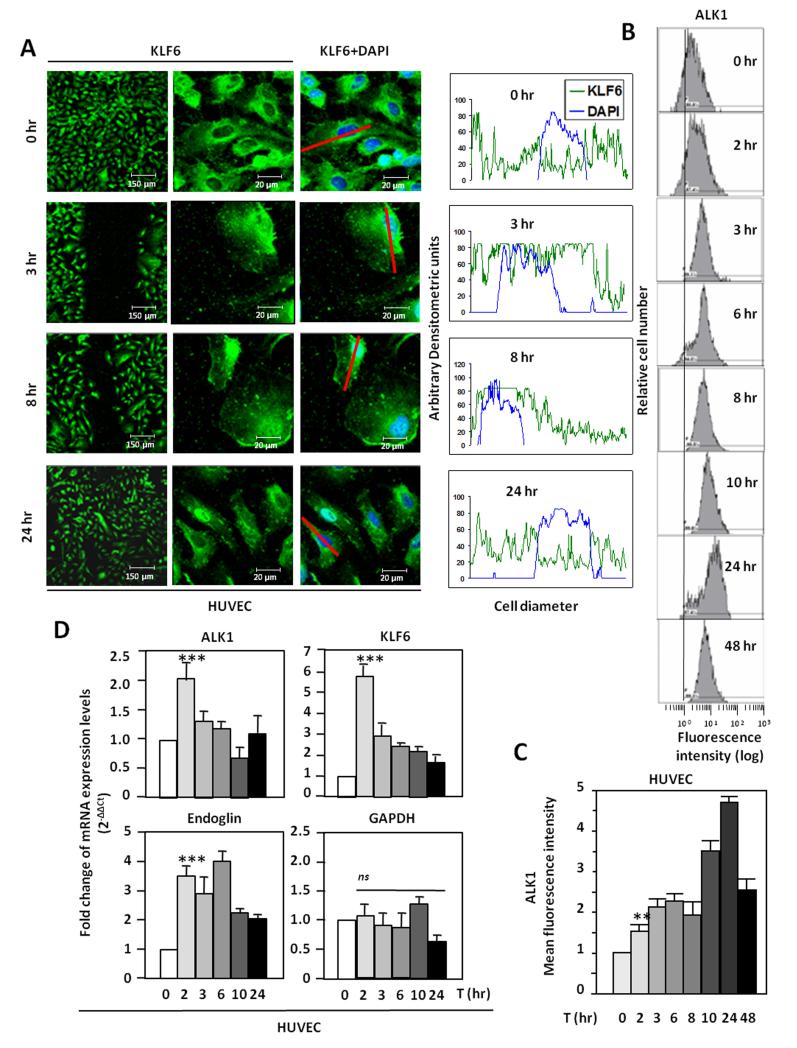
The wounding effect on Alk1 expression was next assessed using a model of endothelial injury in vitro. To this end, monolayers of Human Umbilical Vein Endothelial Cells (HUVECs) were subjected to in vitro denudation and ALK1 levels were measured by flow cytometry. As expected, ALK1 levels were upregulated 2-3 hr after injury, reaching a 5 fold-increase after 24 hr (Fig. 2B). To unravel the molecular mechanism underlying the ALK1 upregulation after wounding, KLF6, an early damage response factor was studied. After 3 hours, KLF6 translocation to the nucleus was observed by fluorescence microscopy (Fig. 2A). The translocation is restricted to cells adjacent to the wound, within 300 μm, and decreases in more distant areas (Supplementary Fig. I). The nuclear localization of KLF6 is still evident after 8 hours, but at 24 hours, KLF6 has been shuttled back to the cytoplasm. In the same experiment, ALK1 surface levels underwent a time-dependent increase that peaked at 24 hours (5-fold upregulation), as measured by flow cytometry (Fig. 2B and 2C). Moreover, the expression of ALK1 and KLF6 transcripts was analyzed by real time PCR using total RNA from denuded HUVEC monolayers (Fig. 2D). KLF6 mRNA levels were markedly upregulated achieving a maximum of 6-fold induction after 2 hours and these levels were slowly decreased reaching basal levels after 24 hours. A similar profile was observed with ALK1, whose mRNA levels were upregulated after 2 hours, returning to basal levels after 10 hours. This kinetics of ALK1 mRNA induction is compatible with the subsequent increase in ALK1 protein levels shown at the cell surface (after 12-24 hours). As a positive control, KLF6-responsive endoglin37 showed a similar upregulation. As negative control, the levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were unaffected. Overall, these results demonstrate that upon in vitro denudation of endothelial monolayers, KLF6 induction precedes ALK1 upregulation in ECs, and this kinetics is compatible with the transcriptional regulation of ALK1 by KLF6.
Figure 2. ALK1 expression is upregulated in vitro in endothelial cells after injury.
A. Left, HUVECs were wounded in vitro and the intracellular location of KLF6 was tracked by immunofluorescence. Right, measurements of KLF6 (green) and DAPI nuclear staining (blue) along a longitudinal section of a representative cell (red line) of each condition. Fluorescence intensities were measured and represented in histograms using Image J™ software tool. Cellular distributions of both signals at distinct time points (0, 3, 8 and 24 hr) are shown. B and C. ALK1 protein levels in the surface of HUVECs from A were analyzed by flow cytometry. The time-dependent increase of the ALK1 mean fluorescence intensity is shown in C. D. Real time RT-PCR analysis of ALK1, KLF6, endoglin and GAPDH mRNA levels in HUVECs at different time points after the in vitro denudation.
In vivo suppression of Klf6 leads to Alk1 downregulation
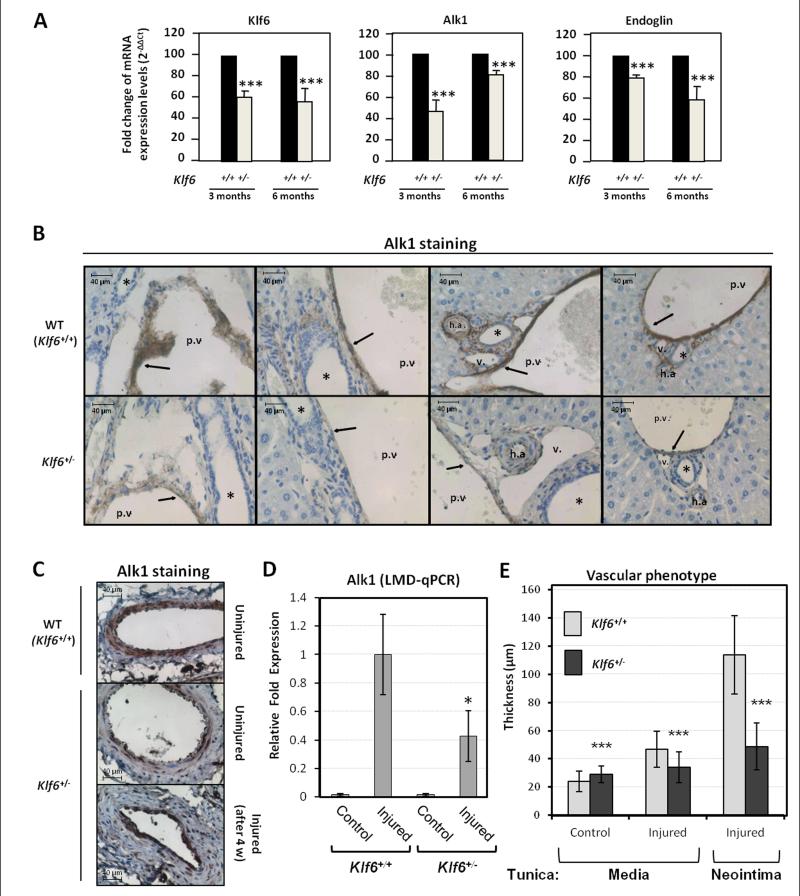
To assess the effect of Klf6 suppression in an in vivo model, Alk1 expression was studied in the liver of heterozygous Klf6+/− mice. This organ was selected because HHT2 patients present a high prevalence of hepatic AVMs.30 Total liver RNA from either Klf6+/+ or Klf6+/− mice was analyzed by real time RT-PCR (Fig. 3A). Levels of Klf6 mRNA in both 3-months and 6-months-old heterozygous mice were clearly diminished (~60% compared to control values) respect to their wild type siblings. Interestingly, basal levels of Alk1 mRNA in Klf6+/− heterozygous mice were expressed at lower levels than in wild type animals. As a positive control, endoglin levels were found to be decreased, although this reduction was more important in 6-months-old mice (Fig. 3A). Further support for the Klf6-dependent regulation of Alk1 was obtained from protein staining in the vessel walls of these mice. Liver sections from Klf6+/− heterozygous and their wild type siblings were paraffin-embedded and analyzed by immunohistochemistry (Fig. 3B). In wild type animals, Alk1 was clearly expressed in the endothelium of liver vessels. By contrast, Alk1 signal was much weaker in Klf6+/− heterozygous mice. Furthermore, Alk1 staining of quiescent endothelium from femoral arteries was found to be lower in Klf6+/− compared to Klf6+/+ mice (Fig. 3C). Also, upon wire-induced endothelial injury, Alk1 protein was induced in both Klf6+/+ and Klf6+/− mice (Fig. 3C), but laser microdissection studies showed that the upregulated Alk1 mRNA levels in Klf6+/− were lower than in Klf6+/+ animals (Fig. 3D). In addition, a distinct vascular phenotype was observed (Fig. 3C,E). Thus, while the increase in the tunica media thickness was ~2-fold in WT mice, only a slight augmentation (1.2-fold) was observed in Klf6+/− mice. Remarkably, upon injury, the neointima of Klf6+/+ mice was more than two-fold thicker than that of Klf6+/− animals. Taken together, these results agree with the crucial role of Alk1 in vascular remodeling and strongly support the involvement of Klf6 in the regulation of Alk1 gene expression.
Figure 3. Klf6+/− heterozygous mice express lower levels of Alk1 in both basal condition and after endothelial injury.
A. Real time RT-PCR of Alk1, Klf6 and endoglin levels from total liver mRNA of Klf6+/− heterozygous mice (3 and 6 months-old) compared to their wild type siblings. B. Immunohistochemistry of Alk1 in hepatic vasculature of Klf6+/− and Klf6+/+ mice livers. Arrows highlight the Alk1 staining in ECs. The asterisks indicate the bile ducts. h.a, hepatic artery; p.v, portal vein; v, vein. C. Immunohistochemical staining of Alk1 protein in 4 weeks-injured femoral arteries from Klf6+/− mice in comparison with wild type littermates. D. Quantification of Ak1 mRNA by qPCR using laser microscopy microdissection (LMD) from tissue sections of femoral arteries. E. Measurement of the tunica media and neointima 4 weeks post-injury. Each value represents the mean of at least 75 different measurements.
ALK1 gene is a transcriptional target of KLF6
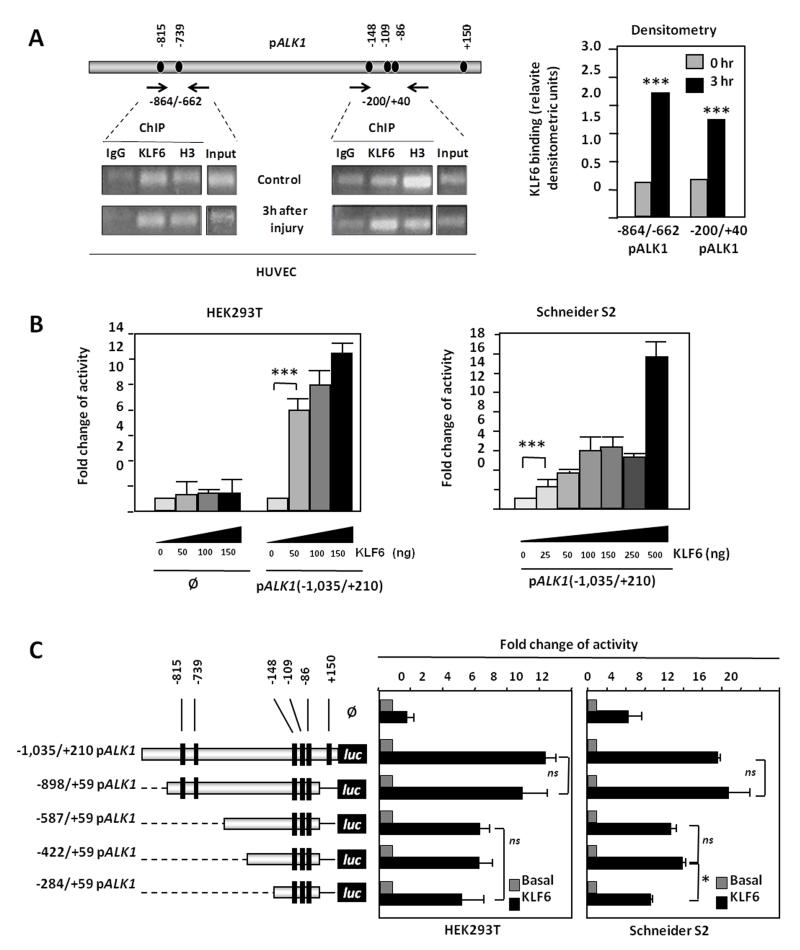
The experiments shown above suggest a transcriptional regulation of ALK1 by KLF6. Supporting this view, various consensus motifs for KLF6 were identified in the −1,035/+210 fragment of the ALK1 promoter, at positions −815, −739, −148, −109, −86 and +150 (Fig. 4A). The physical interaction between KLF6 and the ALK1 promoter was examined by chromatin immunoprecipitation (ChIP). HUVEC monolayers were subjected to endothelial denudation and ChIP experiments were assayed using an anti-KLF6 antibody both in control situation and after 3 hours of endothelial denudation. KLF6-immunoprecipitated chromatin was subjected to PCR using two different couples of primers, encompassing the two clusters of KLF6 motifs present in the ALK1 promoter sequence. As shown in Fig. 4A, KLF6 binding to ALK1 promoter was detected in both amplified fragments (−872/−670 and −208/+38) under basal conditions. Moreover, upon endothelial wounding, the binding of KLF6 to ALK1 promoter was enhanced, as shown in the densitometric analysis (Fig. 4A). This increase was especially evident in fragment −208/+38. These results indicate that, at least one KLF6 motif, within each cluster, is bound to KLF6 in vivo.
Figure 4. KLF6 transactivates ALK1 promoter.
A. KLF6 interacts with ALK1 promoter in HUVECs and this interaction is increased after vascular injury. Left, chromatin immunoprecipitation (ChIP) of KLF6 over the two main KLF6-sites rich regions of the ALK1 5′-proximal promoter in HUVECs. Right, KLF6 binding was measured by densitometry of the individual bands and values of the (KLF6-IgG)/Input ratios were represented in both the control situation and 3 hr after the endothelial denudation B. Dose-response effect of KLF6 on the transcriptional activity of ALK1 promoter in HEK293T cells (left) and Drosophila Schneider S2 cells (right). C. Effect of KLF6 on the transcriptional activity of 5′deleted constructs of ALK1 promoter. Left, a scheme of the different ALK1 promoter constructs shows the distribution of the KLF6 consensus binding sites found along the ALK1 promoter (black boxes). Right, transient co-transfection of KLF6 expression vector with different 5′deleted constructs of ALK1 promoter in both HEK293T and Schneider S2 cells.
To assess the effect of this interaction, transcriptional experiments using ALK1 promoter (pALK1) constructs were performed. Transient transfections of the pGL2-pALK1(−1,035/+210) reporter vector with increasing doses of KLF6 resulted in a marked activation (up to 13-fold) of ALK1 promoter activity in HEK293T cells (Fig. 4B). Moreover, using a KLF6-free cellular model such as Drosophila Schneider S2 cell line (S2), a similar activation (up to 16-fold) of ALK1 promoter activity was obtained. Overall, these results show KLF6 binding to, and transactivation of, the ALK1 promoter. To assess the contribution of the different KLF6 motifs to ALK1 transcription, a set of deletion constructs of the ALK1 promoter were analyzed in HEK293T and S2 cells (Fig. 4C). In both cell lines, the highest KLF6-induced response was obtained with the two largest constructs (−1,035/+210 and −898/+50) containing 6 and 5 KLF6 binding motifs, respectively. The rest of the constructs (−587/+59, −422/+59 and −284/+59), all of them containing 3 KLF6 motifs, showed a proportional reduction in the KLF6-dependent transcriptional activity (~50%) respect to the largest constructs. These results demonstrate that KLF6 is able to interact with the −1,035/+210 fragment of ALK1 promoter, stimulating its expression.
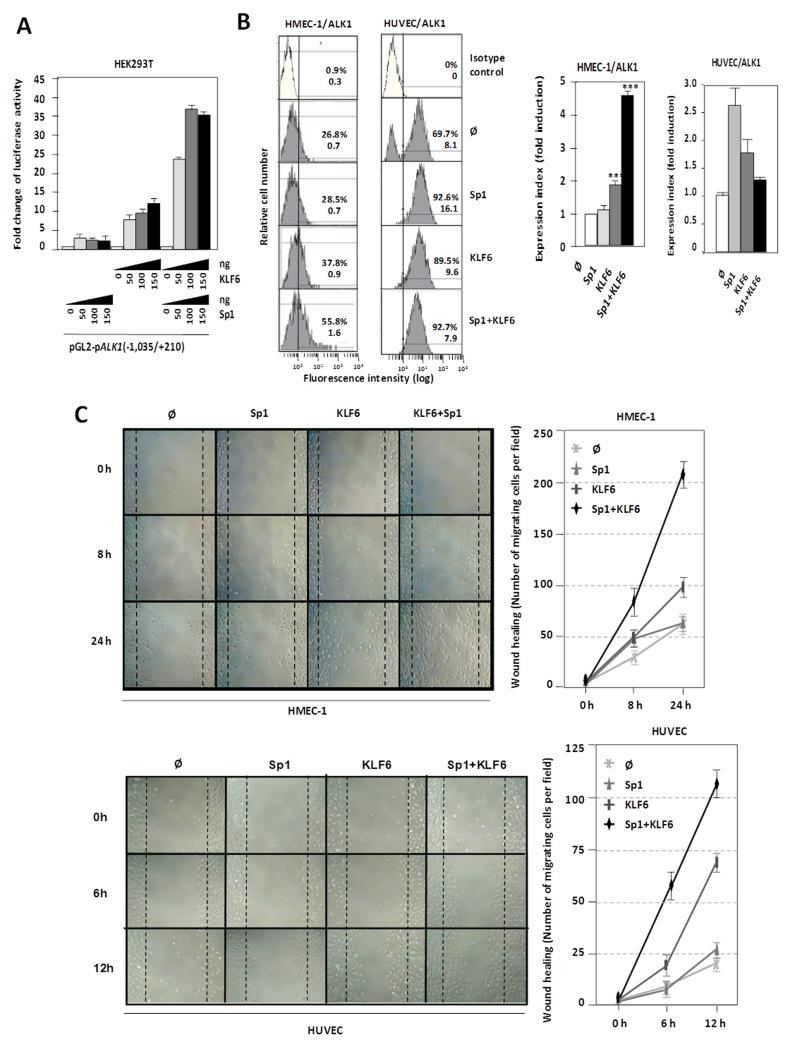
KLF6 upregulates ALK1 expression through a synergistic cooperation with Sp1
Based on our previous work, where we demonstrated that ALK1 gene is Sp1-dependent for transcription initiation41, the transactivator effect of KLF6 on the ALK1 promoter segment −1,035/+210 was assessed in the absence or presence of Sp1 in HEK293T cells. As expected,41 Sp1 overexpression induced (~4-fold) the transcriptional activity of the ALK1 promoter construct (Fig. 5A). Similarly, overexpression of KLF6 achieved a 12-fold transactivating effect over the basal transcription rate of the ALK1 promoter. Interestingly, simultaneous overexpression of KLF6 and Sp1 allowed a maximum transactivating effect of ~38-fold. Comparing the individual effects of Sp1 and KLF6 with the combined effect, it is obvious that the overexpression of both proteins leads to a clear synergistic cooperation, where the activating effect is much higher than the simple addition of each transcription factor independently. Next, the effect of both Sp1 and KLF6 overexpression on ALK1 protein levels was monitored by flow cytometry in ECs. As shown in Fig. 5B, overexpression of Sp1 and KLF6, independently upregulated ALK1 protein at the cell surface. Single transfections of these factors in HMECs increased ALK1 expression between 1.2- and 2-fold for Sp1 and KLF6, respectively, whereas cotransfection experiments showed a clear cooperation between Sp1 and KLF6 reaching an upregulation of 4.5-fold. Similarly, single nucleofections of Sp1 and KLF6 in HUVECs increased ALK1 expression 2.6- and 1.7-fold, respectively, although no synergistic effect was detected, probably due to a cytotoxic/apoptotic effect of the combined treatment for 48 hours in these primary cells (data not shown).
Figure 5. Functional cooperation between KLF6 and Sp1 in ALK1 transactivation.
A. Luciferase activity of the pALK1 (−1,035/+210) reporter in HEK293T cells transiently transfected with the indicated amounts of KLF6 and Sp1 expression vectors after 48hr. B. Left, flow cytometry of ALK1 protein levels at the surface of HMEC-1 and HUVEC cells 48hr after transfection with Sp1 and KLF6, as indicated. Right, quantification of expression index (fluorescence intensity normalized by number of positive cells) in each case is represented in the histograms. C. Wound healing experiments in HMEC-1 and HUVEC cells overexpressing Sp1 and KLF6, as indicated. After endothelial disruption, cells overexpressing both Sp1 and KLF6 close the wound much faster than control cells or those overexpressing only Sp1 or KLF6. Right, quantification of the healing by measurement of the number of cells that have migrated to close the wound in each case.
Because ALK1 is involved in endothelial cell migration,47-51 a hallmark of activated ECs, we analyzed whether the KLF6-dependent upregulation of the ALK1 protein was associated with a migratory phenotype. The human microvascular endothelial cell line-1 (HMEC-1) was transiently transfected with different combinations of Sp1 and KLF6 and then subjected to a wound healing assay. When both Sp1 and KLF6 are overexpressed in these ECs, their migration capacity was stimulated achieving the closure of the wound, after 24 hours (Fig. 5C). The same type of experiment was performed with primary cultures of HUVECs, previously electroporated with Sp1 and KLF6 expression vectors (Supplementary Figure II), yielding a similar wound healing kinetics (Fig. 5C). The toxicity derived from the Sp1/KLF6 combination in HUVECs was not observed in this case because the migration studies were carried out within a short time frame (12 hours). Taken together, these data demonstrate that ALK1 is a KLF6 target gene and support the hypothesis that KLF6 acts cooperatively with Sp1 in order to promote endothelial activation of ALK1 during vascular remodeling.
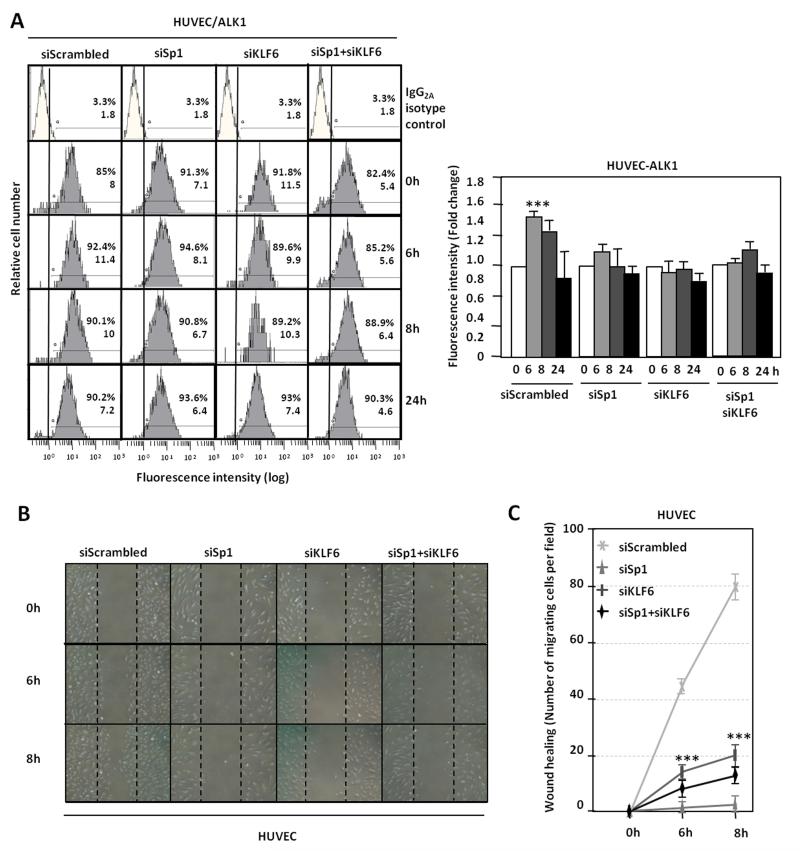
KLF6 and Sp1 knock down prevent ALK1 upregulation during wound healing
The expression of Sp1 and KLF6, independently or in combination, was silenced in HUVECs, during the wounding process by transfection of specific siRNAs (Supplementary Fig. III). Fig. 6A shows that silencing of Sp1, KLF6 or both resulted in the abolishment of the wound-mediated ALK1 induction as determined by flow cytometry analysis. When cellular migration was measured, as a function depending on ALK1 expression, in the presence of siRNA of Sp1, KLF6 or both, HUVECs were unable to close the disrupted monolayer (Fig. 6B) as compared to a correct healing when silencing was made with control scrambled siRNA. These changes are shown in Fig. 6C, where quantification of cell migration at each point time is expressed as number of migrated cells per field. These data corroborate that KLF6 in conjunction with Sp1, are essential for the upregulation of ALK1 during the angiogenic response to endothelial injury.
Figure 6. KLF6 and Sp1 knock down decrease ALK1 expression and inhibit ALK1 upregulation during wound healing.
A. ALK1 levels on HUVEC surface 72h after transfection with siRNA Scrambled or siRNA specific for Sp1, KLF6 or both, at different time points of endothelial denudation in vitro, measured by flow cytometry. Right, representation of fold change in fluorescence intensity by bar histograms. B. Wound healing experiments in HUVEC cells after Sp1 and KLF6 knock down. C. quantification of the healing by measurement of the number of cells migrated to close the wound in each condition.
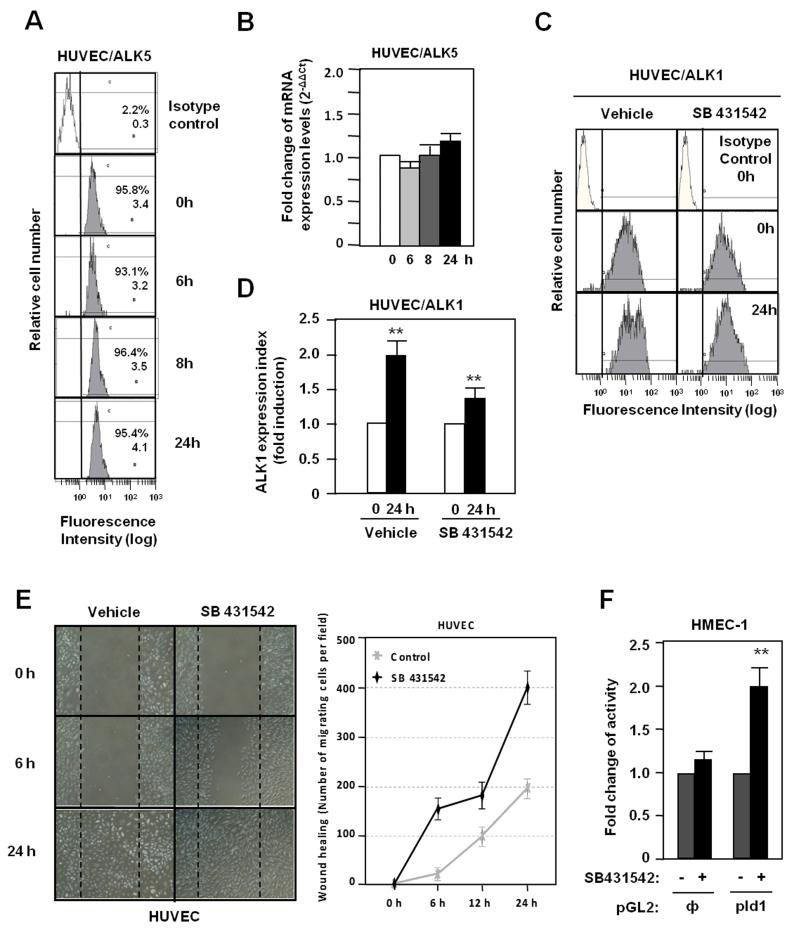
ALK5 is not implicated in ALK1 upregulation induced by vascular injury
In ECs there are two TGF-β type I receptors, the ubiquitous ALK5 and the endothelial ALK1. Because a cross-talk between ALK1 and ALK5 has been postulated,47,50,52 the possible role of ALK5 in the wound-induced ALK1 expression was assessed by measuring changes in ALK5 levels and activity. As shown in Fig. 7A, ALK5 surface protein levels remained unchanged after wound healing and the same was true for the corresponding mRNA levels (Fig. 7B). To analyze the influence of ALK5 signal, ECs were treated with SB431542, a specific ALK5 kinase inhibitor. In spite of the suppression of ALK5 signaling, ALK1 expression was upregulated upon wound healing, although at a slightly lower extent than cells treated with vehicle (Fig. 7C,D). Upon scratching endothelial monolayers, the migration of cells is stimulated when the ALK5 pathway is inhibited (Fig. 7E), whereas the ALK5 inhibitor stimulated the promoter activity of the Id1 target gene in ECs (Fig. 7F). The efficiency of SB431542 treatment is demonstrated in HUVECs by the decrease in mRNA levels of PAI-1, a target gene of ALK5 (Supplementary Fig. IV). These results suggest that while wound-mediated upregulation of ALK1 is mostly independent of the ALK5 signaling pathway, inhibition of ALK5 activity favours signaling through ALK1, as shown by the stimulation of cell migration and activation of the Id1 target.
Figure 7. Role of ALK5 signaling in the wounding-induced ALK1 expression.
A. ALK5 expression levels in HUVEC surface after wound healing measured by flow cytometry. B. ALK5 mRNA levels after wound healing measured by real time PCR. C. ALK1 expression in HUVEC surface after wound healing in absence or presence of the ALK5 kinase inhibitor SB431542, measured by flow cytometry. D. Histogram of ALK1 expression index from panel C. E. Wound healing assay of HUVECs treated or not with SB431542. Right, quantification of the endothelial migration expressed as number of migrating cells per field. F. Activity of Id1 promoter after treatment with SB431542 in HMEC-1 cells previously transfected with the PGL2 reporter containing the Id1 promoter (pId1);, empty PGL2.
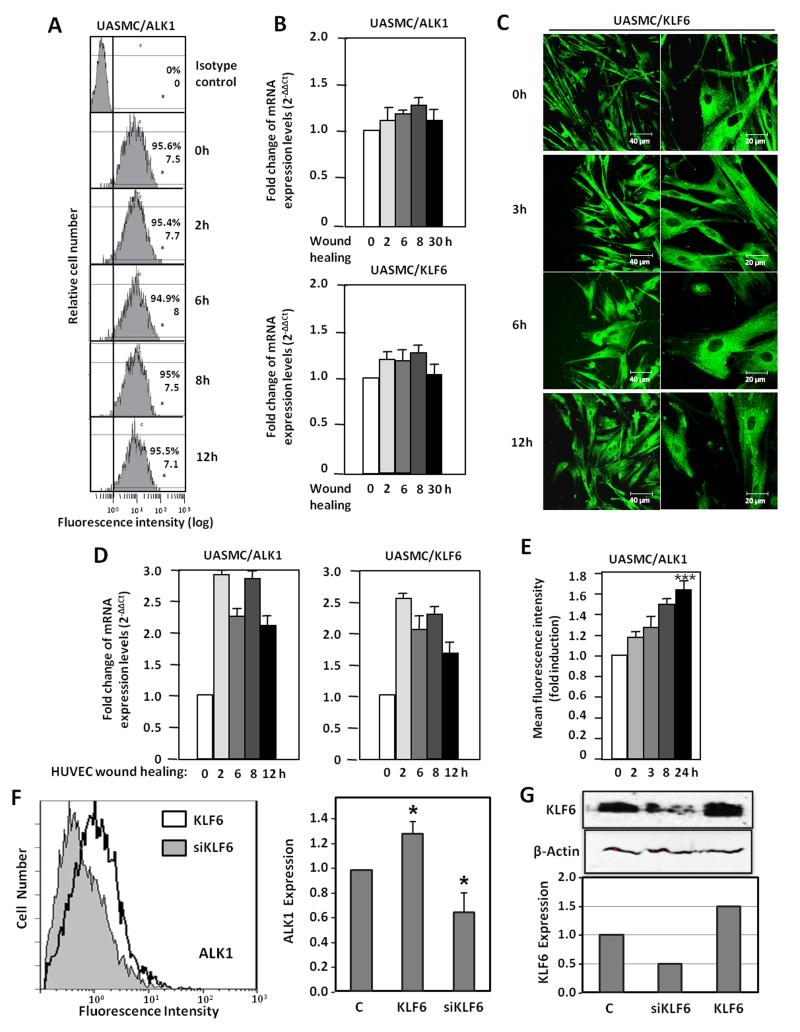
ALK1 is upregulated in smooth muscle cells after wounding through a paracrine communication with endotelial cells
As seen in Fig. 1, endothelial injury induces ALK1 upregulation in cells of the tunica media, suggesting the involvement of vSMC. As vSMCs are in close contact with ECs, we have explored the wounding effect in vSMCs. For this purpose, primary cultures of umbilical artery smooth muscle cells (UASMCs) were subjected to a scratching process and the surface levels of ALK1 were measured by flow cytometry. As observed in Fig 8A, UASMCs express high levels of ALK1 at their surface, but these levels remain unchanged along the wound healing process at variance with the increase of ALK1 in ECs after wounding. Accordingly, levels of ALK1 mRNA were unaffected (Fig. 8B); the same was true for KLF6, as opposed to the increased levels of KLF6 observed in ECs after wound-healing (Fig. 2B,C,D). Staining of KLF6 in UASMC at different times after wound healing, did not reveal any nuclear translocation, either (Fig. 8C), at variance to ECs, where nuclear translocation is evident after 3h (Fig. 2A). Interestingly, when UASMCs were cultured with conditioned media from wounded-monolayers of ECs, a significant increase in ALK1 was evident at mRNA and protein levels, already after 2 hours (Fig. 8D,E). Furthermore, KLF6 mRNA levels were increased in parallel with ALK1 mRNA in UASMCs grown in conditioned media from ECs subjected to wound-healing. The increase in ALK1 protein was sustained up to 24 hours (Fig. 8E). Next, we assessed the contribution of the wound healing-dependent induction of KLF6 in ECs to the upregulated expression of ALK1 in UASMCs. KLF6 was overexpressed (using pCiNeo-KLF6) or suppressed (using pSuperKLF6) in HUVECs and the corresponding conditioned media were used to culture vSMCs. As shown in Fig 8F, culture media from KLF6 overexpressing HUVECs induced a modest, but significant increase (1.3-fold), in the expression levels of ALK1 of UASMCs. By contrast, culture media from HUVECs with a knocked down expression of KLF6 induced a marked reduction of ALK1 levels (0.6-fold) compared to mock treated cells. Of note, changes in ALK1 levels paralleled those of KLF6 in vSMCs (Fig. 8G). In summary, these results suggest that ALK1 is not directly upregulated in vSMCs in response to injury. Instead, endothelial injury triggers indirectly a similar KLF6-dependent stimulation of ALK1, in vSMCs. This response would constitute a paracrine mechanism operating from endothelium (intima) to smooth muscle cells (tunica media). In an attempt to analyse candidates responsible for this paracrine response, several cytokines and growth factors involved in angiogenesis were analyzed by ELISA in conditioned media from ECs following injury. The results showed a sustained increase of interleukin 6 (IL-6) in wounded cultures of HUVECs (from 6 to 24 h after wounding; Supplementary Fig. VI), suggesting the involvement of this cytokine in the upregulated expression of ALK1 in vSMCs after endothelial injury. To investigate the putative regulation of IL-6 by KLF6, immunohistochemistry staining of IL-6 in 4 weeks-injured femoral arteries from Klf6+/− mice in comparison with wild type littermates was carried out (Supplementary Fig. VIIA). In uninjured vessels from wild type and heterozygous animals the presence of IL-6 was almost undetectable. By contrast, IL-6 staining was clearly detected upon wire injury in different layers of the femoral artery. However, the increased signal of IL-6 in wild type mice was higher than that of Klf6+/− littermates, suggesting that KLF6 regulates IL-6 gene expression. Supporting this view, several putative consensus motifs for KLF6 were identified upon in silico analysis of both human and mouse IL-6 proximal promoter sequences (Supplementary Fig. VIII). Furthermore, ectopic expression of KLF6 stimulated more than 3-fold the promoter activity of a luciferase reporter construct driven by the human IL-6 promoter sequence (Supplementary Fig. VIIB). Taken together these experiments suggest that IL-6 is a target gene of KLF6. It can be speculated that KLF6 induction upon vascular injury modulates the expression of a set of target genes, including IL-6, which in turn upregulate ALK1. An in-depth analysis of these genes may shed light on the molecular mechanism triggered to achieve vascular repair.
Figure 8. Paracrine effect of HUVEC denudation on ALK1 expression in vascular SMCs.
A. ALK1 expression in the surface of vSMCs after denudation in vitro, measured by flow cytometry. B. Real time RT-PCR of ALK1 and KLF6 after vSMC denudation. C. Immunofluorescent staining of KLF6 in vSMCs during wound healing. D,E. Effect of conditioned media from HUVEC subjected to denudation at different time points on vSMC treated overnight. D. Real time RT-PCR of ALK1 and KLF6 transcripts. E. ALK1 expression measured by flow cytometry and represented as fold induction. F,G. Effect of conditioned media from HUVEC subjected to KLF6 overexpression (pCiNeoKLF6; KLF6), KLF6 suppression (pSuper-siKLF6; siKLF6) or mock transfection (C) on vSMC treated overnight. F. ALK1 expression measured by flow cytometry (left) and represented as fold induction (right). G. Western blot analysis of KLF6 in total cell lysates. The intensity of the KLF6 band relative to β-actin intensity is represented in the histogram. UASMC, umbilical artery smooth muscle cells.
DISCUSSION
ALK1 functions are closely related to vascular biology.28,53,54 During embryogenesis, ALK1 is highly expressed in the vascular bed due to its critical requirement for a correct vasculogenic process, whereas in the adult life, the endothelium reaches quiescence and ALK1 expression levels drop24. In adults, the signaling network triggered by ALK1 is framed in the angiogenic process, when activated endothelial cell, proliferate and migrate, in order to develop new vessels from the preexistent ones, in response to certain stimuli.23,50 After formation of the new sprouts, a resolution phase is needed, in which TGF-β-mediated signaling blocks the proliferation and stabilizes the new vessel by the deposition of extracellular matrix.47,49 Therefore, TGF-β signaling in the EC during angiogenesis is crucial, and needs to be highly regulated to control the balance between activating and resolving signals in each stage of the process. Having this in mind, a vascular damage, would require an immediate ALK1 upregulation for the correct coordination of the subsequent repair mechanisms, while ALK5-controlled pathway, should remain unchanged immediately after injury.
In Hereditary Hemorrhagic Telangiectasia, patients harboring mutations in ALK1 are able to develop normal vessels, and their vascular system is, overall, indistinguisible from that of a healthy subject. However, when angiogenesis is activated in areas exposed to different stimuli, such as inflammation, injury or hypoxia (second hit), ALK1 haploinsufficiency impairs the angiogenic process and may cause vascular lesions (telangiectases and Arteriovenous Malformations-AVMs),55,56 as it is frequently the case of nasal mucosa, where vessels suffer from mechanical injury. Therefore, identification of molecular mediators implicated in the response to vascular injury may provide new insights for understanding the mechanism involved in the formation of telangiectases and AVMs in HHT patients. In addition, this information may be useful to elucidate the molecular basis of vascular embolotherapy, a common method used to treat pulmonary AVMs in HHT patients that involves vascular damage and remodeling induced with coils or balloons.56
Using both in vitro and in vivo models, we demonstrated that ALK1, but not ALK5 levels become strongly upregulated at the surface of ECs after vascular injury. We observed the existence of a temporal relationship between ALK1 upregulation and KLF6 translocation to the nucleus, in an in vitro endothelial wound healing model; a relationship that is compatible with a transcriptional involvement of KLF6 in ALK1 gene expression regulation. Supporting this observation, ALK1 protein and mRNA levels are much lower in liver vasculature and in femoral arteries of Klf6+/− mice than those of wild type animals, and ectopic expression of KLF6 is able to markedly transactivate the ALK1 promoter. Our recent studies on cloning and characterization of ALK1 gene promoter41 prompted us to explore the potential regulation by KLF6 of the ALK1 gene. Based on the in silico analysis of the ALK1 promoter sequence, six putative consensus binding sites for KLF6 were found along the −1,035/+210 pALK1 fragment. The motifs are located at positions −815, −739, −148, −109, −86 and +150. By chromatin immunoprecipitation we show that KLF6 indeed binds to the ALK1 promoter and we demonstrate that, at least, one KLF6 motif is functional, within each one of the two KLF6 clusters located in −872/−670 and −208/+38, respectively. Of note, KLF6 binding to ALK1 promoter is evident under basal conditions, being this interaction increased after vascular injury. Remarkably, three of the KLF6 motifs are surrounding the major transcriptional start site (TSS) (+1) driven by the transcription factor Sp1 through binding to the GC-rich regions in the TATA-less proximal promoter of ALK141. These characteristics emphasize the importance of Sp1 in the basal mechanism of ALK1 transcription and explain the strong synergistic cooperation observed between KLF6 and Sp1 potentiating ALK1 transcriptional activity. Similarly, we have previously demonstrated the direct physical interaction and functional cooperation between Sp1 and KLF6 on the ENG promoter, in response to vascular injury 37. There are also experimental evidences of similar regulatory mechanisms on the expression of other TGF-β family-related genes involved in vascular repair.36,37 Among them are TGF-β1, TβRI/ALK5, TβR-II,36 as well as other important key regulators of the vascular physiology like Col1A35 and uPA.38 Of note, the transcriptional activation of uPA by KLF6 activates latent TGF-β1 in vascular endothelial cells.38 All these genes are intimately involved in endothelial homeostasis. Thus, even though KLF6 is ubiquitously expressed, following endothelium injury its endothelial expression is markedly increased, playing key roles in vascular biology.34
Recently, we described the TGF-β regulation of KLF6 and its splice variants and the cooperative transactivation effect on common target genes through a Smad3/Sp1/KLF6 interaction,39 highlighting the tight relationship between KLF6 and the TGF-β pathway. ALK1 and Endoglin are involved in a common signaling pathway within the TGF-β system,22,55,56 in agreement with the fact that ALK1 and ENG gene mutations result in similar syndromic diseases, HHT2 and HHT1, respectively.56 HHT is characterized by the presence of vascular lesions associated with fragile vessels and impaired vascular remodelling, likely a consequence of a deficient endothelial signaling in response to TGF-β. Indeed, experiments using heterozygous mouse models, alk1+/− or eng+/− have shown that vascular lesions develop upon an angiogenic stimulus, such as vascular injury, due to an inappropriate EC wound-healing response.23,57 In this sense, endoglin cooperates with ALK1 in the proliferating responses of ECs and opposes to ALK5-promoted responses, including growth arrest, differentiation of ECs, recruitment of pericytes and production of extracellular matrix proteins.50,52
The signal(s) that triggers the KLF6 nuclear translocation remains to be elucidated. It can be postulated that the loss of intercellular contacts sustained by VE-cadherins and integrins might be a primary stimulus for KLF6 translocation in ECs affected by the wound. This nuclear translocation would promote an immediate stimulation of KLF6 target genes, including Endoglin, ALK1, and KLF6 itself, to start the repair process. In the context of vascular homeostasis after endothelial damage, crucial players are the vSMCs in close contact with the EC layer, contributing to vessel stabilization and recovery. Alk1 is highly expressed in the vSMC layers surrounding the tunica intima as seen in Fig. 1, and it is especially conspicuous in the process of neointima formation after femoral denudation. At variance with ECs, neither ALK1 upregulation nor KLF6 translocation in cultured vSMCs was observed upon direct wounding. Thus, a cross-talk between endothelial and smooth muscle cell layers appears necessary for a correct homeostasis of the vessel wall. Indeed, in vitro experiments have shown that ALK1 is induced in vSMCs through a paracrine signal from the wounded endothelium. In this regard, the increase of IL-6 along the wounding process of ECs, suggests that this factor is a putative candidate to contribute to the paracrine effect on vSMCs surrounding the endothelium. Thus, the release of soluble factors, as IL-6, from the injured endothelium would serve to expand the repair signal by upregulating ALK1 from the inner to the more distant layers of the vessel. In addition, among the soluble factors secreted in vivo, it would be worth to analyze the levels of BMP-9, a specific ligand for ALK1 and endoglin.58-60 BMP-9 is synthesized by the liver, circulates in human adult blood, is involved in vascular remodeling and induces vascular quiescence.15,61 However, the in vitro effects of BMP-9 on endothelial proliferation and migration are controversial as some reports have described an inhibition, while others have described a stimulation of these functions.58,59,62 Because upon vascular injury, EC proliferation and migration are increased, it will be of interest to find out whether the upregulation of ALK1 and endoglin in this setting is associated with changes in BMP-9 levels. Overall, the parallelism between ALK1 and endoglin in their pathophysiological functions as well as in their regulated gene expression in response to endothelial damage, support their involvement in the TGF-β-dependent events triggered by a vascular injury to recover the endothelial homeostasis. Because haploinsufficiency is the mechanism of pathogenicity in HHT1 and HHT2,56,63 those stimuli able to upregulate the gene expression of ALK1 and ENG constitute therapeutic targets to counteract the haploinsufficiency. In this regard, the characterization of KLF6 as a mediator of vascular injury in the induction of ALK1 and endoglin expression deserves an independent investigation.
Supplementary Material
Novelty and Significance.
What Is Known?
ALK1 is an endothelial TGF-β receptor involved in vascular remodeling and angiogenesis and whose expression is rapidly increased with angiogenic stimuli or upon vascular injury.
The heterozygous deficiency of ALK1 gives rise to Hereditary Hemorrhagic Telangiectasia type 2 (HHT2), characterized by aberrant dilated vessels, and lack of capillary beds in certain areas.
What New Information Does This Article Contribute?
Upon vascular injury, nuclear translocation of the transcription factor Kruppel-like factor 6 (KLF6) activates ALK1 gene transcription.
The mechanism also involves a paracrine signal from endothelial cells that lead to the upregulation of ALK1 in smooth muscle cells.
Endothelial integrity is essential to regulate angiogenesis and vascular remodeling, but the repair mechanisms involved upon endothelial injury are poorly understood. ALK1 is an endothelial receptor whose expression is rapidly increased with angiogenic stimuli or upon vascular injury. In the present study we find that following endothelial injury, KLF6 translocates to the nucleus binding and activating the ALK1 gene promoter in synergy with Sp1 in endothelial cells. In addition, KLF6 translocation results in the release of soluble factors, including IL-6, which act on smooth muscle cells, increasing their ALK1 levels as well. This work demonstrates a key role of KLF6 in ALK1 upregulation after vascular damage both, in vitro and in vivo. These findings enhance our o understanding of the mechanism involved in vascular remodeling upon angiogenic stimuli or after endothelial denudation during embolotherapy of vascular lesions. Thus, KLF6 may be a therapeutic target to counteract ALK1 deficiency in HHT2.
ACKNOWLEDGEMENTS
We thank Drs. Jorge Allina and Zahra Ghiassi-Nejad for their valuable discussions and help, Dr. Manuel Fresno for IL6 reporter plasmid and Lucía Recio and Carmen Langa for excellent technical assistance. The CIBER de Enfermedades Raras is an initiative of the Instituto de Salud Carlos III (ISCIII) of Spain.
SOURCES OF FUNDING
This work was supported by the Ministerio de Ciencia e Innovación of Spain (MICINN grants SAF2007-61827 and SAF2010-19222 to CB and SAF2008-01218 to LMB, and predoctoral fellowship BES-2005-7974 to EMG-M), Genoma España (MEICA), Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), and National Institutes of Health & National Center for Research Resources (DK56621 and DK37340 to SLF).
Non-standard Abbreviations
- ACVRL1
Activin A receptor type II-like 1
- ALK
Activin receptor-like kinase
- ECs
Endothelial cells
- ENG
Endoglin
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HEK293T
Human embryonic kidney 293T cells)
- HHT
Hereditary haemorrhagic telangiectasia
- HMEC-1
Human microvascular endothelial cell line-1
- HUVEC
Human umbilical vein endothelial cells
- IL-6
interleukin 6
- KLF6
Krüppel-like factor 6
- pALK1
ALK1 promoter
- S2
Drosophila Schneider embryonic cells
- Sp1
Specificity protein 1
- TGF-β
Transforming growth factor-β
- UASMC
Umbilical artery smooth muscle cell
- vSMC
vascular smooth muscle cells.
Footnotes
DISCLOSURES
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Goligorsky MS. Endothelial cell dysfunction and nitric oxide synthase. Kidney Int. 2000;58:1360–1376. doi: 10.1046/j.1523-1755.2000.00292.x. [DOI] [PubMed] [Google Scholar]
- 2.Hack CE, Zeerleder S. The endothelium in sepsis: source of and a target for inflammation. Crit Care Med. 2001;29:S21–27. doi: 10.1097/00003246-200107001-00011. [DOI] [PubMed] [Google Scholar]
- 3.Gotlieb AI, Koo EW. Endothelial injury. CMAJ. 1990;142:349. [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 5.Murakami M, Simons M. Regulation of vascular integrity. J Mol Med (Berl) 2009;87:571–582. doi: 10.1007/s00109-009-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajashekhar G, Willuweit A, Patterson CE, Sun P, Hilbig A, Breier G, Helisch A, Clauss M. Continuous endothelial cell activation increases angiogenesis: evidence for the direct role of endothelium linking angiogenesis and inflammation. J Vasc Res. 2006;43:193–204. doi: 10.1159/000090949. [DOI] [PubMed] [Google Scholar]
- 7.Reidy MA, Irvin C, Lindner V. Migration of arterial wall cells. Expression of plasminogen activators and inhibitors in injured rat arteries. Circ Res. 1996;78:405–414. doi: 10.1161/01.res.78.3.405. [DOI] [PubMed] [Google Scholar]
- 8.Wysocki SJ, Zheng MH, Fan Y, Lamawansa MD, House AK, Norman PE. Expression of transforming growth factor-beta1 (TGF-beta1) and urokinase-type plasminogen activator (u-PA) genes during arterial repair in the pig. Cardiovasc Res. 1996;31:28–36. [PubMed] [Google Scholar]
- 9.Santibanez JF, Quintanilla M, Bernabeu C. TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2011;121:233–251. doi: 10.1042/CS20110086. [DOI] [PubMed] [Google Scholar]
- 10.Frank S, Madlener M, Werner S. Transforming growth factors beta1, beta2, and beta3 and their receptors are differentially regulated during normal and impaired wound healing. J Biol Chem. 1996;271:10188–10193. doi: 10.1074/jbc.271.17.10188. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, O’Brien JE, Jr., Fard A, Zalewski A. Transforming growth factor-beta 1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol. 1996;16:1298–1305. doi: 10.1161/01.atv.16.10.1298. [DOI] [PubMed] [Google Scholar]
- 12.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 13.Wolf YG, Rasmussen LM, Ruoslahti E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J Clin Invest. 1994;93:1172–1178. doi: 10.1172/JCI117070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruse JJ, Floot BG, te Poele JA, Russell NS, Stewart FA. Radiation-induced activation of TGF-beta signaling pathways in relation to vascular damage in mouse kidneys. Radiat Res. 2009;171:188–197. doi: 10.1667/RR1526.1. [DOI] [PubMed] [Google Scholar]
- 15.Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan R, Agrotis A, Bobik A. Understanding the role of transforming growth factor-beta1 in intimal thickening after vascular injury. Cardiovasc Res. 2007;74:223–234. doi: 10.1016/j.cardiores.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Yokote K, Kobayashi K, Saito Y. The role of Smad3-dependent TGF-beta signal in vascular response to injury. Trends Cardiovasc Med. 2006;16:240–245. doi: 10.1016/j.tcm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Meyer C, Meindl-Beinker NM, Dooley S. TGF-beta signaling in alcohol induced hepatic injury. Front Biosci. 2010;15:740–749. doi: 10.2741/3643. [DOI] [PubMed] [Google Scholar]
- 19.Lalazar A, Wong L, Yamasaki G, Friedman SL. Early genes induced in hepatic stellate cells during wound healing. Gene. 1997;195:235–243. doi: 10.1016/s0378-1119(97)00159-5. [DOI] [PubMed] [Google Scholar]
- 20.Saltis J, Bobik A. Regulation by protein kinase C of transforming growth factor-beta 1 action on the proliferation of vascular smooth muscle cells from spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1996;23:573–575. doi: 10.1111/j.1440-1681.1996.tb02783.x. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz SM, Majesky MW, Murry CE. The intima: development and monoclonal responses to injury. Atherosclerosis. 1995;118(Suppl):S125–140. [PubMed] [Google Scholar]
- 22.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoud M, Borthwick GM, Hislop AA, Arthur HM. Endoglin and activin receptor-like-kinase 1 are co-expressed in the distal vessels of the lung: implications for two familial vascular dysplasias, HHT and PAH. Lab Invest. 2009;89:15–25. doi: 10.1038/labinvest.2008.112. [DOI] [PubMed] [Google Scholar]
- 24.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93:682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 25.Roelen BA, van Rooijen MA, Mummery CL. Expression of ALK-1, a type 1 serine/threonine kinase receptor, coincides with sites of vasculogenesis and angiogenesis in early mouse development. Dev Dyn. 1997;209:418–430. doi: 10.1002/(SICI)1097-0177(199708)209:4<418::AID-AJA9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Panchenko MP, Williams MC, Brody JS, Yu Q. Type I receptor serine-threonine kinase preferentially expressed in pulmonary blood vessels. Am J Physiol. 1996;270:L547–558. doi: 10.1152/ajplung.1996.270.4.L547. [DOI] [PubMed] [Google Scholar]
- 27.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nature genetics. 2000;26:328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 30.Letteboer TG, Mager JJ, Snijder RJ, Koeleman BP, Lindhout D, Ploos van Amstel JK, Westermann CJ. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006;43:371–377. doi: 10.1136/jmg.2005.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunha SI, Pardali E, Thorikay M, Anderberg C, Hawinkels L, Goumans MJ, Seehra J, Heldin CH, ten Dijke P, Pietras K. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med. 2010;207:85–100. doi: 10.1084/jem.20091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 33.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 34.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 35.Ratziu V, Lalazar A, Wong L, Dang Q, Collins C, Shaulian E, Jensen S, Friedman SL. Zf9, a Kruppel-like transcription factor up-regulated in vivo during early hepatic fibrosis. Proc Natl Acad Sci USA. 1998;95:9500–9505. doi: 10.1073/pnas.95.16.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q, Kim SJ, Friedman SL. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273:33750–33758. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- 37.Botella LM, Sanchez-Elsner T, Sanz-Rodriguez F, Kojima S, Shimada J, Guerrero-Esteo M, Cooreman MP, Ratziu V, Langa C, Vary CP, Ramirez JR, Friedman S, Bernabeu C. Transcriptional activation of endoglin and transforming growth factor-beta signaling components by cooperative interaction between Sp1 and KLF6: their potential role in the response to vascular injury. Blood. 2002;100:4001–4010. doi: 10.1182/blood.V100.12.4001. [DOI] [PubMed] [Google Scholar]
- 38.Kojima S, Hayashi S, Shimokado K, Suzuki Y, Shimada J, Crippa MP, Friedman SL. Transcriptional activation of urokinase by the Kruppel-like factor Zf9/COPEB activates latent TGF-beta1 in vascular endothelial cells. Blood. 2000;95:1309–1316. [PubMed] [Google Scholar]
- 39.Botella LM, Sanz-Rodriguez F, Komi Y, Fernandez LA, Varela E, Garrido-Martin EM, Narla G, Friedman SL, Kojima S. TGF-beta regulates the expression of transcription factor KLF6 and its splice variants and promotes co-operative transactivation of common target genes through a Smad3-Sp1-KLF6 interaction. Biochem J. 2009;419:485–495. doi: 10.1042/BJ20081434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 41.Garrido-Martin EM, Blanco FJ, Fernandez LA, Langa C, Vary CP, Lee UE, Friedman SL, Botella LM, Bernabeu C. Characterization of the human Activin-A receptor type II-like kinase 1 (ACVRL1) promoter and its regulation by Sp1. BMC Mol Biol. 2010;11:51. doi: 10.1186/1471-2199-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gill G, Pascal E, Tseng ZH, Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci USA. 1994;91:192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courey AJ, Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988;55:887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 44.Lv W, Chen L, Zhou DH, Wei B. Influence of specific blocking of the delta-like ligand 4/notch signal transduction pathway on the biological behavior of human umbilical vein endothelial cells. Cancer Biother Radiopharm. 2010;25:449–454. doi: 10.1089/cbr.2010.0782. [DOI] [PubMed] [Google Scholar]
- 45.Roque M, Fallon JT, Badimon JJ, Zhang WX, Taubman MB, Reis ED. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20:335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 46.Sanz-Gonzalez SM, Barquin L, Garcia-Cao I, Roque M, Gonzalez JM, Fuster JJ, Castells MT, Flores JM, Serrano M, Andres V. Increased p53 gene dosage reduces neointimal thickening induced by mechanical injury but has no effect on native atherosclerosis. Cardiovasc Res. 2007;75:803–812. doi: 10.1016/j.cardiores.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li B, Yin W, Hong X, Shi Y, Wang HS, Lin SF, Tang SB. Remodeling retinal neovascularization by ALK1 gene transfection in vitro. Invest Ophthalmol Vis Sci. 2008;49:4553–4560. doi: 10.1167/iovs.07-0995. [DOI] [PubMed] [Google Scholar]
- 49.Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13:301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 50.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez LA, Garrido-Martin EM, Sanz-Rodriguez F, Pericacho M, Rodriguez-Barbero A, Eleno N, Lopez-Novoa JM, Duwell A, Vega MA, Bernabeu C, Botella LM. Gene expression fingerprinting for human hereditary hemorrhagic telangiectasia. Hum Mol Genet. 2007;16:1515–1533. doi: 10.1093/hmg/ddm069. [DOI] [PubMed] [Google Scholar]
- 52.Blanco FJ, Santibanez JF, Guerrero-Esteo M, Langa C, Vary CP, Bernabeu C. Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J Cell Physiol. 2005;204:574–584. doi: 10.1002/jcp.20311. [DOI] [PubMed] [Google Scholar]
- 53.Lux A, Salway F, Dressman HK, Kroner-Lux G, Hafner M, Day PJ, Marchuk DA, Garland J. ALK1 signalling analysis identifies angiogenesis related genes and reveals disparity between TGF-beta and constitutively active receptor induced gene expression. BMC Cardiovasc Disord. 2006;6:13. doi: 10.1186/1471-2261-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ota T, Fujii M, Sugizaki T, Ishii M, Miyazawa K, Aburatani H, Miyazono K. Targets of transcriptional regulation by two distinct type I receptors for transforming growth factor-beta in human umbilical vein endothelial cells. J Cell Physiol. 2002;193:299–318. doi: 10.1002/jcp.10170. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Novoa JM, Bernabeu C. The physiological role of endoglin in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2010;299:H959–974. doi: 10.1152/ajpheart.01251.2009. [DOI] [PubMed] [Google Scholar]
- 56.Shovlin CL. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 2010;24:203–219. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, Oh SH, Walter G, Raizada MK, Sorg BS, Oh SP. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119:3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 59.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 60.Alt A, Miguel-Romero L, Donderis J, Aristorena M, Blanco FJ, Round A, Rubio V, Bernabeu C, Marina A. Structural and functional insights into endoglin ligand recognition and binding. PLoS One. 2012;7:e29948. doi: 10.1371/journal.pone.0029948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, Watabe T. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 63.Ricard N, Bidart M, Mallet C, Lesca G, Giraud S, Prudent R, Feige JJ, Bailly S. Functional analysis of the BMP9 response of ALK1 mutants from HHT2 patients: a diagnostic tool for novel ACVRL1 mutations. Blood. 2010;116:1604–1612. doi: 10.1182/blood-2010-03-276881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.