Abstract
Objectives
To assess whether sleep apnea severity has an independent relationship with leptin levels in blood after adjusting for different measures of obesity and whether the relationship between OSA severity and leptin levels differs depending on obesity level.
Methods
Cross-sectional study of 452 untreated obstructive sleep apnea (OSA) patients (377 males and 75 females), in the Icelandic Sleep Apnea Cohort (ISAC), age 54.3±10.6 (mean±SD), BMI 32.7±5.3 kg/m2 and apnea-hypopnea index (AHI) 40.2 ± 16.1 events/hour. A sleep study and magnetic resonance imaging of abdominal visceral and subcutaneous fat volume were performed as well as fasting serum morning leptin levels measured.
Results
Leptin levels were more highly correlated with body mass index (BMI), total abdominal and subcutaneous fat volume than visceral fat volume per se. No relationship was found between sleep apnea severity and leptin levels, assessed within three BMI groups (BMI<30, BMI 30–35 and BMI>35 kg/m2). In a multiple linear regression model, adjusted for gender, BMI explained 38.7% of the variance in leptin levels, gender explained 21.2% but OSA severity did not have a significant role and no interaction was found between OSA severity and BMI on leptin levels. However, hypertension had a significant effect on the interaction between OSA severity and obesity (p=0.04). In post-hoc analysis for nonhypertensive OSA subjects (n=249), the association between leptin levels and OSA severity explained a minor but significant variance (3.2%) in leptin levels. This relationship was greatest for nonobese nonhypertensive subjects (significant interaction with obesity level). No relationship of OSA severity and leptin levels was found for hypertensive subjects (n=199).
Conclusion
Obesity and gender are the dominant determinants of leptin levels. OSA severity is not related to leptin levels except to a minor degree in nonhypertensive nonobese OSA subjects.
Keywords: Obstructive sleep apnea, leptin, visceral fat, subcutaneous fat, obesity, hypertension
Introduction
Leptin is an adipokine released peripherally from fat cells that contributes to regulating body adiposity through a feedback loop whereby elevations in leptin are recognized by the central nervous system, acting as a satiety signal to suppress appetite (reviewed by1). Leptin resistance in the brain is thought to be responsible for a failure of the high levels of circulating leptin to suppress appetite, ultimately resulting in increased food intake and adiposity. The central leptin resistance is site-specific since other functions of leptin in regulating inflammation and sympathetic activation remain intact. Therefore, high leptin levels may be a part of the cascade causing the low-grade systemic inflammation and sympathetic nervous system-mediated hypertension commonly seen in obesity1 and be an independent risk factor for cardiovascular disease.2, 3
Since obesity and OSA commonly coexist (reviewed by4), it is important to understand how the two disorders might interact in determining leptin levels. It is conceivable that in the most obese subjects the effects of OSA on leptin levels is small since there is already very high stimulation by obesity. Some studies have found that OSA subjects have higher leptin levels than controls5–18 even when matched for BMI6, 7, 10, 13, 14, 16 and a decrease in leptin levels with continuous positive airway pressure (CPAP) treatment.7, 18–25 There are, however, conflicting data showing no differences in leptin levels between OSA subjects and controls26–32 and no change with CPAP treatment.26, 32–37 Finally, three small studies suggest differential effects of OSA on leptin levels depending on obesity; two studies showing effects of OSA in lean subjects only but the third effects in obese subjects only.28, 38, 39 In none of these studies has the relative role of subcutaneous and visceral fat been adequately addressed which may be an important factor since subcutaneous fat has been found to produce more leptin than visceral fat.40–42
The aim of this study was to assess whether OSA severity has an independent relationship with leptin levels after adjusting for different measures of obesity and whether the relationship between OSA severity and leptin levels differs depending on obesity level. Analyses were performed using a large cross-sectional study of OSA patients with varying degrees of obesity, who had a magnetic resonance imaging (MRI) evaluation of abdominal subcutaneous and visceral fat. The subjects are part of the Icelandic Sleep Apnea Cohort (ISAC). The primary a priori hypothesis for these analyses was that OSA severity would be independently associated with leptin levels adjusting for obesity and differential effects of OSA would be found depending on obesity level.
Methods
Participants and measurements
All patients diagnosed with moderate to severe OSA in Iceland and referred for CPAP treatment to the Landspitali – The National University Hospital of Iceland from September 2005 – September 2008 were invited to join the study (with an apnea-hypopnea index [AHI] ≥15 on original sleep study). This is the only site in Iceland providing CPAP therapy. More than 90% of eligible and approached subjects agreed to participate (n=530). Subjects answered standardized questionnaires, including questions about general medical history and medication. Blood was drawn in the morning after sleep from the antecubital vein of fasting untreated subjects and leptin levels measured in serum. For further details, see supplement.
Biomarker assessment
A radioimmunoassay (RIA) was used to determine serum leptin levels in ng/ml, using the double antibody/PEG technique with I125-labeled human leptin and human leptin antiserum (Millipore, HL-81K). Leptin levels were measured in duplicate. For further details, see supplement.
Whole night study
The subjects had a sleep study while untreated with an Embletta type 3 portable monitor or an Embla 12 channel system (Embla™; Reykjavik, Iceland) recording the same channels. All sleep studies were re-read by a centralized scoring lab using the Somnologica Studio software. An apnea-hypopnea index (AHI) and an oxygen desaturation index (ODI) (≥4%) were calculated (for definition of events, see Supplement). The minimum SaO2 was defined as the lowest oxygen saturation reached during the study. Hypoxia time was defined as the number of minutes with SaO2 <90%. For further details, see supplement.
Magnetic resonance imaging (MRI)
Subjects underwent MRI of the abdomen using a 1.5T scanner (Siemens Avanto, Germany) while untreated. Briefly, the abdominal compartment was defined from the superior aspect of the xiphoid process to the anterior portion of the L5-S1 interspace. MR images were obtained in 1 cm contiguous intervals through the abdominal compartment and summed to assess the total abdominal, subcutaneous and visceral fat volume. Intraclass correlation coefficient (ICC) analyses for visceral and subcutaneous fat volumes, for two trained raters, were both essentially 1.0, showing ignorable technical variability. For further details, see supplement and our previous publication.43
Statistical analyses
Descriptive group comparisons were performed using one-way analysis of variance (ANOVA) and chi-square tests for continuous and categorical variables, respectively. Leptin levels were (natural) log transformed for normality in all analyses.
Initial evaluations of the strengths of linear associations between leptin levels, obesity and OSA severity were based on Pearson and Spearman rank correlations. Statistical tests that compared the strengths of linear associations between leptin levels and different obesity metrics were produced using a non-parametric bootstrap re-sampling procedure (n=1000 replications) that accounted for within subject correlations.44
The goal of the primary analysis was to estimate the simultaneous statistical effects of OSA and obesity severity and their interaction on leptin levels. The parameters of the multiple linear regression model included higher order and interaction terms (response surface modeling45) to permit the association of OSA severity to depend on obesity in both linear and non-linear ways. Different obesity and OSA severity markers were compared for their ability to explain variance in leptin levels. The prediction equation included linear and quadratic terms for selected OSA and obesity severity measures as well as interactions between obesity and OSA linear and higher orders terms as follows: E(log(leptin)) = β0 + β1*OSA + β2*(OSA)2 + β3*Obesity + β4*(Obesity)2 + β5*(OSA*Obesity) + β6*OSA*(Obesity)2 + β7*BMI*(Obesity)2. A p value of ≤0.05 was considered significant for all analyses. For further details on analytical strategies, see our previous publication.46
The primary analysis was performed on n=452 subjects in the study cohort in three strata defined on the basis of BMI categories restricted to have identical ranges of OSA severity. This was done to avoid extrapolation in covariate analyses (see details below). Sensitivity analyses were conducted with all subjects included (n=520) as well as cohorts with further exclusions based on comparable hypoxia severity (hypoxia time and minSaO2). Analyses were also repeated excluding premenopausal women (n=13/75) and women on hormone replacement therapy (HRT) (n=4/75). All sensitivity analyses led to the same conclusions as the primary analysis, see supplemental Table E1.
Individuals who were responsible for scoring of sleep studies and measurements of MRIs and leptin levels were blinded to other data. The consents of the National Bioethics Committee of Iceland, the Data Protection Authority of Iceland and the Institutional Review Board of the University of Pennsylvania were granted for the study. Written consent was obtained from the research subjects.
Results
Demographics and data on sleep-disordered breathing
A total of 530 subjects completed the study. 9 subjects had sleep studies that did not fulfill the quality criteria (inadequate quality of the oximeter signal) and were excluded from further analysis. One subject was excluded due to extreme leptin values (twice as high as the next highest sample, 216 ng/ml). The cohort was divided into three BMI categories; BMI <30, BMI 30–35, and BMI ≥35 kg/m2 for descriptive analysis (Figure 1). Increased OSA severity was apparent with increased BMI (supplemental Figure E1). The primary study cohort therefore included only patients with an AHI 14–80 and an ODI 10–65 (n=452), excluding subjects with OSA severity not found in other BMI groups (n=68, supplemental Figure E1). Analyses including all subjects, showed similar results and are presented in supplemental Table E1. See further details in our previous publication.46
Figure 1.
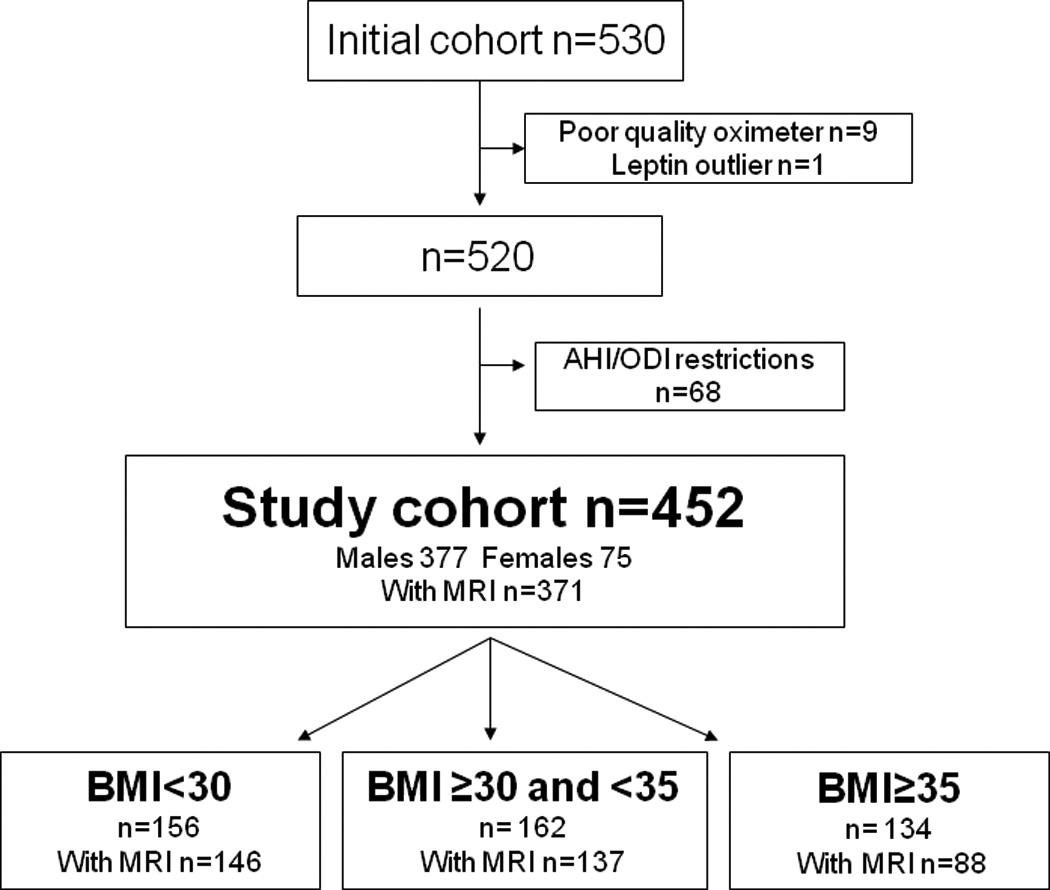
The study cohort. Selection of the cohort by sleep apnea severity and division into BMI tertiles.
The three BMI groups were similar with regard to demographics except the prevalence of hypertension and diabetes increased with increasing BMI (Table 1). Also with increasing BMI, the average degree of hypoxia increased despite having restricted the ranges for AHI and ODI to be equal. The AHI was, however, comparable between BMI groups. Complete abdominal MRI measurements were obtained in 82% of the study cohort. Failure to obtain MRI assessment occurred more commonly in the most obese group, usually due to claustrophobia or image quality issues. 62 out of 75 women were postmenopausal and n=4 were on HRT.
Table 1.
Demographic and sleep-disordered breathing data in the study cohort and in the 3 BMI groups. Data are presented as mean ± standard deviation or % where indicated. Significant differences between the 3 BMI groups are in bold (p<0.05).
| All | BMI < 30 kg/m2 |
BMI 30 −< 35 kg/m2 |
BMI ≥ 35 kg/m2 |
p-value for comparison between groups* |
|
|---|---|---|---|---|---|
| n = 452 | n = 156 | n = 162 | n = 134 | ||
| Age (years) | 54.4 ± 10.6 | 55.1 ± 9.4 | 54.9 ± 10.9 | 52.8 ± 11.3 | 0.13 |
| % of males | 83.4 | 86.5 | 83.3 | 79.9 | 0.31 |
| Body mass index (kg/m2) | 32.7 ± 5.3 | 27.6 ± 2.0 | 32.2 ± 1.4 | 39.2 ± 3.7 | N/A |
| Current smokers (%) | 24.0 | 27.6 | 22.8 | 21.1 | 0.40 |
| Sleep disordered breathing | |||||
| Apnea-hypopnea index (events/hour) | 40.2 ± 16.1 | 38.9 ± 15.2 | 41.4 ± 16.8 | 40.4 ± 16.3 | 0.38 |
| Oxygen desaturation index (events/hour) | 31.5 ± 14.1 | 28.5 ± 12.4 | 31.7 ± 14.9 | 34.8 ± 14.2 | 0.0006 |
| Minimum SaO2 (%) | 77.2 ± 7.2 | 78.7 ± 5.9 | 77.6 ± 7.3 | 74.9 ± 8.0 | <0.0001 |
| Hypoxia time (minutes) | 42.3 ± 60.7 | 29.7 ± 41.7 | 39.0 ± 60.9 | 61.1 ± 73.7 | <0.0001 |
| Epworth Sleepiness Score | 11.6 ± 4.9 | 12.0 ± 4.8 | 11.3 ± 4.8 | 11.7 ± 5.0 | 0.46 |
| Medical history | |||||
| Hypertension (%) | 44.4 | 34.2 | 44.4 | 56.4 | 0.0008 |
| Cardiovascular disease (%) | 17.8 | 16.2 | 17.4 | 20.2 | 0.68 |
| Diabetes (%) | 11.1 | 5.2 | 8.7 | 20.9 | 0.0001 |
| Leptin levels (ng/ml)† | 10.6 (9.9–11.3) | 6.7 (6.0–7.3) | 10.8 (9.8–11.8) | 17.9 (16.1–19.9) | <0.0001 |
One-way ANOVA for continuous variables and Pearson chi-square test for percentages.
Geometric mean and 95% confidence interval determined from exponentiation of log transformed values.
Leptin levels in the cohort
The distribution of leptin levels was highly skewed and therefore log transformed to permit parametric analysis. Leptin levels were lowest in the nonobese OSA group and increased significantly with increased obesity level (Table 1). For males, the geometric mean (95% CI) leptin level was 9.0 (8.5 – 9.6) ng/ml and for females, it was 24.0 (20.7 – 27.8) ng/ml, p<0.0001. Menopausal status was not significantly associated with leptin levels.
Relationship between leptin levels and different measures of obesity
The correlations between leptin levels and different measures of obesity were assessed with and without adjustment for gender (Table 2). The magnitude of correlation between total fat volume and leptin levels, adjusting for gender, was significantly highest of all the obesity measures (r=0.73, p<0.001). The correlations for BMI, waist circumference and subcutaneous fat volume were significantly lower based on bootstrap-based comparison (r=0.64–0.69, p<0.0001). Visceral fat volume, neck circumference and waist-to-hip ratio were significantly less associated with leptin levels than all of the above.
Table 2.
Pearson correlation coefficients between leptin levels in serum to various obesity measurements in all subjects with MRI data (n = 371). The analysis is shown both unadjusted and adjusted for gender. Significant p-values for assessment of correlation are in bold (p<0.05).
| Leptin levels (ng/ml) | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted for gender | |||
| r | p | r | p | |
| Total abdominal fat volume (cm3) | 0.58 | <0.0001 | 0.73 | <0.0001 |
| Subcutaneous fat volume (cm3) | 0.67 | <0.0001 | 0.69* | <0.0001 |
| Waist circumference (cm) | 0.44* | <0.0001 | 0.67* | <0.0001 |
| Body mass index (kg/m2) | 0.61 | <0.0001 | 0.64* | <0.0001 |
| Visceral fat volume (cm3) | 0.24* | <0.0001 | 0.59* | <0.0001 |
| Neck circumference (cm) | 0.04* | 0.50 | 0.44* | <0.0001 |
| Waist-to-hip ratio (cm/cm) | 0.03* | 0.61 | 0.42* | <0.0001 |
Note: Leptin levels are log transformed for analysis. Similar correlations were obtained when assessed using Spearman correlation.
The magnitude of the correlation is significantly smaller than between leptin levels and total abdominal fat volume.
Relationship between leptin levels and OSA severity
The primary hypothesis tested was that OSA severity contributes to explaining the variance in leptin levels after accounting for the role of obesity. Four measures of OSA were assessed; AHI, ODI, hypoxia time and minimum SaO2. A simple correlation analysis for the whole study cohort showed a significant association between leptin levels and both hypoxia time (r=0.20, p<0.0001) and minimum SaO2 (r= −0.17, p=0.003) but not with AHI or ODI (Table 3). However, when the association of leptin levels and OSA severity was assessed within the three BMI categories, no correlation was found with any measure of OSA severity.
Table 3.
Pearson correlation between leptin levels in serum to OSA severity in the study cohort (n = 452) and for the 3 different BMI categories. Significant p-values for assessment of correlation are in bold (p<0.05).
| Pearson correlation coefficients with log(leptin) level | ||||||||
|---|---|---|---|---|---|---|---|---|
| All | BMI < 30 kg/m2 |
BMI ≥30 and <35 kg/m2 |
BMI ≥35 kg/m2 |
|||||
| n = 452 | n = 156 | n = 162 | n = 134 | |||||
| r | p | r | p | r | p | r | p | |
| Apnea-hypopnea index | −0.04 | 0.40 | 0.01 | 0.86 | −0.14 | 0.07 | −0.09 | 0.30 |
| Oxygen desaturation index | 0.05 | 0.32 | 0.02 | 0.85 | −0.13 | 0.10 | −0.07 | 0.43 |
| Minimum SaO2 (%) | −0.17 | 0.0003 | −0.04 | 0.66 | −0.12 | 0.13 | −0.05 | 0.60 |
| Hypoxia time (minutes) | 0.20 | <0.0001 | 0.13 | 0.11 | 0.08 | 0.34 | 0.14 | 0.11 |
Note: Similar correlations were obtained when assessed using Spearman correlation, the complete cohort (n=520) and when analyses were adjusted for gender.
Leptin levels: Role of OSA after adjustment for BMI
To simultaneously estimate the role of OSA severity and obesity in leptin levels as well as the possible interaction between OSA severity and obesity in leptin levels, a multiple linear regression model was employed (as described in Methods). Initial analyses involved BMI as the primary measure of obesity as it was the simplest measure of obesity, with the highest number of subjects available and had a strong correlation with leptin levels. Additional analyses involved waist circumference and fat distribution variables from the MRI analysis. All analyses were adjusted for gender as the gender main effect explained ~20% of the variance in leptin levels. Because the gender main effect was so large, careful attention was paid to the potential for gender to act as a confounding factor and effect modifier (interaction). Although adding gender to the model increased explained variance substantially, its addition did not appreciably change model parameter estimates indicating that gender is not a key confounding factor when estimating simultaneously the relationship between obesity and OSA severity on leptin. Moreover, when the set of gender interactions with obesity and OSA were added to the model, these were not statistically significant (p=0.10) and the increase in explained variance was very small (1.0%). While the relatively small number of females makes estimating gender-specific parameters challenging and reduces power to detect interactions, results implied that controlling for gender as a main effect added power and did not distort the primary findings.
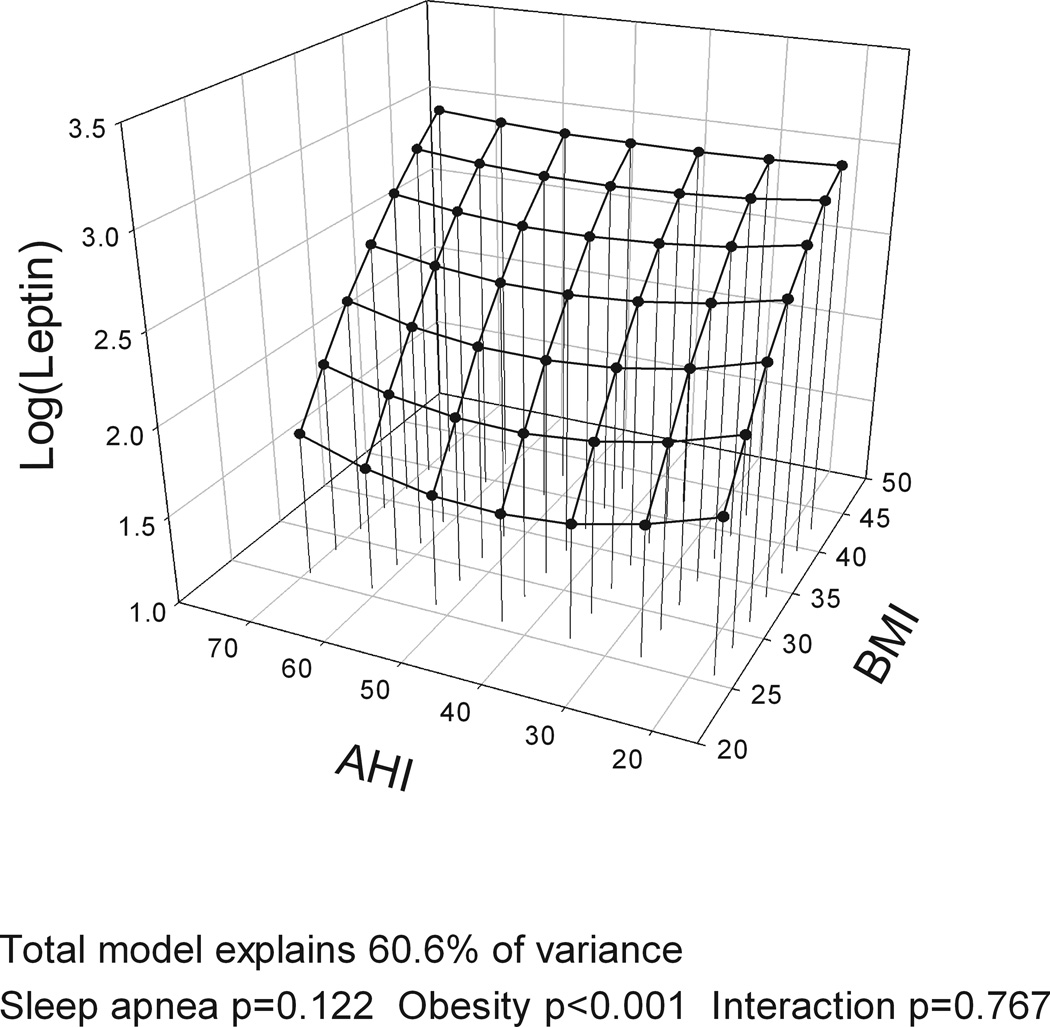
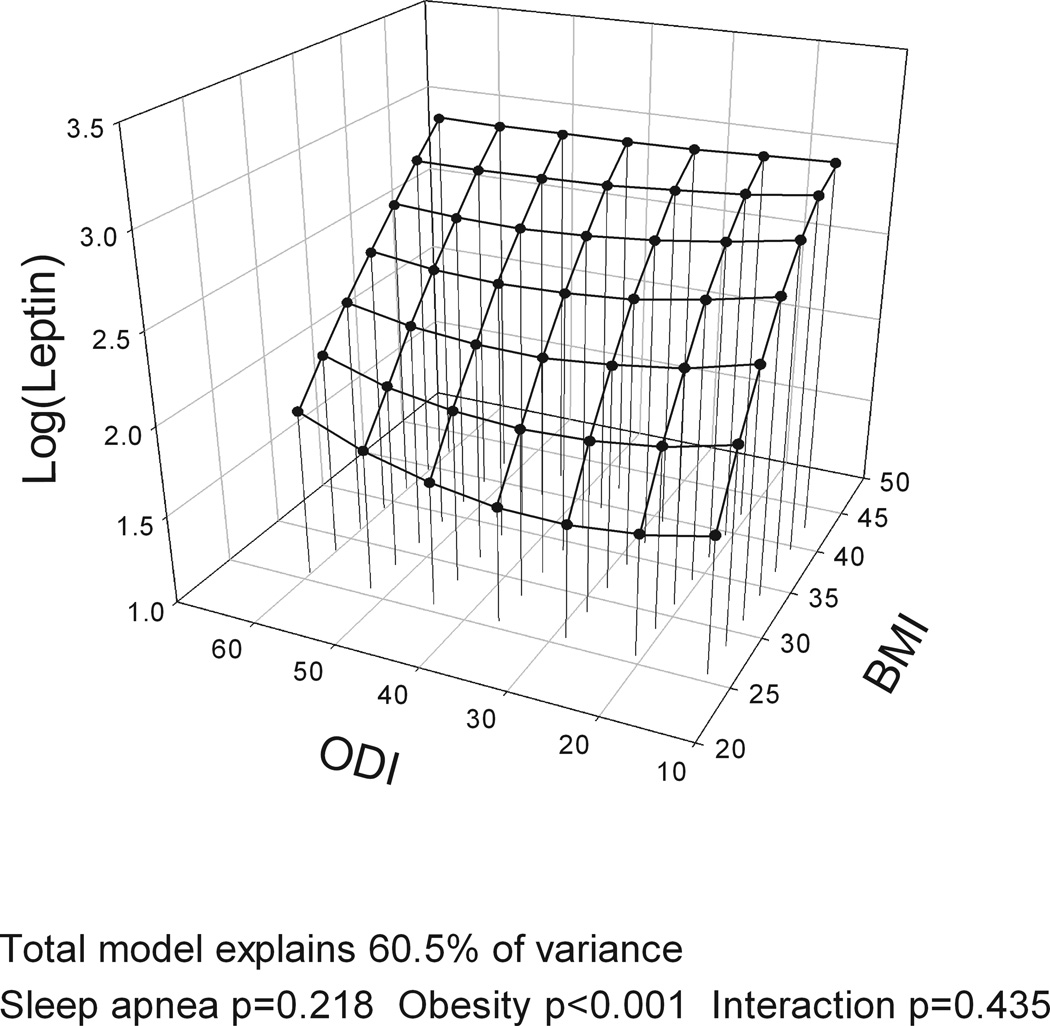
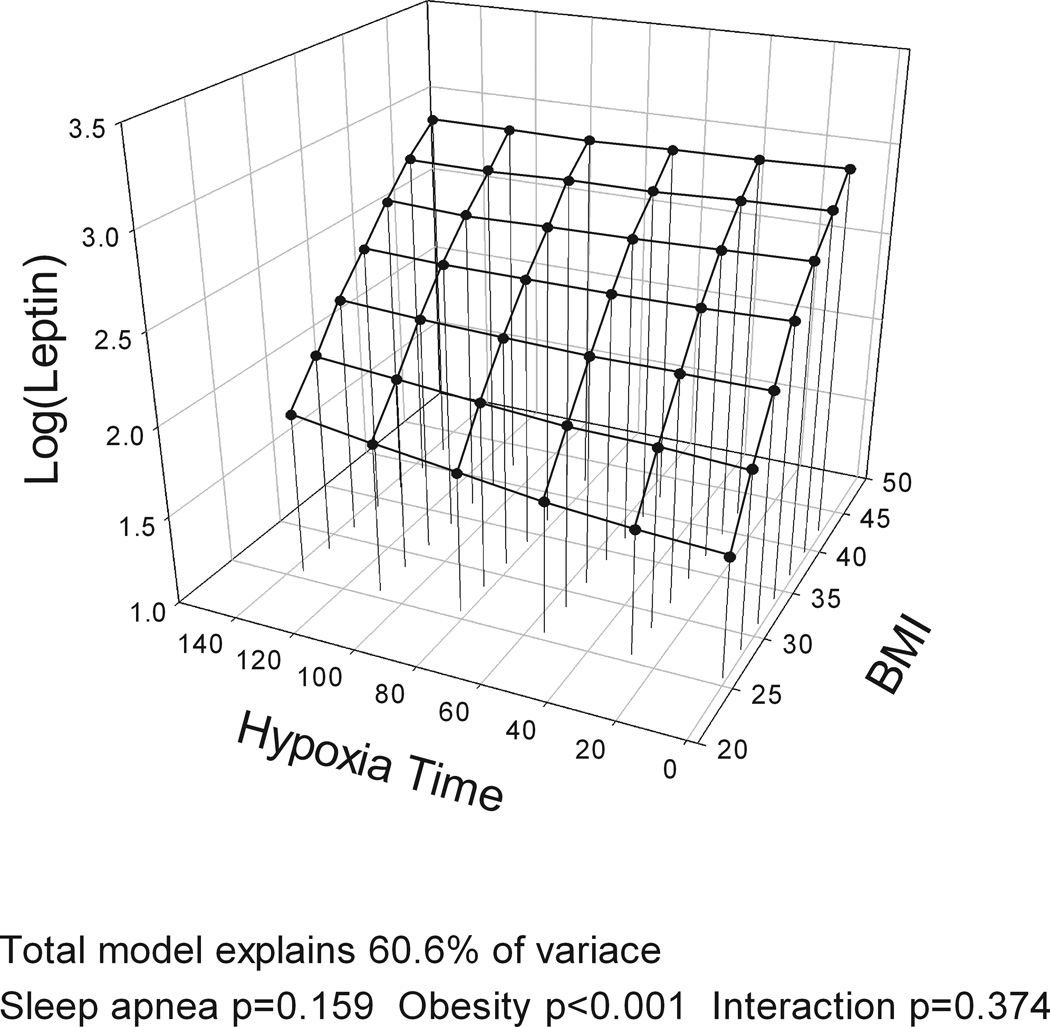
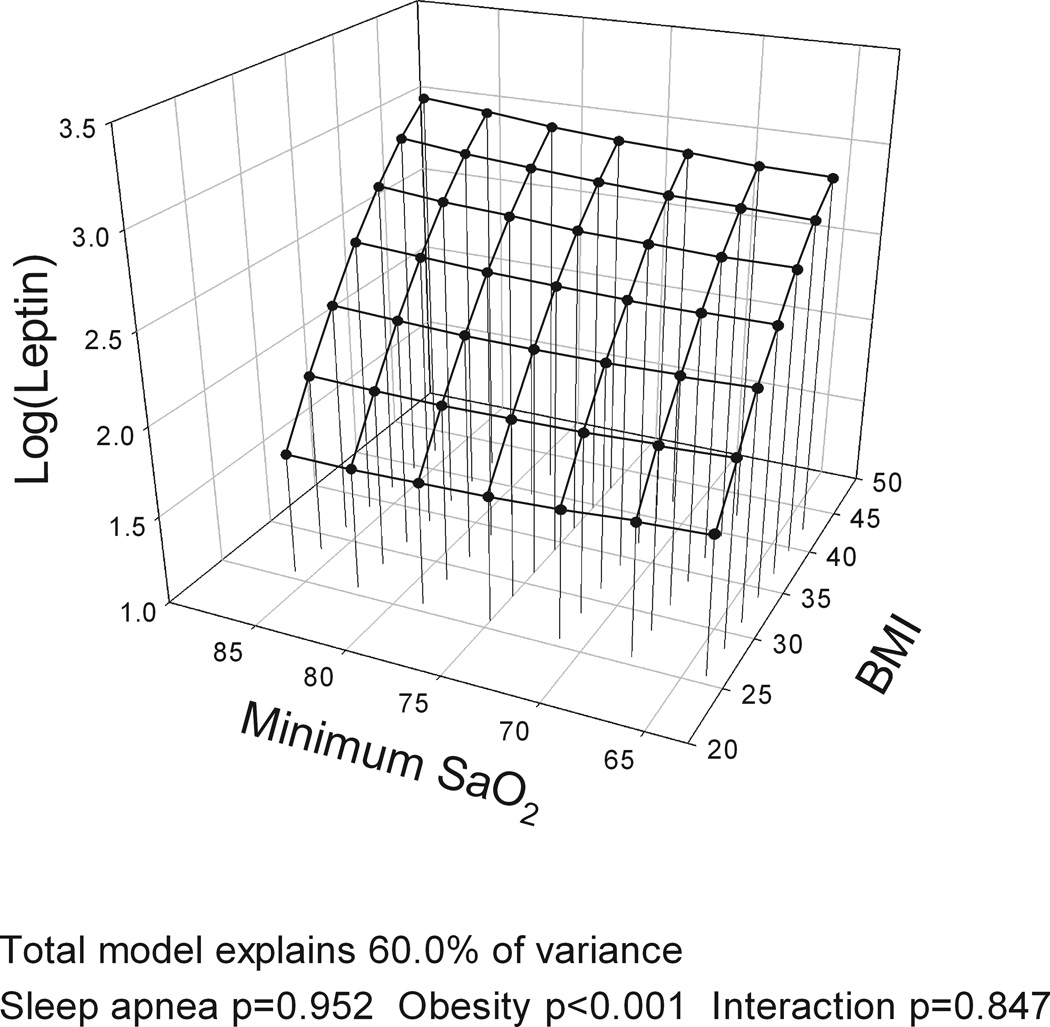
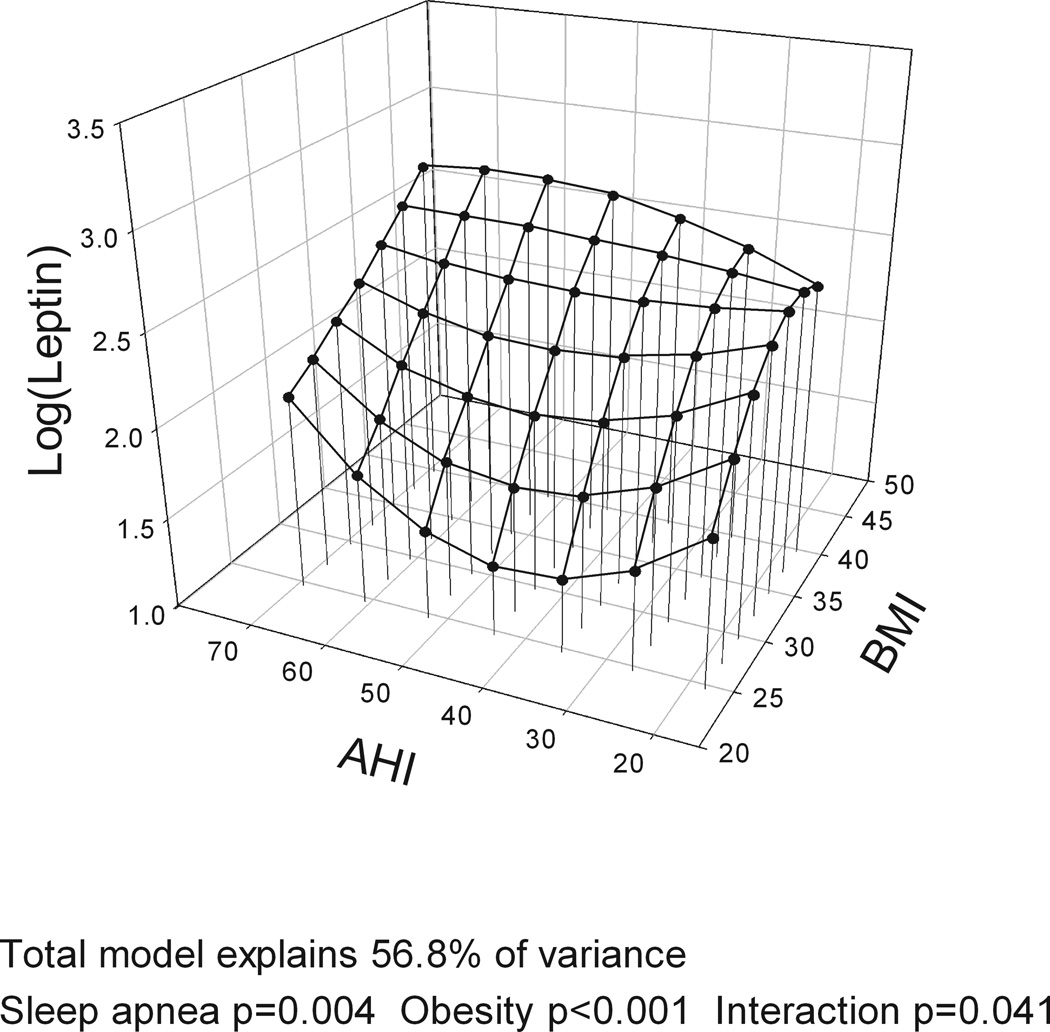
The models with BMI, gender and the four different measures of OSA severity (AHI, ODI, hypoxia time and minSaO2) all explained a similar amount of variance in leptin levels (R2=60.0–60.6%). BMI was significantly associated with leptin levels in all analyses, accounting for over half of explained variance in leptin while gender explained ~20%. However, no measure of OSA severity was significantly associated with leptin levels and no interaction was found between BMI and OSA severity on leptin levels (Table E1 in Supplement). Figure 2 shows a three-dimensional graph of the association of BMI and leptin (large effect) and different measures of OSA severity (no effect) and leptin levels, adjusting for gender. When the same analysis was performed for the complete cohort with no exclusions, AHI explained 1% of the variance in leptin levels indicating a potential very minor independent association of OSA severity and leptin levels.
Figure 2.
Three-dimensional plots for leptin as a function of BMI and four alternative obstructive sleep apnea (OSA) severity measures: a) apnea-hypopnea index (AHI); b) oxygen desaturation index (ODI); c) Hypoxia time (minutes with SaO2 < 90%); and d) minimum SaO2. The significance of associations between leptin levels and sleep apnea severity, obesity and their interaction are shown below the figures. A significant association of obesity and leptin levels is found but no association of OSA and leptin levels and no interaction of OSA and obesity. All plots are adjusted for gender.
Leptin levels: Multivariable models with different measures of obesity
The model with waist circumference and AHI, instead of BMI and AHI, explained a similar percentage of leptin levels (63.2% vs. 60.6%, difference not significant in bootstrap comparison). Also, AHI significantly explained, a very small percentage (1.1%) of the variance of leptin levels with waist circumference in the model, not found for any of the three hypoxia variables or when using BMI as the obesity variable.
To compare how well MRI measures of obesity predict leptin levels in multivariable models, in comparison to anthropometric measures, the n=371 subset with MRI measurements was assessed (gender and OSA severity included in model). The model with total abdominal fat explained the largest amount of variance in leptin levels, 69.0% (significantly highest by bootstrap analysis) while models with BMI and waist circumference explained 61.1% and 62.4%, respectively. The model with subcutaneous fat explained 66.5% but the model with visceral fat explained less than all the models above, i.e., 55.5% of the variance. The association of OSA and leptin levels remained nonsignificant in the models with total abdominal fat as the obesity variable and there was no interaction between total fat volume and OSA severity. Gender continued to explain a large proportion of the variance in leptin levels in these models.
The role of other covariates in leptin levels
The associations of potential confounders on leptin levels were tested separately for each confounder in the model with BMI and AHI (Table E2). Age and cardiovascular disease had statistically significant but small associations with leptin levels and explained some additional variance in leptin levels beyond obesity and gender. Adding to the model an extra fat variable, visceral to subcutaneous fat volume ratio or visceral fat volume alone explained additional variance in leptin levels (6.1% and 2.8%, respectively), further emphasizing the importance of fat distribution in leptin levels. Total fat volume and subcutaneous fat volume could not be assessed as covariates as they were highly correlated with BMI (r>0.85). Two covariates had a significant effect on the interaction between OSA severity and obesity; hypertension (p=0.04) and age group (p=0.04). Subgroup analyses for age category showed minor inconsistent associations of OSA severity and leptin levels. Subgroup analyses for hypertension status are now described.
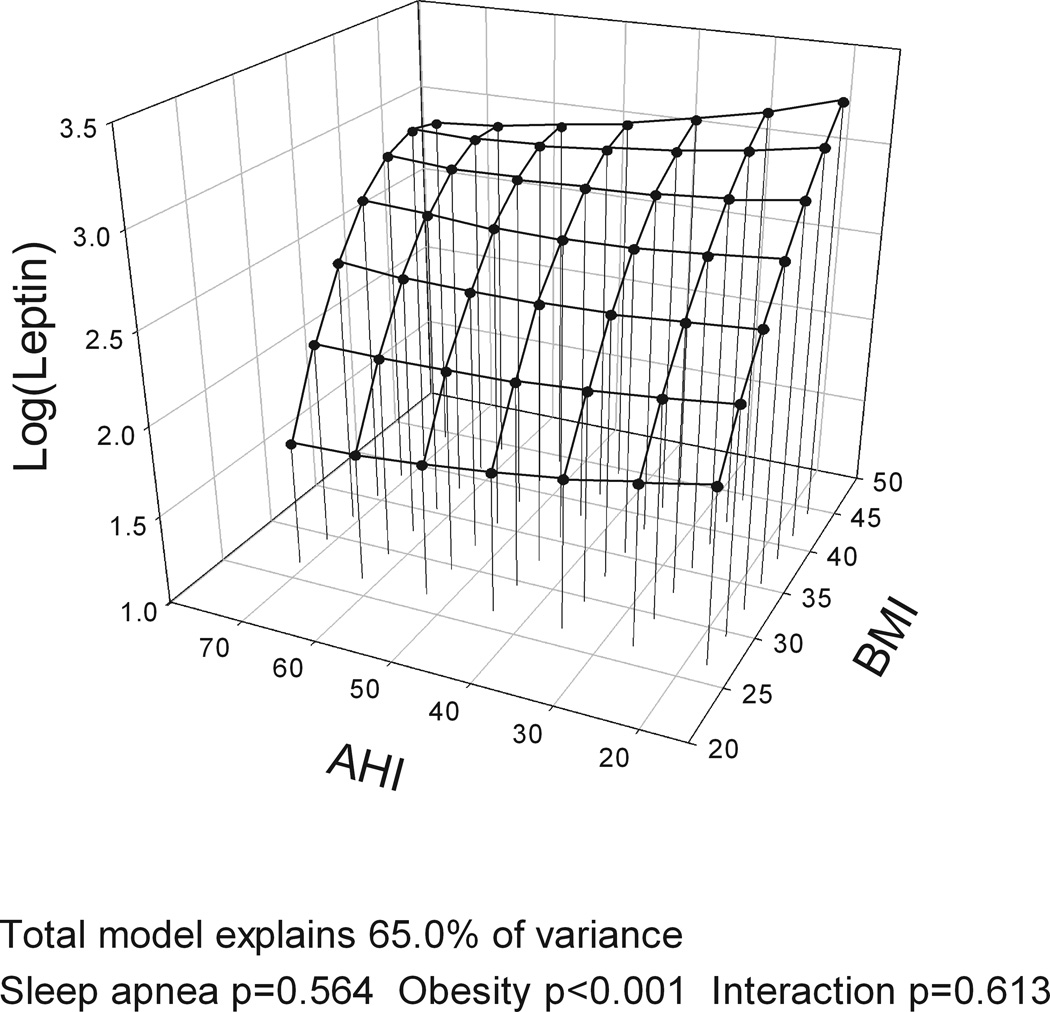
Hypertension and leptin levels
Post-hoc subgroup analyses were performed separately for hypertensive (n=199) and nonhypertensive (n=249) subjects using the same model as above with BMI. Hypertensive subjects had higher leptin levels than the nonhypertensive (12.6 [11.4–14.0] ng/ml vs. 9.3 [8.5–10.1] ng/ml, p<0.0001). They were also 6 years older on average and more obese (by 2.3 BMI units). No gender differences were found. For nonhypertensive subjects, there was a minor but significant effect of OSA severity on leptin levels, explaining 3.2% of the variance in leptin levels as well as a significant interaction between OSA severity and BMI, explaining an additional 1.5% of the variance. For nonhypertensive subjects, the role of increased OSA severity was greatest for nonobese subjects (Figure 3), i.e. a nonhypertensive OSA patient with a BMI of 25 and AHI 30 had expected leptin levels of 4.6 ng/ml in the model but a nonhypertensive subject with the same BMI but an AHI 70 had expected leptin levels of 8.4 ng/ml. No such differences were found for obese nonhypertensive subjects, for hypertensive subjects or those on different classes of antihypertensives. Models with waist circumference or total fat volume instead of BMI had the same findings.
Figure 3.
Three-dimensional plots for leptin as a function of BMI and apnea-hypopnea index (AHI) for a) non hypertensive (n = 249) and b) hypertensive subjects (n = 199). The significance of associations between leptin levels and sleep apnea severity, obesity and their interaction are shown below the figures. For non hypertensive subjects, a significant association of obstructive sleep apnea (OSA) and obesity with leptin levels is found. Also an OSA by obesity interaction is found with greater association of OSA and leptin in leaner subjects. In hypertensive subjects, no association with OSA is found. All plots are adjusted for gender.
Discussion
Our results, in a large cross-sectional clinical sample of OSA subjects, show that obesity and gender have a dominant role in leptin levels. The highest association is with total abdominal fat volume. Models including this measure of obesity explain 69.0% of the total variance in leptin levels. There is an association of OSA severity with leptin levels but not after adjusting for obesity. Subgroup analyses indicate that OSA severity is independently related to leptin levels in nonhypertensive nonobese OSA subjects, but even in this group the role of OSA severity is small and not likely to be clinically meaningful. Thus, the major determinants of leptin levels are obesity and gender and not OSA.
The role of fat
Obesity and OSA commonly coexist as obesity is the most important risk factor for OSA (reviewed by4) making studies assessing their independent effects more difficult. The increased adipose tissue surrounding the upper airway in obesity causes increased volume of upper airway structures, increasing the risk of airway collapse.47 The increased fat mass also decreases lung volume and may affect neuromuscular control (reviewed by48). Visceral fat accumulation is more associated with adverse health effects than other fat deposits (reviewed by49) and may also be more associated with the presence of OSA than other measures of obesity.14, 17, 50
This study showed that obesity level explains the majority of the variance in leptin levels. Our results are in agreement with previous studies showing that subcutaneous fat produces more leptin than visceral fat40–42, as we found a higher correlation between leptin levels and subcutaneous fat volume than with visceral fat volume. However, our study also shows that total abdominal fat volume is the best indicator for leptin levels in sleep apneic subjects. Our results are in contrast to a study on nonobese Japanese OSA subjects (n=96) which found a higher correlation between leptin and visceral fat than with subcutaneous fat and no effect of obesity after adjusting for OSA severity.14 The differences in results are likely due to the large BMI differences between the two studies and the small BMI range in the previous study. Ethnic differences may have also play a role.51–55
The role of gender
Females have, on average, more than twice as high leptin levels than males in this study as has previously been reported.56 These differences are partly explained by higher fat mass and more subcutaneous fat in females compared to males but may also be due to sex steroids (bio-available estrogen and testesterone).56 In our study the number of females was relatively small compared to males and power to detect a gender difference may be limited. However, our study still includes more females (n=75) than the majority of previously published studies on OSA and leptin levels. Most studies have focused on males only8–10, 13–17, 19, 22, 24–26, 28, 29, 32, 38 and many studies include a very low number of females (≤5 per group).7, 11, 18, 20, 23, 34, 35 Four other studies have had a reasonable number of females (n=44–81).5, 12, 30, 39 Our results show that while gender explains a substantial portion of leptin variance, controlling for gender differences did not appreciably change the relationship between obesity, OSA and leptin in our cohort of subjects.
The role of sleep-disordered breathing
The association of OSA severity and leptin levels after adjusting for obesity and gender, was none or very minor in the whole cohort. However, a post-hoc subgroup analysis on nonhypertensive subjects showed a significant, albeit very small, association of OSA severity and leptin levels. This association was found in nonobese subjects.It may be that the oxidative stress and inflammation associated with OSA4 only contribute to an increase in leptin levels in subjects who are not already chronically exposed to these physiological stressors. The obese and hypertensive OSA subjects may already have maximal stimulation of leptin levels. It is important to remember though that there is strong evidence that OSA itself causes hypertension, complicating research when looking at hypertension simply as a confounder to the independent effects of OSA. Those more vulnerable to pathological cardiometabolic effects of OSA, likely due to genetic predisposition, will therefore already have hypertension and be on hypertensive medication (reviewed by4, 57).
In support of our results, other studies comparing obese and nonobese subjects, have shown differential effects of OSA on leptin levels depending on the degree of obesity.28, 38, 39 Two of these studies have comparable results to our study; showing a difference in leptin levels between nonobese OSA subjects and controls only as well as a decrease in leptin levels with CPAP in nonobese subjects only.38, 39 A recent study in mice exposed to intermittent hypoxia also supports these findings, showing increased leptin levels in lean but not obese mice.58 However, in another report, no difference in leptin levels between OSA subjects and controls was found and a decrease in leptin levels with CPAP was found in the obese OSA subjects only.28
The discrepancy in the earlier OSA literature on leptin levels, as discussed in the introduction, is likely partly due to the heterogeneity of leptin levels for different obesity levels and hypertension status in OSA subjects as found in our study. Many of the previous studies have a small sample size (n<30 per group/assessment7, 9–11, 15–20, 22–25, 28, 31–36, 38, 39), the OSA subjects often had higher obesity levels than controls (2–6 BMI units8, 11, 15, 17, 18, 27, 28 or difference in other fat variables between groups6, 8, 14, 30), higher age (3–8 years8, 11, 15, 17, 27, 28, 31, 32), and more comorbidities6, 17, 32. In CPAP studies7, 18–26, 28, 32–38, changes in weight32, 34 and possibly fat distribution with CPAP use25 may also be of importance. Ethnic differences may play an important role in the discrepancy as well, due to different etiology in the onset of obesity-related morbidities, such as type 2 diabetes.51 Asians may have a different susceptibility to OSA52, 53 and a different leptin profile to Caucasians and other ethnicities.54, 55 Many studies reporting effects of OSA on leptin levels have been performed in relatively lean Asians7, 9, 14, 15, 19, 20, 24, while only two studies in Asians have shown no relationship between leptin levels and OSA.30, 32
Strengths and limitations of the study design
The strengths of this study include a large sample of OSA subjects with a broad range of BMI and OSA severity (AHI from 14–80), allowing analyses of the role of OSA severity and obesity level as well as their potential interaction. Also, the sample is a clinical sample with various co-morbidities, representing the whole spectrum of OSA patients. The MRI studies performed to assess visceral and subcutaneous abdominal fat included assessment of the total abdominal volume and both the MRI and sleep studies were read by centralized labs, with high reliability of assessment.
The limitations included the relatively low percentage of females as discussed above, although given the size of the sample we still had a higher number of females than previous studies. The lack of subjects in our study with mild OSA (AHI<15 events/hour) is also a potential limitation as there may be a plateau in the effect of OSA on leptin levels. This type of plateau in OSA severity has been found for another biomarker—plasminogen activator inhibitor-1 (PAI-1).59 Finally, our study did not include controls. Recruiting well-matched controls with regards to obesity level and co-morbidities is very challenging, especially to find non-apneic controls for the more obese OSA subjects. These difficulties are reflected in the limitations of the current literature as discussed above. Instead our study employed the large range of obesity and OSA severity within our study cohort, to evaluate their relative importance on leptin levels. Our study cohort was also large enough to allow for assessment of the effects of various co-morbidities such as hypertension.
Other limitations include the use of a portable type III monitor (Embletta) instead of a full in-lab polysomnography. The study therefore lacks assessment of arousals. However, the Embletta portable monitoring system has been validated for assessing sleep-disordered breathing compared to polysomnography.60 Also, recent recommended scoring rules do not require arousals to assess hypopneas as some previous scoring rules did.61 Furthermore, our study did not include a whole body fat percentage assessment, such as dual energy X-ray absorptiometry (DEXA)62 in the otherwise detailed assessment of obesity level. But we had a very detailed assessment of actual volumes of both visceral and subcutaneous fat in the abdominal compartments. Finally, the blood was not drawn on the morning after the sleep study, but some time before the subject began CPAP therapy. But since the night-to-night variability in OSA severity is significantly smaller in home studies than in-lab polysomnography63 and the variability is smaller in severe OSA patients such as in this study64, we do not believe this to be of major concern.
Conclusions
The results of this study, the largest to date, on leptin levels in sleep apnea subjects show a dominant role of obesity and gender in leptin levels. OSA severity does not play a significant role except for a small association in a subgroup of nonobese nonhypertensive subjects. This finding requires replication and further assessment of its potential for clinical significance. Our results are in contrast to some of the previous literature and raise the question whether ethnicity plays a role in determining the effect of OSA on leptin levels.
Supplementary Material
Acknowledgements
We are grateful to Sigrun Gudmundsdottir, Lovisa Gudmundsdottir, Magdalena Osk Sigurgunnarsdottir, Kristjan Andri Kristjansson, Bethany Staley, Matthew Thorne-Fitzgerald, Robert Hachadoorian and the other staff at the Sleep Centers of Landspitali – The National University Hospital of Iceland and the University of Pennsylvania who helped assemble and analyze the data as well as Heather Collins and the other staff at the Radioimmunoassay and Biomarker Core, Diabetes and Endocrinology Research Center, University of Pennsylvania (NIH DK 19525), who performed the leptin measurements. We would also like to thank Ms. Karen McLaughlin and Mr. Daniel Barrett for their help in preparation of the manuscript.
Support:
This work was supported by NIH grant HL72067 for “A Family Linkage Study of Obstructive Sleep Apnea” and HL94307 for “Endophenotypes of Sleep Apnea and Role of Obesity”, the Eimskip Fund of the University of Iceland and the Landspitali University Hospital Research Fund.
Footnotes
Conflicts of interest:
A. Pack is the John Miclot Professor of Medicine. Funds for this endowment are provided by the Philips Respironics Foundation. Other authors report no conflicts of interest.
Supplementary information is available at IJO's website
References
- 1.Bravo PE, Morse S, Borne DM, Aguilar EA, Reisin E. Leptin and hypertension in obesity. Vasc Health Risk Manag. 2006;2(2):163–169. doi: 10.2147/vhrm.2006.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciccone M, Vettor R, Pannacciulli N, Minenna A, Bellacicco M, Rizzon P, et al. Plasma leptin is independently associated with the intima-media thickness of the common carotid artery. Int J Obes Relat Metab Disord. 2001;25(6):805–810. doi: 10.1038/sj.ijo.0801623. [DOI] [PubMed] [Google Scholar]
- 3.Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, et al. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999;34(5):1047–1052. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures in obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32(4):447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Lawati N, Mulgrew A, Cheema R, vanEeden S, Butt A, Fleetham J, et al. Pro-atherogenic cytokine profile of patients with suspected obstructive sleep apnea. Sleep Breath. 2009;13(4):391–395. doi: 10.1007/s11325-009-0259-1. [DOI] [PubMed] [Google Scholar]
- 6.Basoglu OK, Sarac F, Sarac S, Uluer H, Yilmaz C. Metabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and C-reactive protein in obese patients with obstructive sleep apnea syndrome. Ann Thorac Med. 2011;6(3):120–125. doi: 10.4103/1817-1737.82440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118(3):580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 8.Kapsimalis F, Varouchakis G, Manousaki A, Daskas S, Nikita D, Kryger M, et al. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186(4):209–217. doi: 10.1007/s00408-008-9082-x. [DOI] [PubMed] [Google Scholar]
- 9.Makinodan K, Yoshikawa M, Fukuoka A, Tamaki S, Koyama N, Yamauchi M, et al. Effect of serum leptin levels on hypercapnic ventilatory response in obstructive sleep apnea. Respiration. 2008;75(3):257–264. doi: 10.1159/000112471. [DOI] [PubMed] [Google Scholar]
- 10.McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175(2):190–195. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 11.Ozturk L, Unal M, Tamer L, Celikoglu F. The association of the severity of obstructive sleep apnea with plasma leptin levels. Arch Otolaryngol Head Neck Surg. 2003;129(5):538–540. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 12.Patel SR, Palmer LJ, Larkin EK, Jenny NS, White DP, Redline S. Relationship between obstructive sleep apnea and diurnal leptin rhythms. Sleep. 2004;27(2):235–239. doi: 10.1093/sleep/27.2.235. [DOI] [PubMed] [Google Scholar]
- 13.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi K, Kasahara Y, Kurosu K, Tanabe N, Takiguchi Y, Kuriyama T. Sleep oxygen desaturation and circulating leptin in obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127(3):716–721. doi: 10.1378/chest.127.3.716. [DOI] [PubMed] [Google Scholar]
- 15.Tokuda F, Sando Y, Matsui H, Koike H, Yokoyama T. Serum levels of adipocytokines, adiponectin and leptin, in patients with obstructive sleep apnea syndrome. Intern Med. 2008;47(21):1843–1849. doi: 10.2169/internalmedicine.47.1035. [DOI] [PubMed] [Google Scholar]
- 16.Ulukavak Ciftci T, Kokturk O, Bukan N, Bilgihan A. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration. 2005;72(4):395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 18.Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA. Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit. 2011;17(3):CR159–CR164. doi: 10.12659/MSM.881450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100(7):706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 20.Chin K, Nakamura T, Takahashi K, Sumi K, Ogawa Y, Masuzaki H, et al. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med. 2003;114(5):370–376. doi: 10.1016/s0002-9343(02)01570-x. [DOI] [PubMed] [Google Scholar]
- 21.Cuhadaroglu C, Utkusavas A, Ozturk L, Salman S, Ece T. Effects of nasal CPAP treatment on insulin resistance, lipid profile, and plasma leptin in sleep apnea. Lung. 2009;187(2):75–81. doi: 10.1007/s00408-008-9131-5. [DOI] [PubMed] [Google Scholar]
- 22.Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22(2):251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 23.Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997;6(2):146–147. doi: 10.1046/j.1365-2869.1997.00034.x. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu K, Chin K, Nakamura T, Masuzaki H, Ogawa Y, Hosokawa R, et al. Plasma leptin levels and cardiac sympathetic function in patients with obstructive sleep apnoea-hypopnoea syndrome. Thorax. 2002;57(5):429–434. doi: 10.1136/thorax.57.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trenell MI, Ward JA, Yee BJ, Phillips CL, Kemp GJ, Grunstein RR, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9(5):679–687. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 26.Drummond M, Winck JC, Guimaraes JT, Santos AC, Almeida J, Marques JA. Autoadjusting-CPAP effect on serum leptin concentrations in obstructive sleep apnoea patients. BMC Pulm Med. 2008;8:21. doi: 10.1186/1471-2466-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papaioannou I, Patterson M, Twigg GL, Vazir A, Ghatei M, Morrell MJ, et al. Lack of association between impaired glucose tolerance and appetite regulating hormones in patients with obstructive sleep apnea. J Clin Sleep Med. 2011;7(5) doi: 10.5664/JCSM.1314. 486-92B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-de-la-Torre M, Mediano O, Barcelo A, Pierola J, de la Pena M, Esquinas C, et al. The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath. 2011 Sep 13; doi: 10.1007/s11325-011-0552-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29.Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122(3):829–839. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 30.Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8(1):12–17. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Ursavas A, Ilcol YO, Nalci N, Karadag M, Ege E. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: Role of obesity. Ann Thorac Med. 2010;5(3):161–165. doi: 10.4103/1817-1737.65050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto Y, Fujiuchi S, Hiramatsu M, Nishigaki Y, Takeda A, Fujita Y, et al. Resistin Is Closely Related to Systemic Inflammation in Obstructive Sleep Apnea. Respiration. 2008;76(4):377–385. doi: 10.1159/000141866. [DOI] [PubMed] [Google Scholar]
- 33.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134(4):686–692. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 34.Garcia JM, Sharafkhaneh H, Hirshkowitz M, Elkhatib R, Sharafkhaneh A. Weight and metabolic effects of CPAP in obstructive sleep apnea patients with obesity. Respir Res. 2011;12(1):80. doi: 10.1186/1465-9921-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harsch IA, Schahin SP, Bruckner K, Radespiel-Troger M, Fuchs FS, Hahn EG, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71(3):252–259. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 36.Harsch IA, Koebnick C, Wallaschofski H, Schahin SP, Hahn EG, Ficker JH, et al. Resistin levels in patients with obstructive sleep apnoea syndrome--the link to subclinical inflammation? Med Sci Monit. 2004;10(9):CR510–CR515. [PubMed] [Google Scholar]
- 37.Sanner BM, Kollhosser P, Buechner N, Zidek W, Tepel M. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur Respir J. 2004;23(4):601–604. doi: 10.1183/09031936.04.00067804. [DOI] [PubMed] [Google Scholar]
- 38.Barcelo A, Barbe F, Llompart E, de la Pena M, Duran-Cantolla J, Ladaria A, et al. Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med. 2005;171(2):183–187. doi: 10.1164/rccm.200405-579OC. [DOI] [PubMed] [Google Scholar]
- 39.Carpagnano GE, Resta O, Pergola GD, Sabato R, Foschino Barbaro MP. The role of obstructive sleep apnea syndrome and obesity in determining leptin in the exhaled breath condensate. J Breath Res. 2010;4(3):036003. doi: 10.1088/1752-7155/4/3/036003. [DOI] [PubMed] [Google Scholar]
- 40.Lefebvre AM, Laville M, Vega N, Riou JP, van Gaal L, Auwerx J, et al. Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes. 1998;47(1):98–103. doi: 10.2337/diab.47.1.98. [DOI] [PubMed] [Google Scholar]
- 41.Minocci A, Savia G, Lucantoni R, Berselli ME, Tagliaferri M, Calo G, et al. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord. 2000;24(9):1139–1144. doi: 10.1038/sj.ijo.0801385. [DOI] [PubMed] [Google Scholar]
- 42.Montague CT, Prins JB, Sanders L, Digby JE, O'Rahilly S. Depot-and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46(3):342–347. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- 43.Maislin G, Ahmed MM, Gooneratne N, Thorne-Fitzgerald M, Kim C, Teff K, et al. Single Slice vs. Volumetric MR Assessment of Visceral Adipose Tissue: Reliability and Validity Among the Overweight and Obese. Obesity (Silver Spring) 2012 Mar 7; doi: 10.1038/oby.2012.53. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Efron B, Tibshirani RJ. An Introduction to the Bootstrap, (Monographs on Statistics and Applied Probability,57) New York: Chapman and Hall; 1993. [Google Scholar]
- 45.Myers RH, Montgomery DC. Response Surface Methodology, Process and Product Optimization Using Designed Experiments. New York: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 46.Arnardottir ES, Maislin G, Schwab RJ, Staley B, Benediktsdottir B, Olafsson I, et al. The Interaction of Obstructive Sleep Apnea and Obesity on the inflammatory markers C-reactive protein and interleukin-6: The Icelandic Sleep Apnea Cohort. Sleep. 2012 doi: 10.5665/sleep.1952. Accepted March 6, 2012 for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 50.Shinohara E, Kihara S, Yamashita S, Yamane M, Nishida M, Arai T, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241(1):11–18. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 51.Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34(8):1741–1748. doi: 10.2337/dc10-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Genta PR, Lorenzi-Filho G. Sleep apnoea in Asians and Caucasians: comparing apples and oranges. Eur Respir J. 2011;37(6):1537–1538. doi: 10.1183/09031936.00200510. author reply 1538–9. [DOI] [PubMed] [Google Scholar]
- 53.Lee RW, Vasudavan S, Hui DS, Prvan T, Petocz P, Darendeliler MA, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33(8):1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conroy SM, Chai W, Lim U, Franke AA, Cooney RV, Maskarinec G. Leptin, adiponectin, and obesity among Caucasian and Asian women. Mediators Inflamm. 2011:253580. doi: 10.1155/2011/253580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010;33(7):1629–1634. doi: 10.2337/dc09-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas T, Burguera B, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, Riggs BL, et al. Relationship of serum leptin levels with body composition and sex steroid and insulin levels in men and women. Metabolism. 2000;49(10):1278–1284. doi: 10.1053/meta.2000.9519. [DOI] [PubMed] [Google Scholar]
- 57.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51(5):434–451. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111(3):881–890. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehra R, Xu F, Babineau DC, Tracy RP, Jenny NS, Patel SR, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med. 2010;182(6):826–833. doi: 10.1164/rccm.201001-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21(2):253–259. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 61.Iber C, Ancoli-Israel S, Chesson A, Quan SF, editors. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 62.Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996;12(1):45–51. doi: 10.1016/0899-9007(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 63.Levendowski D, Steward D, Woodson BT, Olmstead R, Popovic D, Westbrook P. The impact of obstructive sleep apnea variability measured in-lab versus in-home on sample size calculations. Int Arch Med. 2009;2(1):2. doi: 10.1186/1755-7682-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wittig RM, Romaker A, Zorick FJ, Roehrs TA, Conway WA, Roth T. Night-to-night consistency of apneas during sleep. Am Rev Respir Dis. 1984;129(2):244–246. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.