Abstract
Purpose
To determine the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), pharmacokinetics (PK), and pharmacodynamics (PD) of sorafenib, bevacizumab, and low-dose oral cyclophosphamide in children and young adults with recurrent/refractory solid tumors.
Patients and Methods
Sorafenib dose was escalated from 90 mg/m2 to 110 mg/m2 twice daily with fixed doses of bevacizumab at 5 mg/kg every 3 weeks and cyclophosphamide at 50 mg/m2 daily. Once sorafenib’s MTD was established, bevacizumab dose was escalated. Each course was 21 days. PK and PD studies were performed during the first course.
Results
Nineteen patients (11 males; median age, 9.2 years) received a median of 4 courses (range, 1 to 23). DLTs during course 1 included grade 3 rash (2), increased lipase (1), anorexia (1), and thrombus (1). With an additional 71 courses of therapy, the most common toxicities ≥ grade 3 included neutropenia (9), lymphopenia (9), and rashes (4). Five of 17 evaluable patients had partial tumor responses, and 5 had disease stabilization (>2 courses). Median day 1 cyclophosphamide apparent oral clearance was 3.13 L/h/m2. Median day 1 sorafenib apparent oral clearance was 44 and 39 ml/min/m2 at the 2 dose levels evaluated, and steady-state concentrations ranged from1.64 to 4.8 mg/L. Inhibition of serum VEGFR2 was inversely correlated with sorafenib steady-state concentrations (p=0.019).
Conclusion
The recommended phase II doses are sorafenib, 90 mg/m2 twice daily; bevacizumab, 15 mg/kg q3 weeks; and cyclophosphamide, 50 mg/m2 once daily. This regimen is feasible with promising evidence of antitumor activity that warrants further investigation.
Keywords: sorafenib, cyclophosphamide, pediatric, phase I, bevacizumab
INTRODUCTION
Angiogenesis is necessary for tumor growth, metastasis, and survival. Vascular endothelial growth factor (VEGF) and its receptors, VEGFR-1 and VEGFR-2, and platelet derived growth factor (PDGF) and its receptors are key regulators of tumor vasculature. In preclinical models, dual inhibition of VEGF and PDGF signaling with low-dose, continuous “metronomic” chemotherapy results in more effective tumor suppression and improved survival.(1, 2) Additionally, more robust inhibition of VEGF signaling may be achieved by redundant inhibition of VEGF receptors and its ligand. This strategy may not only hinder angiogenesis and tumor growth but also circumvent resistance by impeding the feedback loop from elevated VEGF levels resulting from VEGF receptor inhibition.(3–5)
Bevacizumab (Avastin; Genentech, San Francisco, CA) is a VEGF-specific recombinant, humanized monoclonal antibody that binds directly to all four VEGF isoforms with high affinity and is approved for use in adults. In a pediatric phase I study of single-agent bevacizumab in patients with refractory solid tumors, no dose-limiting toxicities (DLTs) were observed when three dose levels (5, 10, and 15 mg/kg every 2 weeks) were studied. No objective responses were observed. Five patients had disease stabilization for more than 3 months.(6)
Sorafenib tosylate (BAY43-9006, Nexavar, Bayer Health Care Pharmaceuticals, Wayne, NJ) is an orally bioavailable multi-target kinase inhibitor of Raf-1, BRAF, FLT-3, p38α, and c-Kit as well as VEGFR-2, VEGFR-3, and PDGFRB. Sorafenib is approved for the treatment of adults with advanced renal cell carcinoma and unresectable hepatocellular carcinoma at 400 mg twice daily. In a pediatric phase I single agent study, the maximum tolerated dose (MTD) of sorafenib was 200 mg/m2 twice daily.(7) Grade 3 DLTs included elevated lipase, hyponatremia, hand-foot syndrome (HFS), rash, hypertension, and elevated ALT. No objective responses were observed.
Cyclophosphamide is a commonly chosen chemotherapy agent for continuous low-dose administration because of its good oral bioavailability, minimal toxicity at low doses, and extensive clinical use. Low-dose continuous oral dosing of cyclophosphamide has been used in adult and pediatric studies, usually in combination with other cytotoxic agents, with minimal toxicity.(8–13)
We conducted a single-institution phase I study of sorafenib, bevacizumab, and low-dose cyclophosphamide to define the toxicity profile, DLTs, and MTD of this combination in children and young adults with refractory or recurrent solid tumors. Pharmacokinetic studies of sorafenib and cyclophosphamide were performed along with pharmacodynamic studies, including serial sampling of angiogenic factors in the plasma and contrast-enhanced ultrasound to assess changes in tumor blood flow during therapy.
PATIENTS AND METHODS
Patient Population
Eligibility criteria included: solid tumor recurrent/refractory to standard therapy; age ≤ 21 years at initial diagnosis, life expectancy ≥ 8 weeks, Karnofsky/Lansky performance score of ≥ 50 and body surface area ≥ 0.3 m2. Laboratory criteria for enrollment included an absolute neutrophil count (ANC) ≥ 1000/m3,a platelet count ≥ 75,000/m3, hemoglobin≥ 8 g/dl, total bilirubin ≤ 1.5 × upper limit of normal (ULN) for age, ALT (SGPT) ≤ 2.5 × ULN for age, albumin ≥ 2 g/dL, PT/PTT/INR ≤ 1.2 × ULN, amylase and lipase ≤ 1.5 × ULN, GFR ≥ 70 ml/min/1.73 m2 or a normal serum creatinine for age, urine protein less than 1+ or ≤500 mg protein/24 hour urine collection. Patients with solid tumors metastatic to bone marrow were eligible for study but not evaluable for hematologic toxicity. Cardiac shortening fraction ≥ 28%, corrected QT interval ≤ 440, and hypertension well controlled for at least 2 weeks were required for study entry. Patient must have fully recovered from the acute toxic effects of all prior therapy; received no myelosuppressive therapy within 2 weeks, no biologic therapy within 7 days, no focal irradiation within 2 weeks, no craniospinal, total body, or whole pelvis irradiation within 3 months, no medications known to inhibit platelet functionor induce cytochrome P450 enzyme within 1 week, no hematopoietic growth factors within 1 week prior to study entry; no current or recent use of full-dose anticoagulants; no allogenic transplant within 3 months of study entry; negative pregnancy test if female; not breast-feeding if female; agreed to use an effective contraceptive method if male or female of reproductive potential. Exclusion criteria included: a history of deep venous or arterial thrombosis within 3 months of study entry, known bleeding diathesis or coagulopathy, known hypersensitivity to recombinant human antibodies, myocardial infarction, severe or unstable angina, or severe peripheral vascular disease or a chronic non-healing wound, ulcer, or bone fracture, or a major surgical procedure or significant traumatic injury within 28 days of study entry, and uncontrolled infection or evidence of intratumoral central nervous system hemorrhage on brain imaging prior to study entry.
Written informed consent was obtained from patients, parents, or legal guardians, with assent as appropriate. The protocol was approved by the institutional review board, which later approved review of the St. Jude medical records of those who continued treatment after removal from protocol therapy.
Drug Administration and Study Design
Bevacizumab was administered over 90 minutes with subsequent doses over 60 minutes and then 30 minutes if tolerated. Cyclophosphamide was administered as liquid or tablet. Sorafenib was administered as a combination of capsules (compounded from the commercially available 200 mg tablets in strengths of 10, 20, 50, and 100 mg). Capsules were opened and sprinkled on low to moderate fat-containing soft foods for administration to children who could not swallow capsules.
The study followed a traditional 3-plus-3 phase I design. The MTD was defined as one dose level below the dose level at which 2 or more of 6 patients experienced DLTs (see below for definition). The first cohort of patients received escalating doses of sorafenib (90, 110, 140 and 180 mg/m2/dose orally twice daily) with fixed doses of bevacizumab (5 mg/kg IV every 3 weeks) and cyclophosphamide (50 mg/m2 orally once daily). Once an MTD of sorafenib (sMTD) was established, then the bevacizumab dose was escalated to 10 mg/kg and 15 mg/kg. There was no intra-patient dose escalation.
Adverse events (toxicities) were graded according to the Common Terminology Criteria for Adverse Events version 3.0. DLT was defined as any left ventricular systolic dysfunction ≥ grade 2 or any nonhematologic toxicity ≥ grade 3 during the first course of therapy except for ,grade 3 nausea and vomiting, grade 3 hypertension well controlled with oral medication, grade 3 infection or fever, grade 3 hypophosphatemia or hypokalemia responsive to oral supplementation, grade 3 elevations in ALT or bilirubin that returned to ≤ 2.5 × ULN for age and ≤ 1.5 × ULN for age, respectively, within 7 days of stopping the drug, and asymptomatic grade 3 elevations in amylase and lipase that resolved to grade 1 within 7 days of drug interruption. Hematologic DLT was defined as an ANC less than 500/mm3 lasting longer than 7 days or platelet count less than 50,000/m3 requiring transfusion on more than 2 occasions in 7 days or grade 3 hemorrhage.
Pretreatment evaluations included a medical history, physical examination, performance status assessment, echocardiogram and electrocardiogram (ECG), complete blood count with differential (CBCD), haptoglobin, reticulocyte count, coagulation profile, amylase, lipase, serum electrolytes, renal and liver function studies, urinalysis, free T4 and thyroid stimulating hormone, bilateral knee radiographs to assess growth plates, and brain MRI or CT to exclude CNS hemorrhage. During the first course of treatment, weekly physical examinations, amylase, lipase, serum electrolytes, renal and liver function studies, urinalysis and twice weekly CBCD and reticulocyte counts were performed. After the first course, only weekly CBCD and reticulocyte counts were required. At the end of courses 1 and 2, and then after every other course, all pretreatment evaluations were repeated.
Disease evaluations were obtained at baseline, at the end of courses 1 and 2, and then at every other course. Tumor response was reported using the original Response Evaluation Criteria for Solid Tumors (RECIST).(14) Participants were required to complete a medication diary for every course of treatment.
A course could be repeated if the patient had at least stable disease and had recovered from the prior course of therapy such that the hematologic entry criteria had been met, hypertension and proteinuria were adequately controlled, and other drug-related adverse events were ≤ grade 1 or baseline, whichever was highest. Patients with grade 3 nonhematologic toxicity, with the specific exception of hypertension, nausea/vomiting, or electrolyte imbalances adequately controlled with medications/supplementations, which did not resolve to ≤ grade 1 or baseline within 2 weeks after completion of a course, were not permitted to resume therapy. Patients could continue on study treatment for a maximum of 24 courses if there was no disease progression or unacceptable toxicity.
Pharmacokinetic Studies
Pharmacokinetic studies for sorafenib and cyclophosphamide were performed in consenting patients.
Peripheral blood (1.5 mL) was collected after the first dose on day 1 before and 0.5, 2.0, 4.5, 6.0, 7.5, 24, and 48 h after sorafenib administration. After the first dose, sorafenib was withheld until the morning of day 3 (after the 48 h blood sample was obtained). Blood samples were also obtained during course 1 before sorafenib treatment on days 7, 13, and 21. Samples were centrifuged for 10 min at 3000×g and plasma was stored at −20°C until analysis. Sorafenib and the active metabolite sorafenib N-oxide were measured in human plasma using a validated analytical method based on high-performance liquid chromatography with tandem mass spectrometric detection, as previously described.(15) Sorafenib first-dose pharmacokinetic parameters were determined by nonlinear mixed-effects modeling via MONOLIX 3.2 (MONOLIX 3.2 User Guide, http://software.monolix.org; 2011) using the stochastic approximation of expectation-maximization algorithm. A one-compartment model with first-order oral absorption was used to describe the sorafenib plasma concentration-versus-time data. Individual sorafenib pharmacokinetic parameters were determined from the population pharmacokinetic model post hoc analysis, and individual values for the area under the concentration time curve (AUC) were determined from the concentration time profile simulated using the individual model-estimated pharmacokinetic parameters. The sorafenib AUC from time 0 to 12 h (AUC0–12h) was estimated for comparison with previously published sorafenib pharmacokinetic data in adults, and the AUC from time 0 to 24 h (AUC0–24h) was estimated for comparison with data obtained in a phase I study of sorafenib in children with solid tumors. The extent of metabolic conversion of sorafenib was determined as the ratio of sorafenib N-oxide concentration to sorafenib concentration on days 7, 13, and 21.
On day 1 of course 1, serial blood samples (1 mL) for pharmacokinetic studies of cyclophosphamide and its metabolites (4–hydroxy-cyclophosphamide and carboxyethylphosphoramide mustard) were collected before the first dose, and0.25, 0.5, 1.5, 2, and 6 hours after the dose. Samples were analyzed by an online extraction high-performance liquid chromatography method with tandem mass spectrometry.(16) The cyclophosphamide and metabolite concentration-time data were modeled by nonlinear mixed-effects modeling as implemented in NONMEM VII.(17) A two-compartment model with first-order absorption (ADVAN 5) was fit to plasma concentration-time data for cyclophosphamide while 4-hydroxy-cyclophosphamide and carboxyethylphosphoramide mustard were represented by separate compartments linked sequentially to the cyclophosphamide central compartment. After estimation of the population parameters, individual pharmacokinetic parameters were obtained using a post hoc analysis. Pharmacokinetic parameters estimated included apparent volume of the central compartment for cyclophosphamide and both measured metabolites, apparent oral clearance for cyclophosphamide and both measured metabolites, and absorption rate constant. The estimate of the AUC for each patient was calculated as the dose divided by the post hoc estimate of the apparent oral clearance.
Pharmacodynamic Studies
Contrast-enhanced ultrasound (CEUS) of a single target lesion was performed at baseline, on days 3 and 7 (± 2 days), at the end of courses 1 and 2, and then every other course and off treatment in consenting patients who had tumor that was visible on non-contrast enhanced sonography and met our institutional CEUS screening criteria. Our institutional CEUS criteria require patients to have a normal twelve lead ECG and echocardiogram with no evidence of right-to-left or bidirectional intracardiac shunting or pulmonary hypertension, an oxygen saturation of at least 92% on room air and no history or allergy to perflutren. In eligible patients, a perflutren contrast agent consisting of an injectable suspension of human serum albumin microspheres encapsulating octafluoropropane gas (Optison, General Electric Heath Care, Princeton, NJ) was administered at a dose of 0.3 mL to children weighing < 20 kg and 0.5 mL to those ≥ 20 kg. The contrast injection was followed by a 5 mL flush of normal saline. Because the contrast agent microspheres approximate the size of a red blood cell (range, 2–4.5 µm) they remain in the intravascular space and serve as a surrogate marker for tumor blood flow. A region of interest (ROI) within each tumor was identified for analysis. Using contrast-specific software, each ROI was evaluated for change in signal intensity from pre-contrast baseline to initial post-contrast peak (ΔSI, in decibels) and rate of signal intensity increase from baseline to initial peak (RSI, decibels per second). The examination was considered successful if a 5 dB increase in signal intensity was detected. When < 5 dB was detected, the dose was doubled and the examination repeated. If the second dose did not result in at least a 5 dB increase, the study was considered unsuccessful, and the patient did not undergo further CEUS during follow-up. Imaging parameters at baseline and at follow-up were compared to determine whether a change occurred that might indicate altered tumor blood flow in response to protocol therapy.
In consenting patients whose tumor evaluations were suitable for MRI imaging, dynamic contrast-enhanced MR imaging (DCE-MRI) was obtained at baseline, on day 7, at the end of courses 1 and 2, and then every other course. Dynamic MR studies consist of rapid sequential T1-weighted imaging before, during, and after delivery of a paramagnetic contrast agent into the tumor capillaries and its subsequent diffusion into the extravascular space. These sequential imaging sets were then analyzed using a pharmacokinetic model accounting for microvascular transport (Ktrans), vascular volume (vp), and extracellular/extravascular space (ve). Because the CEUS agent remains in the intravascular space, we anticipated a correlation between the DCE-MRI measure of vp and the CEUS measure of ΔSI.
Plasma VEGF, soluble VEGFR2 (sVEGFR2), soluble VEGFR3 (sVEGFR3), and PDGF-AB were quantified at baseline and on days 3, 7, 14, and 21 of course 1 using commercially available validated ELISA kits (RayBiotech, Inc., Norcross, GA) that included positive and negative controls, and quantified proteins with which to generate a standard curve. At the same time points, blood samples were also obtained to measure circulating endothelial cells (CECs: CD45-, CD34+, CD31+, CD133-) and circulating endothelial progenitors (CEPs: CD45-, CD34+, CD31+, CD133+). CECs and CEPs were measured by six-color flow cytometry using FACS LSR II machine (Becton Dickson, San Jose, CA) as previously described.(18) The antibodies used were againstCD31, CD45, CD133, and CD34.
Statistical Methods
The exact Wilcoxon sign test was used to test for differences in plasma protein levels and CECs and CEPs from baseline to the end of course 1. The association between sorafenib steady-state concentrations and inhibition of pharmacodynamics endpoints was assessed using the Spearman rank correlation. The response rate was estimated and reported with a 95% Blyth-Still-Casella confidence interval. Duration of response was defined as the time the RECIST criteria were met for objective response to the date of disease progression.
RESULTS
Patient Characteristics
Nineteen eligible patients were enrolled, all of whom were evaluable for toxicity. Patient characteristics are listed in Table 1. The median number of courses administered per patient was 4 (range, 1–23) and the total number of courses completed was 86.
Table 1.
Patient Characteristics
| No. patients | 19 | |
| Age on study (years) | ||
| Median (Range) | 9.2 (1.2–24.5) | |
| Sex | ||
| Male:Female | 11:8 | |
| Histologic diagnosis | ||
| Rhabdoid tumor | 5 | |
| Neuroblastoma | 3 | |
| Osteosarcoma | 2 | |
| Rhabdomyosarcoma | 2 | |
| Synovial sarcoma | 2 | |
| Other* | 5 | |
| Prior Therapy | ||
| No. prior chemotherapy regimen [median (range)] | 3 (1–6) | |
| Prior radiotherapy | 13 | |
| Prior doxorubicin | 18 | |
Wilms tumor (1), malignant peripheral nerve sheath tumor (1), adrenocortical carcinoma (1), epithelioid sarcoma (1), medulloblastoma (1)
Toxicity and MTD
No DLTs were observed in 3 patients at the first dose level (sorafenib 90 mg/m2, bevacizumab 5 mg/kg and cyclophosphamide 50 mg/m2). At the second dose level (sorafenib increased to 110 mg/m2), two DLTs were observed in 2 patients (grade 3 HFS, grade 3 elevated lipase). Thus, the sMTD was declared to be 90 mg/m2 and enrollment proceeded with dose escalation of bevacizumab to 10 mg/kg. Among the first cohort of 3 patients, 1 patient experienced DLT (grade 3 thrombus). Three additional patients were enrolled and none experienced DLT. The dose of bevacizumab was increased to 15 mg/kg. One of 6 patients experienced DLT (grade 3 HFS and anorexia). Although the MTD was not reached, the recommended phase II dose was defined as 90 mg/m2 of sorafenib, 15 mg/kg of bevacizumab, with 50 mg/m2 of cyclophosphamide.
The most common (> 60% of patients) nonhematologic toxicities were grade 1/2 elevation of AST, pain, vomiting, proteinuria, fatigue, and HFS. Grade 3/4 toxicities observed during and after course 1 are shown in Table 2. Five patients were taken off study therapy for unacceptable toxicities, including one each with pneumothorax (course 3), hemorrhagic cystitis (course 7), thrombosis (course 1), HFS (course 5), and HFS and anorexia (course 1). A total of 5 patients had dose modification in cyclophosphamide and/or sorafenib. Sorafenib was modified from 90 mg/m2 twice daily to once daily in three patients, all for HFS. Dose modification resulted in improvement of HFS in all three patients; however, one patient had an exacerbation after 2 courses. Because no further dose reduction was allowed by protocol, this patient was removed from the study. Four patients had cyclophosphamide dose reduction (from 50 to 25 mg/m2), 3 for neutropenia and one for thrombocytopenia.
Table 2.
Grade 3, 4 or 5 Toxicities Possibly or Probably Related to Treatment During and After Course 1*
| Course 1 | After Course 1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Level | Dose Level | |||||||||||||||
| Adverse Event† | 1 (n = 3) | 2 (n = 4) | 5 (n = 6) | 6 (n = 6) | 1 (n = 3) | 2 (n = 4) | 5 (n = 5) | 6 (n = 2) | ||||||||
| Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | |||||||||
| 3 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 5 | 3 | 4 | 3 | 4 | |
| Elevated lipase | 1 | |||||||||||||||
| Hypophosphatemia | 2 | 1 | ||||||||||||||
| Hypokalemia | 1 | |||||||||||||||
| Hyponatremia | 1 | |||||||||||||||
| Anorexia | 1 | |||||||||||||||
| Weight loss | 1 | |||||||||||||||
| Vomiting | 1 | |||||||||||||||
| Hand-foot syndrome | 1 | 1 | 1 | 1 | 2 | |||||||||||
| Pneumothorax | 1 | |||||||||||||||
| Decreased leukocytes (total) | 1 | 2 | 2 | 1 | 1 | |||||||||||
| Lymphopenia | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | |||||
| Neutropenia | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | |||||
| Anemia | 1 | 2 | 1 | |||||||||||||
| Thrombocytopenia | 1 | |||||||||||||||
| Thrombosis | 1 | |||||||||||||||
| Febrile neutropenia | 1 | 1 | ||||||||||||||
| Infection with neutropenia | 1 | |||||||||||||||
| Bladder | 1 | |||||||||||||||
Grades are according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Each adverse event was counted once (any course, highest grade) for each patient.
Three of 12 patients with lung nodules developed pneumothorax. Pneumothorax was first noted on CT chest performed at the end of course 1 in two patients and the end of course 2 in one patient. One patient was treated at the second dose level (sorafenib 110 mg/m2, cyclophosphamide 50 mg/m2 and bevacizumab 5 mg/kg) and two patients were treated with the same dose of cyclophosphamide, but with the sMTD of sorafenib of 90 mg/m2 and an escalated dose of bevacizumab of 10 mg/kg. In all cases, the development of pneumothorax was associated with tumor response. Two patients required chest tube placement, and one died as a complication of pneumothorax.
With the exception of HFS, other significant toxicities frequently described in adults receiving bevacizumab and/or sorafenib were uncommon. In most cases, bleeding was limited to self-limited epistaxis, and complaints of fatigue did not interfere with activities of daily living. One patient had grade 2hypertension which was well controlled with amlodipine and one had grade 2 left ventricular dysfunction requiring interruption of study medications for one week. One patient had a thrombus at the tumor site 5 days after the initiation of therapy that was most likely related to inflammation and swelling from tumor necrosis. This patient received an additional 10 courses of therapy off study without complications until disease progression. Five patients had elevated thyroid stimulating hormone, and all but one of these patients had a free T4 level within the normal range. The patient with abnormal free T4 (low) was treated with levothyroxine. No growth plate abnormalities were observed.
Two patients developed cystitis, one during course 2 and the other during course 5. Intravenous or oral fluids to facilitate hydration and medications for bladder spasms/pain were administered with symptomatic improvement. However, one patient elected to discontinue study therapy because of this complication.
Pharmacokinetics Studies
Sorafenib first-dose pharmacokinetic studies were performed in 15 patients (Table 3). Substantial inter-patient exposure variability was observed (6- to 8-fold from the minimum to maximum AUC values). Median apparent oral sorafenib clearance at 90 mg/m2 was similar to that at 110 mg/m2 (44 vs 39 mL/min/m2, p > 0.6). Median sorafenib steady-state concentrations sorafenib N-oxide steady-state concentrations and sorafenib N-oxide metabolic ratios on days 7, 13, and 21 are shown in Table 4 and were similar at both dose levels. There was no correlation between the sorafenib steady-state plasma concentrations during the first course and the development of DLT.
Table 3.
Sorafenib first-dose pharmacokinetic parameters.
| Dose (mg/m2 ) |
n | Ka (h−1) | Cmax (mg/L) | Tmax (h) | AUC0–12h (mg*h/L) |
AUC0–24h (mg*h/L) |
t1/2 (h) | CL/F (mL/min/m2) |
|---|---|---|---|---|---|---|---|---|
| 90 | 11 | 0.10 (0.038 – 0.72) | 1.1 (0.35 – 2.5) | 8.7 (3.4 – 17) | 10 (2.5 – 24) | 20 (6.6 – 41) | 5.5 (0.73 – 113) | 44 (7.0 – 146) |
| 110 | 4 | 0.093 (0.080 – 0.15) | 1.6 (0.92 – 2.4) | 11 (8.2 – 14) | 14 (8.0 – 20) | 30 (16 – 47) | 10 (3.9 – 12) | 39 (25 – 80) |
Values are the median (range).
Abbreviations: AUC0–12h, area under the plasma concentration-time curve from time zero to 12 hours; AUC0–24h, area under the plasma concentration-time curve from time zero to 24 hours; Cmax, maximum plasma concentration; CL/F, apparent oral clearance; Ka, absorption rate constant; Tmax, time to maximum plasma concentration; t1/2, half-life.
Table 4.
Sorafenib and sorafenib N-oxide steady-state concentrations.
| Sorafenib Ctrough (mg/L) | Sorafenib N-oxide Ctrough (mg/L) | N-oxide metabolic ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/m2) |
n | Day 7 | Day 13 | Day 21 | Day 7 | Day 13 | Day 21 | Day 7 | Day 13 | Day 21 |
| 90 | 13 | 3.5 (1.2 – 14.4) | 2.5 (1.2 – 15.7) | 4.8 (1.1 – 11.7) | 0.50 (0.11 – 4.5) | 0.54 (0.17 – 3.4) | 0.72 (0.17 – 4.0 | 0.14 (0.066 – 0.31) | 0.21 (0.12 – 0.37) | 0.20 (0.11 – 0.34) |
| 110 | 4 | 3.0 (3.0 – 3.5) | 2.1 (1.4 – 4.9) | 1.6 (1.4 – 5.7) | 0.57 (0.37 – 1.5) | 0.37 (0.33 – 0.92) | 0.40 (0.20 – 0.78) | 0.18 (0.12 – 0.49) | 0.19 (0.18 – 0.23) | 0.14 (0.12 – 0.29) |
Values are the median (range).
Cyclophosphamide pharmacokinetic studies were performed in 18 patients. Of these 18 patients, 7 received the cyclophosphamide dose in tablet form versus 11 patients who received the dose as a liquid formulation. The median (range) cyclophosphamide absorption rate constant, apparent oral clearance, and apparent volume of distribution were 0.17 hours−1 (0.15 to 0.21 hours−1), 3.13 L/h/m2 (2.42 to 3.89 L/h/m2), and 2.28 L/m2 (0.58 to 13.09 L/m2), respectively. The median (range) apparent oral clearance and apparent volume of distribution for 4-hydroxy-cyclophosphamide were 44.75 L/h/m2 (26.12 to 92.99 L/h/m2) and 3.01 mL/m2 (2.99 to 3.03 mL/m2) while the median (range) apparent clearance and apparent volume of distribution for carboxyethylphosphoramide mustard were 65.10 L/h/m2 (39.01 to 91.83 L/h/m2) and 116.60 L/m2 (5.10 to 942.90 L/m2). The median cyclophosphamide,4-hydroxy-cyclophosphamide, and carboxyethylphosphoramide mustard AUC0-∞ were 62.5µM*h (49.2 to 80.9µM*h), 4.3 µM*h (1.9 to 7.6µM*h), and 3.0 µM*h (1.9 to 5.5 µM*h), respectively. The AUC0-∞ for the active metabolite, 4-hydroxy-cyclophosphamide, was on average ~5% higher when the cyclophosphamide oral formulation was administered as a liquid vs. tablet, but the difference was not found to be statistically significant (p = 0.82).
Pharmacodynamics Studies
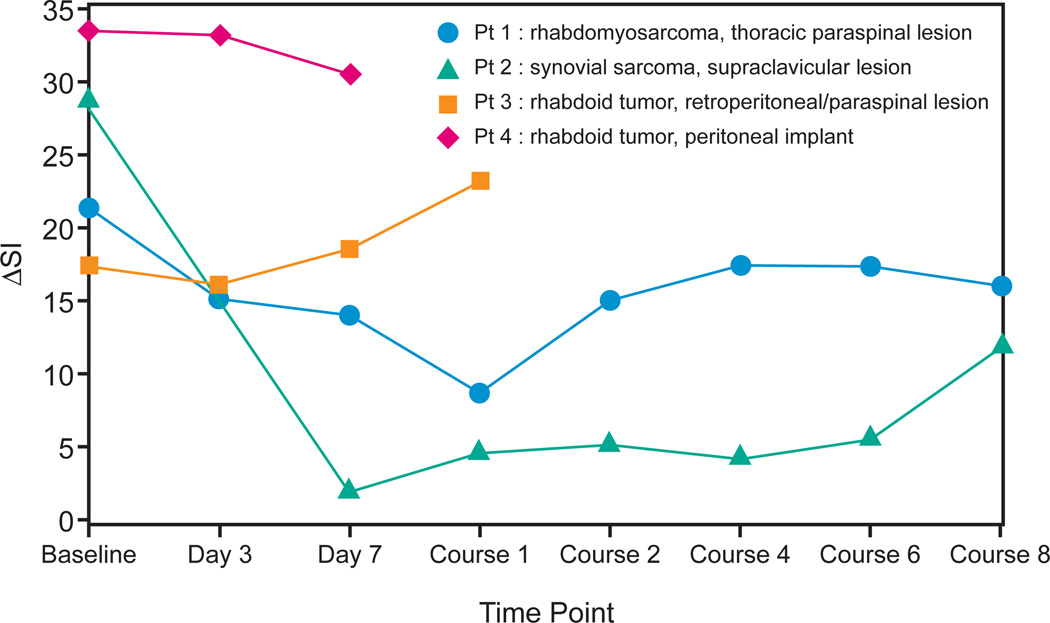
Seven patients consented to CEUS. Two baseline CEUS examinations were excluded for technical difficulties; 1 was excluded because the femoral node evaluated was not biopsy-proven to represent metastatic disease. The remaining 4(age range, 21 months to 15 years) underwent a total of 22 CEUS examinations of target lesions. Results are shown in Figure 1 and suggest that greater decreases in tumor blood flow, measured by CEUS early in therapy, may predict better outcome. The RSI for these patients did not predict time to progression and was not a valuable parameter.
Figure 1.
Change in signal intensity from pre-contrast baseline to initial post-contrast peak (ΔSI, in decibels) through therapy in 4 patients who underwent contrast-enhanced ultrasound (CEUS) of target lesions. Patient 1 and 2 showed substantial decreases in ΔSI on days 3 and 7 and at the end of course 1, and both had long times to progression. The maximum decline in ΔSI in these two patients was 12.6 and 26.8 decibels, respectively. In contrast, patient 3 and 4 showing minimal decrease or an increase in ΔSI at the same early time points both had progressive disease by the end of course 1. The maximum decline in ΔSI in these two patients was 1.1 and 2.9 decibels, respectively.
Only two patients had serial DCE-MRI examinations. While both patients exhibited a rapid decrease in Ktrans and vp in the first 7 days, one patient plateaued with relatively stable DCE-MRI measures while the other patient continued to have progressive decreases in both Ktrans and vp with additional courses. One of these patients had both longitudinal CEUS and DCE-MRI examinations. Both quantitative DCE-MRI measures (Ktrans, vp, ve) and ΔSI rapidly decreased from baseline to day 7 and then maintained a relatively stable appearance thereafter.
Fifteen patients had samples available for analysis of plasma proteins, CECs and CEPs at baseline and during course 1. Only sVEGFR3 levels and CEPs showed a statistically significant change (decrease) from baseline to the end of course 1 (p = 0.007 and p = 0.026, respectively). Sorafenib steady-state concentrations on day 21 were inversely correlated with inhibition of VEGFR2 on day 21 (p = 0.019) and CECs on day 21 (p = 0.01). No correlation between response and change in protein levels or CECs and CEPs was detected in the small number of patients tested.
Tumor Response
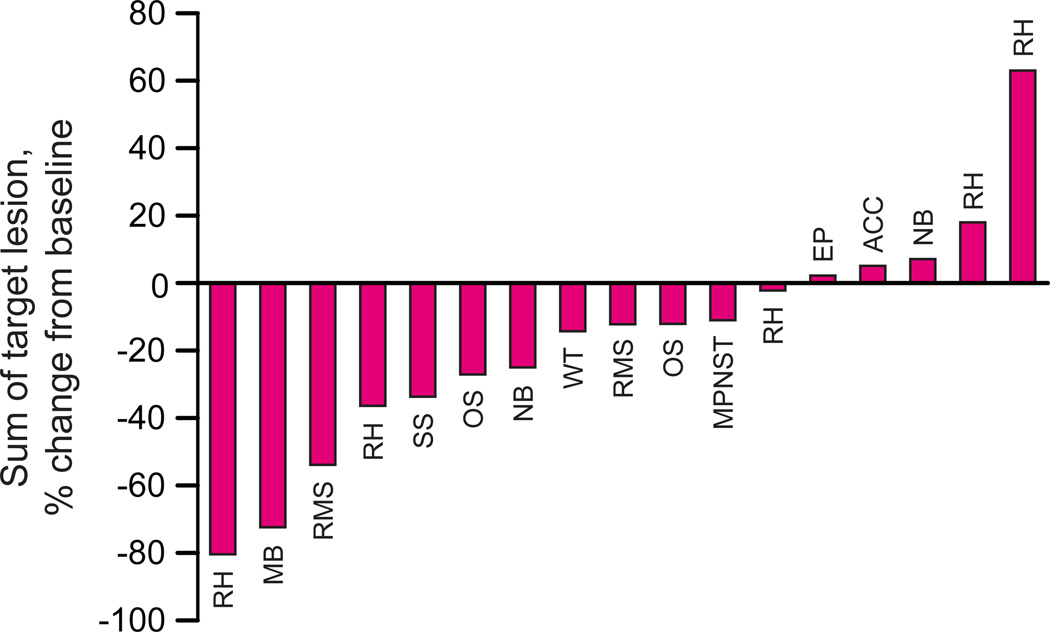
Seventeen patients were evaluable for tumor response by RECIST. Figure 2 shows the percent changes in the sum of the longest diameters of all target lesions for each patient from baseline to maximum (best) percent change.
Figure 2.
Waterfall plot of percent change in the sum of the longest diameter of all target lesions from baseline in 17 patients with evaluable for tumor response by RECIST. Abbreviations: RH, rhabdoid tumor; MB, medulloblastoma; RMS, rhabdomyosarcoma; SS, synovial sarcoma; NB, neuroblastoma; WT, Wilms tumor; MPNST, malignant peripheral nerve sheath tumor; EP, epithelioid sarcoma; ACC, adrenocortical carcinoma.
Five patients had partial responses. Responses were confirmed in 3 patients (one each with rhabdomyosarcoma, rhabdoid tumor, and medulloblastoma), with a median duration of response of 12 weeks (range, 12 – 23 weeks). Two additional patients achieved a PR that was not confirmed for at least 4 weeks.
Five patients (two with osteosarcoma and one each with neuroblastoma, MPNST, and adrenocortical carcinoma) had stable disease lasting more than 2 courses (median, 4 courses; range, 3–7 courses). Three patients had progressive disease after one course and 4 patients after 2 courses. Considering any partial response (confirmed or unconfirmed), the observed response rate was 29.4% (5/17) (95% CI, 12.4%–54.4%). Considering only confirmed PR, the observed response rate was 17.6% (3/17) (95% CI, 5.0%–41.7%).
DISCUSSION
In this study, the recommended dosage of the combination tested was found to be 90 mg/m2 of sorafenib by mouth twice daily, 15 mg/kg of bevacizumab IV every 3 weeks, and 50 mg/m2 of cyclophosphamide by mouth once daily. Although we were able to escalate the bevacizumab to its single-agent dose, we were unable to escalate the dose of sorafenib beyond approximately half of its single-agent dose due to HFS and elevated lipase. This finding is in contrast with the adult study of the combination sorafenib and bevacizumab in which neither drug was tolerable at the single-agent dose. The DLTs in adults included proteinuria and thrombocytopenia.(3)
Overall, the study therapy was well tolerated and the toxicities manageable. Notable toxicities observed were dermatologic reactions and pneumothoraces. Skin reactions, primarily HFS attributed to sorafenib, have been reported to occur in 20% to 100% of patients.(19, 20) Thirteen of the 19 patients in our study had grade 1 or higher HFS. We learned as the study progressed that early initiation of emollients and initiation of pyridoxine therapy was beneficial and that interruption or dose reduction of sorafenib in most patients with grade 2 or 3 HFS prevented further progression or recurrence of skin toxicity.
Three patients in our study had a pneumothorax associated with tumor shrinkage and cavitation of pulmonary lesions. All three had a history of thoracotomies, but none had a history of spontaneous pneumothorax. Two of the patients were asymptomatic, and one patient died as a complication of pneumothorax following chest tube placement and pleurodesis. Spontaneous pneumothorax as a complication of anti-angiogenic therapy has been reported in the literature.(21–23) The mechanism is unknown but since the development of the pneumothorax seems to always be associated with necrosis and cavitation of the tumor lesion, we suspect that an air leak is generated as a consequence.
Objective responses and prolonged stabilization of disease in a variety tumor types were observed in our cohort of heavily pretreated patients, many of whom had rapidly progressive disease at study entry. The anti-tumor activity observed with the combination of sorafenib, bevacizumab, and low-dose cyclophosphamide appears to be greater than that observed with the reported activity of these drugs as single agents.(6, 24–27) However, it would not be possible to affirm this with certainty in the absence of a larger or randomized phase II study comparing each of the drugs as single agents and in various combinations. The latter would not be feasible in the pediatric setting.
Although cyclophosphamide has been used clinically for several decades, very few publications are available related to the disposition of this compound in children. To the best of our knowledge, this is the first report describing oral cyclophosphamide pharmacokinetics in this population. Several pharmacokinetic investigations have been conducted in adults, but most studies report only intravenous pharmacokinetics.(28–34) In this report, the cyclophosphamide apparent oral clearance of 3.13 L/h/m2 is consistent with previous intravenous administration values(35–37) obtained in children (2.9 to 4.23 L/h/m2), suggesting that the compound is well absorbed from the gastrointestinal tract at this dosage. In one of the few adult studies describing oral pharmacokinetics, the mean apparent oral clearance was reported as 6.18 L/h.(33) Assuming an average adult body surface area of 1.73 m2, this corresponds to a BSA normalized value of 3.57 L/h/m2,also in good agreement with the data presented here. To our knowledge, no oral pharmacokinetic data has been reported in adults at a dosage of 50 mg/m2, but a published study at a 12-fold higher oral dosage of 600 mg/m2 resulted in a mean AUC of 699.6 µM*h which is about ~11 times higher than the median AUC value observed in our study.(34) We also observed no statistically significant difference in the AUC0-∞ for the active metabolite, 4-hydroxy-cyclophosphamide, when the cyclophosphamide oral formulation was administered as a liquid versus tablet. The median AUC ratio for 4-hydroxy-cyclophosphamide/cyclophosphamide of 0.07 (range: 0.03 – 0.14) was similar to previous findings in adult patients (0.04 to 0.09) suggesting hepatic metabolism of cyclophosphamide was unaltered by co-administration of sorafenib.(19, 38, 39) Overall, the pharmacokinetic findings from our study are similar to those previously reported.
In this study, median sorafenib exposure (AUC0–24h) after the first dose when given in combination with oral cyclophosphamide was approximately twice as high as that reported in a preliminary report in children with solid tumors receiving single-agent sorafenib at a similar dose of 105 mg/m2.(7) The apparently higher sorafenib exposure that was observed in our study could be due to co-administration with oral cyclophosphamide, interaction with food, use of an alternative formulation of sorafenib, or wide inter-patient sorafenib pharmacokinetic variability. However, median sorafenib steady-state concentrations observed at the two dose levels evaluated (range, 1.6 to 4.8 mg/L) were similar to steady-state concentrations achieved in adults receiving an approximately equivalent sorafenib dose of 200 mg twice daily (range, 1.6 to 4.2 mg/L), but were lower than children with relapsed/refractory acute myeloid leukemia receiving a higher sorafenib dose of 200 mg/m2 twice daily (6.5 mg/L).(20, 40) In our study, the conversion of sorafenib to the active metabolite sorafenib N-oxide ranged from 14% to 21%. Metabolic conversion was higher than that reported in adult patients receiving single-agent sorafenib (mean, <10%),(41) but lower than in children with AML receiving single-agent sorafenib (mean, 33%).(20) Therefore, it is unlikely that co-administration with oral cyclophosphamide alters sorafenib CYP3A4-mediated metabolism to sorafenib N-oxide.
Levels of angiogenic factors known to be targeted by the study therapy, VEGF, sVEGFR2, sVEGFR3, and PDGF, were variable through the first course of therapy and showed no correlation with response or disease stabilization in this small cohort of patients (data not shown). Interestingly, the only factor that showed a significant change from baseline to the end of the first course was sVEGFR3. VEGFR3 is expressed on the surface of lymphatic endothelial cells and is implicated in tumor lymphangiogenesis. We also detected a significant decrease in CEPs from baseline to end of course 1. Whether a change in plasma sVEGFR3 or CEPs could serve as a predictor for response, development of resistance or metastases, or outcome will require validation in a larger cohort of patients.
CEUS is emerging as reliable method to assess tumor vascularity in preclinical models and clinical trials.(42–46) We explored the use of CEUS as a noninvasive, less costly technique to assess tumor microcirculation in response to our anti-angiogenic therapy. We found that CEUS was well tolerated and easy to perform in children and adolescents. A limitation of this technique was that not all sites of disease were amenable to sonographic visualization. Nonetheless, although only 4 patients were fully evaluated by CEUS, it is noteworthy that the 2 patients who had a rapid decline in change of contrast flow through the tumor vasculature by day 7 had prolonged disease control in contrast to the 2 patients that did not have a rapid decline. Furthermore, in the single patient who had serial CEUS and DCE-MRI performed, there was good correlation between the vascular flow measurements in the two modalities. These findings will require validation in a larger cohort of patients. If confirmed, then changes in tumor blood flow using CEUS could serve as an early surrogate imaging marker of response and as a useful tool in defining an optimal dose of anti-angiogenic therapy.
We have defined a recommended dose of sorafenib, bevacizumab, and low-dose cyclophosphamide for phase II testing. The combination is tolerable and shows promising anti-tumor activity in a variety of tumors; however, it should be used with caution in patients with pulmonary metastatic disease. This type of anti-angiogenic therapy would be ideal for maintenance therapy in the setting of minimal residual disease, although further investigation is needed to assess its degree of activity in various tumor types.
TRANSLATIONAL RELEVANCE.
Angiogenesis is necessary for tumor growth, metastasis, and survival. Vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF) and their receptors are key regulators of tumor vasculature. Based on preclinical studies demonstrating tumor suppression and survival advantage in mouse models when targeting both of these pathways combined with low dose cyclophosphamide, we performed a dose finding and pharmacokinetic study of sorafenib, low-dose oral cyclophosphamide and bevacizumab in children and young adults with refractory/recurrent solid tumors. Our results show that this drug combination is tolerable, has promising anti-tumor activity in a variety of tumors, and would be well-suited for maintenance therapy in the setting of minimal residual disease.
ACKNOWLEDGEMENTS
We thank all of the patients and their families, research nurses and clinical and laboratory personnel, study coordinators, and operations staff who participated in this study.
GRANT SUPPORT
Cancer Center Grant CA23099 and Cancer Center Support CORE Grant P30 CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities
Footnotes
Conflict of Interest: The authors declare no potential conflict of interest.
REFERENCES
- 1.Kumar S, Mokhtari RB, Sheikh R, Wu B, Zhang L, Xu P, et al. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res. 2011;17:5656–5667. doi: 10.1158/1078-0432.CCR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose "chemo-switch" regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 3.Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–5362. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- 5.Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487–6501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 6.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 7.Widemann BC, Fox E, Adamson PC, Baruchel A, Kim A, Ingle AM, et al. Phase I study of sorafenib in children with refractory solid tumors: a Children's Oncology Group phase I consortium trial. ASCO. 2009 doi: 10.1158/1078-0432.CCR-11-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolis G, Bortolozzi G, Carinelli G, D'Incalci M, Gramellini F, Morasca L, et al. Low-dose cyclophosphamide versus adriamycin plus cyclophosphamide in advanced ovarian cancer. A randomized clinical study. Cancer Chemother Pharmacol. 1980;4:129–132. doi: 10.1007/BF00254034. [DOI] [PubMed] [Google Scholar]
- 9.Casanova M, Ferrari A, Bisogno G, Merks JH, De Salvo GL, Meazza C, et al. Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: pilot study for the upcoming European Rhabdomyosarcoma Protocol. Cancer. 2004;101:1664–1671. doi: 10.1002/cncr.20544. [DOI] [PubMed] [Google Scholar]
- 10.Glode LM, Barqawi A, Crighton F, Crawford ED, Kerbel R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer. 2003;98:1643–1648. doi: 10.1002/cncr.11713. [DOI] [PubMed] [Google Scholar]
- 11.Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–581. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 12.Maurer HM, Beltangady M, Gehan EA, Crist W, Hammond D, Hays DM, et al. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Maurer HM, Gehan EA, Beltangady M, Crist W, Dickman PS, Donaldson SS, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Zhao M, Navid F, Pratz K, Smith BD, Rudek MA, et al. Quantitation of sorafenib and its active metabolite sorafenib N-oxide in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B, Analyt Technol Biomed Life Sci. 2010;878:3033–3038. doi: 10.1016/j.jchromb.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai F, Fraga CH, Tagen M, Schaiquevich P, Hagedorn N, Stewart CF. Simultaneous determination of cyclophosphamide and carboxyethylphosphoramide mustard in human plasma using online extraction and electrospray tandem mass spectrometry (HTLC-ESI-MS/MS) J Chromatogr B, Analyt Technol Biomed Life Sci. 2009;877:1709–1715. doi: 10.1016/j.jchromb.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User's Guide (1989 – 2009) Ellicott City, MD, USA: Icon Development Solutions; 2009. [Google Scholar]
- 18.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen TL, Kennedy MJ, Anderson LW, Kiraly SB, Black KC, Colvin OM, et al. Nonlinear pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide/aldophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Drug Metab Dispos. 1997;25:544–551. [PubMed] [Google Scholar]
- 20.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29:3293–3300. doi: 10.1200/JCO.2011.34.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox E, Aplenc R, Bagatell R, Chuk MK, Dombi E, Goodspeed W, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28:5174–5181. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladoire S, Beynat C, Diaz P, Coudert B, Favier L, Ghiringhelli F. Spontaneous pyopneumothorax in patients treated with mTOR inhibitors for subpleural pulmonary metastases. Med Oncol. 2010;27:938–941. doi: 10.1007/s12032-009-9311-z. [DOI] [PubMed] [Google Scholar]
- 23.Tamura T, Tamura S, Nasu H, Fujimoto T, Kinoshita T. [A case of intractable pneumothorax in a patient with pulmonary adenocarcinoma during bevacizumab-containing chemotherapy] Nihon Kokyuki Gakkai Zasshi = The Journal of the Japanese Respiratory Society. 2011;49:702–706. [PubMed] [Google Scholar]
- 24.Grignani G, Palmerini E, Dileo P, Asaftei SD, D'Ambrosio L, Pignochino Y, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23:508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 25.Maki RG, D'Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacey S, Ratain MJ, Flaherty KT, Kaye SB, Cupit L, Rowinsky EK, et al. Efficacy and safety of sorafenib in a subset of patients with advanced soft tissue sarcoma from a Phase II randomized discontinuation trial. Invest New Drugs. 2011;29:481–488. doi: 10.1007/s10637-009-9367-9. [DOI] [PubMed] [Google Scholar]
- 27.Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: After 10 years of experience, where do we stand and where are we going? Critl Rev in Oncol Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Ayash LJ, Wright JE, Tretyakov O, Gonin R, Elias A, Wheeler C, et al. Cyclophosphamide pharmacokinetics: correlation with cardiac toxicity and tumor response. J Clin Oncol. 1992;10:995–1000. doi: 10.1200/JCO.1992.10.6.995. [DOI] [PubMed] [Google Scholar]
- 29.D'Incalci M, Bolis G, Facchinetti T, Mangioni C, Morasca L, Morazzoni P, et al. Decreased half life of cyclophosphamide in patients under continual treatment. Eur J Cancer. 1979;15:7–10. doi: 10.1016/0014-2964(79)90198-1. [DOI] [PubMed] [Google Scholar]
- 30.D'Incalci M, Facchinetti T, Bolis G, Mangioni C, Morasca L. Pharmacokinetics of cyclophosphamide after prolonged low dose treatment in ovarian cancer patients. Eur J Drug Metab Pharmacokinet. 1979;4:83–85. doi: 10.1007/BF03189405. [DOI] [PubMed] [Google Scholar]
- 31.Egorin MJ, Forrest A, Belani CP, Ratain MJ, Abrams JS, Van Echo DA. A limited sampling strategy for cyclophosphamide pharmacokinetics. Cancer Res. 1989;49:3129–3133. [PubMed] [Google Scholar]
- 32.Haubitz M, Bohnenstengel F, Brunkhorst R, Schwab M, Hofmann U, Busse D. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int. 2002;61:1495–1501. doi: 10.1046/j.1523-1755.2002.00279.x. [DOI] [PubMed] [Google Scholar]
- 33.Juma FD, Rogers HJ, Trounce JR. Pharmacokinetics of cyclophosphamide and alkylating activity in man after intravenous and oral administration. Br J Clin Pharmacol. 1979;8:209–217. doi: 10.1111/j.1365-2125.1979.tb01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Struck RF, Alberts DS, Horne K, Phillips JG, Peng YM, Roe DJ. Plasma pharmacokinetics of cyclophosphamide and its cytotoxic metabolites after intravenous versus oral administration in a randomized, crossover trial. Cancer Res. 1987;47:2723–2726. [PubMed] [Google Scholar]
- 35.McCune JS, Salinger DH, Vicini P, Oglesby C, Blough DK, Park JR. Population pharmacokinetics of cyclophosphamide and metabolites in children with neuroblastoma: a report from the Children's Oncology Group. J Clin Pharmacol. 2009;49:88–102. doi: 10.1177/0091270008325928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yule SM, Boddy AV, Cole M, Price L, Wyllie R, Tasso MJ, et al. Cyclophosphamide pharmacokinetics in children. Br J Clin Pharmacol. 1996;41:13–19. doi: 10.1111/j.1365-2125.1996.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 37.Yule SM, Price L, McMahon AD, Pearson AD, Boddy AV. Cyclophosphamide metabolism in children with non-Hodgkin's lymphoma. Clin Cancer Res. 2004;10:455–460. doi: 10.1158/1078-0432.ccr-0844-03. [DOI] [PubMed] [Google Scholar]
- 38.Joy MS, La M, Wang J, Bridges AS, Hu Y, Hogan SL, et al. Cyclophosphamide and 4-hydroxycyclophosphamide pharmacokinetics in patients with glomerulonephritis secondary to lupus and small vessel vasculitis. Br J Clin Pharmacol. 2012;74:445–455. doi: 10.1111/j.1365-2125.2012.04223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slattery JT, Kalhorn TF, McDonald GB, Lambert K, Buckner CD, Bensinger WI, et al. Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol. 1996;14:1484–1494. doi: 10.1200/JCO.1996.14.5.1484. [DOI] [PubMed] [Google Scholar]
- 40.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 41.Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24:1437–1444. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 43.Lamuraglia M, Escudier B, Chami L, Schwartz B, Leclere J, Roche A, et al. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced doppler ultrasound. Eur J Cancer. 2006;42:2472–2479. doi: 10.1016/j.ejca.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Lassau N, Lamuraglia M, Chami L, Leclere J, Bonvalot S, Terrier P, et al. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol. 2006;187:1267–1273. doi: 10.2214/AJR.05.1192. [DOI] [PubMed] [Google Scholar]
- 45.McCarville MB, Streck CJ, Dickson PV, Li CS, Nathwani AC, Davidoff AM. Angiogenesis inhibitors in a murine neuroblastoma model: quantitative assessment of intratumoral blood flow with contrast-enhanced gray-scale US. Radiology. 2006;240:73–81. doi: 10.1148/radiol.2401050709. [DOI] [PubMed] [Google Scholar]
- 46.Sirsi SR, Flexman ML, Vlachos F, Huang J, Hernandez SL, Kim HK, et al. Contrast ultrasound imaging for identification of early responder tumor models to anti-angiogenic therapy. Ultrasound Med Biol. 2012;38:1019–1029. doi: 10.1016/j.ultrasmedbio.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]