Abstract
The co-occurrence of alcoholism and depression is highly prevalent and difficult to treat. In an animal model of binge drinking that exhibits abstinence-induced behaviors reminiscent of negative affective states, the triple monoamine uptake inhibitor, amitifadine, produced a selective, dose dependent attenuation of binge drinking. Amitifadine also reversed abstinence-induced increases in the intracranial self-stimulation threshold, a model of anhedonia, and immobility in the forced swim test, reflecting behavioral despair. In view of the safety profile of amitifadine in humans, including low risk for weight gain, lack of sexual side effects, and low potential for abuse, we hypothesize that amitifadine will be effective in treating co-occurring alcoholism and depression.
Keywords: alcoholism, depression, triple uptake inhibitor, anhedonia, negative affective states, behavioral despair
1. INTRODUCTION
Alcoholism and depression often co-occur, but no single agent is effective in treating both conditions. Administration of antidepressants (e.g. selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs)) to depressed alcoholics reduces depressive symptomatology, but in most cases has little direct effect on drinking compared to placebo (Nunes and Levin, 2004; Pettinati, 2004). Thus, six of eight studies (75%) found a relationship between the medication and a reduction in depressive symptomatology (Pettinati, 2004); while only three of the eight studies (38%) found a significant advantage for the medication to reduce drinking in depressed alcoholics (Cornelius et al., 1997; Hernandez-Avila et al., 2004; Mason et al., 1996). A more recent multisite study demonstrated that sertraline (an SSRI), neither provided an advantage over placebo in reducing depressive symptoms, nor did it reduce drinking (Kranzler et al., 2006). Given the disappointing outcomes of treating depressed alcoholics with currently available antidepressants, it is generally accepted that newer treatment approaches are warranted (see Papakostas et al., 2007; Pettinati, et al., 2010). One of the most salient conclusions emerging from this body of literature is that adding a pharmacotherapy that “directly impacts drinking” to the antidepressant regimen may be necessary for successful treatment (see Pettinati, et al., 2010; Boden and Fergusson, 2011).
In an effort to develop a “single” pharmacotherapy to treat co-occurring alcoholism and depression, our laboratory has employed a series of compounds referred to as both “broad spectrum” antidepressants and triple uptake inhibitors (TUIs) (Skolnick and Basile, 2007; Skolnick, 2012). Unlike currently available antidepressants, these agents inhibit the uptake of norepinephrine, serotonin, and dopamine with varying potencies via a direct action at the respective monoaminergic transporters (see Skolnick, 2012). The development of TUIs as antidepressants is grounded on the hypothesis that such compounds would be superior to current monoamine-based agents (i.e., single or dual inhibitors) in one or more dimensions, including efficacy, speed of onset, and tolerability. At the core of this hypothesis is that addition of a dopaminergic component to a dual reuptake inhibitor will activate mesocorticolimbic dopaminergic pathways that are linked to anhedonia, a core symptom of depression (Tran et al., 2012; Skolnick, 2005; Skolnick, 2012).
Clinical studies indicate that binge drinking, a form of excessive drinking wherein blood alcohol levels (BAL) exceed/equal 80 mg % in a 2-hr period (NIAAA, 2004), is associated with the development of depressive symptoms (Paljarvi, et al., 2009; Choi and Dinitto, 2011). Hence, studies that identify compound effective in treating comorbid binge drinking and depression-like symptoms may have significant public health implications. In the present study, we examined the ability of the TUI, amitifadine (aka DOV 21, 947 or EB-1010), to reduce binge drinking and depressive symptomatology, inferred via negative affective states, in alcohol preferring (P) rats. A chemically related TUI, DOV 102, 677 (Popik et al., 2006), has previously been shown to reduce volitional consumption and operant responding for alcohol in alcoholic rats (McMillen et al., 2007; Yang et al., 2012). Nonetheless, amitifadine was selected for evaluation because it is safe and well tolerated (Skolnick and Basile, 2007), and produced a robust antidepressant effect in a multisite, double blind, placebo controlled trial (Tran et al., 2012). Moreover, because amitifadine is currently in clinical development, there is a potential for successful translation of preclinical findings to the treatment of co-occurring alcoholism and depression.
2. MATERIALS AND METHODS
2.1 Subjects
P rats (males; N = 270) were used to model binge alcohol drinking (Bell et al., 2006; Naimi et al., 2003) and secondary depression (Pettinati, 2004; Pettinati, et al., 2010) in humans. Animals were approximately 4 – 5 months of age at the beginning of the experiment and weighed between 410 to 487 grams. Subjects were individually housed. The treatment of all subjects was approved by the institutional review board of the University of Maryland School of Medicine. All procedures were conducted in strict adherence with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Oral drugs and administration procedures
Amitifadine (DOV Pharmaceutical, Somerset, N.J.) and imipramine (Sigma Aldrich, St. Louis, MO) were mixed immediately before the experimental test sessions in a volume of 1 ml/kg in deionized water. Drugs were given by oral gavage 25 min prior to all experimental sessions. This 25 min time period is based on the Tmax of amitifadine, which has been reported to be 1 hour after oral administration in rats (Tizzano et al., 2008). Animals were habituated to the gavage procedures by administering deionized water alone over a number of experimental sessions. The gavage procedure was used to model oral drug taking in humans.
2.3 Binge Drinking Apparatus
Animals were tested in 30 standard operant chambers (Coulbourn Instruments, Inc., Lehigh Valley, PA) enclosed in an isolated chamber as previously described (June et al., 2001; June and Gilpin, 2010; Liu et al., 2011). The operant apparatus contained two levers, two dipper manipulanda, a cue light over each lever, and a house light. The dipper cup size which contained the 10% (v/v) alcohol or 0.1 % (w/v) sucrose reinforcers was 0.1 ml (Liu et al., 2011). The Coulbourn Graphic State “3” operant software was used (Liu et al., 2011).
2.4 Drinking in the Dark Multiple Scheduled Access (DIDMSA) Paradigm
To initiate excessive “binge” alcohol drinking, we employed the drinking-in-the dark-multiple-scheduled-access (DIDMSA) protocol (Bell et al., 2006) with P rats. First, the procedure entailed adapting the rats to a 12 h:12 h light/dark cycle which began at 4:30 pm (lights off) and lasted to 4:30 am (lights on). Rats were trained to orally self-administer EtOH for daily 30 min sessions under an FR1 schedule employing the sucrose fading technique (Harvey et al., 2002; June et al., 2003). After a period of stabilization on the FR1 schedule, the response requirement was then increased to an FR4 schedule. For each schedule, responding was considered stable when responses were within ± 20% of the average responses for five consecutive days. Stabilization on the FR4 schedule took approximately 8 days. During the stabilization procedures, the animals were never deprived of food or fluid. These procedures are well established in our laboratory (June and Eiler, 2007; Liu et al., 2011). Other cohorts of rats were given a 1% (w/v) concentration of sucrose and trained in an identical manner under the FR1, then FR4, schedule. Following stabilization on the FR4 schedule for EtOH/sucrose, the DIDMSA protocol began using an FR4 where the rats were given access to 10% (v/v) alcohol, or 1% sucrose (w/v) on both the left and right levers. To initiate the DIDMSA protocol during the dark phase, rats were given a 30 min operant session. After the initial 30 min session had elapsed, rats were then placed in the home cage with food and water ad libitum for 1 h. Rats then received two additional 30 min alcohol access periods, spaced 1 h apart. In total, animals received three daily 30 min access periods, each spaced 1 h apart, across the dark cycle. Please note that, during the non-session binge period, rats received food and water ad libitum as well. Rats engaged in the binge drinking for 21 consecutive days. Using this protocol, the P rats in our laboratory produced consistent BACs of 144 ± 22 mg%/dL. Thus, our BAC levels approximated those of Bell et al. (2006), although our procedures were slightly modified. Other cohorts of rats were trained in a similar manner; however, they lever pressed for a 1% (w/v) sucrose concentration over the 21 day period. The sucrose control rats allowed for evaluation of reinforcer specificity following administration of the amitifadine treatments. The 21-day time course was employed and produced sustainable and highly reliable measures of BACs, and negative affective measures of withdrawal following abstinence (see below). As observed in our prior research on binge drinking (Liu et al., 2011), responding increased over the 3 week period from approximately 280 to 325 lever presses per 90 min to 750 – 800 lever presses per 90 min.
2.5 Blood Alcohol Concentration (BAC) Measurement
To ensure animals were consuming pharmacologically relevant amounts of EtOH to model human binge drinking (Naimi et al., 2003; also see Bell et al., 2006), BACs were collected as previously reported (Liu et al., 2011). BACs were determined in duplicates at 30 and 90 min. Measurements taken at the 90 min period were used to characterize the BAC levels immediately: 1) prior to evaluation of amitifadine and imipramine on binge drinking (see section 2.9.1); 2) prior to withdrawal in the FST assay (see section 2.9.2); and 3) prior to withdrawal in the ICSS assay (see section 2.9.4). In all cases, BAC measurements exceeded ≥ 80 mg % IdL at the 90 min operant session. This BAC measurement was consistent with the NIAAA definition of binge alcohol consumption in humans (NIAAA, 2004; Bell et al., 2006).
2.6 Forced Swim Test (FST)
The FST test comprised a two-day procedure, as described by Lucki and colleagues (Detke et al. 1995; Hemby et al., 1997; Yang et al., 2012). Swimming sessions were conducted by placing rats in individual glass cylinders (46 cm tall, 20 cm in diameter) containing 25 ± 0.5 °C water at a depth o f 30 cm, so that rats could not support themselves by touching the bottom with their feet. The two swimming sessions were conducted, between 12 pm and 7 pm: an initial 15 min pretest followed 24 h later by a 5 min test. The Clever Scan, System, (Reston VA), which allows for the accurate quantification of the depressive-like behaviors, was employed in the present study. This system uses video analysis to automate the entire observation process of the FST. It analyzes the video of each experiment and automatically calculates the time the rat spent struggling/escaping/climbing or immobile/floating/passively swimming.
2.7 Locomotor activity studies
Locomotor activity in the open field was recorded individually over a 30 min session in a Plexiglas chamber (42 cm X 42 cm X 30 cm) using the Columbus Electronics System (Columbus, Ohio, USA) (June et al. 2007; Liu et al., 2011). Experiments were conducted under dim lighting conditions. Immediately after each rat completed its session, the entire activity chamber was cleaned with a mild, non-bleach detergent to eliminate odors stimuli to avoid influencing the behavior of the next subject.
2.8 Intracranial self-stimulation (ICSS)
2.8.1 Stereotaxic Surgery
Subjects selected for ICSS were anesthetized through isoflurane/O2 gas inhalation and placed in a stereotaxic apparatus. The isoflurane was delivered at a concentration of 1 – 2% of the air mixture through a nose cone by a gas vaporizer. To evaluate ICSS, bipolar platinum electrodes 0.3 mm in diameter were implanted into the left hemisphere of the medial forebrain bundle (Paxinos and Watson, 1998) at the level of the lateral hypothalamus as previously reported (Eiler, et al., 2005; 2007). The coordinates for the medial forebrain bundle were AP = −2.3 mm, ML = +1.6 mm, and DV = −8.8 mm from bregma. The animals were monitored, weighed and examined daily to ensure recovery from surgery, with at least 6 – 7 days of post-surgical care prior to experimental manipulations. After completion of experimental procedures each rat was sacrificed and electrode placement was determined.
2.8.2 Apparatus
All training and testing for ICSS was conducted in 20 standard operant chambers (Coulbourn Instruments, Allentown, PA), each equipped with two removable levers and enclosed in a sound-attenuating cubicle. On top of each operant chamber, a commutator (Model: SL2C) (Plastics One, Inc., Roanoke, VA) was positioned to allow for the free movement of the attached downward-projecting electrode cable. Each lever was connected to an Insteck DC power supply (SPS-3610) that provided a constant current to the lever. The details of the apparatus have been extensively described in previous literature (Eiler et al., 2005; 2007).
2.8.3 Parameter Calculation
The ICSS setup and calculation of ICSS parameters were based on the earlier work by Kling-Petersen and Svensson (1993) (Eiler et al., 2005; 2007). To calculate the effective frequency (EF50) that produces half-maximal responding, the rate/frequency function was converted to a straight line using Probit analyses; the EF50 typically falls in the mid-range of this function (Stellar and Stellar 1985). The minimum frequency threshold was defined as either (a) the frequency that produces the lowest amount of responding, or (b) the first frequency that produces zero responses (Stellar and Stellar 1985). The maximum frequency (not shown here) was defined as the frequency that produces the maximum response rate or the upper asymptote of BSR responding. Response rates were also evaluated (see Eiler et al., 2005; 2007).
2.8.4 Training Phase
Rats were trained to lever press for electrical current on an FR1 schedule of reinforcement. Frequency was held constant at 100 Hz while the current started at 50 μA and was incrementally increased each session by 50 μA until a current that initiated and maintained ICSS responding was determined. Rats that initiated lever pressing were then shaped to press on the FR1 and then FR6 schedules of reinforcement for 100 Hz at a current level that maintained high levels of responding (e.g., 50 – 300 μA) (see Eiler et al., 2005; 2007).
2.9 Experimental Design
2.9.1 Evaluation of amitifadine and imipramine effects on binge drinking
After 21 days of binge drinking of alcohol or sucrose solutions, rats were randomly divided into drug or vehicle treatment groups. BACs were taken on day 21 from a subset of rats randomized into the drug treatment groups. On Day 22, rats in the drug treatment groups were administered either amitifadine (3.1, 6.3, 12.5, 25, or 50 mg/kg), vehicle (deionized water) (N = 4 – 10/group), imipramine (6.3, 12.5, 25, or 50 mg/kg) or vehicle (N = 4 – 8/group).
2.9.2 Evaluation of amitifadine on performance in the FST
2.9.2.1 Phase I
Following 19 consecutive days of 90 minute binge alcohol (N = 8) and sucrose (N = 10) sessions, rats participated in the initial 15 min swim session on Day 20. Two hours after the initial swim, rats were given their 90 min binge alcohol and sucrose sessions. One day after the binge sessions (Day 21), the alcohol and sucrose abstinence phase began, where all rats were given access to deionized water only. Following the 90 min deionized water consumption session, rats participated in their second, 5 min swim session in the FST. Data on immobility were collected during this 5 min session.
2.9.2.2 Phase II
(Amitifadine administration) Two additional cohorts of rats (N = 5/group) were trained to binge drink alcohol for 20 consecutive days. On Day 20, prior to their 90 min session, rats were given their initial 15 min swim. Two hours after the 15 min swim, rats were given their 90 min binge alcohol session. BACs were taken from all rats at the conclusion of the 90 min session on Day 20. On Day 21, 24 h after the 90 min session, rats were withdrawn from alcohol, and randomized into 12.5 mg/kg amitifadine + 24 h withdrawal (N = 5) and 25 mg/kg amitifadine + 24 h withdrawal (N = 5) treatment groups. Rats received deionized water during the 90 min withdrawal session, after which they completed their final 5 min swim session. Data on immobility in the amitifadine treatment groups were then compared against the sucrose control and 24 h abstinent groups from Phase I.
2.9.3 Evaluation of amitifadine on locomotor behaviors
Cohorts of rats (N = 25) were trained to binge drink alcohol for 20 consecutive days. On Day 21, 24 h after the 90 min session, rats were withdrawn from alcohol, and randomized into a 12.5, 25, 40, and 50 mg/kg treatment groups (n = 5/group). Amitifadine was given by oral gavage 25 min prior to a 90 min session to evaluate horizontal activity and stereotypy.
2.9.4 Evaluation of the effects of amitifadine on ICSS
2.9.4.1 Phase I. Time course of alcohol withdrawal
Following stabilization on an FR6 ICSS schedule, rats were trained to binge drink alcohol (N = 9) or sucrose (N = 9). Following 21 days of binge drinking, abstinence was induced and ICSS parameters were measured over a period of 6 to 84 hours. During the abstinence period, both groups were given access to deionized water.
2.9.4.2 Phase II. Amitifadine effects on alcohol withdrawal
To evaluate the effects of the amitifadine treatments, rats (N = 41) were stabilized on the FR6 ICSS schedule. Following stabilization on this schedule, 32 of the 41 rats were trained to binge drink alcohol for 21 consecutive days while 9 rats were trained to binge drink sucrose. After samples for determining BACs were taken from a small cohort of animals, the 32 rats were randomly divided into the following four treatment groups: 24 h withdrawal only (N = 8); 12.5 mg/kg amitifadine + 24 h withdrawal (N = 8); 25 mg/kg amitifadine + 24 h withdrawal (N = 8); and 50 mg/kg amitifadine + 24 h withdrawal (N = 8). One day after the last 90 min drinking session on Day 21, rats were withdrawn from alcohol and received deionized water during the 90 min session. Amitifadine (12.5, 25, or 50 mg/kg, PO) were administered to their respective treatment groups 30 min prior to the 24 h abstinence period, while the 24 h withdrawal rats received deionized water. The four cohorts were then compared with the sucrose control treated group from Phase I that did not undergo abstinence.
2.9.4.3 Phase III. Evaluation of amitifadine
Following stabilization on the FR6 ICSS schedule, three additional cohorts of rats were randomly divided into vehicle (N = 12), and amitifadine 12.5 mg/kg (N = 8) and 25 mg/kg (N = 8) treatment groups. Amitifadine and vehicle were given by gavage 25 min prior to a 20 min ICSS session.
2.10 Statistical Analyses
Data were analyzed by both between-group, and mixed-design group ANOVAs. Significant ANOVAs were followed by the Newman-Keuls post-hoc tests. All analyses were performed using the Sigma Plot 11.2 software program (Systat Software Inc., San Jose, CA).
3. RESULTS
3.1 Effects of amitifadine and imipramine on binge drinking
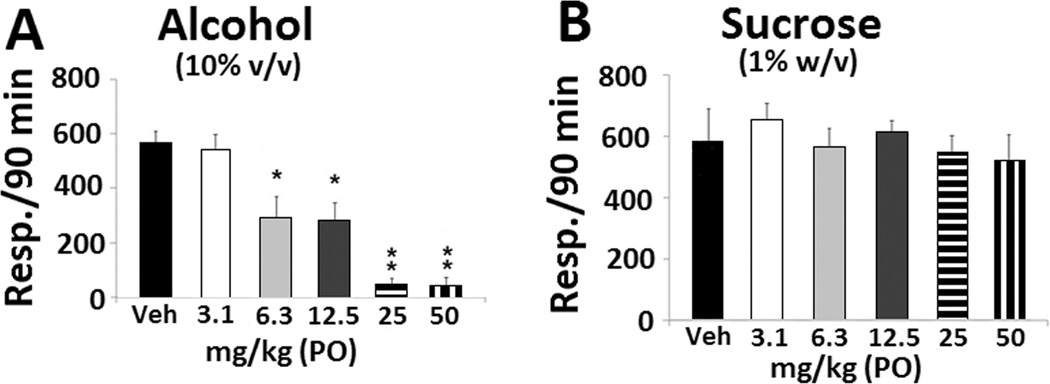
The rates of binge responding maintained by alcohol were significantly (F(5,35) = 11. 095, p < 0.001) suppressed by all but the lowest dose of amitifadine. A significant dose effect relationship was manifested for amitifadine (F(5,35) = 11. 095, p < 0.001), compared with the control group (p < 0.01, Figure 1A). In contrast, amitifadine did not alter sucrose responding (F(5,29) = 0.292, p > 0.05, Figure 1B).
Figure 1. Oral amitifadine attenuates binge alcohol, but not binge sucrose, drinking.
(A) Binge alcohol drinking of P rats following administration of vehicle (N = 10), 3.1 mg/kg (N = 4), 6.3 mg/kg (N = 9), 12.5 mg/kg (N = 9), 25 mg/kg (N = 4), and 50 mg/kg (N = 5) of amitifadine. (B) Binge sucrose drinking of P rats following administration of vehicle (N = 8), 3.1 mg/kg (N = 4), 6.3 mg/kg (N = 4), 12.5 mg/kg (N = 5), 25 mg/kg (N = 9), and 50 mg/kg (N = 5) of amitifadine. All treatments were randomly administered in this, and all subsequent experiments below. Drinking is measured as lever presses over 90 min (three 30 min sessions), with data being presented as mean ± SEM. *, p ≤ 0.01, ** p ≤ 0.001, compared with vehicle control. On Day 21, the BAC of the amitifadine group was 128 ± 36 mg%/dL.
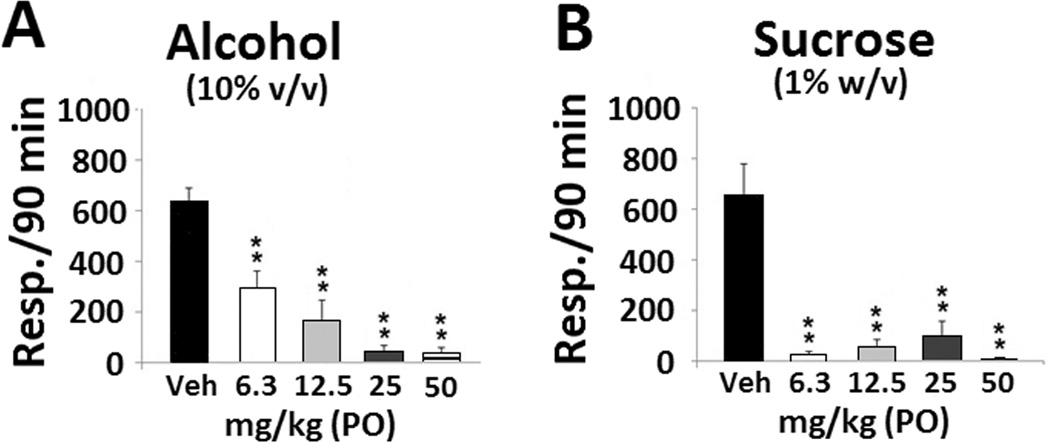
The SNRI imipramine was also evaluated for its effects on binge alcohol drinking, and was found to be highly effective in suppressing it (F(4,21) = 21.560, p < 0.001, Figure 2A). However, unlike amitifadine, imipramine also profoundly reduced binge sucrose responding (F(4,19) = 13.330, p < 0.001; Figure 2B). On Day 21, the BACs in the amitifadine and imipramine groups were 128 ± 36 mg%/dL and 144 ± 29 mg%/dL, respectively.
Figure 2. Oral imipramine attenuates binge alcohol and binge sucrose drinking.
(A) Effects of oral administration of vehicle (N = 8), 6.3 mg/kg (N = 4), 12.5 mg/kg (N = 5), 25 mg/kg (N = 5), and 50 mg/kg (N = 4) of imipramine on binge alcohol drinking in P rats. (B) Effects of oral administration of vehicle (N = 6), 6.3 mg/kg (N = 4), 12.5 mg/kg (N = 5), 25 mg/kg (N = 5), and 50 mg/kg (N = 4) of imipramine on binge sucrose drinking in P rats. Drinking is measured as lever presses over 90 min (three 30 min sessions), and the data are presented as mean ± SEM. ** p ≤ 0.001 compared with vehicle control. On Day 21, the BAC of the imipramine group was 144 ± 29 mg%/dL.
3.2 Amitifadine effects on FST during withdrawal
3.2.1 Phase I
On Day 20, BACs in rats averaged 137 ± 33 mg%/dL. Withdrawal from alcohol in the 24 h abstinent rats significantly increased immobility compared with the sucrose control rats (see F value in Phase II).
3.2.2 Phase II
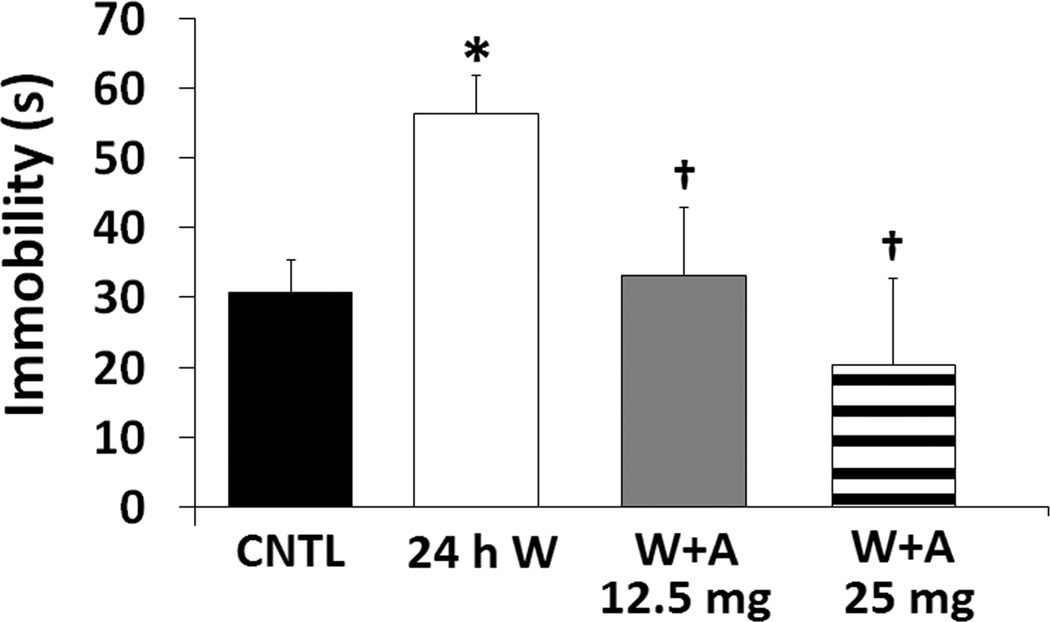
Oral amitifadine (12.5 and 25 mg/kg) significantly and dose-dependently antagonized the 24 h abstinence-induced elevation of immobility (Figure 3). Significant between group effects were seen for immobility (F(3,24) = 4.519, p = 0.012) and were confirmed by post-hoc analyses (p ≤ 0.01).
Figure 3. Oral amitifadine attenuates the effects of 24 h alcohol withdrawal.
Immobility in the forced swim test (FST) following sucrose control (N = 10), 24 h alcohol withdrawal (N = 8), and 12.5 (N = 5) and 25 (N = 5) mg/kg of amitifadine in 24 h alcohol withdrawn rats after 21days of consecutive binge alcohol/sucrose intake (*, p ≤ 0.05 compared with the sucrose control; †, p ≤ 0.05 compared with the 24 h post-alcohol withdrawal). The data are presented as mean ± SEM. On Day 20, BACs in the alcohol-withdrawn rats averaged 137 ± 33 mg%/dL.
3.3 Effects of amitifadine on locomotor activity
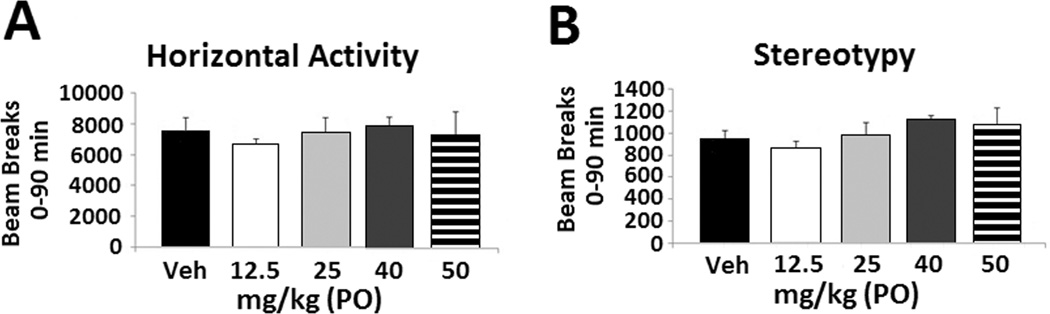
To determine if the attenuation of immobility in the FST by amitifadine is due to secondary elevations/reductions in locomotor behaviors, P rats were examined in the open field. No significant between groups effects were seen during the full 90 min (Figure 4A; F(4,35) = 1.015, p = 0.413) session on ambulatory behavior (i.e., horizontal activity). Similarly, no between group effects were seen for amitifadine on stereotypy during the 90 min session (Figure 4B; F(4,35) = 2.046, p = 0.112).
Figure 4. Oral amitifadine does not alter locomotor activity in 24 hr abstinence P rats.
Effects of amitifadine on horizontal activity and stereotypy following 12.5 mg/kg (N = 7), 25 mg/kg (N = 5), 40 mg/kg (N = 5), and 50 mg/kg (N = 4) compared with vehicle (N = 8) in 24 hr alcohol withdrawn P rats. Activity was measured for the 90 minutes of testing (A and B). The data are presented as mean ± SEM.
3.4 Effect of amitifadine on ICSS during withdrawal
3.4.1 Phase I. Time-dependent threshold elevations in abstinent rats: 6 to 84 h time course
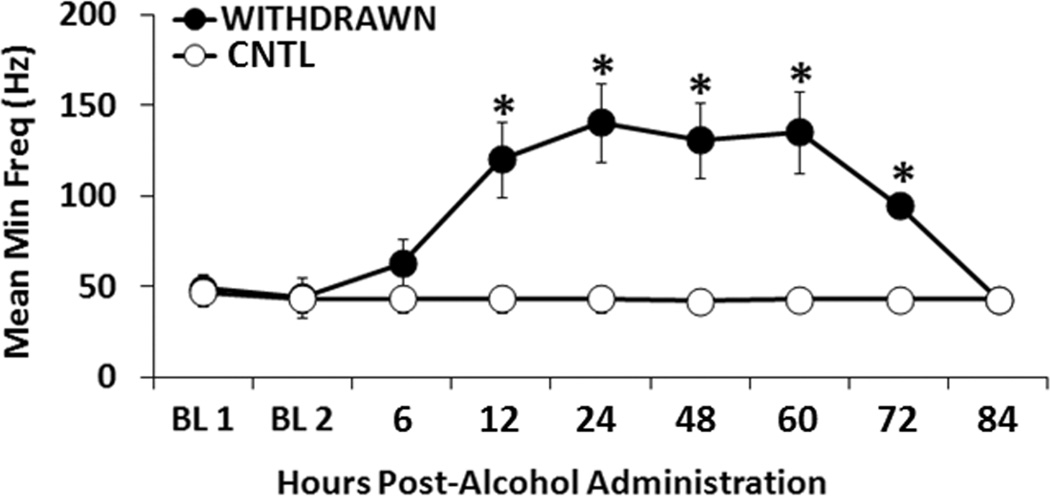
On Day 21, BACs averaged 148 ± 32 mg%/dL over the 90 min drinking period. Compared with the sucrose control animals, the alcohol abstinent rats displayed highly significant threshold elevations (at minimum frequency) between 12 and 72 h. However, by 84 h post-alcohol administration, threshold elevations had dissipated (Figure 5). Alcohol withdrawal produced significant main effects of time (F(8,144) = 5.586, p < 0.001), group (F(1,144) = 67.601, p < 0.001), and group×time interaction (F(8,144) = 5.926, p < 0.001). Post-hoc analyses confirmed the effect of time course (p ≤ 0.01).
Figure 5. Time-dependent elevations of alcohol withdrawal on the minimum frequency parameter of the ICSS assay.
P rats were withdrawn from alcohol (10% v/v) (n=9) and the control sucrose solution (1% w/v) (n=9) over a 6 to 84 h time course following 21 days of consecutive binge alcohol/sucrose drinking. As noted in the methods, a daily binge operant session comprised “90 min”. The average BAC on Day 21 was 148 ± 32 mg%/dL. (* p ≤ 0.05 compared with the control condition). The data are presented as mean ± SEM.
3.4.2 Phase II. Effect of amitifadine at 24 h of abstinence
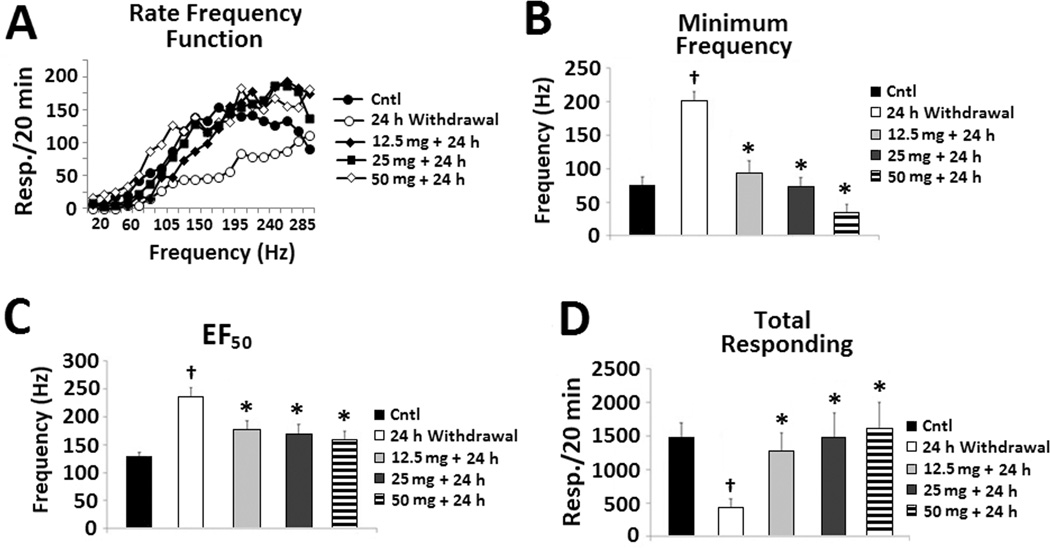
Evaluation of the rate frequency function (Figure 6A) revealed significant main effects of treatment group (F(4,19) = 12.913, p < 0.001) and frequency (F(19,76) = 18.019, p < 0.001), but no interaction. Rats exhibited a significant elevation in minimum frequency threshold at 24 hours of abstinence compared with sucrose control (Figure 6B). However, amitifadine (12.5-50 mg/kg) attenuated these threshold elevations. This resulted in a highly significant effect of treatment group (F(4,36) = 14.762, p < 0.001), confirmed by post-hoc analyses (p ≤ 0.001). As with the minimum threshold, 24 h of abstinence resulted in a marked elevation in the half-of-the-maximum-frequency (EF50) threshold. These effects were significantly attenuated by amitifadine, resulting in a significant treatment group effect (F(4,36) = 7.718, p < 0.001, Figure 6C). Post-hoc analyses confirmed the abstinence-induced elevations in threshold, and attenuation by amitifadine on the EF50 threshold parameter (p ≤ 0.01). Consistent with the elevations in threshold measures, rats in the 24 h abstinence group evidenced a significant rightward shift in the rate-frequency function relative to the sucrose control group. In contrast, consistent with the reduction in the abstinence-induced elevations on threshold measures by the amitifadine treatments (e.g., 12.5, 25, 50 mg/kg), rats in the amitifadine treatment groups evidence a significant leftward shift in the rate-frequency function relative to the abstinence-induced rats (Figure 6A). Compared with the sucrose control group, 24 h of alcohol abstinence resulted in a profound reduction in total responding (Fig. 6D). However, when amitifadine was administered immediately prior to the 24 h abstinence period, it produced a dose-related reversal of this reduction in responding. This resulted in a significant effect of treatment group F(4,36) = 4.24, p < 0.05), confirmed by post-hoc analyses (p ≤ 0.05).
Figure 6. Oral amitifadine attenuates the effects of 24 h alcohol withdrawal on ICSS parameters.
Effects of amitifadine following the 12.5 mg/kg (N = 8), 25 mg/kg (N = 8), and 50 mg/kg (N = 8) doses compared with the 24 h alcohol withdrawal (N = 8) and sucrose control treated (N = 9) P rats. Effects of amitifadine are depicted for (A) the rate-frequency function, (B) minimum frequency, (C) EF50, and (D) total responding. *, p ≤ 0.05 compared with 24 h alcohol withdrawal; †, p ≤ 0.05 compared with vehicle. The data are presented as mean ± SEM.
3.4.3 Phase III. Effect of amitifadine alone
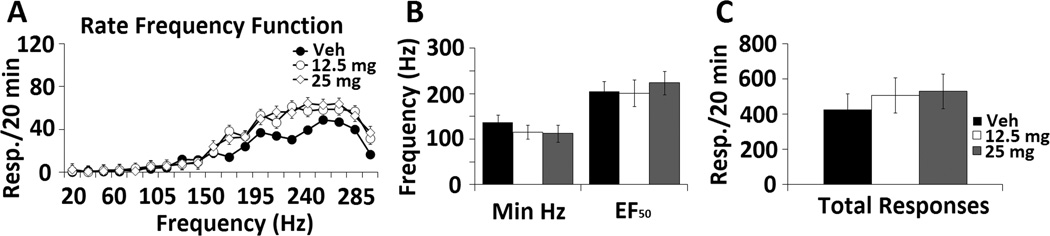
Acute administration of amitifadine alone, at doses that profoundly affected both binge drinking and abstinence-related behavior, failed to significantly alter any ICSS parameter (Figure 7 A-C; p ≥ 0.05).
Figure 7. Effects of oral amitifadine alone on ICSS parameters.
Rats were administered 12.5 mg/kg (N = 8) and 25 mg/kg (N = 8) compared with the PBS vehicle treatment (N = 12). Rats were then tested during a 20 min ICSS session. The rate frequency function over a 300 – 20 Hz descending frequency schedule (A), minimum frequency, EF50 (B), and total responding (C) parameters were evaluated.
4. DISCUSSION
Binge drinking is associated with depressive symptomatology in individuals with comorbid alcoholism and depression (Paljarvi, et al., 2009; Choi and Dinitto, 2011), a condition that is difficult to treat (Pettinati et al., 2010). Using an established binge model (Bell et al., 2006; Liu et al., 2011), we demonstrated the TUI amitifadine, effectively reduces binge alcohol, but not binge sucrose drinking. In contrast, imipramine, a tricyclic antidepressant reported to attenuate the comorbid condition (Mason et al., 1996), attenuated binge alcohol drinking, but also reduced binge sucrose drinking at comparable doses. One explanation for this difference is that the rate of responding of the rats used may have been sensitive to the doses of imipramine. However, the sucrose concentration was selected so response rates would be relatively similar to both alcohol and sucrose, eliminating the potential confound of a “difference in reinforcer efficacy” (see June et al., 2003; also June and Gilpin, 2010). Other biogenic amine-based antidepressants have also been reported to non-selectively reduce alcohol drinking in dependent rats (Sellers et al., 1992; Simon O’Brien et al., 2011). Thus, taken together, these data indicate that unlike SSRIs and SNRIs, amitifadine appears to selectively suppress alcohol-motivated behaviors in alcohol-dependent rats at doses that exert antidepressant-like actions in behavioral despair models (Skolnick et al., 2003).
The development of immobility when rats are placed in an inescapable cylinder of water has been linked to both behavioral despair and the development of passive behavior that disengages the animal from active forms of coping with stressful stimuli, forming the basis of the FST (Lucki, 1997; Porsolt et al., 1992). Consistent with previous findings, withdrawal from drugs of abuse increases immobility in the FST (Paterson and Markou, 2007). Twenty-four hours of abstinence increased immobility relative to sucrose controls. As previously observed in outbred, nondependent rats and mice (Skolnick et al., 2003), amitifadine dose-dependently reduced immobility in alcohol-withdrawn rats. These reductions in immobility were not secondary to elevations in motor behaviors, because consistent with previous findings, amitifadine (at doses of up to 50 mg/kg) failed to increase either locomotor or stereotypic behaviors (Figure 4) (Skolnick et al., 2003; Skolnick and Basile, 2007; Golembiowska, et al., 2012).
Elevations in ICSS threshold operationally parallel anhedonia (Paterson and Markou, 2007), a condition characterized by a diminished pleasure or interest (American Psychiatric Association (APA), 2000) that is a core symptom of depression and drug withdrawal (Markou et al., 1998; Paterson and Markou, 2007). Thus, elevations in ICSS threshold have been used to model negative affective states (Der-Avakian and Markou, 2012). As observed in both depressed and drug-withdrawn states, ICSS measures of total responding also provide an assessment of psychomotor performance (Eiler et al., 2005; Paterson and Markou, 2007). Moreover, increased 5-HT tone has been associated with a reduced potential for reinforcement among drugs which increase monoaminergic neurotransmission (Wee et al., 2006; Howell et al., 2007). Thus, given the greater relative engagement of the 5-HT, relative to the NE and DA transporters (Skolnick and Basile, 2007; Skolnick, 2012), these findings are consistent with a profile of monoamine uptake inhibitors with little, or no abuse liability (Nicholson et al., 2009).
The effects of amitifadine on ICSS thresholds were not due to secondary elevations in response rates above basal levels (Liebman, 1983; Wise and Rompre, 1989; Markou and Koob, 1991). Thus, when given alone, amitifadine did not alter any ICSS parameters (see Figure 7) indicating that amitifadine may “normalize” monoaminergic neurotransmission in abstinent rats, restoring the alcohol-deficient monoamine systems to appropriately regulate reward-related behaviors. The mechanism(s) by which amitifadine restores reward-related behaviors in abstinent rats is unclear. However, because anhedonia has been suggested to be due to a functional deficit of dopaminergic transmission in the DA reward system, and P rats also exert a hypodopaminergic tone due to their genetic predisposition (McBride and Li, 1998), a strong possibility exist that amitifadine may normalizes abstinence-induced and innate hypodopaminergic activity. In addition, P rats also exert an innate hyposerotonergic state (McBride and Li, 1998); hence, amitifadine’s pharmacological profile may be ideal for elevation of the two monoamines under such conditions. Heinz et al., (1994) and Volkow et al., (1996) have demonstrated a reduction in DA activity is linked to the anhedonia seen in abstinence alcoholics. A number of authors have suggested “anhedonia” is a key component of the abstinence symptomatology (Gawin and Kleber, 1986), and is also an important factor in relapse (Koob and Le Moal, 2001). In both the ICSS and FST models, amitifadine was effective in reducing alcohol-induced negative affective states which have been suggested to emulate depression-like behaviors in humans (Heinz et al., 1994; Paterson and Markou, 2007).
The doses of amitifadine used in the present studies are consistent with previous reports demonstrating antidepressant-like activity in the 5 – 20 mg/kg range (Skolnick et al., 2003). Moreover, plasma concentrations following these doses correspond to those produced by antidepressant doses of amitifadine in the clinic (Tran et al., 2012). Thus, translation of the present findings to the clinic may be possible at doses in the range previously reported to be safe and well tolerated (Skolnick and Basile, 2007). Both preclinical (Skolnick and Basile, 2007) and clinical studies (reviewed in Skolnick and Basile, 2007; Tran et al., 2012) with amitifadine indicate this compound should have a low potential for abuse. Whilst further studies are required to fully assess abuse liability, many drugs of abuse lower ICSS threshold (Liebman, 1983; Wise and Rompre, 1989; Markou and Koob, 1991). The lack of effect of amitifadine on ICSS at the doses tested is consistent with available clinical and preclinical data of a low abuse potential, albeit the testing of amitifadine over a wider dose range is needed to unequivocally resolve the issue of significant abuse liability.
4.1 Conclusions
In summary, amitifadine was effective in attenuating binge alcohol drinking at BACs exceeding 120 mg%/dl. In addition, amitifadine was effective in attenuating two measures of negative affect induced by abstinence: one hypothesized to model behavioral despair, and the anhedonia (see Paterson and Markou, 2007). The profile of locomotor behaviors in the open field, and the responding profiles in the ICSS assay suggest that the effects of amitifadine on negative affective states are not secondary to altering general activity. Thus, given the ability of the FST and ICSS to model aspects of the depressive symptomatology (Paterson and Markou, 2007), we propose that amitifadine may be effective in attenuating depression secondary to alcohol-induced abstinence. Given the safety profile of amitifadine, low risk for weight gain, lack of sexual side effects, and low abuse liability, we propose amitifadine would be effective in treating the co-occurrence of alcoholism and depression, and at doses that are safe and well tolerated in humans.
Highlights.
We examined the effect of amitifadine on binge drinking and negative affective states in P rats.
Amitifadine produced a selective, dose dependent attenuation of binge drinking.
It also reversed abstinence-induced increases in the intracranial self-stimulation threshold and immobility in the forced swim test.
ACKNOWLEDGEMENTS
The studies in this manuscript were financed by grant AA10406 to HLJSr from the National Institute of Alcohol Abuse and Alcoholism (NIAAA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
The authors declare that over the past 5 years DOV Pharmaceutical, Inc., the original manufacturer of amitifadine (DOV 21,947), has provided funding for past projects performed in the laboratory of HLJSr. HLJSr is currently a consultant for Euthymics Bioscience, the current licensee of amitifadine. The work by PS and ASB was performed as part of their duties as employees of DOV Pharmaceutical, Inc. Neither PS nor ASB have financial interests in amitifadine or Euthymics Bioscience.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) Virginia: American Psychiatric Publishing; 2000. [Google Scholar]
- 2.Bell RL, Z A, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 3.Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106(5):906–914. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi NG, Dinitto DM. Heavy/binge drinking and depressive symptoms in older adults: gender differences. Int J Geriatr Psychiatry. 2011;26(8):860–868. doi: 10.1002/gps.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, et al. Fluoxetine in depressed alcoholics: A double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54:700–705. doi: 10.1001/archpsyc.1997.01830200024004. [DOI] [PubMed] [Google Scholar]
- 6.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 8.Eiler WJ, Woods JE, Masters J, McKay PF, Hardy L, Goergen JJ, Mensah-Zoe B, Cook JB, Johnson NJ, June HL. Brain stimulation reward performance and sucrose maintained behaviors in alcohol-preferring and non-preferring rats. Alcohol Clin Exp Res. 2005;29:571–583. doi: 10.1097/01.alc.0000158934.50534.b7. [DOI] [PubMed] [Google Scholar]
- 9.Eiler WJ, 2nd, Hardy L, 3rd, Goergen J, Seyoum R, Mensah-Zoe B, June HL. Synapse. Responding for brain stimulation reward in the bed nucleus of the stria terminalis in alcohol-preferring rats following alcohol and amphetamine pretreatments. 2007;61(11):912–924. doi: 10.1002/syn.20437. [DOI] [PubMed] [Google Scholar]
- 10.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43(2):107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 11.Golembiowska K, Kowalska M, Bymaster FP. Effects of the triple reuptake inhibitor amitifadine on extracellular levels of monoamines in rat brain regions and on locomotor activity. Synapse. 2012;66(5):435–444. doi: 10.1002/syn.21531. 2012. [DOI] [PubMed] [Google Scholar]
- 12.Harvey SC, Foster KL, McKay PF, Carroll Mm, Seyoum R, Woods JE, II, Grey C, McCane S, Cummings R, Mason D, Jones CM, Ma C, Cook JM, June HL. The GABAAreceptor a1 subtype in the ventral pallidum regulates EtOH-seeking behaviors. Journal of Neuroscience. 2002;22:3766–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinz A, Schmidt LG, Reischies FM. Anhedonia in schizophrenic, depressed, or alcohol-dependent patients--neurobiological correlates. Pharmacopsychiatry. 1994;(Suppl 1):7–10. doi: 10.1055/s-2007-1014317. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Avila CA, Modesto-Lowe V, Feinn R, Kranzler HR. Nefazodone treatment of comorbid alcohol dependence and major depression. Alcohol Clin Exp Res. 2004;28:433–440. doi: 10.1097/01.alc.0000118313.63897.ee. [DOI] [PubMed] [Google Scholar]
- 15.Hemby SE, Lucki I, Gatto G, Singh A, Thornley C, Matasi J, Kong N, Smith JE, Davies HM, Dworkin SI. Potential antidepressant effects of novel tropane compounds, selective for serotonin or dopamine transporters. J Pharmacol Exp Ther. 1997;282(2):727–733. [PubMed] [Google Scholar]
- 16.Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320(2):757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- 17.June HL, Harvey SC, Foster KL, McKay PF, Cummings R, Garcia M, Mason D, Grey C, McCane SL, Williams L, Durr LF, Johnson TB, Xiaohui He, Rock S, Cook JM. GABAA-receptors containing α5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: An extended ethanol reward circuitry. Journal of Neuroscience. 2001;21:2166–2177. doi: 10.1523/JNEUROSCI.21-06-02166.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.June HL, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, Harvey SC, Eiler WJA, II, Grey C, McCane S, Garcia M, Jones CM, Mason D, Cummings R, Yin W, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- 19.June HL, Eiler WLA. Dopaminergic and GABAergic regulation of alcohol-motivated behaviors: Novel neuroanatomical substrates. In: Sibley DR, Hanin I, Kuhar M, Skolnick P, editors. Handbook of Contemporary Neuropharmacology. New York: Wiley & Sons; 2007. pp. 1–72. [Google Scholar]
- 20.June HL, Sr, Foster KL, Eiler WJA, II, Goergen J, Cook JB, Johnson N, Mensah-Zoe B, Simmons JO, June HL, Jr, Yin W, Cook JM, Homanics GE. Dopamine and benzodiazepine-dependent mechanisms regulate the EtOH-enhanced locomotor stimulation in the GABAA alpha1 subunit null mutant mice. Neuropsychopharmacology. 2007;32:137–152. doi: 10.1038/sj.npp.1301097. [DOI] [PubMed] [Google Scholar]
- 21.June HL, Gilpin NW. Current Protocols in Neuroscience. Vol. 9. NY: John Wiley and Sons, NY; 2010. “Operant self-administration models for testing the neuropharmaco-logical basis of ethanol consumption in rats”; pp. 1–26. 9.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kling-Peterson T, Svensson K. A simple computer-based method for performing and analyzing intracranial self-stimulation experiments in rats. J Neurosci. 1993;47:215–225. doi: 10.1016/0165-0270(93)90084-5. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 24.Kranzler HR, Mueller T, Cornelius J, Pettinati HM, Moak D, Martin PR, Anthenelli R, Brower KJ, O'Malley S, Mason BJ, Hasin D, Keller M. Sertraline treatment of co-occurring alcohol dependence and major depression. J Clin Psychopharmacol. 2006;26(1):13–20. doi: 10.1097/01.jcp.0000194620.61868.35. [DOI] [PubMed] [Google Scholar]
- 25.Liebman JM. Discriminating between reward and performance: a critical review of intracranial self-stimulation methodology. Neurosci Biobehav Rev. 1983;7(1):45–72. doi: 10.1016/0149-7634(83)90007-6. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, Elnabawi A, Merchenthaler I, Sieghart W, June HL, Sr, Aurelian L. Binge alcohol drinking is associated with GABAA α2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proceedings of the National Academy of Science USA. 2011;108(11):4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8(6–7):523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 29.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18(3):135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 30.Mason BJ, Kocsis JH, Ritvo EC, Cutler RB. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA. 1996;275:761–767. [PubMed] [Google Scholar]
- 31.McMillen BA, Shank JE, Jordan KB, Williams HL, Basile AS. Effect of DOV 102,677 on the volitional consumption of ethanol by Myers' high ethanol-preferring rat. Alcohol Clin Exp Res. 2007;31(11):1866–1871. doi: 10.1111/j.1530-0277.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 32.Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289(1):70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 33.NIAAA. National Institute on Alcohol Abuse and Alcoholism Council approves definition of binge drinking. NIAAA Newsletter. 2004;Vol. 3 (cited 2012 Jul 20). Available from: http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf. [Google Scholar]
- 34.Nicholson KL, Balster RL, Golembiowska K, Kowalska M, Tizzano JP, Skolnick P, Basile AS. Preclinical evaluation of the abuse potential of the analgesic bicifadine. J Pharmacol Exp Ther. 2009;330(1):236–248. doi: 10.1124/jpet.109.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- 36.Paljärvi T, Koskenvuo M, Poikolainen K, Kauhanen J, Sillanmäki L, Mäkelä P. Binge drinking and depressive symptoms: a 5-year population-based cohort study. Addiction. 2009;104(7):1168–1178. doi: 10.1111/j.1360-0443.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 37.Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biol Psychiatry. 2007;62(11):1217–1227. doi: 10.1016/j.biopsych.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 40.Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56:785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, Dackis CA, O'Brien CP. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167(6):668–675. doi: 10.1176/appi.ajp.2009.08060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popik P, Krawczyk M, Golembiowska K, Nowak G, Janowsky A, Skolnick P, Lippa A, Basile AS. Pharmacological profile of the "triple" monoamine neurotransmitter uptake inhibitor, DOV 102,677. Cell Mol Neurobiol. 2006;26(4–6):857–873. doi: 10.1007/s10571-006-9012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porsolt RD, Lenegre A. Behavioral models of depression. In: Elliott M, Heal Dl, Marsden CA, editors. Experimental Approaches to Anxiety and Depression. Chichester, UK: Wiley; 1992. pp. 73–85. [Google Scholar]
- 44.Sellers EM, Higgins GA, Tompkins DM, Romach MK. Serotonin and alcohol drinking. NIDA Res Monogr. 1992;119:141–145. [PubMed] [Google Scholar]
- 45.Simon O'Brien E, Legastelois R, Houchi H, Vilpoux C, Alaux-Cantin S, Pierrefiche O, André E, Naassila M. Fluoxetine, desipramine, and the dual antidepressant milnacipran reduce alcohol self-administration and/or relapse in dependent rats. Neuropsychopharmacology. 2011;36(7):1518–1530. doi: 10.1038/npp.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. Antidepressant-like actions of DOV 21-947: a 'triple' reuptake inhibitor. EurJPharmacol. 2003;461:99–104. doi: 10.1016/s0014-2999(03)01310-4. [DOI] [PubMed] [Google Scholar]
- 47.Skolnick P. Dopamine and depression. Chapter 9. In: Schmidt W, editor. Dopamine and Glutamate in Psychiatric Disorders. Totowa, NJ: Humana Press; 2005. pp. 199–214. [Google Scholar]
- 48.Skolnick P, Basile AS. Triple reuptake inhibitors ("broad spectrum" antidepressants) CNS Neurol Disord Drug Targets. 2007;6:141–149. doi: 10.2174/187152707780363285. [DOI] [PubMed] [Google Scholar]
- 49.Skolnick P. Triple-Uptake Inhibitors (Broad Spectrum Antidepressants) In: Peters JU, editor. Polypharmacology in Drug Discovery. New York: Wiley & Sons Inc; 2012. pp. 361–380. [Google Scholar]
- 50.Stellar JR, Stellar E. The neurobiology of motivation and reward. New York: Springer-Verlag; 1985. Chapter 6: The neuroanatomy of brain stimulation reward; pp. 121–156. [Google Scholar]
- 51.Tizzano JP, Stribling DS, Perez-Tilve D, Strack A, Frassetto A, Chen RZ, Fong TM, Shearman L, Krieter PA, Tschöp MH, Skolnick P, Basile AS. The triple uptake inhibitor (1R,5S)-(+)-1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0] hexane hydrochloride (DOV 21947) reduces body weight and plasma triglycerides in rodent models of diet-induced obesity. J Pharmacol Exp Ther. 2008;324(3):1111–1126. doi: 10.1124/jpet.107.133132. [DOI] [PubMed] [Google Scholar]
- 52.Tran P, Skolnick P, Czobor P, Huang NY, Bradshaw M, McKinney A, Fava M. Efficacy and tolerability of the novel triple reuptake inhibitor amitifadine in the treatment of patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. J Psychiatr Res. 2012;46(1):64–71. doi: 10.1016/j.jpsychires.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20(9):1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 54.Wee S, Carroll FI, Woolverton WL. A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants. Neuropsychopharmacology. 2006;31:351–362. doi: 10.1038/sj.npp.1300795. [DOI] [PubMed] [Google Scholar]
- 55.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 56.Yang ARST, Heon SY, Warnock KT, Mamczarz J, June HL, Jr, Mallick N, Krieter PA, Tonelli T, Skolnick P, Basile AS, June HL., Sr Effects of the triple monoamine uptake inhibitor DOV 102,677 on alcohol-motivated responding and antidepressant activity in P rats. Alcohol Clin Exp Res. 2012;36(5):863–873. doi: 10.1111/j.1530-0277.2011.01671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]