Abstract
Cardiovascular disease is a primary cause of morbidity and mortality in captive chimpanzees. Four years of blood pressure data was analyzed from a captive former laboratory population of 201 healthy adult chimpanzees with assessment of age and obesity on elevated blood pressure. Five different measures of obesity were compared: abdominal girth, basal metabolic rate, body-mass index (BMI), body weight and surface area. Systolic BP varied by sex. Obesity did not influence male BP. For females, obesity was a significant determinant of BP. The best measure of female obesity was basal metabolic rate and the worst was BMI. Median systolic BP of healthy weight females (<54.5 Kg) was significantly lower (128 mmHg) than overweight or obese females (140 mmHg), but both were lower than all males (147 mmHg). For diastolic BP, neither sex nor any of the 5 obesity measures was significant. But age was highly significant, with geriatric chimpanzees (> 30 years) having higher median diastolic blood pressure (74 mmHg) than young adults of 10–29 years old (65 mmHg). By these criteria, 80% of this population is normotensive, 7% pre-hypertensive and 13% hypertensive. In summary, systolic BP intervals required adjustment for obesity among females but not males. Diastolic BP required adjustment for advanced age (≥30 years). Use of these reference intervals can facilitate timely clinical care of captive chimpanzees.
Keywords: blood pressure, body weight, geriatric, normal range, normotension, pre-hypertension
Introduction
Hypertension is a classic risk factor for cardiovascular disease (CVD) in humans (Chobanian et al., 2003; Kazaam et al., 2005; National High Blood Pressure Education Program, 1997; O’Donnell and Kannel, 2002). In humans, hypertension increases the risk of cardiac failure 2 to 3-fold (Kannel and Stokes, 1985; Kenchaiah et al., 2004). Hypertension doubles the risk of CVD-related death for every 20/10mm increase in blood pressure (BP), and no critical threshold exists below which an individual is risk-free (Lewington et al., 2002). High blood pressure is also associated with increased heart size, particularly left-ventricular hypertrophy (LVH), and cardiac arrhythmias (Jokiniitty et al., 2001; Sparrow et al., 1985; Tanase et al., 1982; Yiu and Tse, 2008). Consequently, the monitoring and prevention of blood pressure has become a primary concern in human medicine (Chobanian et al., 2003), and the primary risk reduction strategy is treatment of hypertension (Botdorf et al., 2011; Kazzam et al., 2005).
Among captive chimpanzees, cardiac disease is a primary cause of morbidity and mortality (Ely et al., 2010, 2011a, b; Lammey et al., 2008; Seiler et al., 2009; Sleeper et al., 2005; Varki et al., 2009). A survey of four chimpanzee laboratory facilities showed that 35% of all reported deaths were due to cardiac disease (Varki et al., 2009). Interstitial myocardial fibrosis (IMF) characterizes cardiac pathology in chimpanzees but is rare in humans (Varki et al., 2009). Yet it resembles the etiology of human hypertensive cardiac disease, wherein LVH develops as an adaptive structural changes from pressure overload (hypertension), leading eventually to remodeling changes (myocardial fibrosis) and heart failure (Diez et al., 2005; Diez, 2007). Thus the high prevalence of sudden cardiac death characterized post-mortem by IMF was hypothesized to result from long-term hypertension precipitated by malignant arrhythmias in chimpanzees (Ely et al., 2010, 2011a,b; Doane et al., 2006; Lammey, 2008; Seiler et al., 2009).
These considerations have made the understanding of chimpanzee hypertension a priority. Until recently, the lack of reliable blood pressure reference values for healthy adult chimpanzees prevented reliable and objective identification of high blood pressure and stymied rigorous analysis of the association between hypertension and CVD. The first study of chimpanzee blood pressure focused on subadults, included only 5 chimpanzees above 30 years of age, did not define health status, used inappropriate statistical methods to infer “normal” ranges, and did not distinguish normotension from pre-hypertension or hypertension (Eichberg and Shade, 1987). Later studies incorporated numerous analytical or design shortcomings, including arbitrary definitions of hypertension based on multiple human reference standards (Elliot et al., 2007; Sleeper et al., 2005); indiscriminately combined subadults (<10 yr old) with adults (Elliot et al., 2007); failed to distinguish the effects of different sedatives on BP (Videan et al., 2007); and failed to account for the effects of induction by projectile dart sedation versus voluntary manual injection (Videan et al., 2007). Recent experimental evidence demonstrated that chimpanzee blood pressure increased by 0.09–0.13 mmHg per 1 mmol/day of dietary salt (Elliot et al., 2007). Elevated blood pressure is a serious public health concern because it is a risk factor for heart disease, stroke and renal disease (Henney et al., 2010). However, that study did not attempt to define blood pressure reference intervals for healthy chimpanzee (Elliott et al., 2007). Preliminary evidence that high blood pressure was related to age and obesity and increased the risk of all–cause mortality in chimpanzees was limited by sample size restrictions (Ely et al., 2011b). Given the increasingly geriatric (≥30 yr) and obesogenic profile of captive U.S. laboratory chimpanzees, and the association of hypertension with CVD, the impact of these determinants of hypertension remain of concern (Andrade et al., 2011; Grundy et al., 2004; McTighe et al., 2011; Miller et al., 2011; Seaberg et al., 2005; Videan et al., 2007).
To resolve these questions, the current study assembled blood pressure measurements collected during routine physical examinations on healthy adult chimpanzees over a four year period. This expanded dataset was used to statistically investigate the effects of age, sex and obesity on blood pressure. Analytical methods from human laboratory medicine were used to define reference intervals for systolic and diastolic blood pressure (Harris and Boyd, 1995). Results allowed more precise definition of normotensive, pre-hypertensive and hypertensive categories in healthy adult chimpanzees and are applicable in similar clinical veterinary settings involving adult chimpanzees.
Materials/Methods
Facility
The Alamogordo Primate Facility (APF) is a research reserve chimpanzee colony. No APF chimpanzees have been involved in laboratory research since contract inception in 2001. The population consisted of captive-born descendants of founders from the West African subspecies, Pan troglodytes verus (Ely et al., 2005). Chimpanzees were housed in same-sex group housing, plus several mixed-sex groups involving vasectomized males, to comply with the NIH breeding moratorium (ILAR, 1997). At the beginning of this study, the colony consisted of 201 animals (107 males and 94 females). Animals were fed a commercial primate diet (5LR2 containing 0.077% salt, Purina Labs, St. Louis, MO, USA) supplemented by fresh fruit and vegetables. Animals were maintained in indoor dens (180 ft2), with 24 hr access to outdoor exercise areas (242 ft2) or 802 ft2 PrimadomesR. All APF chimpanzees participated in the daily enrichment program, involving novel foods and activities, to maintain psychological well-being. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (ILAR, 1996). The facility and its program were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC).
Health Status and physical examinations
All APF chimpanzees received annual physical examinations (PEs). The time frame of this study (21 January 2004 to 2 April 2008) used a single consistent PE protocol to assess blood pressure (BP). After overnight fasting, animals were sedated by voluntary intramuscular injection of 3.0 mg/Kg Telazol (Tiletamine, Fort Dodge Animal Products, Fort Dodge, IA). A total of 408 PEs were performed on 201 healthy adult chimpanzees (107 males, 94 females) during the study period. Of these, 207 PEs were repeated on the same individuals, for an average of 2.1 PEs/chimpanzee. PEs included hematology, clinical chemistry, body temperature, heart rate, ECG, pulse oxygen and blood pressure. Chimpanzees were evaluated for health status at the time of the PE and were considered healthy if they had no known morbid condition and were not on any medications (Ely et al., 2011b). Analysis was restricted to clinically healthy adult chimpanzees, aged 10 years or more (Ely et al., 2011b; Lee and Guhad, 2001).
Data collection and calculations
All data were collected with full prior approval by the APF IACUC. Body weight (in Kg) was measured on calibrated scales (Avery Weigh-Tronix, model E10, Fairmont, MN). Body height was measured with a device that permitted reliable objective measurement of the crown-pubic bone length. Abdominal girth was measured at the umbilicus using a calibrated tape measure. The Quetelet formula (weight [in Kg]/height2 [in meters]) was used to estimate body-mass index (Berman and Schwartz, 1988; Eknoyan, 2008).] Blood pressure (BP) was measured using a non-invasive automatic oscillometry monitoring device (Passport 2 patient monitor, Datascope Corporation, Montvale, NJ), with an air-inflatable cuff and hose. BP data were collected within 15 min of sedation (Denton et al., 1995). Three consecutive blood pressure readings were recorded at 2.5 min intervals, during which time animals were not manipulated. Potential laterality differences were avoided by exclusive use of the right arm for BP readings (Kim et al., 2003). Human studies often drop the initial BP reading, to account for the “white coat” effect, then use the remaining two consecutive readings to estimate mean systolic and diastolic BP (McAllister & Strauss, 2001; NHBPEP, 1997; Vasan et al., 2001). Chimpanzee BP readings that had only two readings were downward biased and had greater variability (Ely et al., 2011b). Therefore, data from PEs was only included if there were three consecutive BP readings. Previously published data on Gabonese sanctuary chimpanzees that were fed a naturalistic low-salt diet were re-analyzed and used as a standard of comparison (Elliot et al., 2007).
Five different measures of obesity were estimated and evaluated. First, abdominal girth was measured (in cm) at the umbilicus using a calibrated plastic tape-measure. Second, body weight was measured in Kg. Third, Quetelet’s body-mass index (BMI) was estimated as body weight (in Kg) divided by the square of crown-rump length, in meters (Berman and Schwartz, 1988; Eknoyan, 2008). Fourth, body surface area (SA) was estimated using Meeh’s allometric equation, which defines SA (a measure of thermal homeostasis) as proportional to the two-thirds power of body weight (Calder, 1984; Livingston and Lee, 2001). Phylogenetic divergence time estimates of humans and rhesus macaques (Macaca mulatta) from chimpanzees were used to interpolate the constant (c=1049) and exponent (x=0.70) of the allometric equation for chimpanzee surface area (Burgess and Yang, 2008). The resulting formula, SA=1049*Mass0.70, was used to estimate chimpanzee body surface area. Finally, basal metabolic rate (BMR), which is proportional to body size, was estimated using the Brody-Proctor allometric equation as BMR=3.41*Mass0.73 (Calder, 1984; Livingston and Kohlstadt, 2005).
Statistical Methods
Statistical modeling was performed on SYSTAT version 11.0 (SYSTAT Software, Inc., Richmond, CA). The analysis of variance (ANOVA) was used, with statistical significance determined by omnibus F-statistics (Snedecor and Cochran, 1967) or single degree-of-freedom comparisons (Rosenthal and Rosnow, 1982). Covariates believed to influence blood pressure included sex and age (Ely et al., 2011b). Three consecutive BP readings were averaged to produce a single mean systolic and mean diastolic BP values (National High Blood Pressure Education Program, 1997). The Shapiro-Wilks omnibus goodness-of-fit test was used to verify the assumption of a Normal (Gaussian) distribution (Conover, 1980). Non-Gaussian data were normalized using a natural logarithm transformation (Ely et al., 2011b; Elliot et al., 2007). Outliers were identified using Studentized residuals and eliminated from statistical modeling.
Reference Intervals
Statistically significant covariates do not automatically identify clinically important sub-groups (Harris and Boyd, 1995; McGeechan et al., 2008). Two criteria were used to determine when to partition into subgroups with separate reference intervals: 1) a z* statistic > 5.0; or 2) a z* statistic > 3.0 and either a ≥10% reduction of subgroup standard deviations relative to pooled data, or a ratio of standard deviations > 1.5 (Harris and Boyd, 1990, 1995; Horn and Pesce, 2005). Outliers were identified and eliminated using the robust Tukey inter-quartile method (Dixon, 1953; Harris and Boyd, 1995; Horn and Pesce, 2005; Tukey, 1977). Reference intervals were estimated with commercial software (MedCalc version 11.6, MedCalc Software, Mariakerke, Belgium) using the Harrell-Davis bootstrap method or the robust method, depending on sample size (CLSI, 2008; Harrell and Davis, 1982; Harris and Boyd, 1995; Horn and Pesce, 2005; Hutson and Ernst, 2000). Normotension was defined as BP readings below the 90th percentile. Pre-hypertension was defined as BP readings between the 90th and 95th percentiles. Hypertension was defined as readings greater than or equal to the 95th percentile.
Results
There was no trend for the chimpanzees to increase in weight (X23=0.964, p=0.810), decade of life (X23=2.124, p=0.346), or in systolic and diastolic BP (X25=8.185, p=0.184) in subsequent PEs.
Systolic Blood Pressure
It was hypothesized that age and sex would influence systolic BP. Sex was a highly significant predictor of systolic BP (F1,406 =30.404, p<0.000) and separate reference intervals by sex were necessary. Therefore, all subsequent statistical modeling was performed separately for males and females.
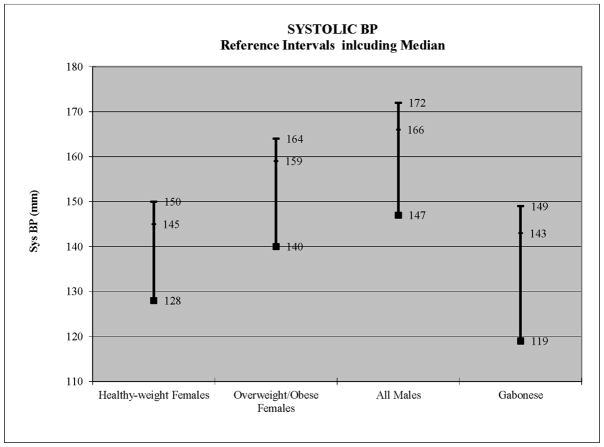
For females, age was not significant (Table 1). The five different measures of obesity (body weight, abdominal girth, BMI, Meeh surface area, and BMR) were fit in separate models and compared results. Results demonstrated that BMI was not significant but the other four obesity measures (abdominal girth, body weight, Meeh surface area, and BMR) were significant (Table 1). Among the 5 measures of obesity, BMR provided the best fit to the data (Table 1). This suggested that it is not body weight per se but the role of body weight in determining basal metabolic rate (BMR) that influences systolic blood pressure among females. When divided into healthy weight, overweight and obese categories, there was a significant increase in mean systolic BP with increasing body weight (healthy weight =128 mmHg, overweight =137 mmHg, obese =139 mmHg). Partitioning tests indicated that healthy weight females had significantly lower systolic BP than the overweight and obese females, which did not differ from each other. Therefore, two separate reference intervals were required, one for healthy weight females (<54.5 Kg) and one for overweight and obese females (≥54.5 Kg) (Figure 2). For healthy-weight females, the median systolic BP was 128 mmHg, the normotensive interval ranged up to 144 mmHg, the pre-hypertensive interval was from 145–149 mmHg, and the hypertension interval was 150+ mmHg (Figure 1, Table 2). For overweight or obese females, the median systolic BP was 140 mmHg (Figure 1, Table 2). The normotensive interval ranged up to 158 mmHg, the pre-hypertensive interval ranged from 159–163 mmHg, and the hypertension interval) was 164+ mmHg (Figure 1, Table 2).
Table 1.
ANOVA Results for Systolic BP in Female and Male Chimpanzees
| FEMALES | MALES | |||||||
|---|---|---|---|---|---|---|---|---|
| FACTOR | F | df | p | R2 | F | df | P | R2 |
| Age | 3.401 | 1, 180 | 0.067 | 1.9% | 1.104 | 1,223 | 0.295 | 0.5% |
| 5 Obesity measures: | ||||||||
| Body Weight | 16.281 | 1,180 | <0.000** | 8.3% | 0.795 | 1,222 | 0.373 | 2.3% |
| Abdominal Girth | 6.278 | 1,175 | 0.013* | 3.5% | 0.004 | 1,213 | 0.949 | 2.4% |
| BMI | 3.843 | 1,175 | 0.052 | 2.1% | 0.249 | 1,213 | 0.618 | 2.5% |
| Meeh Surface Area | 6.676 | 1,175 | 0.011* | 3.7% | 1.581 | 1,213 | 0.210 | 3.1% |
| Basal metabolic rate | 18.057 | 1,180 | <0.000** | 9.1% | 0.540 | 1,222 | 0.463 | 2.2% |
Legend:
p<0.05,
p<0.001
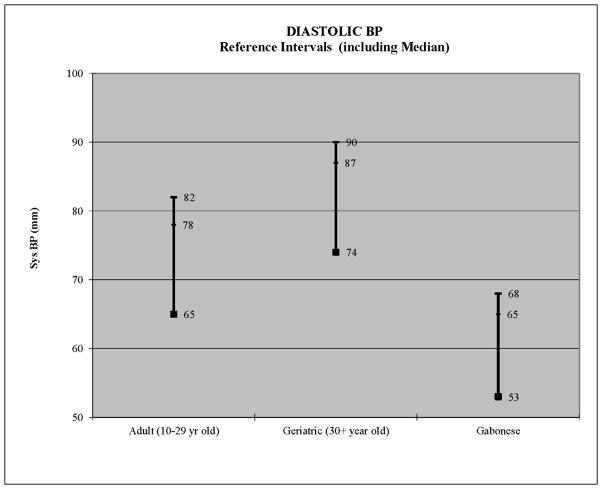
Figure 2.
Median, 90th and 95th percentiles depicted per group
Figure 1.
Median, 90th and 95th percentiles depicted per group
Table 2.
ANOVA Results for Diastolic BP in Chimpanzees
| All Chimpanzees (Male & Female) | ||||
|---|---|---|---|---|
| FACTOR | F | df | P | R2 |
| Age | 46.687 | 1,406 | <0.000** | 10.3% |
| Sex | 0.024 | 1, 405 | 0.632 | 10.5% |
| 5 Obesity measures: | ||||
| Body Weight | 0.413 | 1, 404 | 0.521 | 10.5% |
| Abdominal Girth | 1.716 | 1, 390 | 0.191 | 9.9% |
| BMI | 0.392 | 1, 390 | 0.531 | 9.6% |
| Meeh Surface Area | 0.089 | 1, 390 | 0.766 | 9.5% |
| Basal metabolic rate | 0.408 | 1, 404 | 0.523 | 10.5% |
Legend:
p<0.001
For males, age was not significant a significant predictor of systolic BP (Table 1). Separate models were fit for all 5 measures of obesity, none of which approached statistical significance in males (Table 1). This resulted in a single group consisting of all adult males for estimation of systolic BP reference intervals. This interval for all healthy adult males had a median systolic BP of 147 mmHg. The normotensive interval ranged up to 165 mmHg, the pre-hypertensive interval from 166–171 mmHg, and the hypertension interval was 172 mmHg and above (Figure 1, Table 2).
Diastolic Blood Pressure
The same analytical procedures were repeated for diastolic blood pressure. It was again hypothesized that age, sex and obesity would influence diastolic BP. Results indicated that there was no influence of sex (F1,405=0.024, p=0.632; Table 2). None of the 5 obesity measures was significant, although abdominal girth performed best (Table 2). Age was highly significant (Table 2). When categorized by decade of life, age maintained a highly significant effect on diastolic BP (Table 2). Mean diastolic BP increased linearly, from 61 mmHg among 10–19 year olds, to 65 mmHg among 20–29 year olds, to 73 mmHg among geriatric (≥30 year old) chimpanzees. This represented an average increase of 6.3 mmHg per decade of life.
Partitioning tests indicated that the difference between 10–19 year olds and 20–29 year olds was too small (only 4 mmHg) to justify partitioning into separate subgroups. But separation of geriatric (≥30 years old) animals from both younger age groups was strongly justified. Therefore, age was categorized as young adult (10–29 years old) and geriatric (≥30 years old) chimpanzees. Separate reference intervals were then constructed for both age groups. For young adults, the median diastolic BP was 65 mmHg and the normotensive interval ranged up to 77 mmHg (Figure 2, Table 3). The pre-hypertension interval ranged from 78–81, and the hypertension interval was 82 mmHg and above (Figure 2, Table 3). For geriatric animals (30+ years old), the median diastolic BP was 74 mmHg and the normotensive interval ranged up to 86 mmHg (Figure 2, Table 3). The pre-hypertensive interval ranged from 88–89, and the hypertension interval was 90 mmHg and above (Figure 2, Table 3). Thus, at every point, geriatric chimpanzees had 8–9 mmHg higher diastolic blood pressure than younger adult chimpanzees.
Table 3.
Normotensive, pre-Hypertensive and Hypertensive Reference Intervals for Healthy Adult Chimpanzees sedated with Telazol (mmHg).
| BP | Sub-group | Median | Normotensive | Pre-Hypertensive | Hypertensive |
|---|---|---|---|---|---|
| Systolic | Healthy Weight Females (<54.5 Kg) | 128 | ≤144 | 145–149 | 150+ |
| Overweight/Obese Females (≥54.5 Kg) | 140 | ≤158 | 159–163 | 164+ | |
| All Males | 147 | ≤165 | 166–171 | 172+ | |
| Diastolic | Adult (10–29 yr old) | 65 | ≤77 | 78–81 | 82+ |
| Geriatric (30+ yr old) | 74 | ≤86 | 87–89 | 90+ |
Comparison to Gabonese sanctuary chimpanzees on low-salt diet
For purposes of comparison, reference intervals were also estimated for young adult (10–16 year old) Gabonese chimpanzees eating a low-salt diet (Elliot et al., 2007). Their median systolic BP was 119, with a pre-hypertension category from 143–148.9 mmHg, and a hypertension category from 149 mmHg and above (Figure 1). Their median diastolic BP was 53 mmHg, with a pre-hypertension category from 65–67.9 mmHg, and a hypertension category from 68 mmHg and above (Figure 2). By comparison, captive females of healthy body weight had pre-hypertension and hypertension categories that were virtually identical to the Gabonese chimpanzees, although their median systolic BP was 9 mmHg higher (Figure 1). By contrast, the overweight/obese females plus all males had systolic BP levels that were 15–28 mmHg higher at every point (Figure 1). For diastolic BP, the Gabonese chimpanzees had diastolic BP levels that were 12–22 mmHg lower, compared to all captive chimpanzees, both young adult and geriatric (Figure 2).
Discussion
Statistical analysis of a large dataset drawn from four years of physical examinations on healthy adult chimpanzees was used to clarify the effects of age, sex and obesity on blood pressure. Obesity increased systolic BP in females but not males. Diastolic BP increased with age in both sexes. Reference intervals for systolic and diastolic BP were adjusted for age and sex. Separate systolic BP reference intervals were required for females by body weight. Separate diastolic BP intervals were needed for geriatric chimpanzees (≥30 years old). These intervals defined normotension, pre-hypertension and hypertension (Table 3; Figures 1, 2) and are recommended for healthy adult chimpanzees (>10 yo) sedated with Telazol.
High blood pressure was not uncommon in this colony. Overall, 80% of the chimpanzees were normotensive, 7% pre-hypertensive and 13% hypertensive (Table 4). Ignoring the effects of sex and age (Table 3), 14 individuals would be reclassified, resulting in 74% normotensive, 7% pre-hypertensive and 19% hypertensive. This 6% difference in the prevalence of hypertension represents the increased risk of hypertension due to increased body weight among females and to advanced age in both sexes.
TABLE 4.
Prevalence (%) of Systolic and Diastolic Normotension, Pre-hypertension and Hypertension.
| Diastolic BP
| ||||
|---|---|---|---|---|
| Systolic BP | Normotensive | Pre-hypertensive | Hypertensive | Total |
|
| ||||
| Normotensive | 161 (80%) | 8 (4%) | 11 (5.5%) | 180 |
| Prehypertensive | 2 (1%) | 4 (2%) | 1 (0.5%) | 7 |
| Hypertensive | 8 (4%) | 1 (0.5%) | 5 (2.5%) | 14 |
|
| ||||
| Total | 171 | 13 | 17 | 201 |
This work corroborates and extends earlier findings. First, there is a sex difference in the effects of obesity, which increased systolic blood pressure among female but not male chimpanzees (Andrade et al., 2011; Ely et al., 2011b). Secondly, these body weight categories (healthy weight ≤ 54.5 Kg; overweight =55 to 65.9, obese ≥66 Kg) correspond closely to suggestions drawn from a leaner chimpanzee population (Videan et al., 2007). Third, advanced age (≥30 yr old) significantly increased diastolic BP in both sexes (Ely et al., 2011b). These are important considerations in the medical management of increasingly obese geriatric captive chimpanzee populations (Ely et al., 2011a,b; McTighe et al., 2011).
The 5 different measures of obesity (body weight, body mass index, abdominal girth, surface area, and basal metabolic rate) performed differently. First, no measure of obesity influenced male systolic BP (Table 1). Other authors reported similar sex differences (Adrade et al., 2011; Videan et al., 2007). Nor did any measurement of obesity influence diastolic BP (Table 2). For females, the only obesity measure not associated with systolic BP was BMI (Table 1). Despite its widespread usage, BMI is a poor surrogate for body fat composition (Heymsfield et al., 2007; Prentice and Jebb, 2001). Abdominal girth and body surface area showed only weak associations to systolic BP. Other authors reported correlations of waist circumference with female (but not male) diastolic BP (Adrade et al., 2011). Waist circumference is an important marker of metabolic syndrome in humans but an inadequate marker of visceral obesity (Després et al., 2008; Grundy et al., 2004). This exemplifies the differences in cardiometabolic risks and cardiac disease between chimpanzees and humans (Varki et al., 2009). BMI, abdominal girth and body surface area were poor measures of obesity. The best measurement of obesity was body weight. Its strong similarity to BMR suggested that it is not body weight per se, but the role of body weight in determining basal metabolic rate that influences female systolic blood pressure. The three categories of body weight used here (healthy-weight <54.5 Kg, over-weight 55–65.9 Kg, and obese ≥66 Kg) are recommended as guidelines.
The comparison of blood pressure reference intervals for these captive chimpanzees to Gabonese sanctuary chimpanzees was instructive. Captive females of healthy weight had very similar systolic BP compared to the naturalistic Gabonese population (Figure 1). But overweight and obese females and all males had higher systolic BP (Figure 1). This suggests that captive chimpanzee populations can emulate a natural low systolic blood pressure by maintaining a healthy body weight. In contrast, all captive chimpanzees had diastolic BP that was 15–22 mmHg higher than the naturalistic Gabonese population (Figure 2). Since the Gabonese population was both younger (mean age=14.6 yr, range 10.5 to 17.9 yr old) and consumed a low-salt diet, the observed differences in BP could be due to lower age, lower dietary salt, or both. Strictly speaking, other explanations cannot be ruled out, including potential subspecies genetic differences (Ely et al., 2005) or unidentified feature(s) of captivity (Terio et al., 2011). In any case, the husbandry lesson is clear: systolic BP can be minimized in captive settings by maintaining healthy body weight (54.5 Kg).
Several limitations of this work should be noted. First, these data were obtained from chimpanzees sedated by Telazol. It is not known how these reference intervals would compare to intervals obtained from alert chimpanzees or from chimpanzees sedated with a different sedative. Secondly, the best measure of obesity was basal metabolic rate (BMR), as estimated allometrically from body weight. Allometric estimates of BMR from body size in humans, while generally accurate, required adjustment for effects of age and sex (Livingston and Kohlstadt, 1999). Yet calorimetric estimates of BMR are independent of body weight (Snodgrass et al., 2008). It would be worthwhile to benchmark these morphometric measures of obesity against more precise calculations of total body fat and lean body mass using methods such as D2O dilution (Kanto and Clawson, 1980) or dexascan (Haarbo et al., 1991). This suggests a need for more detailed characterization of obesity, including body-condition scores, in chimpanzees. Finally, the association between elevated BP and CVD risk requires more extensive study in chimpanzees (Kannel and Stokes, 1985; Kenchaiah et al., 2004; Lewington et al., 2002). Evidence that chimpanzees with systolic BP above the 90th% ile had a 3.6-fold increased risk of CVD (Ely et al., 2011b) was the first indication that high BP could cause heart failure and potentially lead to myocardial fibrosis in chimpanzees (Ely et al., 2011b). This is the model of human hypertensive heart disease (Diez, 2005; Diez et al., 2007; Ely et al., 2011b; Lammey et al., 2008; Kenchaiah et al., 2004; Weber, 2000). Although a pre-hypertension category was not supported by the data (Ely et al., 2011b), it remains a distinct possibility given the elevated risk of progression to CVD in pre-hypertensive humans (Elliot and Black, 2007). Consequently, there is a need for prospective longitudinal studies on chimpanzee cardiac disease (Varki et al., 2009). Larger and more detailed epidemiological studies are needed to fully characterize the association between hypertension, interstitial myocardial fibrosis, and the risk of cardiac disease and CVD-related mortality (Ely et al., 2010, 2011a,b, Lammey et al., 2008; Terio et al., 2011). Prospective cohort studies are feasible in any large captive chimpanzee population and in collections of smaller populations, if conducted using standardized methods across institutions (Dindo, 2012). Such studies should assess the impact of elevated BP, as defined here, on cardiac disease and other disorders disease related to hypertension, including end-stage renal disease and hypertensive nephrosclerosis (Bergersen, 2006; Botdorf et al., 2011; Jung et al., 2004; Luke, 1999).
This is only the third application to veterinary medicine (Ely et al., 2011b; McTighe et al., 2011) of analytical methods for reference intervals developed for human laboratory medicine 40 years ago (Grasbeck and Saris, 1969; Solberg, 1987; Solberg and Gräsbeck, 1989; Sunderman, 1975). These methods are demonstrably superior to statistical methods based on Normal (Gaussian) theory and should be used in all veterinary medical situations requiring the determination of reference intervals for healthy animals, such as hematology, clinical chemistry, organ sizes, and disease biomarkers. A key rationale underlying the theory of reference intervals is the need to define a reference population, particularly their health status (Ely et al., 2011b). Harris and Boyd, 1995; Horn et al., 2001). Best practice is to clearly define the reference population (here, captive adult chimpanzees free of trauma and medication and sedated with Telazol), make health determinations during the physical examination itself, and exclude data on unhealthy subjects from all subsequent analyses.
Conclusions
Analysis of four years of blood pressure data on 201 captive adult chimpanzees allowed clear definitions of chimpanzee normotension, pre-hypertension and hypertension, controlling for sex and age.
The best measure of obesity was body weight and is best understood as a proxy for basal metabolic rate.
Overweight or obese females and all males had higher systolic BP than healthy-weight females, which were comparable to BP expected on a low-salt diet.
Younger adult chimpanzees (10–29 years old) have lower diastolic BP than geriatric (>30 year old) chimpanzees.
The blood pressure reference intervals defined here are appropriate for the average of 3 consecutive readings on healthy adult chimpanzees sedated with Telazol.
Acknowledgments
This study was supported by NIH contract HHSN268201100065C. We thank Dr. Brian Corning, D.V.M., for comments on the manuscript, Dr. Derek Denton, M.B.B.S., for valuable discussions of earlier drafts of this manuscript and for sharing rare blood pressure data from Gabonese chimpanzees, Ms. Dawn Myers for graphical assistance, and two anonymous reviewers for helpful comments on the manuscript.
References
- Andrade MCR, Higgins PB, Mattern VL, De La Garza M, Brasky KM, Vorundanti VS, Comuzzie AG. Morphometric variables related to metabolic profile in captive chimpanzees (Pan troglodytes) Comp Med. 2011;61:457–461. [PMC free article] [PubMed] [Google Scholar]
- Berman CM, Schwartz S. A nonintrusive method for determining relative body fat in free-ranging monkeys. Amer J Primatol. 1988;14:53–64. doi: 10.1002/ajp.1350140105. [DOI] [PubMed] [Google Scholar]
- Bergersen BM. Cardiovascular risk in patients with HIV infection: Impact of antiretroviral therapy. Drugs. 2006;66:1971–1987. doi: 10.2165/00003495-200666150-00006. [DOI] [PubMed] [Google Scholar]
- Botdorf J, Chaudhary K, Whaley-Connell A. Hypertension in cardiovascular and kidney disease. Cardiorenal Med. 2011;1:183–192. doi: 10.1159/000329927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R, Yang Z. Estimation of hominoid ancestral population sizes under Bayesian coalescent models incorporating mutation rate variation and sequencing errors. Molec Biol Evol. 2008;25:1979–1994. doi: 10.1093/molbev/msn148. [DOI] [PubMed] [Google Scholar]
- Calder W. Size function and life history. Cambridge, MA: Harvard University Press; 1984. p. 431. [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The national high blood pressure education program coordinating committee, seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- [CLSI] Clinical and Laboratory Standards Institute. CLSI document C28-A3. 3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline; p. 76. [Google Scholar]
- Conover WJ. Practical nonparametric tests. 2. New York, NY: Wiley; 1980. [Google Scholar]
- Denton D, Weisinger R, Mundy NI, Wickings EJ, Dixson A, Moisson P, Pingard AM, Shade R, Carey D, Ardaillou R, Paillard F, Chapman J, Thillet J, Michel JP. The effect of increased salt intake on blood pressure in chimpanzees. Nature Medicine. 1995;1:1009–1016. doi: 10.1038/nm1095-1009. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux JB, Bergeron J, Pibarot M, Larose E, Rodes-Cabau J, Bertrand O, Poipier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- Diez J, Gonzales A, Lopez B, Querejeta R. Mechanisms of disease: Pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nature Clinical Practice Cardiovascular Medicine. 2005;2:209–216. doi: 10.1038/ncpcardio0158. [DOI] [PubMed] [Google Scholar]
- Diez J. Mechanisms of cardiac fibrosis in hypertension. Journal of Clinical Hypertension. 2007;9:546–550. doi: 10.1111/j.1524-6175.2007.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo M. Ape cardiac exams & the GAHP database. The Great Ape Heart Project. 2012 Feb 27;2012:2. [Google Scholar]
- Dixon WJ. Processing data for outliers. Biometrics. 1953;9:74–89. [Google Scholar]
- Doane CJ, Lee DR, Sleeper MM. Electrocardiogram abnormalities in captive chimpanzees (Pan troglodytes) Comp Med. 2006;56:485–491. [PubMed] [Google Scholar]
- Eichberg JE, Shade R. “Normal” blood pressure in chimpanzees. J Med Primatol. 1987;16:317–321. [PubMed] [Google Scholar]
- Eknoyan G. Adolphe Quetelet (1796–1874)-The average man and indices of obesity. Nephrol Dial Transplant. 2008;23:47–51. doi: 10.1093/ndt/gfm517. [DOI] [PubMed] [Google Scholar]
- Elliot P, Walker LL, Little MP, Blair-West JR, Shade RE, Lee DR, Rouquet P, Leroy E, Jeunemaitre X, Ardaillou R, Paillare F, Meneton P, Denton DA. Change of salt intake affects blood pressure of chimpanzees: implications for human populations. Circulation. 2007;116:1563–1568. doi: 10.2215/01.CJN.0000926952.00563.65. [DOI] [PubMed] [Google Scholar]
- Elliot WJ, Black HR. Prehypertension. Nature Clin Prac Cardiovascular Med. 2007;4:538–548. doi: 10.1038/ncpcardio0989. [DOI] [PubMed] [Google Scholar]
- Ely JJ, Dye B, Frels WI, Fritz J, Gagneux P, Khun HH, Switzer WM, Lee DR. Subspecies composition and founder contribution of the captive U.S. chimpanzee (Pan troglodytes) population. Am J Primatol. 2005;67:223–242. doi: 10.1002/ajp.20179. [DOI] [PubMed] [Google Scholar]
- Ely JJ, Bishop MA, Lammey ML, Sleeper MM, Steiner JM, Lee DR. Use of biomarkers of collagen types I and III fibrosis metabolism to detect cardiovascular and renal disease in chimpanzees (Pan troglodytes) Comp Med. 2010;60:154–158. [PMC free article] [PubMed] [Google Scholar]
- Ely JJ, Zavaskis T, Lammey ML, Sleeper MM, Lee DR. Association of brain-type natriuretic protein and cardiac troponin I with incipient cardiovascular disease in chimpanzees (Pan troglodytes) Comp Med. 2011a;61:163–9. [PMC free article] [PubMed] [Google Scholar]
- Ely JJ, Zavaskis T, Lammey ML, Lee DR. Blood pressure reference intervals for healthy adult chimpanzees (Pan troglodytes) J Med Primatol. 2011b;40(3):171–180. doi: 10.1111/j.1600-0684.2011.00467.x. [DOI] [PubMed] [Google Scholar]
- Gräsbeck R, Saris N-E. Establishment and use of normal values. Scand J Clin Lab Invest. 1969;24(Suppl 171):62–63. [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Haarbo J, Gotfredsen A, Hassager C, Christiansen C. Validation of body composition by dual energy X-ray absorptiometry (DEXA) Clin Physiol. 1991;11:331–341. doi: 10.1111/j.1475-097x.1991.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Davis CE. A new distribution-free quantile estimator. Biometrika. 1982;69:635–640. [Google Scholar]
- Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference intervals. Clin Chem. 1990;36:265–270. [PubMed] [Google Scholar]
- Harris EK, Boyd JC. Statistical bases of reference values in laboratory medicine. New York: Marcel Dekker, Inc; 1995. p. 384. [Google Scholar]
- Henney JE, Taylor CL, Boon CS Committee on Strategies to Reduce Sodium Intake. Strategies to Reduce Sodium Intake in the United States. Washington, D.C: National Academies Press; 2010. [PubMed] [Google Scholar]
- Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Amer J Clin Nutr. 2007;86:82–91. doi: 10.1093/ajcn/86.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn PS, Feng L, Li Y, Pesce AJ. Effect of outliers and nonhealthy individuals on reference intervals. Clin Chem. 2001;47:2137–3145. [PubMed] [Google Scholar]
- Horn PS, Pesce AJ. Reference intervals: A user’s guide. Washington, DC: AACC Pr; 2005. p. 115. [Google Scholar]
- Hutson AD, Ernst MD. The exact bootstrap mean and variance of an L-estimator. J R Stat Soc B. 2000;62:89–94. [Google Scholar]
- [ILAR]Institute for Laboratory Animal Research. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; 1996. p. 140. [Google Scholar]
- [ILAR]Institute for Laboratory Animal Research. Chimpanzees in research- strategies for their ethical care, management and use. Washington (DC): National Academies Press; 1997. p. 108. [PubMed] [Google Scholar]
- Jokiniitty JM, Majahalme SK, Kahonen MA, Tuomisto MT, Turjanmaa VM. Pulse pressure is the best predictor of future left ventricular mass and change in left ventricular mass: 10 years of follow-up. J Hypertens. 2001;9:2047–2054. doi: 10.1097/00004872-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Jung O, Bickel M, Ditting T, Rickerts V, Welk T, Helm EB, Staszewski S, Geiger H. Hypertension in HIV-1-infected patients and its impact on renal and cardiovascular integrity. Nephrology Dialysis Transplantation. 2004;19:2250–2258. doi: 10.1093/ndt/gfh393. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Stokes JS., III . Hypertension as a cardiovascular risk factor. In: Bulpitt CJ, editor. Handbook of Hypertension. Amsterdam: Elsevier Scientific; 1985. pp. 15–34. [Google Scholar]
- Kanto U, Clawson AJ. Use of deuterium oxide for the in vivo prediction of body composition in female rats in various physiological states. J Nutr. 1980;110:1840–1848. doi: 10.1093/jn/110.9.1840. [DOI] [PubMed] [Google Scholar]
- Kazaam E, Ghurbana BA, Obineche EN, Nicholl MG. Hypertension—still an important cause of heart failure? J Hum Hypertension. 2005;19:267–275. doi: 10.1038/sj.jhh.1001820. [DOI] [PubMed] [Google Scholar]
- Kenchaiah S, Narula J, Vasan RS. Risk factors for heart failure. Med Clin N Am. 2004;88:1145–1172. doi: 10.1016/j.mcna.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Won CW, Ann ES, Jung JJ, Kim BS, Choi HR. Blood pressure difference between right and left arms of some college freshman. J Korean Acad Fam Med. 2003;24:166–171. [Google Scholar]
- Lammey ML, Baskins GB, Gigliotti AP, Lee DR, Ely JJ, Sleeper MM. Interstitial myocardial fibrosis in a captive chimpanzee (Pan troglodytes) population. Comp Med. 2008;58:389–394. [PMC free article] [PubMed] [Google Scholar]
- Lee DR, Guhad FA. Chimpanzee medicine and health care program. The care and management of chimpanzees. In: Brent L, editor. Special topics in primatology. Vol. 2. San Antonio, TX: American Society of Primatologists; 2001. pp. 83–118. [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective studies collaboration, Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Livingston EH, Lee S. Body surface area prediction in normal-weight and obese patients. Am J Physiol Endocrinol Metab. 2001;281:E586–91. doi: 10.1152/ajpendo.2001.281.3.E586. [DOI] [PubMed] [Google Scholar]
- Livingston EH, Kohlstadt I. Simplified resting metabolic rate-predicting formulas for normal-sized and obese individuals. Obesity Research. 2005;13(7):1255–1262. doi: 10.1038/oby.2005.149. [DOI] [PubMed] [Google Scholar]
- Luke RG. Hypertensive nephrosclerosis: pathogenesis and prevalence. Neprology Dialysis Transplantation. 1999;14:2271–2278. doi: 10.1093/ndt/14.10.2271. [DOI] [PubMed] [Google Scholar]
- McAlister F, Straus S. Evidence based treatment of hypertension: Measurement of blood pressure: an evidence based review. Brit Med J. 2001;322:908–911. doi: 10.1136/bmj.322.7291.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeechan K, Macaskill P, Irwig L, Liew G, Wong TY. Assessing new biomarkers and predictive models for use in clinical practice. Arch Intern Med. 2008;168:2304–2310. doi: 10.1001/archinte.168.21.2304. [DOI] [PubMed] [Google Scholar]
- McTighe M, Hansen B, Ely J, Lee DR. Determination of hemoglobin A1c and fasting blood glucose reference intervals in captive chimpanzees (Pan troglodytes) J Am Assoc Lab Anim Sci. 2011;50:165–170. [PMC free article] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- [NHBPEP] National High Blood Pressure Education Program. The sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- O’Donnell CJ, Kannel WB. Epidemiologic appraisal of hypertension as a coronary risk factor in the elderly. Am J Geriatric Cardiology. 2002;11:86–92. doi: 10.1111/j.1076-7460.2002.00996.x. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Jebb SA. Beyond body mass index. Obesity Reviews. 2001;2(3):141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow R. Contrast analysis: Focused comparisons in the analysis of variance. New York, NY: Cambridge University Press; 1985. p. 107. [Google Scholar]
- Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP. Multicenter AIDS cohort study. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- Seiler BM, Dick EJ, Guardado-Mendoza R, VandeBerg JL, Williams JT, Mubiru JN, Hubbard GB. Spontaneous heart disease in the adult chimpanzee (Pan troglodytes) J Med Primatol. 2009;38:51–58. doi: 10.1111/j.1600-0684.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeper MM, Doane CJ, Langner PH, Curtis S, Avila K, Lee DR. Successful treatment of idiopathic dilated cardiomyopathy in an adult chimpanzee (Pan troglodytes) Comp Med. 2005;55:80–84. [PubMed] [Google Scholar]
- Snedecor GW, Cochrane WG. Statistical methods. 6. Ames, Iowa: Iowa State University Press; 1967. p. 593. [Google Scholar]
- Snodgrass JJ, Leonard W, Sorenson MV, Tarskaia L, Mosher MJ. The influence of basal metabolic rate among indigenous Siberians. American Journal of Anthropology. 2008;137:145–155. doi: 10.1002/ajpa.20851. [DOI] [PubMed] [Google Scholar]
- Solberg HE. Approved recommendations (1986) on the theory of reference values. Part 1. The concept of reference values. J Clin Chem Clin Biochem. 1987;25:337–342. [PubMed] [Google Scholar]
- Solberg H, Gräsbeck R. Reference values. Adv Clin Chem. 1989;27:1–79. doi: 10.1016/s0065-2423(08)60181-x. [DOI] [PubMed] [Google Scholar]
- Sparrow D, Tifft CP, Dibbs E, Saini M, Rosner B, Weiss ST. The relationship of various indices of heart size on chest x-ray to the 10-year incidence of hypertension. The Normative Aging Study. Am J Epidemiol. 1985;122:782–788. doi: 10.1093/oxfordjournals.aje.a114161. [DOI] [PubMed] [Google Scholar]
- Sunderman FW. Current concepts of “normal values,” “reference values,” and “discrimination values” in clinical chemistry. Clin Chem. 1975;21:1873–1877. [PubMed] [Google Scholar]
- Tanase H, Yamori Y, Hansen CT, Lovenberg W. Heart size in inbred strains of rats. Part 1. Genetic determination of the development of cardiovascular enlargement in rats. Hypertension. 1982;4:864–872. doi: 10.1161/01.hyp.4.6.864. [DOI] [PubMed] [Google Scholar]
- Terio KA, Kinsel MJ, Raphael J, Mlengeva T, Lipende I, Kirchhoff CA, Gilagiza B, Wilson ML, Kamenva S, Estes JD, Keele BF, Rudicell RS, Liu W, Patton S, Colllins A, Hahn BH, Travis DA, Lonsdorf EV. Pathologic lesions in chimpanzees (Pan troglodytes schweinfurthii) from Gombe National Park, Tanzania, 2004–20120. J Zoo Wildlife Med. 2011;42:597–607. doi: 10.1638/2010-0237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. New York: Addison-Wesley; 1977. p. 688. [Google Scholar]
- Varki N, Anderson D, Herndon JG, Pham T, Gregg CJ, Cheriyan M, Murphy J, Strobert E, Fritz J, Else JG, Varki A. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evolutionary Applications. 2009;2:101–112. doi: 10.1111/j.1752-4571.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan R, Larson M, Leip E, Evans J, O’Donnell C, Kannel W, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Eng J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- Videan EN, Fritz J, Murphy J. Development of guidelines for assessing obesity in captive chimpanzees (Pan troglodytes) Zoo Biol. 2007;26:93–104. doi: 10.1002/zoo.20122. [DOI] [PubMed] [Google Scholar]
- Weber KT. Fibrosis and hypertensive heart disease. Curr Opinion Cardiology. 2000;15:264–272. doi: 10.1097/00001573-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Yiu K-H, Tse H-F. Hypertension and cardiac arrhythmias: a review of the epidemiology, pathophysiology and clinical implications. J Hum Hypertens. 2008;22:380–388. doi: 10.1038/jhh.2008.10. [DOI] [PubMed] [Google Scholar]