Abstract
Engagement in regular aerobic exercise is associated with cognitive benefits, but information on the mechanisms governing these changes in humans is limited. The goal of the current study was to compare neurometabolite concentrations relating to cellular metabolism, structure, and viability in endurance-trained and sedentary middle-aged adults. Twenty-eight endurance-trained and 27 sedentary adults, aged 40–65 years, underwent general health assessment, cardiorespiratory fitness measurement, neuropsychological testing, and proton magnetic resonance spectroscopy (1H MRS). 1H MRS was used to examine N-acetyl-aspartate (NAA), creatine (Cr), myo-inositol (mI), choline (Cho), and glutamate (Glu) concentrations in frontal and occipitoparietal grey matter. Group differences in concentrations of NAA, Cho, mI, and Glu, calculated as ratios over Cr, were explored using ANOVA. There were no significant differences in global cognitive function, memory, and executive function performance between the groups. In comparison to sedentary adults, the endurance-trained group displayed significantly higher NAA/Cr in the frontal grey matter (F(1, 53) = 5.367, p = 0.024) and higher Cho/Cr in the occipitoparietal grey matter (F(1, 53) = 5.138, p = 0.028). Within our middle-aged sample, endurance-trained adults demonstrated higher levels of NAA/Cr in the frontal grey matter and higher Cho/Cr in the occipitoparietal grey matter. Higher levels of NAA may indicate greater neuronal integrity and higher cerebral metabolic efficiency in association with cardiorespiratory fitness, whereas increased Cho may represent increased phospholipid levels secondary to neural plasticity.
Keywords: Aerobic fitness, 1H MRS, N-acetyl-aspartate, Choline, Endurance exercise
Introduction
Normal human aging induces pervasive changes in brain morphology such as global mass reduction, ventricle expansion, and loss of myelination (Pakkenberg and Gundersen 1997; Skullerud 1985). Age-related structural changes couple with decrements in cognitive abilities (Park et al. 2001), placing older adults at increased risk for institutionalization and all-cause mortality (St John et al. 2002). Fortunately, the trajectory towards cognitive decline is not immutable as recent research indicates that the simple practice of engaging in regular aerobic exercise lowers the incidence of cognitive impairment and dementia (Barnes et al. 2003; Larson et al. 2006). These findings provide an unprecedented opportunity to enhance our understanding of cognitive aging. Systematic examination of the mechanisms underlying exercise-related cognitive benefits may yield unique insights into the physiological underpinnings of successful cognitive aging, paving the way for new therapeutic interventions.
To date, the majority of research on the impact of fitness on the central nervous system has been conducted using animal models. Within these models, running wheel access robustly increases trophic factors and enhances the number of newly labeled cells within the dentate gyrus of the hippocampus, a brain structure crucial for successful declarative memory performance (Neeper et al. 1996; Van Praag et al. 1999). However, while neurogenesis in rodent studies appears to be restricted to the hippocampal region, the largest cognitive benefits of regular exercise in humans have been noted in the domain of executive function (Colcombe and Kramer 2003), which is not dependent on the hippocampus. Thus, current evidence suggests that the mechanisms driving fitness-related cognitive enhancement in human and non-human animals may be different.
Fortunately, fitness-related changes in the central nervous system can be noninvasively assessed in humans in vivo with the help of magnetic resonance imaging (MRI). In particular, proton magnetic resonance spectroscopy (1H MRS) can provide quantitative measures of neurometabolites relating to cellular metabolism, structure, and viability (Ross and Sachdev 2004), which may shed light on how aerobic exercise alters brain composition in humans. Using a cross-sectional design, neurochemical concentrations were compared between sedentary and endurance-trained middle-aged adults in two voxels of interest, the frontal gray matter, including anterior cingulate gyrus, and occipitoparietal gray matter including, posterior cingulate. The frontal area was selected based on substantial evidence of fitness-related increases in brain structure and function in this region (Colcombe et al. 2003b, 2004; Prakash et al. 2010). The posterior region was chosen because neurochemical alterations that relate to cognition in this area are well-documented (Friedman et al. 1999; Kantarci et al. 2000; Griffith et al. 2007). Based on the findings of increased brain volume in association with higher cardiorespiratory fitness (Colcombe et al. 2003a; Gordon et al. 2008) it was hypothesized that higher concentrations of N-acetyl-aspartate (NAA), a marker of neuronal viability, would be observed in the endurance-trained adults.
Materials and Methods
Participants
Sedentary and endurance-trained adults between the ages of 40 and 65 years were recruited through flyers and newspaper advertisements. Participants were classified as endurance-trained if they reported cycling and/or running at a moderate or strenuous exercise intensity at least 4 days per week on the International Physical Activity Questionnaire Short Form (Craig et al. 2003). Sedentary participants reported engaging in no regular physical exercise in the past year. Self-reported exercise training status was verified by maximal oxygen consumption (Wilson and Tanaka 2000). The age range of the sample was restricted to middle-age adults in accordance with the World Health Organizations (2005) recommendations for implementing early interventions. All participants were apparently healthy and free of overt coronary artery disease, neurological disease (e.g., stroke, Parkinson’s disease, clinically significant traumatic brain injury), major psychiatric illness (e.g. schizophrenia, bipolar disorder), and substance abuse (i.e., diagnosed abuse and/or previous hospitalization for substance abuse) as assessed by the medical history questionnaire. All participants were nonsmoking and were not taking any cardiovascular-acting medications. Sixty-two participants completed the initial screen and were enrolled in the study after providing written consent. Seven participants were excluded from analyses due to poor quality MRS data (Cramér-Rao Lower Bounds for NAA/creatine [Cr], myo-inositol [mI]/Cr, choline [Cho]/Cr or glutamate [Glu]/Cr > 12). The removed participants did not differ in age (p = 0.668), education (p = 0.502), cardiorespiratory fitness (VO2 max) (p = 0.331), or sex distribution (p = 0.677) from the remaining sample. The final sample consisted of 28 participants in the endurance-trained group and 27 participants in the sedentary group. Participants identified themselves as follows: 83.6 %, Caucasian; 5.5 %, Hispanic; 3.6 %, African American; 1.8 %, Asian; and 5.5 %, Other/Did Not Specify.
Procedures
The study was conducted in accordance with the Helsinki Declaration of 1975 and with approval from the local Institutional Review Board. All volunteers provided written informed consent before enrollment. Participants completed a medical history questionnaire in which medical conditions and treatments were coded as either present or absent based on participants’ self-report. Participants then underwent a general health assessment, cardiorespiratory fitness assessment, and neuropsychological/brain imaging assessment. Assessments were conducted on separate days and participants completed the study within 2 months.
General Health Assessment
Participants abstained from caffeine and fasted for at least 12 h prior to the assessment. Body mass in kilograms and height in centimeters were measured on a physician’s beam balance scale for the subsequent calculations of body mass index (BMI). BMI was calculated by dividing body mass in kilograms by height in meters squared. Following 15 min of rest, participants sat upright while brachial blood pressure was measured using a semi-automated device (VP-2000, Omron Healthcare, Bannockburn, IL). Approximately 3 ml of fasting blood was collected from the antecubital vein by venipuncture. The plasma concentrations of glucose and total-cholesterol were measured using standard enzymatic technique.
Cardiorespiratory Fitness Assessment
Participants abstained from caffeine and physical exercise for 24 h and fasted for at least 4 h prior to the visit. Maximal oxygen consumption (VO2 max) was assessed with a graded treadmill exercise test during a modified Bruce protocol. Following a 5-min warm up period, participants ran or walked at a speed that corresponded to 70–80 % of their age-predicted maximal heart rate. The treadmill slope was increased 2 % every 2 min until volitional exhaustion. Oxygen consumption (indirect calorimetry via respiratory gas measurements; Physio-Dyne, Quogue, NY), heart rate, and ratings of perceived exertion (the original Borg scale) (Borg 1982) were measured throughout the protocol. At the end of each stage, participants rated their perceived exertion using the Borg scale (Borg 1982) and heart rate was recorded. The gas analyzer was calibrated with standard gases of known concentrations before each trial.
Neuropsychological/Brain Imaging Assessment
Participants completed a battery of standard clinical neuropsychological instruments with established reliability and validity (Lezak et al. 2004). In order to reduce the number of multiple comparisons, neuropsychological measures were grouped into one of three domains—global cognition, memory, or executive function. For the domain scores, raw test scores were converted to z scores based on the study sample’s mean and standard deviation. Timed test scores were multiplied by −1 so that higher scores indicate better performance. Domain scores were calculated for each participant by averaging the z scores within the domain as follows: (1) global: Mini-Mental Status Exam (Folstein et al. 1975) and Wechsler Test of Adult Reading (2001); (2) memory: California Verbal Learning Test II immediate recall, delayed recall, and recognition discrimination (Delis et al. 1987); (3) executive: Trail Making Test Parts A and B time to completion (Reitan 1958), Controlled Oral Word Associations Test (Ruff et al. 1996), and Wechsler Adult Intelligence Scale III Digit Span Subtest (1997). All tests were administered and scored by a trained research assistant using standard administration and scoring criteria.
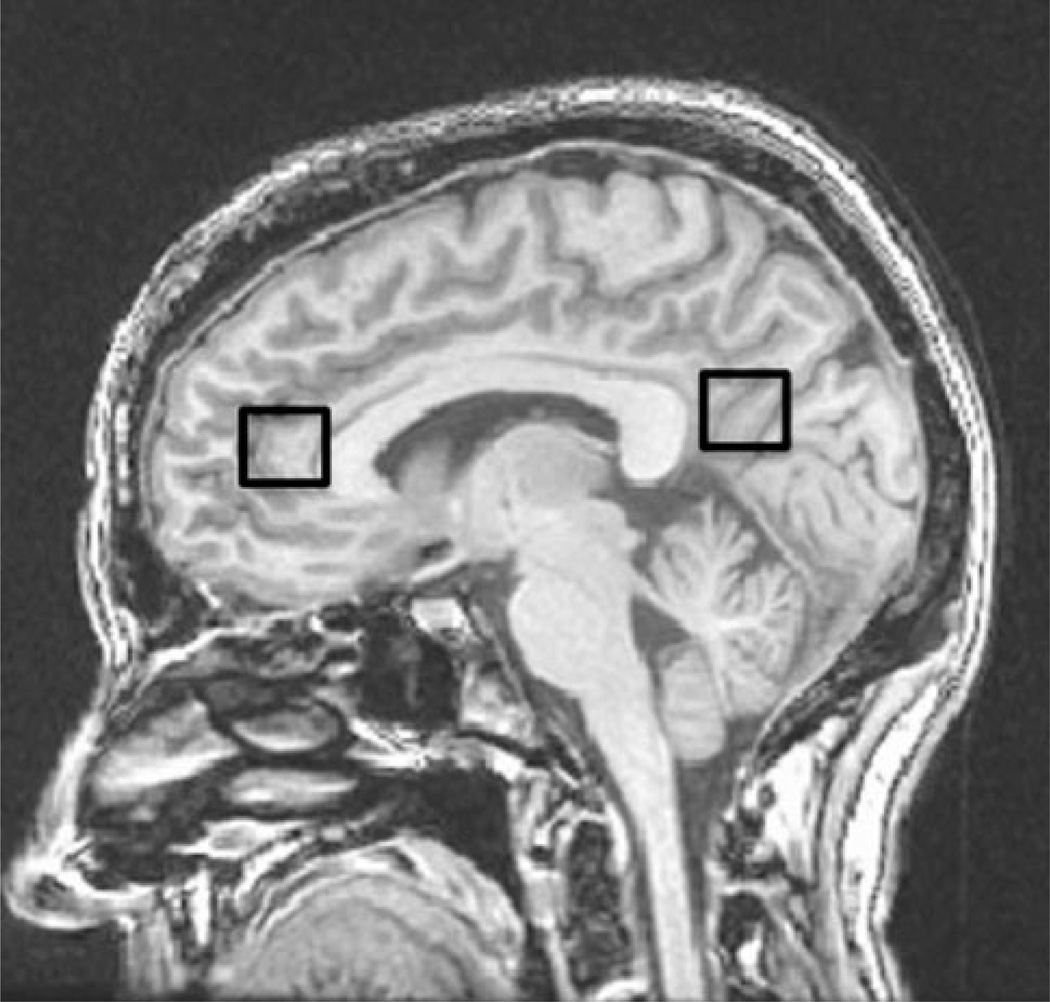
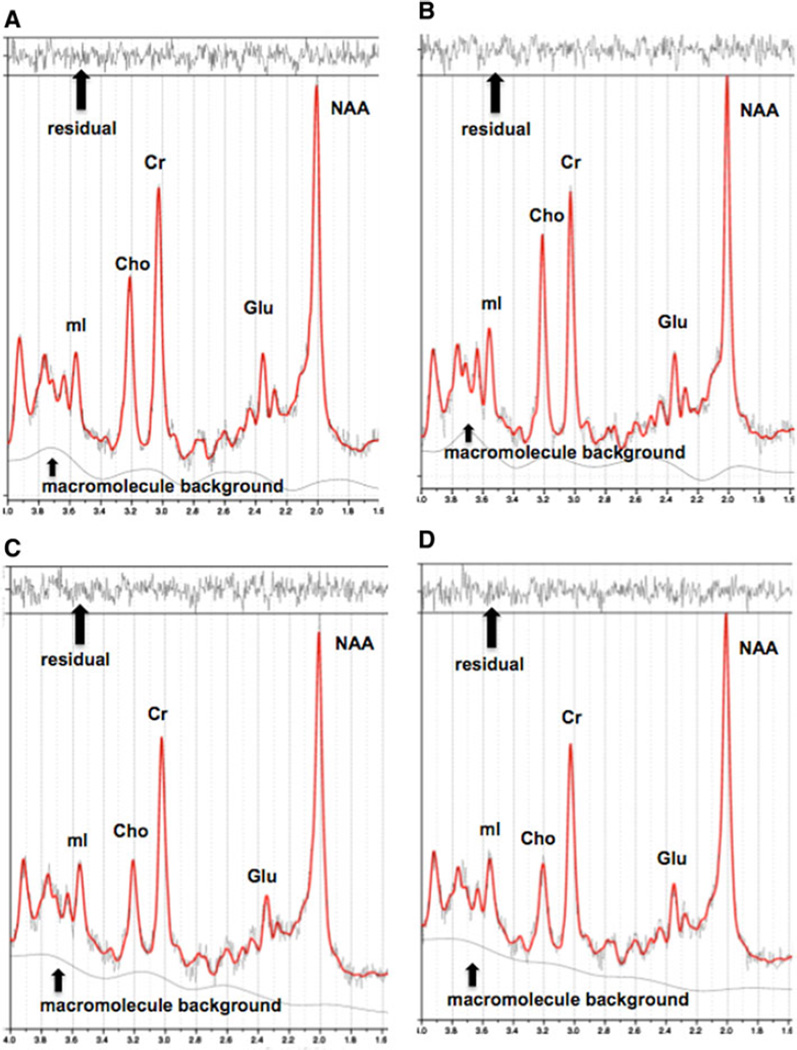
MRS data for each participant were acquired in a single session on a 3T GE Signa Excite MRI scanner equipped with a standard head coil. As described in previous studies (Haley et al. 2010a, b), imaging included single voxel proton MRS performed using the GE pulse sequence PROBE-P, an automated point resolved spectroscopy sequence with chemical shift selected water suppression. Each spectroscopic voxel was prescribed from 3D high-resolution spoiled gradient echo sagittal images (256 × 256 matrix, FOV = 24 × 24 cm2, 1 mm slice thickness, 0 gap) of the entire brain. 1H MRS parameters were as follows: echo time/repetition time = 35/3,000 ms, 128 excitations, 5,000 Hz spectral width, volume ~6 cm3, 15mm × 20 mm × 20 mm from the occipitoparietal gray matter including posterior cingulate and the frontal gray matter including anterior cingulate gyrus (Fig. 1). In order to ensure the quality of the data, a single experimenter localized the voxel placement on all subjects. A digital archive of the placement was saved and reviewed for placement consistency before analysis. The concentrations of five neurochemical were assessed: NAA, a marker of neuronal viability; Cho-containing compounds (phosphocholine and glycerophosphocholine, Cho), an indicator of phospholipid composition; mI, an organic osmolyte and a precursor of inositol triphosphate; Glu, a marker of excitatory neurotransmission; and Cr, an indicator of energy metabolism (Ross and Sachdev 2004). Commercially available software, LCModel, was used to quantify and separate the metabolite resonances from the macromolecule background (Provencher 1993) (Fig. 2). The main metabolites were quantified at the following resonance frequencies: NAA, 2.02 ppm; total Cho, 3.26 ppm; Cr, 3.03 ppm; mI, 3.56 ppm; and Glu, 2.34 ppm.
Fig. 1.
Anatomical image with superimposed voxel outlines indicating MRS volumes in the frontal and occipitoparietal gray matter
Fig. 2.
NAA N-acetyl-aspartate, Glu glutamate, Cr creatine + phosphocreatine, Cho = choline + phosphocholine, mI myo-inositol. a Representative 1H MRS spectrum from the frontal grey matter in the sedentary group. b Representative 1H MRS spectrum from the frontal grey matter in the endurance-trained group. c Representative 1H MRS spectrum from the occipitoparietal grey matter in the sedentary group. d Representative 1H MRS spectrum from the occipitoparietal grey matter in the endurance-trained group
Statistical Analyses
All variable distributions were assessed using the Shapiro–Wilk test of normality recommended for small samples. Group differences in demographic and physiological variables were assessed using non-parametric χ2 or Mann–Whitney U tests since many physiological variables have naturally skewed distributions. Group differences for the cognitive domain scores were assessed with ANOVA. In line with standard protocols (Kantarci et al. 2000; Staffen et al. 2005), the concentrations of NAA, Cho, mI, and Glu were reported as ratios relative to Cr. Group differences in neurometabolite ratios (NAA/Cr, Cho/Cr, mI/Cr, and Glu/Cr) were assessed using ANOVA in each voxel regions. Regression was used to explore the relationship between cardiorespiratory fitness (VO2 max) and neurochemical concentrations with significant group differences (NAA/Cr in the frontal grey matter and Cho/Cr in the occipitoparietal grey matter), controlling for age. All statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL). An α level of 0.05 was set as the criterion for statistical significance.
Results
Descriptive Statistics
Means and standard deviations of the demographic and physiological variables for each group are reported in Table 1. As expected, the two groups differed significantly for VO2 max (Table 1). The groups also differed in BMI, and there was a near significant effect for total cholesterol. However, there were no significant group differences in gender (sex) distribution, age, years of education, systolic and diastolic blood pressure, and fasting blood glucose concentration (Table 1). Cognitive domain scores are reported in Table 2. There were no significant differences in global cognitive function, memory, and executive function scores between endurance-trained and sedentary groups (Table 2).
Table 1.
Selected demographic and physiological characteristics
| Sedentary N = 27 |
Endurance- trained N = 28 |
p Value | |
|---|---|---|---|
| Male/Female | 6/21 | 12/16 | 0.103 |
| Education (years) | 16.4 ± 2.0 | 17.2 ± 2.1 | 0.250 |
| Age (years) | 53.1 ± 5.6 | 51.3 ± 5.4 | 0.252 |
| BMI (kg/m2) | 26.2 ± 4.9 | 23.6 ± 2.8 | 0.021 |
| Systolic blood pressure (mmHg) | 120 ± 13 | 123 ± 12 | 0.411 |
| Diastolic blood pressure (mmHg) | 73 ± 96 | 72 ± 7 | 0.924 |
| Total cholesterol (mg/dl) | 203.3 ± 31.3 | 188.0 ± 36.3 | 0.051 |
| Blood glucose (mg/dl) | 90.5 ± 13.0 | 89.9 ± 7.9 | 0.678 |
| VO2 max (ml/kg/min) | 26.5 ± 5.1 | 45.4 ± 8.3 | <0.001 |
VO2 max maximal oxygen consumption
Table 2.
Cognitive domain z scores
| Cognitive domain | Sedentary N = 27 |
Endurance-trained N = 28 |
p Value |
|---|---|---|---|
| Global | −0.006 ± 0.83 | 0.006 ± 0.58 | 0.948 |
| Memory | −0.18 ± 1.12 | 0.15 ± 0.63 | 0.193 |
| Executive function | −0.08 ± 0.69 | 0.08 ± 0.62 | 0.360 |
Cerebral Metabolism in the Frontal Grey Matter and Cardiorespiratory Fitness
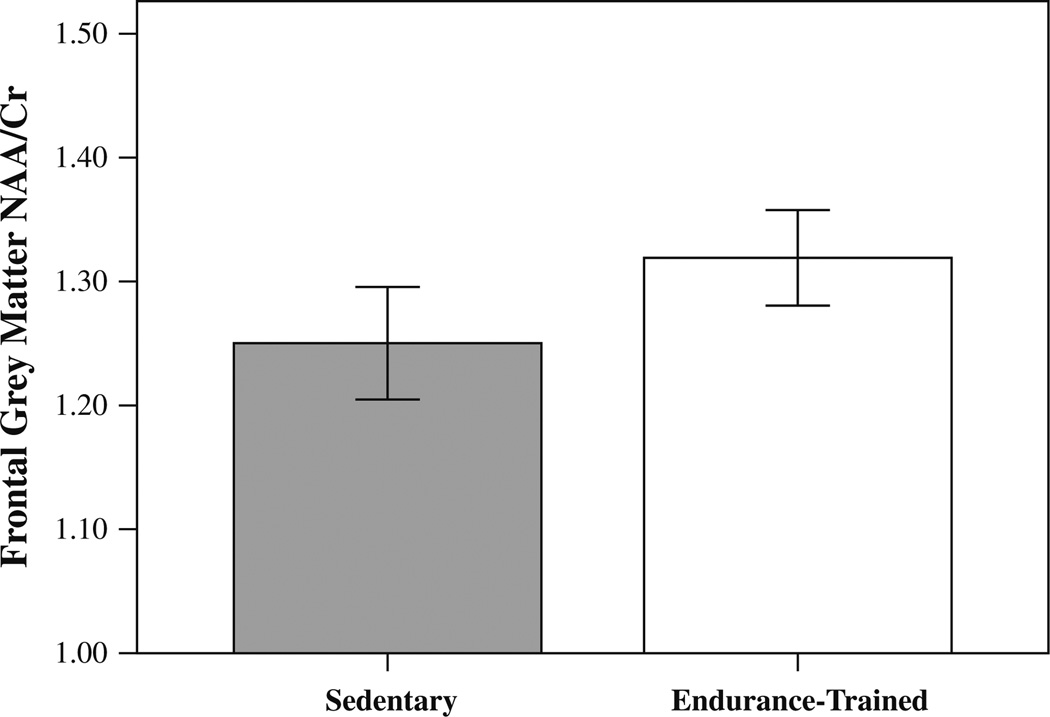
Analysis of variance revealed that endurance-trained adults had significantly higher NAA/Cr in the frontal grey matter (F(1, 53) = 5.367, p = 0.024) (Fig. 3). Endurance-trained and sedentary adults did not significantly differ for frontal grey matter Glu/Cr (F(1, 53) = 0.247, p = 0.621), mI/Cr (F(1, 53) = 0.073, p = 0.787) or Cho/Cr (F(1, 53) = 1.539, p = 0.220) levels. The means and standard deviations of neurochemical concentrations for each group are presented in Table 3.
Fig. 3.
Bar graph with standard errors showing significantly higher frontal grey matter NAA/Cr in the endurance-trained group as compared with sedentary controls
Table 3.
Neurochemical concentrations
| Neurochemical | Sedentary N = 27 |
Endurance- trained N = 28 |
p Value |
|---|---|---|---|
| mI/Cr (frontal) | 0.83 ± 0.12 | 0.84 ± 0.12 | 0.787 |
| Glu/Cr (frontal) | 1.50 ± 0.13 | 1.52 ± 0.17 | 0.621 |
| Cho/Cr (frontal) | 0.29 ± 0.04 | 0.30 ± 0.05 | 0.220 |
| NAA/Cr (frontal) | 1.25 ± 0.12 | 1.32 ± 0.10 | 0.024* |
| mI/Cr (occipitoparietal) | 0.65 ± 0.09 | 0.67 ± 0.08 | 0.306 |
| Glu/Cr (occipitoparietal) | 1.41 ± 0.15 | 1.47 ± 0.15 | 0.146 |
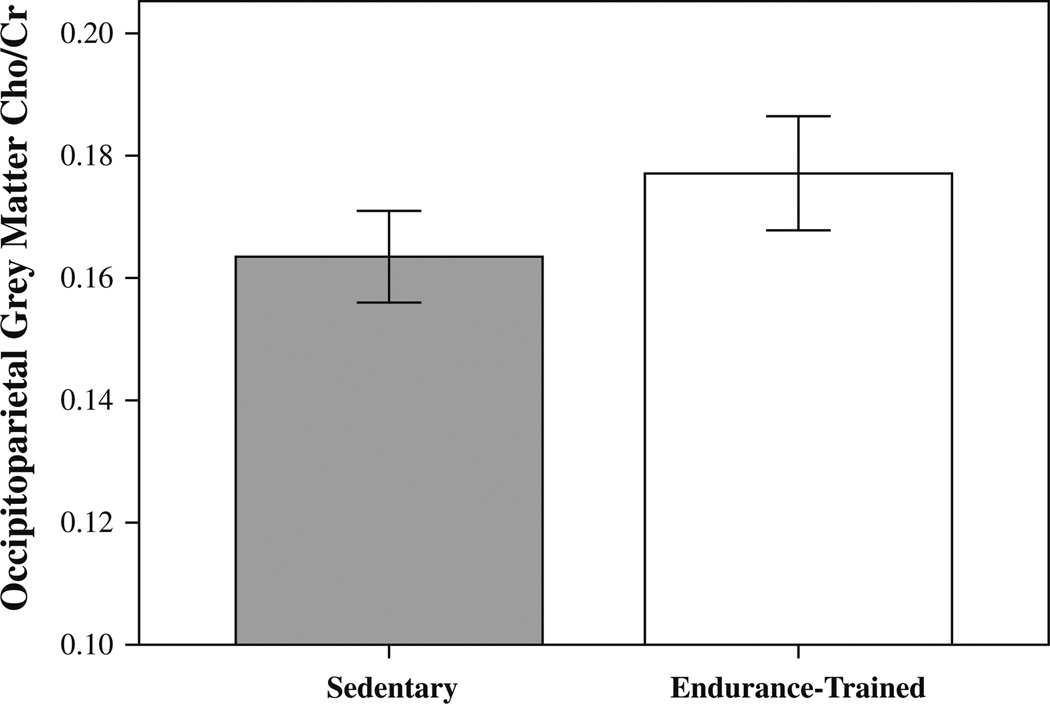
| Cho/Cr (occipitoparietal) | 0.16 ± 0.02 | 0.18 ± 0.02 | 0.028* |
| NAA/Cr (occipitoparietal) | 1.47 ± 0.13 | 1.51 ± 0.08 | 0.180 |
p < 0.05
Cerebral Metabolism in the Occipitoparietal Grey Matter and Cardiorespiratory Fitness
Analysis of variance indicated that endurance-trained had significantly higher Cho/Cr in the occipitoparietal grey matter (F(1, 53) = 5.138, p = 0.028) (Fig. 4). The groups did not differ significantly for NAA/Cr (F(1, 53) = 1.848, p = 0.180), Glu/Cr (F(1, 53) = 2.172, p = 0.146), or mI/Cr (F(1, 52) = 1.069, p = 0.306) occipitoparietal grey matter levels. The means and standard deviations of neurochemical concentrations for each group are presented in Table 3.
Fig. 4.
Bar graph with standard errors showing significantly higher occipitoparietal grey matter Cho/Cr in the endurance-trained group as compared with sedentary controls
Cerebral Metabolism and VO2 max
Independent of age, VO2 max successfully predicted NAA/Cr in the frontal grey matter (adjusted R2 = 0.107, β = 0.338, p = 0.044) and Cho/Cr in the occipitoparietal grey matter (adjusted R2 = 0.227, β = 0.452, p = 0.001).
Discussion
To our knowledge, this is the first study to examine neurochemical differences in endurance-trained and sedentary middle-aged adults. We found that endurance-trained adults displayed higher NAA/Cr in frontal grey matter and higher Cho/Cr in occipitoparietal grey matter. Additionally, VO2 max successfully predicted NAA/Cr in frontal grey matter and Cho/Cr in occipitoparietal grey matter independent of age. These findings significantly extend knowledge based on animal models where the majority of exercise-related changes have been restricted to the hippocampus (Neeper et al. 1996; Van Praag et al. 1999). Additionally, they support a prior study, which found that higher VO2 max was associated with increased right frontal cortex NAA/Cr in a sample of older, sedentary adults (Erickson et al. 2012). Our results indicate that aerobic fitness may have distinctive effects on the human brain, preferentially affecting neuronal viability in the frontal brain regions and cellular membrane composition in the posterior regions. The observed pattern of results is consistent with the cognitive literature documenting the largest exercise-related benefits in humans in the frontally-mediated executive function domain (Colcombe and Kramer 2003).
NAA is found almost exclusively within neurons (Unger et al. 1991), leading to the suggestion that NAA concentrations may be directly reflective of neuronal number (Cheng et al. 2002). Moreover, NAA levels decrease in conditions associated with neuronal loss such as healthy aging (Angelie et al. 2001) and Alzheimer’s disease (Kantarci et al. 2000). Extensive evidence from the animal literature indicates that aerobic exercise is neuroprotective. In rodents, exposure to running wheel access enhances the number of newly labeled cells in the dentate gyrus of the hippocampus (Van Praag et al. 1999). The exercise-induced neurogenesis is believed to be secondary to the upregulation of brain-derived neurotrophic factor (BDNF) (Neeper et al. 1996), which supports the growth and differentiation of neurons. While evidence is more limited in humans, higher cardiorespiratory fitness was associated with attenuated age-related declines in frontal and parietal grey matter volumes (Colcombe et al. 2003). Moreover, a 6-month aerobic exercise intervention was found sufficient to increase brain volume in several regions including the dorsal anterior cingulate, the supplementary motor cortex, the frontal gyrus, and the superior temporal lobe (Colcombe et al. 2006). Therefore, the higher frontal grey matter NAA in endurance-trained adults that we observed may be a reflection of greater neuronal integrity in the response to enhanced cardiorespiratory fitness.
Alternatively, higher NAA/Cr levels may indicate mitochondrial health and metabolic efficiency secondary to cardiorespiratory fitness. NAA is synthesized from acetyl-coA and aspartate within the mitochondria and is therefore, directly related to mitochondrial viability (Clark 1998). NAA levels are known to diminish in response to pharmacological mitochondrial damage (Demougeot et al. 2001) and recover upon restoration of cerebral energy balance (Signoretti et al. 2008). With advancing age, mitochondria in the brain show reductions in oxidation capabilities and higher reactive oxygen species production (Navarro and Boveris 2007). In contrast, habitual exercise has been shown to improve mitochondrial oxidative capacity and reduce oxidative stress levels in the brain (Navarro et al. 2004). Thus, higher NAA/Cr levels in endurance-trained adults may reflect enhanced mitochondrial functioning and potential exercise-related protection from age-related declines in cerebral metabolic efficiency.
While higher NAA in endurance-trained adults was an anticipated result, the outcome of higher Cho/Cr in the occipitoparietal region was an unexpected and intriguing finding. The Cho peak in the MR spectrum is largely comprised of the phospholipids phosphocholine and glycerophosphocholine (Ross and Sachdev 2004). Phosphocholine is a precursor for phosphatidylcholine, the most ubiquitous phospholipid component of cell membranes, whereas glycerophosphocholine is a by-product of phosphatidylcholine breakdown (Klein 2000). Therefore, higher Cho/Cr is often interpreted as either enhanced phospholipid membrane synthesis or heighted membrane turnover and breakdown. For example, higher Cho/Cr levels are documented in demyelinating diseases (Inglese et al. 2003) as well as in conditions of enhanced cellular growth such as tumors (Herminghaus et al. 2002).
In relation to aerobic fitness, higher Cho levels are more likely to represent upregulation of phospholipids secondary to dendritic and axonal growth. Phosphocholine is significantly enhanced during periods of rapid neuritic growth such as in the developing brain (Pettegrew et al. 1999). Additionally, surges in phosphatidylcholine synthesis are observed when axonal growth is stimulated by nerve growth factor (Araki and Wurtman 1997). Supplementation of the phosphatidylcholine precursor, citicoline, increases cerebral levels of phosphatidylcholine (Adibhatla et al. 2001) and improves functional outcomes after stroke by increasing neurogenesis, promoting dendritic spine growth, and enhancing the expression of synaptic proteins (Gutiérrez-Fernández et al. 2011; Hurtado et al. 2007). With 1H MRS, higher Cho levels have been observed in the rat hippocampus following electroconvulsive shock treatment (Sartorius et al. 2003), a procedure associated with neurogenesis (Madsen et al. 2000). Additionally, frontal grey matter Cho levels have been found to positively correlate with greater overall cortical, frontal, and temporal gray matter volumes in patients with HIV (Cohen et al. 2010). Interestingly, many structural brain changes reflecting increased neuroplasticity have been observed in association with regular aerobic exercise. In addition to neurogenesis (Gutiérrez-Fernández et al. 2011; Hurtado et al. 2007), habitual exercise upregulates the synaptic protein, synapsin I (Vaynman et al. 2004) and enhances dendritic arborization and spinal density in the dentate gyrus and entorhinal cortex (Redila and Christie 2006; Stranahan et al. 2007). Thus, higher Cho/Cr levels in endurance-trained adults may represent enhanced neural plasticity stimulated by the upregulation of neurotrophic factors such as BDNF. However, this hypothesis will need verification from future studies of fitness and neurochemical concentrations, particularly those combined with histological examination.
Given that different neurometabolites were elevated in association with fitness in the frontal and occipitoparietal areas, it appears that impact of cardiorespiratory fitness may show regional influence. In particular, aerobic fitness may have a larger impact on neuronal viability in the frontal regions and greater implications for phospholipid composition in the posterior regions. Regional variability of the impact of fitness is supported by the finding that aerobic fitness has the greatest cognitive benefit for frontally-mediated executive function tasks (Redila and Christie 2006; Stranahan et al. 2007). Similarly, a structural MRI study found that higher aerobic fitness was associated with greater frontal, temporal, and parietal grey matter volumes, but had no relation with volumetry in the occipital regions (Colcombe et al. 2003).
The findings in our present study are limited by the cross-sectional study design. It is possible that individuals who choose to engage in regular aerobic exercise may have other fundamental differences that account for the results. However, the fact that the groups did not differ in terms of age, education, and global cognitive ability helps to minimize this possibility. Importantly, longitudinal studies will be critical for determining if exercise interventions can alter neurometabolite concentrations. Given that reductions in NAA are reversible in other conditions (Signoretti et al. 2008), aerobic exercise interventions may be capable of enhancing NAA levels in previously sedentary adults. Another limitation was the 1H MRS methodology, which does not enable tissue segmentation for grey matter, white matter, and cerebrospinal fluid. Finally, future studies with larger sample sizes should assess the influence of other cardioprotective factors (e.g. low BMI, high HDL-cholesterol levels) on the relationship between cardiorespiratory fitness and neurochemical concentrations.
In summary, we found that endurance-trained adults displayed higher levels of NAA/Cr in the frontal grey matter and higher Cho/Cr in the occipitoparietal grey matter than sedentary controls. Higher levels of NAA could be indicative of greater neuronal integrity and higher cerebral metabolic efficiency in association with cardiorespiratory fitness, whereas increased Cho may represent enhanced phospholipid levels secondary to neural plasticity. Our results support a growing body of literature suggesting that engagement in regular aerobic exercise may influence central nervous system structure and function, which may ultimately help to preserve cognitive functioning throughout the lifespan.
Acknowledgments
This work was funded in part by grants from the American Heart Association (09BGIA2060722, APH), American Federation for Aging Research (8A0024, APH), the National Institutes of Health (NS075565, APH) and the University of Texas at Austin (APH). The authors thank the Imaging Center Staff for their help with the participants.
Contributor Information
Mitzi M. Gonzales, Department of Psychology, The University of Texas at Austin, 108 East Dean Keeton, Stop A8000, Austin, TX 78712, USA
Takashi Tarumi, Department of Kinesiology and Health Education, The University of Texas at Austin, 1 University Station, D3700, Austin, TX 78712, USA.
Sonya Kaur, Department of Psychology, The University of Texas at Austin, 108 East Dean Keeton, Stop A8000, Austin, TX 78712, USA.
Nantinee Nualnim, Department of Kinesiology and Health Education, The University of Texas at Austin, 1 University Station, D3700, Austin, TX 78712, USA.
Bennett A. Fallow, Department of Kinesiology and Health Education, The University of Texas at Austin, 1 University Station, D3700, Austin, TX 78712, USA
Martha Pyron, Department of Kinesiology and Health Education, The University of Texas at Austin, 1 University Station, D3700, Austin, TX 78712, USA; Medicine in Motion, 13805 Research Blvd, Ste 150, Austin, TX 78750, USA.
Hirofumi Tanaka, Department of Kinesiology and Health Education, The University of Texas at Austin, 1 University Station, D3700, Austin, TX 78712, USA.
Andreana P. Haley, Email: haley@psy.utexas.edu, Department of Psychology, The University of Texas at Austin, 108 East Dean Keeton, Stop A8000, Austin, TX 78712, USA; University of Texas Imaging Research Center, Austin, TX, USA.
References
- Adibhatla RM, Hatcher JF, Dempsey RJ. Effects of citicoline on phospholipid and glutathione levels in transient cerebral ischemia. Stroke. 2001;32:2376–2381. doi: 10.1161/hs1001.096010. [DOI] [PubMed] [Google Scholar]
- Angelie E, Bonmartin A, Boudraa A, Gonnaud PM, Mallet JJ, Sappey-Marinier D. Regional differences and metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. Am J Neuroradiol. 2001;22:119–127. [PMC free article] [PubMed] [Google Scholar]
- Araki W, Wurtman RJ. Control of membrane phosphatidylcholine biosynthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc Natl Acad Sci USA. 1997;94:11946–11950. doi: 10.1073/pnas.94.22.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Cheng LL, Newell K, Mallory AE, Hyman BT, Gonzalez RG. Quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn Reson Imaging. 2002;20:527–533. doi: 10.1016/s0730-725x(02)00512-x. [DOI] [PubMed] [Google Scholar]
- Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- Cohen PA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, Paul R, Taylor M, Thompson P, Tate D, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B HIV Neuroimaging Consortium. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol. 2010;16:435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund ULF, Yngve A, Sallis JF, Oja P. International physical activity questionnaire 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test: adult version. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- Demougeot C, Garnier P, Mossiat C, Bertrand N, Giroud M, Beley A, Marie C. N-Acetylaspartate, a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. J Neurochem. 2001;77:408–415. doi: 10.1046/j.1471-4159.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Sutton BP, Prakash RS, Voss MW, Chaddock L, Szabo A, Mailey E, White SM, Wojcicki TR, McAuley E, Kramer AF. Beyond vascularization: aerobic fitness on n-acetylaspartate and memory. Brain Behav. 2012;2:32–41. doi: 10.1002/brb3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Brooks WM, Jung RE, Chiulli SJ, Sloan JH, Montoya BT, Hart BL, Yeo RA. Quantitative proton MRS predicts outcome after traumatic brain injury. Neurology. 1999;52:1384–1391. doi: 10.1212/wnl.52.7.1384. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith H, Randall GH, Hollander JA, Okonkwo O, Evanochko WT, Harrell LE, Zamrini EY, Brockington JC, Marson DC. Executive function is associated with brain proton magnetic resonance spectroscopy in amnestic mild cognitive impairment. J Clin Exp Neuropsychol. 2007;29:599–609. doi: 10.1080/13803390600826595. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Fernández M, Rodríguez-Frutos B, Fuentes B, Vallejo-Cremades MT, Álvarez-Grech J, Expósito-Alcaide M, Diez-Tejedor E. CDP-choline treatment induces brain plasticity markers expression in experimental animal stroke. Neurochem Int. 2011;60:310–317. doi: 10.1016/j.neuint.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Haley A, Gonzales M, Tarumi T, Miles S, Goudarzi K, Tanaka H. Elevated cerebral glutamate and myo-inositol levels in middle-aged adults with metabolic syndrome. J Cereb Blood Flow Metab. 2010a;4:397–405. doi: 10.1007/s11011-010-9221-y. [DOI] [PubMed] [Google Scholar]
- Haley AP, Tarumi T, Gonzales MM, Suwagara J, Tanaka H. Subclinical atherosclerosis is related to lower neuronal viability in middle-aged adults: a 1H MRS study. Brain Res. 2010b;1344:54–61. doi: 10.1016/j.brainres.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herminghaus S, Pilatus U, Möller-Hartmann W, Raab P, Lanfermann H, Schlote W, Zanella FE. Increased choline levels coincide with enhanced proliferative activity of human neuroepithelial brain tumors. NMR Biomed. 2002;15:385–392. doi: 10.1002/nbm.793. [DOI] [PubMed] [Google Scholar]
- Hurtado O, Cárdenas A, Pradillo JM, Morales JR, Ortego F, Sobrino T, Castillo J, Moro MA, Lizasoain I. A chronic treatment with CDP-choline improves functional recovery and increases neuronal plasticity after experimental stroke. Neurobiol Dis. 2007;26:105–111. doi: 10.1016/j.nbd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Inglese M, Li BSY, Rusinek H, Babb JS, Grossman RI, Gonen O. Diffusely elevated cerebral choline and creatine in relapsingremitting multiple sclerosis. Magn Reson Med. 2003;50:190–195. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurology. 2000;55:210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm. 2000;107:1027–1063. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Loring D, Hannay H, Fischer J. Neuropsychological assessment. 4th edn. New York: Oxford University Press; 2004. [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive shock therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. J Physiol Cell Physiol. 2007;292:C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marshuetz C. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialog Clin Neurosci. 2001;3:151–166. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Withers G, McKeag D, Strychor S. Changes in brain energy and phospholipid metabolism during development and aging in the Fischer 344 rat. J Neuropathol Exp Neurol. 1999;49:237–249. doi: 10.1097/00005072-199005000-00005. [DOI] [PubMed] [Google Scholar]
- Prakash R, Snook EM, Motl RW, Kramer AF. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 2010;1341:41–51. doi: 10.1016/j.brainres.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Ross AJ, Sachdev PS. Magnetic resonance spectroscopy in cognitive research. Brain Res Rev. 2004;44:83–102. doi: 10.1016/j.brainresrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338. [PubMed] [Google Scholar]
- Sartorius A, Neumann-Haefelin C, Vollmayr B, Hoehn M, Henn FA. Choline rise in the rat hippocampus induced by electroconvulsive shock treatment. Biol Psychiatry. 2003;53:620–623. doi: 10.1016/s0006-3223(02)01600-1. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Marmarou A, Aygok GA, Fatouros PP, Portella G, Bullock RM. Assessment of mitochondrial impairment in traumatic brain injury using high-resolution proton magnetic resonance spectroscopy. J Neurosurg Pediatr. 2008;108:42–52. doi: 10.3171/JNS/2008/108/01/0042. [DOI] [PubMed] [Google Scholar]
- Skullerud K. Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol Scand Suppl. 1985;102:1–94. [PubMed] [Google Scholar]
- St John PD, Montgomery PR, Kristjansson B, McDowell I. Cognitive scores, even within the normal range, predict death and institutionalization. Age Ageing. 2002;31:373–378. doi: 10.1093/ageing/31.5.373. [DOI] [PubMed] [Google Scholar]
- Staffen W, Zauner H, Mair A, Kutzelnigg A, Kapeller P, Stangl H, Raffer E, Niederhofer H, Ladurner G. Magnetic resonance spectroscopy of memory and frontal brain region in early multiple sclerosis. J Neuropsychiatry Clin Neurosci. 2005;17:357–363. doi: 10.1176/jnp.17.3.357. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol. 1991;36:343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. 3rd edn. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler test of adult reading: WTAR. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: relation to training status. Am J Physiol Heart Circ Physiol. 2000;278:H829–H834. doi: 10.1152/ajpheart.2000.278.3.H829. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva: WHO Global Report; 2005. Preventing chronic diseases: a vital investment. [Google Scholar]