Abstract
Both anti-viral and anti-bacterial host defense mechanisms involve TRIF signaling. TRIF provides early clearance of pathogens and coordination of a local inflammatory ensemble through an interferon cascade, while it may trigger organ damage. The multipotentiality of TRIF-mediated immune machinery may direct the fate of our continuous battle with microbes.
Keywords: Host defense mechanism, innate immunity, toll-like receptor, TRIF
1. Introduction
One aspect of human life is fighting against infectious diseases. In spite of the successful development of current antibiotics and vaccination strategies, infectious diseases are still rated as a major cause of human mortality at the present day. Disruptions of microbial ecology may result in selection of certain microbes or evolve new pathogens that can adapt to environmental changes. Previous establishment of the antibiotics with broad-spectrum bactericidal effect has been overcome by many pathogens that have acquired resistance to those drugs. Advanced globalization, food supply and human travels increase the risk of epidemic outbreaks. Pathogen transmission from animals to humans or occurrence of the hybrid vectors may emerge new infectious diseases that had not been previously experienced in humans [1, 2]. These emerging and re-emerging infectious diseases have been identified as important public health problems worldwide. In addition, epidemic potential of some microbes may be misused for bioterrorism purposes, which has become a new threat to our lives. In order to achieve the major improvements in this continuous battle with infectious diseases, it would be required for us to advance our understanding of the pathology and defense mechanisms in our body in a face of pathogenic microbes.
The discovery of pattern recognition receptors (PRRs) has lead to a major paradigm shift in infectious diseases over the last two decades. PRRs are the class of germ line-encoded receptors that trigger innate immune responses by recognizing pathogen-associated molecular patterns (PAMP)s that are conserved molecular structures of microbial components. Upon pathogen recognition, PRRs initiate several intracellular signaling pathways that lead to multiple gene induction required for activation of host defense programs in both arms of innate and adaptive immunity. Innate immunity provides the first line of defense consisting of immediate microbial killing, initiation of local inflammation, and direction and activation of adaptive immunity. Adaptive immunity in turn provides antigen-specific microbial killing and regulation of inflammation through gene rearrangement of T cells and B cells. These antigen non-specific responses in innate immunity do not require the gene rearrangement process that allows immediate defense to variety of microbes. This indicates that PRRs are evolutionally conserved receptors that have been positively selected to sense only molecular structures that are highly conserved in microbes. Although PRRs may inherent risks to be evaded by pathogens carrying mutations in genes encoding their PAMPs, those pathogens can still be recognized by other PRRs because most microbes carry multiple PAMPs. Therefore, recognition of pathogens by PRRs is the essential element of our innate immune system, which has been shaped through long time interaction with microbes to form full defense programs against invading pathogens.
Increased understanding of innate immunity has given rise to a new concept that “we live in host-microbial interactions”. This idea led us to realize that infectious diseases result from disruption of host-microbial interactions, thus microbes are one component of our defense mechanism against pathogenic microbial infection. In addition, substantial parts of the organ pathology in infectious diseases are caused by host immune responses to responsible pathogens. For example, exterior interface of our body such as skin or mucosal surfaces carry diverse but certain groups of microorganisms. These microorganisms seem to be co-evolved with our innate immunity and have established commensal flora, which composes an integrated defense system known as colonization resistance. In fact, germ-free animals have multiple defects in the development of mucosal immune system [3, 4]. In addition, innate immune signaling in epithelial cells leads to the production of antimicrobial peptides in response to commensal bacteria, which contribute to the regulation of commensal composition and protection from harmful foreign pathogens [4, 5]. Therefore, the interplay between host immune system and commensal microorganisms formulates a mutually beneficial symbiotic state in our body. In this review, we discuss a unique aspect of host defense mechanism against microbial pathogens through the viewpoint of TIR-domain-containing adapter-inducing interferon-β (TRIF)-mediated innate immune responses.
2. Infectious disease: an evolutionary arms race
Infectious diseases are the state of illnesses that are caused by invasion of a host by pathogenic microbes, such as viruses, bacteria, fungi and parasites. It is normally triggered by transmission of virulent microbes directly or indirectly from one host to another. Direct transmission includes physical contact of mucosa or wounds to pathogens, mother to fetus or breastfeeding. Indirect transmission can be airborne, food or water borne, blood transfusion, or through vectors such as mosquitoes. Infections may also be triggered by contaminated medical devices e.g., used needles and syringes. The symptoms of an infectious disease are caused by both pathogens and host immune responses. Pathogens produce toxins or utilize virulence factors to invade the host and disrupt structure or function of the organs. By contrast, host immune responses to invading pathogens lead to the production of peroxidase, cytokines, eicosanoids, and bradykinin, resulting in local tissue damage, pyrexia, dilatation of blood vessels, and alteration of clotting, which ultimately can induce SIRS (systemic inflammatory response syndrome) and multiple organ failure. Therefore, most devastating symptoms in infectious diseases are associated with host humoral substances rather than the direct damage caused by pathogens or their toxins.
During our long history of fighting with infectious diseases, host genetic alterations have evolved to minimize the morbidity and mortality from the infections. By contrast, microbes may evolve more rapidly than vertebrates and achieve ways to colonize efficiently and evade host immune defenses. For example, enterohemorrhagic Escherichia coli O157:H7 has emerged in 1982 as a causal pathogen for foodborne illness and has become a major threat to global public health because of its lethal potential by inducing hemolytic uremic syndrome [6]. Recent genetic and evolutionary studies have identified that E. coli O157:H7 is evolved from an enteropathogenic O55:H7 strain, which does not carry several virulence-associated genes in O157:H7 [7]. Bacteria can gain new genetic materials not only by acquiring a natural mutation but also by their competency to uptake DNA from other microbes, which helps them adapt host environment [1]. Specifically in the gastrointestinal tract, the interactions with commensal microorganisms may provide foreign pathogens the chance to acquire an ability to colonize the host through horizontal gene transfer [8]. Therefore, the mutual evolution among host immunity, pathogenic, and non-pathogenic microbes forms a unique ecosystem, which decides the course of our future battle with infectious diseases.
Proper initiation of host immune response is crucial to efficiently defend against pathogenic infections, which largely depends on local innate immunity. The most important task of innate immunity is identification of the pathogenic invasion by PRRs. Although PRRs are thought to be highly conserved in general, recent identification of PRR polymorphisms suggests that the genes encoding PRRs in current generation may have resulted from positive selection through survival from infectious diseases. Furthermore, recent genetic studies have outlined that the intracellular toll-like receptors (TLR)s, especially nucleotide sensing TLRs (TLR3, TLR7, TLR8 and TLR9), have been under stronger selection than have membrane bound TLRs [9]. This biased selection may result from the availability of the PAMPs per pathogen and the location of TLRs. Nucleotide sensing TLRs are mainly responsible for the detection of viral pathogens which carry fewer PAMPs than other types of pathogens, and allowing insertion of foreign nucleotides within the cell is more dangerous than handling pathogens outside of the plasma membrane. Therefore, adaptive evolution by historical constrains including infections and non-adaptive genetic drift shape our innate immune responses in order to survive the evolutionary arms race of humans and microbes. This interaction between host immunity and microbes would be crucial to the understanding of the host defense mechanisms and the pathogenesis of individual infectious diseases.
3. Innate immunity
Innate immunity is the primary host defense program against microbes and tissue damage. It induces generic immune responses to pathogens by recognizing microbial molecular patterns i.e., PAMPs. This response is normally established within hours and continued until the antigen-specific adaptive immune responses are mounted. While the individual arms of these immune pathways play distinct roles to form an organized immune response against invading pathogens, the precise interplay between these two arms of immunity relies on innate immunity. In order to customize host immune response to individual pathogens, innate immunity uses several types of PRRs, which are distributed in the plasma membrane or cytosol of the cells. By sensing each PAMP through a PRR, host antigen-presenting cells (APC)s induce a cascade of cytokines and chemokines to mobilize other types of immune cells to the infection site. The location of individual PRRs in the cell also associates with other cell functions including phagocytosis, intracellular vesicle trafficking, and the expression of cell surface markers. The combination of the types of cells involved and signaling pathways from responsible PRRs shape an innate immune response to infection.
4. TRIF and its signaling
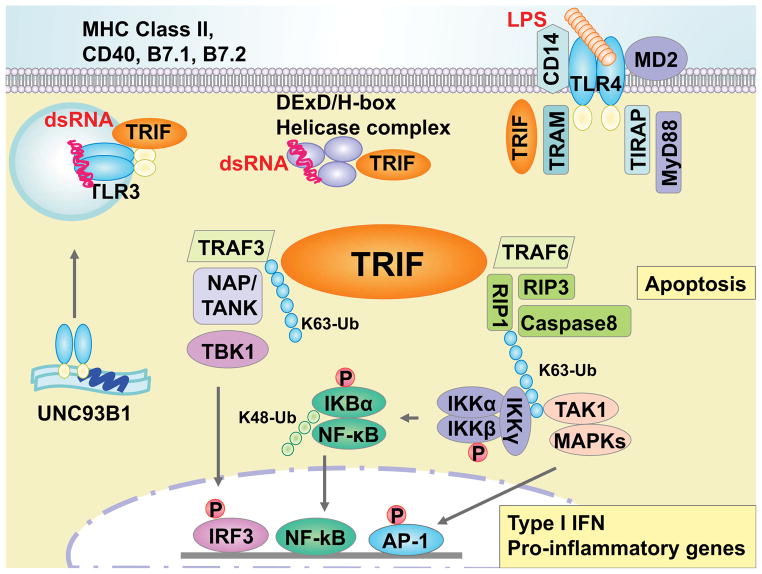
TRIF was discovered as a toll/interleukin-1 receptor-like (TIR) domain-containing adaptor protein of TLR3 and TLR4 as a strong inducer of type I IFNs [10, 11]. Not only type I IFNs, TRIF signaling has been shown to regulate the gene expression of RANTES, IP-10, MCP-1 and IL-12p40 in response to the upstream TLRs [10, 11]. Activation of TRIF signaling also results in cell apoptosis and generation of reactive oxygen species (ROS). Recently, cytosolic DDX1, DDX21, and DHX36 helicases were found as another TRIF interacting PRRs in myeloid DCs that forms a complex in response to dsRNA [12](Figure 1).
Figure 1. TRIF-induced intracellular signaling pathways.
In responds to specific ligands for TLR3, TLR4, or DDX1-DDX21-DX36 (DExD/H-box helicase) complexes, an adaptor protein TRIF activates the transcription factors, IRF3, NF-κB and AP-1 to induce various genes. A dsRNA virus is recognized by TLR3 in endosome or DExD/H-box helicase complex in cytosol. TRL3 is located in endoplasmic reticulum (ER) and move to the endosome membrane through UNC93B1. On the other hand, recognition of Gram-negative bacterial LPS induces TLR4 homodimerization, which transmits the signal through MyD88 and TRIF. During this transmission, TLR4 interacts with TRIF using TRAM. These interactions between TLR3 or TLR4-TRAM with TRIF are madiated thorough TIR:TIR hemophilic interactions, which leads to the oligomerization of TRIF. Activated TRIF then binds to TRAF3, leading to self ubiquitination, which is capable to adapt the scaffolding proteins NAP and/or TANK. In addition, an essential IRF kinase TBK1 is recruited to TRIF-TRAF3 complex that phosphorylate IRF3, resulting in nuclear translocation of IRF3. On the other hand, RIP1 associates with TRIF through RHIM:RHIM hemophilic interactions that is mediated by polyubiquitin by TRAF6. During K63 linked ubiqutinilation, RIP1 binds to IKKγ and TAK1, leading to phosphorylation of IKKβ. Activated IKKβ induces degradation of IKBα that allows translocation of NF-κB heterodimer complex of p50 and p65 to the nucleous. Meantime, TAK1 also binds to MAPKs and activate AP-1 transcription. Transcriptional activation of IRF-3 together with activated of NF-κB and AP-1 induces type I IFNs and other proinflmmatory cytokines and chemokines. In addition to the gene expression, TRIF signaling induces cellular apoptosis through RIP1-induced caspase-8 pathway and necrosis thrugh RIP3 by reactive oxigen species (ROS) accumulation. TRIF also interacts with small GTPases that regulate the surface expression of costimulatory molecues and MHC class II in professional APCs.
Upon activation of upstream PRRs, TRIF serves as a molecular platform for signal transmission and forms physical interactions with several adaptor molecules. The initial step of TRIF signaling is intracellular trafficking to endosomes. This process is mediated through an adaptor TRAM (TRIF-related adaptor molecule) in case of TLR4 stimulation [13]. After receiving upstream inputs by interacting individual Toll/interleukin-1 receptor-like (TIR) domains, TRIF undergoes conformation changes and recruits the downstream TNF receptor-associated factor (TRAF)3 and TRAF6 [14]. TRAF6 facilitates the recruitment and ubiquitination of Receptor-interacting protein (RIP)1, which leads to a complex formation with TAK1 (Transforming growth factor-β-activated kinase 1) and MAPKs (mitogen-activated protein kinase) resulting in nuclear translocation of NF-κB and AP-1, respectively. By contrast, polyubiquitylation of TRAF3 triggers the activation of TANK-binding kinase 1 (TBK1) leading to nuclear translocation of IFN regulatory factor 3 (IRF3).
In addition to the role of TRIF as a signaling adaptor, a recent report has described that TRIF induces mRNA stabilization of IFN-β through MAPK-activated protein 2 [14]. TRIF is also known to mediate apoptotic cell death upon TLR3 and TLR4 stimulation. TRIF-induced cell apoptosis is induced by RIP-mediated caspase-3 activation through caspase-8 but not the mitochondrial pathway [15].
Lastly, the essential roles of TRIF signaling in T cell activation have been reported. TRIF is known to regulate surface expression of costimulatory molecules, CD80 (B7-1), CD86 (B7-2), and CD40 in professional APCs, as well as CTLA-4 in CD4+ T cells [11, 16]. TRIF has also been shown to induce MHCII surface expression in bone marrow derived DCs by interacting with RhoB GTPase after TLR4 stimulation [17]. In addition, TRIF signaling preferentially supports effector T cell accumulation through induction of CXCR3 in vivo [18]. Therefore, TRIF signaling in both APCs and T cells contributes to proper induction of T cell-mediated adaptive immune responses, highlighting TRIF as an important matchmaker between innate and adaptive immunity.
5. TRIF in anti-viral immunity
5.1 Overview
The basic instinct of viruses is passing their genome to the host in order to replicate themselves within the host cells. Major viral pathogens gain access to the host through mucosa and use local cells for viral replication niches. The respiratory tract may be the most prevalent infection site for the viral pathogens such as influenza virus, respiratory synctial virus (RSV), vesicular stomatitis virus and rhinovirus, which cause respiratory and systemic symptoms. The gastrointestinal tract is another major route of occasional viral infection and also involved in the vertical transmission of viruses through breastfeeding. The viruses associated with the vertical transmission include retrovirus HIV, HTLV, Hepatitis A/B, and Herpes simplex viruses (HSV) [19]. Furthermore, many viruses use the gastrointestinal tract as a primary replication site or as a reservoir prior to spreading systemically. The immunomodulatory nature of intestinal mucosa provides a unique environment for pathogenic viruses that is favorable for viral replication. In this regard, symbiotic gut microbiota may contribute to viral colonization and replication as has been shown in mouse infection with retrovirus, mammary tumor virus, and poliovirus [20].
TRIF is an adaptor component of TLR3 that is known to recognize dsRNA viruses, but it plays important roles in infection with other types of viruses, such as HSV (DNA virus) and West Nile virus (ssRNA virus). This is because most viruses form a dsRNA intermediate during replication process in the cytosol. Therefore, TRIF may have multiple roles in anti-viral host defense mechanism.
5.2 Key programs
There are several key programs in the host defense mechanism against viral infection. First, cells nonpermissive to viruses are equipped with intrinsic restriction factors that limit viral replication immediately upon infection. Intrinsic restriction factors include cytosolic enzymes, proteins, and RNA interference that directly inhibit viral fusion, virion release, and viral transcription or translation [21]. For example, APOBEC3G inhibits HIV viral reverse transcription by altering C to U of HIV negative stand, and interferon-inducible transmembrane proteins block entry of the virus, including influenza virus or flaviviruses [21]. These intrinsic factors are unable to control viral infection by themselves but are enough to buy time until the interferon (IFN) system becomes ready.
The IFN system is one of the indispensable host anti-viral programs, which inhibits viral replication and activates several key functions of innate and adaptive anti-viral immunity. IFNs are a prominent cytokine family as it provides early anti-viral response and interactive network through induction of IFN-stimulated genes. Upon viral infection, host cells sense viruses or viral products mainly via nucleotide sensing TLRs, RLRs, or cytosolic DNA sensors resulting in induction of primary responding cytokine, type I IFNs. Secreted type I IFNs bind to cell surface type I IFN receptors (IFNAR1 and IFNAR2) and lead to expression of a variety of genes in an autocrine and paracrine manner. In addition, IFN-stimulated genes inhibit viral replication by degrading viral RNA using 2′-5′ oligoadeylase and RNase L, and shutting down host protein translation through PKR activation [22].
The third anti-viral program is the expression of other cytokines and chemokines by virally infected cells. Secreted cytokines and chemokines help recruit other types of immune cells such as monocytes and NK cells to the infected local area, which processes dead cells and formulate integrated immune responses. Depending on the type of viral antigens, activated NK cells or NKT cells remove the infected cells by releasing Granzymes and IFN-γ. CD8+ cytotoxic T cells also directly kill the infected cells after receiving antigen cross-presentation from APCs. The combination of these anti-viral programs finally reaches to the establishment of antigen-specific adaptive immunity that terminates the infection and memorizes the viral pathogen, which allows the host to rapidly clear a second infection by the same viruses.
5.3 The role of TRIF signaling in antiviral immunity
As one of the major innate immune signaling pathways, the demand of TRIF signaling in host defense against viral infection is greater in mucosal organs including the respiratory or gastrointestinal tract. Airway epithelial cells have been shown to express high levels of TLR3, which is further enhanced during infection with dsRNA viruses [23]. TRIF-mediated induction of an intrinsic factor Mx-GTPase has been associated with host defense against influenza A virus (IVA) infection [24]). However, the TRIF pathway also contributes to the pathogenesis of IVA-associated pneumonia as TRIF-induced inflammatory mediators causes the destruction of airway mucosa [25]. Similarly, TLR3 is the most abundantly expressed TLR in human intestine and can be found in surface epithelial cells and lamina propria mononuclear cells [26, 27]. TLR3-mediated TRIF signaling has been implicated in host defense against rotavirus infection that causes gastroenteritis typically in children under 6 years old. A part of the age specificity may be explained by the fact that the expression levels of TLR3 in intestinal epithelial cells increase along with aging. TLR3 mRNA expression in endoscopically sampled duodenal mucosa was greater in 5 to 20 years old individuals compared to children under 5 years old, which correlates with their resistance to rotavirus infection [28]. In addition to TRIF, other cytosolic viral sensors like retinoic acid-inducible gene I (RIG-I) or melanoma differentiation-associated gene 5 (MDA5) have shown to induce type I IFNs in response to rotavirus infection, suggesting the cooperative or redundant roles of these PRRs in IFN responses to rotavirus infection [29]. In addition, absence of TRIF signaling results in increased mortality in a mouse model of myocarditis caused by coxsackievirus group B [30]. Furthermore, hepatitis A virus blocks interferon signaling through degrading TRIF by viral protease 3CD during infection [31]. A similar strategy is used by hepatitis C virus that nullifies IFN signaling by cleaving TRIF and RIG-I during infection [32]. Therefore, TRIF-mediated IFN signaling is crucial in host defense against infection with these viruses.
In addition to the mucosal organs, TRIF has been shown to play a unique role in host response to viral infection in the central nervous system (CNS). The importance of TRIF in anti-viral defense in the CNS has been highlighted in genetic association studies, which demonstrate strong association of TRIF polymorphism to pediatric herpes simplex virus (HSV) encephalitis [33]. Poliovirus, a causal viral pathogen of poliomyelitis, is a single-stranded RNA virus but forms dsRNA intermediate in the cytosol during viral replication [34]. In animal studies, deficiency of TRIF increased mortality in response to poliovirus infection due to impaired type I IFN expression, indicating an importance of TRIF signaling in protection against poliovirus infection [35]. It has been shown that TLR3-mediated TRIF signaling is required for clearance of West Nile virus from the CNS [36, 37], while others have reported that TRIF signaling is involved in development of encephalitis [38]. These results indicate that TRIF signaling is required for the control of viral load but strong induction of TRIF-mediated pro-inflammatory response may be detrimental in local tissue especially in the CNS.
6. Antibacterial immunity
6.1 Overview
Establishment of infection by bacterial pathogens has several steps. The first step is bacterial colonization of the host tissue. Most of the bacterial pathogens target mucosal organs or dermal wounds where the pathogens can enter our body. Therefore, interfering colonization of pathogenic bacteria in the external and internal interfaces is our primary protection against bacterial infections. Since the mucosal organs normally carry commensal flora, disruption of microbial ecology in mucosal interface may results in increased susceptibility to bacterial infection. For example, germ-free mice showed less capacity to limit the colonization of pathogenic bacteria, relative to conventional mice, suggesting the importance of commensal bacteria for space occupancy. In addition, secreted components of commensal bacteria inhibit pathogenic bacterial growth thus participate in the restriction of pathogenic colonization.
In addition to the territorial competition between commensals and pathogens, host factors also contribute to the colonization resistance that include mucus secretion, peristaltic movement, antimicrobial peptides and secretory IgA (sIgA). The homeostasis of these host factors is regulated by host-commensal interactions and thus involves innate immunity. Once colonization is established, bacterial pathogens locally grow and are eventually recognized by host APCs via PRRs. Therefore, the second step of bacterial infection is to overwhelm local innate immune defense through the pathogen-specific strategies including physical invasion to deeper tissue, toxin secretion, or evasion of host immune responses. In the final step, pathogens that overcame local immune defenses disseminate and colonize other organs. Since bacterial infection bases colonization of the host organs, the major symptom of bacterial infection is mediated by local inflammation of the infected organs, which largely depends on innate immune responses. Therefore, effective suppression of local pathogenic growth by host immunity is the key for successful defense against bacterial infection.
6.2 Bacterial elimination strategy
Prior to identifying pathogens, secretion of antimicrobial peptides and sIgA by host cells prevent local bacterial growth and contact to the mucosal surface. The antimicrobial peptides are secreted from epithelial cells or neutrophils in response to PRR signaling [39]. These peptides are classified by the net change or structure [39]. Generally, these peptides have broad-spectrum bactericidal activity, which is composed of rupturing bacterial cell wall by inhibiting cell wall synthesis through sequestration of membrane component lipid II or inducing the ion channel formation in bacterial cell wall [39].
In addition to the antimicrobial peptide, sIgA confers more specific defense. The antigen information of invading pathogens is transferred from local APCs to T cells in regional lymph nodes. Those T cells are differentiated and activate B cells in the lymphoid organs. The activated B cells then undergo terminal differentiation to plasma cells and are recruited to the infection site by the guide of chemokines induced by local APCs, where plasma cells secrete IgA. This T cell-dependent IgA secretion takes five to seven days. However, B cells can rapidly undergo class switch recombination in a T cell-independent manner in response to B cell-activating factors induced by APCs and epithelial cells and secrete antigen-nonspecific sIgA [40]. This antigen-nonspecific sIgA has multiple cross-reactions and contributes to host defense against a variety of pathogens. Importantly, many aspects of sIgA secretion are regulated by TLR signaling [41, 42].
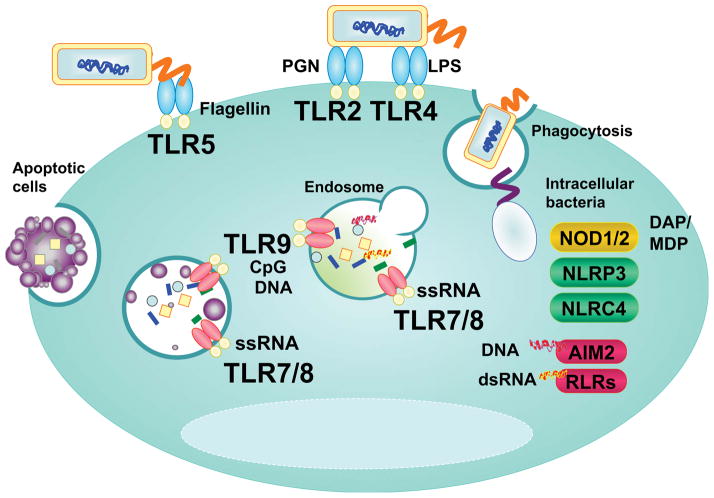
Once bacterial pathogens invade host tissue, pathogens are trapped by regional phagocytes, which is an important process of antibacterial host defense as it provides direct killing of the pathogen and induces cytokines and chemokines to form a localized inflammation. Bacterial pathogens normally carry multiple PAMPs that are sequentially exposed to PRRs within APCs (Figure 2). Because many bacterial pathogens have evolutionally acquired the skills to escape phagocytosis and following endosomal degradation, this redundancy of bacterial recognition systems increase the host capacity to establish the directed immune responses to a variety of pathogens. For example, Yersinia type III secretion system (T3SS) nullifies the phagocytic entrapment by GTPase-activating protein Yops [43]. Shigella escapes from the phagosomes into the cytoplasm and adjacent cells using its T3SS [43]. Salmonella can also interfere with endosomal fusion with lysosomes by modulating vesicle trafficking and creating replication vacuoles through T3SS effector SopB [44]. Pathogens that evade phagocytic elimination by APCs establish an infection and colonize in local tissue, which induces localized inflammation leading to the organ symptoms.
Figure 2. Bacterial PAMPs recognition by multiple PRRs.
Extracellular bacterial PAMPs are sensed by membrane TLRs: Gram-positive bacterial –peptidoglycan, -lipoteichoic acid and –lipoprotein, and lipopoyssacharide, and flagellin are sensed by TLR2, TLR4, and TLR5, respectively. During phagocytosis, engulfed bacteria undergo lysosomal degradation and degraded bacterial DNA and RNA are recognized by TLR9 and TLR7/8 within the phagolysosomes, respectively. Some bacteria such as shigella escape the phagosome by breaking the vesicle membrane and intracellular bacterial components are detected by NODs and NLRs in cytoplasm. Cytosolic nucleotide sensor, AIM2 and RLRs are involved in degraded bacteria DNA and RNA recognition which are leaked out from the phagolysosome. Apoptotic cells carrying debris of bacterial pathogen are engulfed through endocytosis and release DNA or RNA fragments that can be sensed by endosomal TLR9 or TLR7/8.
The induction of local inflammation helps avoid bacterial overgrowth and prevents systemic dissemination. During this process, neutrophils and macrophages are recruited by PRR-induced chemokines. PRRs signaling also accelerate phagocytosis and granular enzyme release of neutrophils. After phagocytosis, the pathogen-carrying APCs move to the regional lymph nodes in order to formulate an organized immune response and activate adoptive immunity. Pathogens, especially intracellular pathogens, in turn may utilize the APCs as a carrier to transport them to other organs [45]. On the other hand, PRR-induced apoptosis has been implicated in host defense against bacterial dissemination. Apoptotic death of the infected cells restricts the pathogenic spreading by conferring enforced death of the cytosolic pathogens. In addition, bacterial PAMPs can be recognized by other APCs through phagocytosing the apoptotic cells, which facilitates induction of adaptive immune responses. It has been shown that TRIF signaling promotes apoptosis via FADD and caspase 8 activation upon Yersinia enterocolitica infection [46]. Therefore, the course of bacterial infections depends on the host phagocytosis and virulence of the pathogens, which relies on PRR signaling.
6.3 The role of TRIF signaling in antibacterial immunity
TRIF-dependent protective immunity is initiated by recognition of invading Gram-negative bacteria by TLR4, which mainly provides the induction of acute inflammatory cell influx to infection sites. Although it is associated with organ pathology, adequate inflammation is necessary to prevent systemic dissemination of pathogens. There are several differences between TRIF-mediated antivirus and antibacterial immunity (Table 1). During infection with Gram-negative bacteria, bacterial PAMPs are recognized by TLR4 that triggers two signaling pathways of MyD88 and TRIF. These two pathways induce distinct cytokines and chemokines that are important for the clearance of pathogens. It has been shown that the TRIF pathway contributes to bacterial elimination during systemic infection with Salmonella typhimurium [47]. Mice deficient in TRIF showed increased bacterial burden and greater mortality than WT mice in a lung infection with Klebsiella pneumoniae due to impaired neutrophil influx and local cytokine responses [48]. Similarly, requirement of TRIF signaling in host resistance to infection was shown in other Gram-negative pathogens such as Pseudomonas aeruginosa, Campylobacter jejuni, and Escherichia coli [49–51]. These results suggest that not only MyD88 but also TRIF signaling are crucial for recruiting neutrophil influx and thus contribute to host defense against infection with Gram-negative pathogens. Selection of the dominant pathway of TLR4 to induce local inflammation during Gram-negative pathogens may be depending on the structure of their lipopolysaccharide (LPS) [52]. In a human study, the TRIF polymorphisms did not show disease preference in selected patients with urinary tract infections [53]. Therefore, weight of the individual pathways in host defense mechanism may differ depending on pathogens or organs.
Table 1.
TRIF-dependent antiviral vs. antibacterial protective immunity.
| Anti-viral immunity | Anti-bacterial immunity |
|---|---|
|
|
Recently, we have found an important aspect of TRIF signaling in intestinal host resistance to infection with Y. enterocolitica [54]. After oral infection, TRIF deficient mice succumb to massive bacterial dissemination within the initial three days. Greater susceptibility is associated with impaired phagocytosis and intracellular bacterial killing in regional macrophages and defective induction of chemokine CXCL10 in Peyer’s patches. We found this local immune defect is further associated with the lack of IFN-β induction from infected macrophages. TRIF-induced macrophage IFN-β is required for IFN-γ production from NK cells in the mesenteric lymph nodes, which contributes to the prevention of bacterial dissemination by enhancing local bacterial killing. Therefore, TRIF-dependent intestinal defense against Gram-negative Y. enterocolitica infection comprises of macrophage activation and induction of local inflammation in Peyer’s patches, and sequential production of IFNs in the mesenteric lymph nodes.
TRIF-mediated host defense mechanisms against Gram-negative bacterial infection differ between intestinal and systemic infection, as TRIF deficient mice show different susceptibility to intestinal vs. systemic infection with Y. enterocolitica [55]. During systemic infection, it is likely that there are multiple compensatory mechanisms that can replace the TRIF dependent protective immunity. In fact, survived TRIF deficient mice show increased production of TNF-α in the spleen during systemic dissemination of Y. enterocolitica [54, 55]. Similarly, others have shown that splenocytes in TRIF deficient mice produce more IFN-γ than WT mice after intravenous infection with S. typhimurium [47]. Therefore, TRIF-dependent protective immunity may be specific for mucosal defense against selected Gram-negative pathogens.
7. The TRIF pathway, a pharmacological target
Elucidation of the substantial immune regulation by TLR3 and TLR4 has shed light on the important role played by TRIF signaling. Several agonists and antagonists for TLR3 and TLR4 have been developed, and many of them are currently in clinical trials (Table 2). TLR3 agonists Poly-ICLC and Ampligen (poly I:C12U) are the stabilized poly I:C analogs, which have shown to be well tolerated without major adverse events in phase III clinical trials in recurrent pediatric glioma and chronic fatigue syndrome, respectively [56, 57]. Ampligen is also currently in phase II clinical trials as a monotherapy for patients with acquired immune deficiency syndrome (AIDS) [58]. Since HIV suppresses IRF3 to evade host innate defense mechanism, and TRIF signaling directly activates IRF3, TRIF stimulation may compete with HIV infection through restoring IRF3 activity [59]. We have recently described that the TRIF pathway can be a novel target for the treatment and prevention of enteric infection with Gram-negative pathogens [54]. We showed subcutaneous application of poly I:C protected mice from dissemination of the Gram-negative bacterial pathogens Y. enterocolitica and S. typhimurium after orogastric inoculation. The protective effect of poly I:C was mediated through induction of IFN-β and activation of regional macrophages during infection. The strategy boosting anti-bacterial innate immunity is advantageous because it provides immediate defense in an antigen-independent manner, which provides a broader clinical applicability.
Table 2.
Current challenges in pharmacological manipulation of TRIF signaling.
| Generic Name | TLR Target | Pathway | Status | Primary Target |
|---|---|---|---|---|
| Poly-ICLC [56] | TLR3 agonist | TRIF | Phase II | Cancer |
| Ampligen poly I:C12U [57, 58] | TLR3 agonist | TRIF | Phase II | HIV, Chronic fatigue syndrome |
| CQ-07001 [63] | TLR3 agonist | TRIF | Preclinical | Under investigation |
| IPH-31XX (dsRNA) [63] | TLR3 agonist | TRIF | Preclinical | Lupus erythematodes |
| MCT-465-dsRNA [63] | TLR3 agonist | TRIF | Preclinical | Adjuvant for virus or oncology |
| CRX-675 [63] | TLR4 agonist | TRIF | Phase II | Allergic rhinitis |
| CRX-547 [52] | TLR4 agonist | TRIF | Preclinical | Vaccine adjuvant |
| Monohosphoryl Lipid A (MPL) [61, 62] | TLR4 agonist | TRIF/MyD88 | Phase III | Adjuvant for virus or oncology |
| Aminoalkyl glucosaminide 4 -phosphate (AGP) (RC529 and RC524) [71] | TLR4 agonist | TRIF | Preclinical | Adjuvant for virus or oncology |
| Bufalin [72] | TLR 3&4 antagonist | TRIF | Preclinical | Under investigation |
In addition to the TLR3-mediated TRIF pathway, TRIF can be induced by TLR4 activation. It has been challenging to establish a less toxic agonist of TLR4. Since major toxicity caused by TLR4 stimulation involves MyD88-dependent induction of multiple pro-inflammatory cytokines, the TLR4 agonist has been specifically designed to induce TRIF signaling (CRX-675 and CRX-547, and monohosphoryl lipid A: MPL), which has shown to induce strong resistance against viral and bacterial infections. The TLR4 agonist CRX-675 has shown to improve nasal symptoms in patients with asthma without major adverse effects [60]. In addition, MPL has been widely used as a vaccine adjuvant for hepatitis B and is currently in phase III clinical trial for the prevention of cervical cancer through vaccination against papillomavirus 16 and 18 [61, 62]. On the other hand, several TLR4 antagonists have been developed to block excessive production of pro-inflammatory cytokines and applied to treat the patients with endotoxin shock [63]. As we discussed earlier, TLR-mediated TRIF signaling may contribute to the organ pathology in several infectious diseases, blocking upstream TLR signaling may be useful to control the organ symptoms if we can simultaneously control the pathogen load.
The TRIF manipulation strategy appears to have met the safety requirements. Given type I IFNs, a major effector of TRIF signaling, have been utilized as therapeutic means for variety of infectious diseases as well as immune disorders, the TRIF stimulation strategy may apply for the treatment option of these clinical settings [64, 65]. In order to maximize the effect of TRIF stimulation strategy with reduced adverse effects, future applications would be target stimulation of cell type specific TRIF signaling. Several delivery means have been developed to achieve targeted stimulation of TLR signaling [66]. In addition, the TRIF stimulation strategy can be used as a potent adjuvant for vaccines as it accelerates antigen presentation to T cells. In fact, TRIF targeted vaccine adjuvant has already been tried against several infectious microbes including tuberculosis, influenza viruses and HIV [67–69].
8. Concluding remarks
Infectious diseases are a serious global threat to human survival. Effective prevention of pandemics has become an urgent issue with the current dramatic increase of international air travel, food trade, and tourism. Recent advancement of technology and civilization unexpectedly brought emerging and re-emerging infectious diseases and bioterrorism threat to our daily life. On the other hand, increased understanding of innate immunity, and evolutionary genetic approaches to study infectious diseases have established a new concept of host-microbial interactions. TRIF mediates both anti-viral and anti-bacterial host defense mechanism, which consist of an immediate clearance of viral pathogens and organization of innate as well as adaptive inflammatory cascade, respectively. Thinking of the evolutionary arms race between humans and microbes, an interesting question is why only TRIF has been assigned to be responsible for both bacteria and viruses. Evolutionary studies have highlighted the significance of TRIF rather than upstream TLRs as it has been evolved under purifying selection yet carried the greatest nucleotide diversity in humans [70]. TRIF might be a new strategy of our immunity to overwhelm microbial infections or a compromise for better symbiosis with them. This question may be answered in the next several centuries, but it should be in the ecological dynamics of host-microbial interactions.
Acknowledgments
This study was supported by National Institute of Allergy and Infectious Diseases (R56 AI095255-01 and 1R01AI095255-01A1) for M.F.
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jackson RW, Johnson LJ, Clarke SR, Arnold DL. Bacterial pathogen evolution: breaking news. Trends Genet. 2011;27:32–40. doi: 10.1016/j.tig.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Couzin J. Genetics. Hybrid mosquitoes suspected in West Nile virus spread. Science. 2004;303:1451. doi: 10.1126/science.303.5663.1451a. [DOI] [PubMed] [Google Scholar]
- 3.Goto Y, Kiyono H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol Rev. 2012;245:147–163. doi: 10.1111/j.1600-065X.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- 4.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 5.Frick IM, Nordin SL, Baumgarten M, Morgelin M, Sorensen OE, Olin AI, Egesten A. Constitutive and inflammation-dependent antimicrobial peptides produced by epithelium are differentially processed and inactivated by the commensal Finegoldia magna and the pathogen Streptococcus pyogenes. J Immunol. 2011;187:4300–4309. doi: 10.4049/jimmunol.1004179. [DOI] [PubMed] [Google Scholar]
- 6.Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett NT, Blake PA, Cohen ML. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 7.Kyle JL, Cummings CA, Parker CT, Quinones B, Vatta P, Newton E, Huynh S, Swimley M, Degoricija L, Barker M, Fontanoz S, Nguyen K, Patel R, Fang R, Tebbs R, Petrauskene O, Furtado M, Mandrell RE. Escherichia coli serotype O55:H7 diversity supports parallel acquisition of bacteriophage at Shiga toxin phage insertion sites during evolution of the O157:H7 lineage. J Bacteriol. 2012;194:1885–1896. doi: 10.1128/JB.00120-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, Pickard DJ, Barthel M, Westendorf AM, Krogfelt KA, Walker AW, Ackermann M, Dobrindt U, Thomson NR, Hardt WD. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc Natl Acad Sci U S A. 2012;109:1269–1274. doi: 10.1073/pnas.1113246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea MG, Wijmenga C, O’Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nat Immunol. 2012;13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto A, Kumakura S, Uchida M, Barrett JC, Tsutsui T. Immortalization of normal human embryonic fibroblasts by introduction of either the human papillomavirus type 16 E6 or E7 gene alone. Int J Cancer. 2003;106:301–309. doi: 10.1002/ijc.11219. [DOI] [PubMed] [Google Scholar]
- 11.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, Qin J, Cheng G, Liu YJ. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34:866–878. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 15.Estornes Y, Toscano F, Virard F, Jacquemin G, Pierrot A, Vanbervliet B, Bonnin M, Lalaoui N, Mercier-Gouy P, Pacheco Y, Salaun B, Renno T, Micheau O, Lebecque S. dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ. 2012;19:1482–1494. doi: 10.1038/cdd.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaddis DE, Michalek SM, Katz J. TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B. J Immunol. 2011;186:5772–5783. doi: 10.4049/jimmunol.1003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamon H, Kawabe T, Kitamura H, Lee J, Kamimura D, Kaisho T, Akira S, Iwamatsu A, Koga H, Murakami M, Hirano T. TRIF-GEFH1-RhoB pathway is involved in MHCII expression on dendritic cells that is critical for CD4 T-cell activation. EMBO J. 2006;25:4108–4119. doi: 10.1038/sj.emboj.7601286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAleer JP, Rossi RJ, Vella AT. Lipopolysaccharide potentiates effector T cell accumulation into nonlymphoid tissues through TRIF. J Immunol. 2009;182:5322–5330. doi: 10.4049/jimmunol.0803616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence RM, Lawrence RA. Breast milk and infection. Clin Perinatol. 2004;31:501–528. doi: 10.1016/j.clp.2004.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan N, Chen ZJ. Intrinsic antiviral immunity. Nat Immunol. 2012;13:214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusch L, Zhou A, Silverman RH. Caspase-dependent apoptosis by 2′,5′-oligoadenylate activation of RNase L is enhanced by IFN-beta. J Interferon Cytokine Res. 2000;20:1091–1100. doi: 10.1089/107999000750053762. [DOI] [PubMed] [Google Scholar]
- 23.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 24.Haller O, Staeheli P, Kochs G. Protective role of interferon-induced Mx GTPases against influenza viruses. Rev Sci Tech. 2009;28:219–231. doi: 10.20506/rst.28.1.1867. [DOI] [PubMed] [Google Scholar]
- 25.Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 26.Furrie E, Macfarlane S, Thomson G, Macfarlane GT. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–574. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 28.Pott J, Stockinger S, Torow N, Smoczek A, Lindner C, McInerney G, Backhed F, Baumann U, Pabst O, Bleich A, Hornef MW. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog. 2012;8:e1002670. doi: 10.1371/journal.ppat.1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sen A, Pruijssers AJ, Dermody TS, Garcia-Sastre A, Greenberg HB. The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J Virol. 2011;85:3717–3732. doi: 10.1128/JVI.02634-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riad A, Westermann D, Zietsch C, Savvatis K, Becher PM, Bereswill S, Heimesaat MM, Lettau O, Lassner D, Dorner A, Poller W, Busch M, Felix SB, Schultheiss HP, Tschope C. TRIF is a critical survival factor in viral cardiomyopathy. J Immunol. 2011;186:2561–2570. doi: 10.4049/jimmunol.1002029. [DOI] [PubMed] [Google Scholar]
- 31.Qu L, Feng Z, Yamane D, Liang Y, Lanford RE, Li K, Lemon SM. Disruption of TLR3 signaling due to cleavage of TRIF by the hepatitis A virus protease-polymerase processing intermediate, 3CD. PLoS Pathog. 2011;7:e1002169. doi: 10.1371/journal.ppat.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imran M, Waheed Y, Manzoor S, Bilal M, Ashraf W, Ali M, Ashraf M. Interaction of Hepatitis C virus proteins with pattern recognition receptors. Virol J. 2012;9:126. doi: 10.1186/1743-422X-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sancho-Shimizu V, Perez de Diego R, Lorenzo L, Halwani R, Alangari A, Israelsson E, Fabrega S, Cardon A, Maluenda J, Tatematsu M, Mahvelati F, Herman M, Ciancanelli M, Guo Y, AlSum Z, Alkhamis N, Al-Makadma AS, Ghadiri A, Boucherit S, Plancoulaine S, Picard C, Rozenberg F, Tardieu M, Lebon P, Jouanguy E, Rezaei N, Seya T, Matsumoto M, Chaussabel D, Puel A, Zhang SY, Abel L, Al-Muhsen S, Casanova JL. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J Clin Invest. 2011;121:4889–4902. doi: 10.1172/JCI59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egger D, Teterina N, Ehrenfeld E, Bienz K. Formation of the poliovirus replication complex requires coupled viral translation, vesicle production, and viral RNA synthesis. J Virol. 2000;74:6570–6580. doi: 10.1128/jvi.74.14.6570-6580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe Y, Fujii K, Nagata N, Takeuchi O, Akira S, Oshiumi H, Matsumoto M, Seya T, Koike S. The toll-like receptor 3-mediated antiviral response is important for protection against poliovirus infection in poliovirus receptor transgenic mice. J Virol. 2012;86:185–194. doi: 10.1128/JVI.05245-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blazquez AB, Saiz JC. West Nile virus (WNV) transmission routes in the murine model: intrauterine, by breastfeeding and after cannibal ingestion. Virus Res. 2010;151:240–243. doi: 10.1016/j.virusres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 39.Hazlett L, Wu M. Defensins in innate immunity. Cell Tissue Res. 2011;343:175–188. doi: 10.1007/s00441-010-1022-4. [DOI] [PubMed] [Google Scholar]
- 40.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 41.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Schneeman TA, Bruno ME, Schjerven H, Johansen FE, Chady L, Kaetzel CS. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J Immunol. 2005;175:376–384. doi: 10.4049/jimmunol.175.1.376. [DOI] [PubMed] [Google Scholar]
- 43.Reis RS, Horn F. Enteropathogenic Escherichia coli, Samonella, Shigella and Yersinia: cellular aspects of host-bacteria interactions in enteric diseases. Gut Pathog. 2010;2:8. doi: 10.1186/1757-4749-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez LD, Hueffer K, Wenk MR, Galan JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304:1805–1807. doi: 10.1126/science.1098188. [DOI] [PubMed] [Google Scholar]
- 45.Wong CE, Sad S, Coombes BK. Salmonella enterica serovar typhimurium exploits Toll-like receptor signaling during the host-pathogen interaction. Infect Immun. 2009;77:4750–4760. doi: 10.1128/IAI.00545-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruckdeschel K, Pfaffinger G, Haase R, Sing A, Weighardt H, Hacker G, Holzmann B, Heesemann J. Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J Immunol. 2004;173:3320–3328. doi: 10.4049/jimmunol.173.5.3320. [DOI] [PubMed] [Google Scholar]
- 47.Talbot S, Totemeyer S, Yamamoto M, Akira S, Hughes K, Gray D, Barr T, Mastroeni P, Maskell DJ, Bryant CE. Toll-like receptor 4 signalling through MyD88 is essential to control Salmonella enterica serovar typhimurium infection, but not for the initiation of bacterial clearance. Immunology. 2009;128:472–483. doi: 10.1111/j.1365-2567.2009.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183:6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Power MR, Li B, Yamamoto M, Akira S, Lin TJ. A role of Toll-IL-1 receptor domain-containing adaptor-inducing IFN-beta in the host response to Pseudomonas aeruginosa lung infection in mice. J Immunol. 2007;178:3170–3176. doi: 10.4049/jimmunol.178.5.3170. [DOI] [PubMed] [Google Scholar]
- 50.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol. 2007;178:3153–3160. doi: 10.4049/jimmunol.178.5.3153. [DOI] [PubMed] [Google Scholar]
- 51.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, Dasti JI, Zautner AE, Munoz M, Loddenkemper C, Gross U, Gobel UB, Heimesaat MM. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowen WS, Minns LA, Johnson DA, Mitchell TC, Hutton MM, Evans JT. Selective TRIF-dependent signaling by a synthetic toll-like receptor 4 agonist. Sci Signal. 2012;5:ra13. doi: 10.1126/scisignal.2001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawn TR, Scholes D, Li SS, Wang H, Yang Y, Roberts PL, Stapleton AE, Janer M, Aderem A, Stamm WE, Zhao LP, Hooton TM. Toll-like receptor polymorphisms and susceptibility to urinary tract infections in adult women. PLoS One. 2009;4:e5990. doi: 10.1371/journal.pone.0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sotolongo J, Espana C, Echeverry A, Siefker D, Altman N, Zaias J, Santaolalla R, Ruiz J, Schesser K, Adkins B, Fukata M. Host innate recognition of an intestinal bacterial pathogen induces TRIF-dependent protective immunity. J Exp Med. 2011;208:2705–2716. doi: 10.1084/jem.20110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sotolongo J, Kanagavelu S, Hyun J, Ruiz J, Fukata M. TRIF mobilizes unique primary defense against Gram-negative bacteria in intestinal interface. Gut Microbes. 2012;3:437–441. doi: 10.4161/gmic.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morse MA, Chapman R, Powderly J, Blackwell K, Keler T, Green J, Riggs R, He LZ, Ramakrishna V, Vitale L, Zhao B, Butler SA, Hobeika A, Osada T, Davis T, Clay T, Lyerly HK. Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clin Cancer Res. 2011;17:4844–4853. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenfeld MR, Chamberlain MC, Grossman SA, Peereboom DM, Lesser GJ, Batchelor TT, Desideri S, Salazar AM, Ye X. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol. 2010;12:1071–1077. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 59.Doehle BP, Hladik F, McNevin JP, McElrath MJ, Gale M., Jr Human immunodeficiency virus type 1 mediates global disruption of innate antiviral signaling and immune defenses within infected cells. J Virol. 2009;83:10395–10405. doi: 10.1128/JVI.00849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casale TB, Kessler J, Romero FA. Safety of the intranasal toll-like receptor 4 agonist CRX-675 in allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:454–456. doi: 10.1016/S1081-1206(10)60934-9. [DOI] [PubMed] [Google Scholar]
- 61.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 62.Cluff CW. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Adv Exp Med Biol. 2010;667:111–123. doi: 10.1007/978-1-4419-1603-7_10. [DOI] [PubMed] [Google Scholar]
- 63.Gearing AJ. Targeting toll-like receptors for drug development: a summary of commercial approaches. Immunol Cell Biol. 2007;85:490–494. doi: 10.1038/sj.icb.7100102. [DOI] [PubMed] [Google Scholar]
- 64.Hensley LE, Fritz LE, Jahrling PB, Karp CL, Huggins JW, Geisbert TW. Interferon-beta 1a and SARS coronavirus replication. Emerg Infect Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lazear HM, Pinto AK, Vogt MR, Gale M, Jr, Diamond MS. Beta interferon controls West Nile virus infection and pathogenesis in mice. J Virol. 2011;85:7186–7194. doi: 10.1128/JVI.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tel J, Beenhakker N, Koopman G, Hart B, Mudde GC, de Vries IJ. Targeted delivery of CpG ODN to CD32 on human and monkey plasmacytoid dendritic cells augments IFNalpha secretion. Immunobiology. 2012;217:1017–1024. doi: 10.1016/j.imbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 68.Ichinohe T, Kawaguchi A, Tamura S, Takahashi H, Sawa H, Ninomiya A, Imai M, Itamura S, Odagiri T, Tashiro M, Chiba J, Sata T, Kurata T, Hasegawa H. Intranasal immunization with H5N1 vaccine plus Poly I:Poly C12U, a Toll-like receptor agonist, protects mice against homologous and heterologous virus challenge. Microbes Infect. 2007;9:1333–1340. doi: 10.1016/j.micinf.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 69.Stevceva L. Toll-like receptor agonists as adjuvants for HIV vaccines. Curr Med Chem. 2011;18:5079–5082. doi: 10.2174/092986711797636027. [DOI] [PubMed] [Google Scholar]
- 70.Fornarino S, Laval G, Barreiro LB, Manry J, Vasseur E, Quintana-Murci L. Evolution of the TIR domain-containing adaptors in humans: swinging between constraint and adaptation. Mol Biol Evol. 2011;28:3087–3097. doi: 10.1093/molbev/msr137. [DOI] [PubMed] [Google Scholar]
- 71.Baldridge JR, Cluff CW, Evans JT, Lacy MJ, Stephens JR, Brookshire VG, Wang R, Ward JR, Yorgensen YM, Persing DH, Johnson DA. Immunostimulatory activity of aminoalkyl glucosaminide 4-phosphates (AGPs): induction of protective innate immune responses by RC-524 and RC-529. J Endotoxin Res. 2002;8:453–458. doi: 10.1179/096805102125001064. [DOI] [PubMed] [Google Scholar]
- 72.Ye J, Chen S, Maniatis T. Cardiac glycosides are potent inhibitors of interferon-beta gene expression. Nat Chem Biol. 2011;7:25–33. doi: 10.1038/nchembio.476. [DOI] [PMC free article] [PubMed] [Google Scholar]