Abstract
INTRODUCTION
Melanoma with recurrent loco-regional metastases to limbs often makes difficult a second surgical approach because of the adhesions affecting the vascular access. Our aim was to evaluate whether the placement of a polytetrafluoroethylene (PTFE) membrane around vessels might facilitate a surgical re-approach.
PRESENTATION OF CASE
We reported a case of a 64-year-old male with a melanoma on the left foot who developed in transit metastases after LND. While performing the inguinopelvic LND we coated the iliac vessels with PTFE patch to facilitate the vascular access in case of re-intervention for a ILP. In the second surgical approach we made a cutaneous incision in the left iliac region and we proceeded through the subcutaneous tissue until detection of iliac vessels, more clearly visible because of the PTFE patch fixed around vascular walls to minimize adhesions. We removed the PTFE coating and easily performed arteriotomy and venotomy for the completion of the ILP.
DISCUSSION
This case report seems to demonstrate the efficacy of a PTFE membrane applied in a patient around iliac vessels during inguinopelvic dissection, to reduce adhesion density. In fact this membrane provided a barrier to adhesions of the iliac vessels, decreasing the risk of vascular injury thereby facilitating a subsequent vascular access. Re-coating of the iliac vessels with PTFE could be preparatory to a better identification of the vascular structures in cases of a surgical re-approach.
CONCLUSION
The use of PTFE effectively simplifies the second approach to vessels in event of a melanoma metastasizing to limbs.
Keywords: Melanoma, Surgery, Polytetrafluoroethylene, Vessels
1. Introduction
The standard of care for cutaneous melanoma (CM) classified at stage III by the American Joint Committee on Cancer (AJCC)1 is a radical lymph-node dissection (LND) in case of regional lymph-node involvement or an isolated limb perfusion (ILP) in presence of in transit metastases.2–4 A significant rate of stage III melanomas undergo to new recurrences after the first surgical treatment so that a further surgical approach has to be considered for the management of these cases of bulky lymph-node or recurrent in transit metastases, in absence of extra-regional disease. The main difficulty in treating these patients is connected to the previous surgical treatment they underwent, that could have caused surgical adhesions affecting vascular fragility and sometimes the inability of a new access to vessels.5–7 Our aim was to evaluate the ability of a device, the polytetrafluoroethylene (PTFE), applied as a membrane around vascular walls, to minimize the formation of adhesions between the vessels thereby providing a plane of dissection (separation of tissues) in event of needing a further surgical approach.
2. Presentation of case
A 64-year-old male was first seen in March 2004 at our Institute by the Melanoma Unit. The physical examination revealed the presence, on the plantar region of the left foot, of an irregular, heterochromic pigmented lesion of 1 cm in diameter; no palpable loco-regional groin lymphadenopathy were detected.
We performed an excisional biopsy of the pigmented skin lesion; the histopathology revealed an acral lentiginous melanoma with a Breslow thickness of 1.48 mm, the presence of ulceration and of a high mitotic rate (AJCC Stage IIA). The resection margins were negative for malignancy. Three weeks later the patient underwent to a wider excision in the plantar region, with a plastic reconstruction by skin graft, and to a groin sentinel lymph-node biopsy (SLNB). Histopathology was negative in both cases. The SLNs were reviewed as permanent sections; multiple sections were carefully examined by immunohistochemical staining for S-100, HMB-45, and later MART-1 or Melan-A.8–10
The patient was submitted to a regular follow-up and 15 months later a groin lymphadenopathy of 2.5 cm was detected, the cytology after a fine needle aspiration confirmed the clinical suspicion of a regional metastasis. Thus a complete inguinopelvic LND was performed. Inguinal dissection was completed via a longitudinal curvilinear incision beginning medial to the anterior superior iliac spine and terminating at the apex of the femoral triangle inclusive of the SLN biopsy site. Prior SLN biopsy incisions were incorporated and removed. Dissection was carried to the pubic tubercle medially, sartorius laterally, femoral triangle apex distally, and inguinal ligament proximally. The saphenous vein and lymphatic vessels were ligated. Contents of the inguinofemoral triangle were cleared off the femoral vessels and submitted. Pelvic dissection was performed via the same incision. The inguinal ligament was divided 1–2 cm lateral to the femoral vessels. The external oblique, internal oblique, and transversalis fascia were also divided. Preperitoneal fat and peritoneum were swept medially. External iliac nodes were then removed and submitted. The pelvic side wall was then dissected with retraction of the external iliac vessels. The obturator fossa was entered with preservation of the ureter, obturator nerve, and hypogastric branches. Obturator node specimens were submitted. The histopathology showed the presence of metastases to 6 crural inguinal lymph-nodes over 15 examined and no evidence of metastases in 5 external iliac and obturatory lymph-nodes.
Considering that the patient had a IIIC stage melanoma, related with an high risk of recurrence, and that the disease was still having a loco-regional involvement, without distant metastases, while performing the groin lymph-node dissection we decided to protect the iliac vessels from surgical adhesions, in case of needing a second surgical approach to this district for a new loco-regional relapse. A membrane of PTFE, commonly experimented as an effective solution to minimize the tissue adhesion in surgical reconstructive procedures11–13 was used; composed of the inert biomaterial expanded, it features a microporous structure allowing for host tissue incorporation. The biological inertness of the prosthesis, combined with softness and conformability in vivo, should minimize foreign-body response and adhesions to surrounding structures. The PTFE membrane was hydrated in sterile saline at room temperature for 15 min; then we proceeded to cut strips about 5 cm long × 3.5 cm high for use during the surgery, with small variations in size due to the caliber of the vessels. A segment (5 cm in length) of the iliac vessel was covered, in order to be able to cannulate the vases in a hypothetical second surgical approach to perform a ILP. The vessels were lined by strip that was inserted using a synthetic monofilament of non-absorbable polypropylene suture between the two extremities of the long side, leaving a few millimeters between the membrane and the vessel. The iliac artery and vein were wrapped separately; no sutures are placed between the vessel wall and the membrane. The technique performed is illustrated in Figs. 1.1 and 1.2.
Fig. 1.
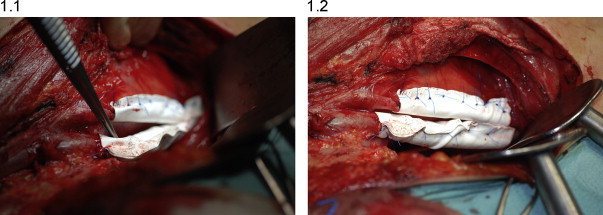
(1.1) Operative photograph showing the technique of coating the iliac vein and artery during inguinopelvic lymph-node dissection. The PTFE membrane was hydrated in sterile saline at room temperature for 15 min; then we proceeded to cut strips about 5 cm long × 3.5 cm high for use during the surgery, with small variations in size due to the caliber of the vessels. (1.2) The vessels were lined by strip that was inserted using a synthetic monofilament of non-absorbable polypropylene suture between the two extremities of the long side, leaving a few millimeters between the membrane and the vessel. No sutures are placed between the vessel wall and the membrane.
Thirteen months after the inguinopelvic dissection a physical examination performed during a follow-up, showed multiple in transit to left lower limb (Fig. 2), for which a conventional surgical approach was not suitable. The patient was so candidate for a ILP of the left lower limb with an iliac vascular access.
Fig. 2.
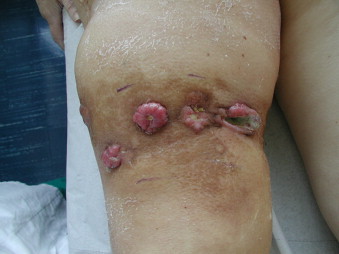
Multiple cutaneous – subcutaneous in transit metastases to left lower limb.
Therefore in this case the technique of limb perfusion was complex; having locally the tissues already subjected to previous surgery, but was more facilitated by positioning the PTFE to cover vessels. This solution allowed us to identify and use a cleavage plane obtaining a second vascular approach less risky, we removed the PTFE coating and easily performed arteriotomy and venotomy, the vessels were cannulated and the ILP was completed (Figs. 3.1 and 3.2).
Fig. 3.
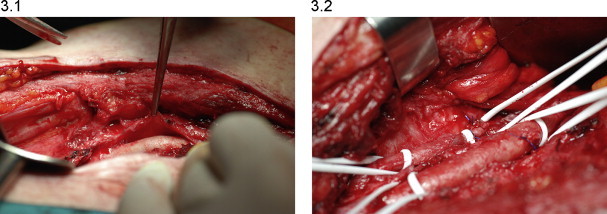
(3.1) We exceeded the sub-cutaneous tissues searching for the iliac vessels till we identified the PTFE patch fixed around vascular walls that allowed us to detect the iliac artery and vein. Furthermore the PTFE membrane gave us the possibility to identify and use a cleavage plane obtaining a less risky approach to vessels walls. (3.2) We removed the PTFE patch in order to have an easier access to the vascular structures. We performed arteriotomy and venotomy and cannulated the vessels.
The patient had no significant post-operative complications and currently he is disease-free and is undergoing to a close and regular follow-up.
3. Discussion
This case report seems to demonstrate the efficacy of a PTFE membrane applied in a patient around iliac vessels during inguinopelvic dissection, to reduce adhesion density. In fact this membrane provided a barrier to adhesions of the iliac vessels, decreasing the risk of vascular injury thereby facilitating a subsequent vascular access.
The impact of post-operative adhesions on the surgical patient and on re-operative surgery are increasingly realized.14,15 Their presence may impose re-operative problems in both cardiac and general surgery, as well as producing post-operative complications peculiar to the type of primary surgery. A vascular approach in melanoma patients is commonly used in the surgical treatment of regional lymph-node metastases or in transit metastases as primary melanoma of the limbs often shows a spreading of disease long the lymphatic ways.2–4 However, while treatment of a positive regional lymph-nodes with a therapeutic lymph-node dissection (TLND) is a highly diffuse procedure, ILP in case of in transit metastases to limbs is only used in a few centers for the principal reason that although the procedure is simple in concept, it is surgically and technically complex, and demanding in practice, and related to a high risk of post-operative vascular complications.16 Even more difficult may be the approach to vessels when performing an ILP in patients already submitted to a TLND or to a previous ILP that caused adhesions on the vascular structures. The best treatment for adhesions should be prevent or minimize their formation. Nevertheless, in the absence of a universally accepted method for preventing adhesions, several techniques and disease states have been suggested to predispose to adhesion formation and various experiences have been performed to document this. They include incomplete haemostasis, foreign bodies, tissue injury, type of suture utilized, amount of crushing and tissue destruction from instrumentation, tissue desiccation, and underlying infection. Many practical techniques may be utilized to minimize aberration from optimal techniques: the use of gauze and minimally moistened dry sponges may cause significant surface injury as well as he use of frequent irrigation has been recommended to limit tissue desiccation and keep tissues moistened. The use of the most acceptable minimally reactive sutures and an effort to not suture unless necessary helps avoid tissue reactions.
In the last years some new materials were tested in order to reduce the extent and severity of adhesion formation between the vessels walls.17–19 The PTFE membrane, which became commercially available for clinical use in 2007, is flexible, biocompatible, and similar to PTFE materials that have been used successfully in clinical procedures as interpositional membranes.20
4. Conclusion
Our preliminary experience suggests that the placement of PTFE patch membrane may represent an effective method for reducing the extent and severity of the adhesion formation as well as for preventing the adverse outcomes for re-operations in case of recurrent melanoma to limbs. In fact the recoating of the vessels walls with PTFE could be preparatory to a better identification of the vascular structures in the event of subsequent surgical approach like an ILP. A large number of cases as well as a careful criteria in eligible patients selection are needed to confirm our findings and validate this experimental method.
Conflict of interest statement
All authors disclose that there are no financial and personal relationship with other people or organizations that could inappropriately influence their work.
Funding
All authors declare that there is no funding for this research.
Ethical approval statement
Authors obtained written and signed consent from the patient to publish anonymously the case report.
Authors’ contribution
Roberta Ruggeri had done acquisition of data, analysis and interpretation of data along with Tiziana Camerini, Riccardo Pirovano, Ilaria Mattavelli, Federica Crippa, Daniele Moglia and Annabella Di Florio. Besides, Roberta Ruggeri had done the critical revision together with Roberto Patuzzo, Andrea Maurichi and Elena Tolomio, who also helped in critical revision of the article. Mario Santinami had approved the final version for submission.
References
- 1.Balch C.M., Gershenwald J.E., Soong S.J., Thompson J.F., Atkins M.B., Byrd D.R. Final version of 2009 AJCC melanoma staging and classification. Journal of Clinical Oncology. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garbe C., Peris K., Hauschild A., Saiag P., Middleton M., Spatz A. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. European Journal of Cancer. 2010;46:270–283. doi: 10.1016/j.ejca.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Kroon H.M., Moncrieff M., Kam P.C., Thompson J.F. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Annals of Surgical Oncology. 2008;15:3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 4.Turley R.S., Raymond A.K., Tyler D.S. Regional treatment strategies for in-transit melanoma metastasis. Surgical Oncology Clinics of North America. 2011;20:79–103. doi: 10.1016/j.soc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies D., Ellis H. Intestinal obstruction from adhesions – how big is the problem? Annals of The Royal College of Surgeons. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis H., Moran B.J., Thompson J.N., Parker M.C., Wilson M.S., Menzies D. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476–1480. doi: 10.1016/S0140-6736(98)09337-4. [DOI] [PubMed] [Google Scholar]
- 7.Attard J.A., MacLean A.R. Adhesive small bowel obstruction: epidemiology, biology and prevention. Canadian Journal of Surgery. 2007;50:291–300. [PMC free article] [PubMed] [Google Scholar]
- 8.Cochran A.J., Wen D.R., Morton D.L. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. American Journal of Surgical Pathology. 1988;12:612–618. doi: 10.1097/00000478-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Morton D.L., Thompson J.F., Essner R., Elashoff R., Stern S.L., Nieweg O.E. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma. Annals of Surgery. 1999;230:453–463. doi: 10.1097/00000658-199910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cochran A.J., Wen D.R., Herschman H.R. Occult melanoma in lymph nodes detected by antiserum to S-100 protein. International Journal of Cancer. 1984;34:159–163. doi: 10.1002/ijc.2910340204. [DOI] [PubMed] [Google Scholar]
- 11.Fritschy W.M., Kruse R.R., Frakking T.G., Van Geloven A.A., Blomme A.M. Performance of ePTFE-covered endograft in patients with occlusive disease of the superficial femoral artery: a three-year clinical follow-up study. The Journal of Cardiovascular Surgery. 2010;51:783–790. [PubMed] [Google Scholar]
- 12.Stone G.W., Goldberg S., O'Shaughnessy C., Midei M., Siegel R.M., Cristea E. 5-Year follow-up of polytetrafluoroethylene-covered stents compared with bare-metal stents in aortocoronary saphenous vein grafts the randomized BARRICADE (barrier approach to restenosis: restrict intima to curtail adverse events) trial. JACC: Cardiovascular Interventions. 2011;4:300–309. doi: 10.1016/j.jcin.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Lindholt J.S., Gottschalksen B., Johannesen N., Dueholm D., Ravn H., Christensen E.D. The Scandinavian Propaten(®) trial – 1-year patency of PTFE vascular prostheses with heparin-bonded luminal surfaces compared to ordinary pure PTFE vascular prostheses – a randomised clinical controlled multi-centre trial. European Journal of Vascular and Endovascular Surgery. 2011;41:668–673. doi: 10.1016/j.ejvs.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Gabbay S. The need for intensive study of pericardial substitution after open heart surgery. Transactions ASAIO. 1990;36:789–791. doi: 10.1097/00002480-199010000-00002. [DOI] [PubMed] [Google Scholar]
- 15.DeCherney A.H., DiZerega G.S. Clinical problem of intraperitoneal postsurgical adhesion formation following general surgery and the use of adhesion prevention barriers. Surgical Clinics of North America. 1997;77:671–688. doi: 10.1016/s0039-6109(05)70574-0. [DOI] [PubMed] [Google Scholar]
- 16.Trezzi M., Parolari A., Loardi C., Alamanni F. Vascular complications following isolated limb perfusion for local recurrence of extremity melanoma: a case report and literature review. International Journal of Vascular Medicine. 2011;2011:2041–2048. doi: 10.1155/2011/204148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haney A.F., Hesla J., Hurst B.S., Kettel L.M., Murphy A.A., Rock J.A. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7+) in preventing adhesions. Fertility and Sterility. 1995;63:1021–1026. [PubMed] [Google Scholar]
- 18.Nkere U.U. Postoperative adhesion formation and the use of adhesion preventing techniques in cardiac and general surgery. ASAIO Journal. 2000;46:654–656. doi: 10.1097/00002480-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kaushal S., Patel S.K., Goh S.K., Sood A., Walker B.L., Backer C.L. A novel combination of bioresorbable polymeric film and expanded polytetrafluoroethylene provides a protective barrier and reduces adhesions. Journal of Thoracic and Cardiovascular Surgery. 2011;141:789–795. doi: 10.1016/j.jtcvs.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Ivanic G.M., Pink P.T., Schneider F., Stuecker M., Homann N.C., Preidler K.W. Prevention of epidural scarring after microdiscectomy: a randomized clinical trial comparing gel and expanded polytetrafluoroethylene membrane. European Spine Journal. 2006;15:1360–1366. doi: 10.1007/s00586-006-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


