Abstract
INTRODUCTION
Hepatocellular carcinoma, the most frequent primary hepatic tumor, metastasizes in more than 50% of cases. However, parotid gland metastatic HCCs are very uncommon. We report a patient in whom the finding of a left parotid mass revealed metastatic HCC.
PRESENTATION OF CASE
A thirty-six-year-old male presented with a round palpable left neck mass that persisted for 3 months. He had received right hemihepatectomy for hepatocellular carcinoma (HCC). Preoperative evaluation revealed a benign tumor of the parotid gland. We performed superficial parotidectomy. Metastatic hepatocellular carcinoma of the parotid gland was diagnosed.
DISCUSSION
Although HCC metastases to the oral cavity have been reported, to date, only 4 cases HCC metastasis to the parotid gland have been reported. Although clinicians and cytopathologists alike both agree that salivary gland fine needle aspiration biopies (FNABs) are highly useful and safe diagnostic alternatives to biopsies and resections, we believe that in specific clinical situations, awareness of potential diagnostic pitfalls in salivary gland FNAB is a necessary part of the microscopic interpretations of these lesions.
CONCLUSION
Although rare, since HCC can metastasize to the parotid gland, high suspicion should be maintained in a patient presenting with a parotid mass with a history of HCC. In addition, since potential diagnostic pitfalls in salivary gland fine-needle aspiration (FNA) biopsies exist, incisional or excisional biopsy may be necessary for definite diagnosis of metastatic HCC to the parotid gland.
Abbreviations: HCC, hepatocellular carcinoma; FNA, fine-needle aspiration
Keywords: Metastatic hepatocellular carcinoma, Parotid gland, Fine-needle aspiration
1. Introduction
Hepatocellular carcinoma (HCC) spreads via the hematogenous route, the lymphatic route, or by direct invasion into adjacent organs.1 The common extrahepatic metastatic sites of HCC are the lungs, peritoneum, adrenal glands, and bone, although there are rare reports of metastases to the heart, nasal cavity, orbital cavity, skin, external auditory canals, and pharynx.2–8 Metastatic involvement of the parotid glands is uncommon, comprising 8% of all parotid gland malignancies.9 These metastases usually arise from a primary mucosal or cutaneous cancer, generally melanomas and squamous cell carcinomas, located in the ipsilateral head and neck region.10,11 Metastases arising from a primary cancer outside the head and neck region are also possible.12 Hepatocellular carcinoma (HCC), the most frequent primary hepatic tumor, metastasizes in more than 50% of cases. However, parotid gland metastatic HCCs are very uncommon with 4 cases reported in the english literature.12–15 We report a patient in whom the finding of a left parotid mass revealed metastatic HCC.
2. Presentation of case
A thirty-six-year-old Korean male presented to our otolaryngology clinic with a round 2 cm × 2 cm sized palpable, firm and painless left neck mass that persisted for 3 months. Past medical history revealed that he had received right hemihepatectomy for hepatocellular carcinoma (HCC) 8 months before presenting to the clinic. The initial pathology of the liver showed multiple lesions in the right lobe and the largest one was 9.3 cm × 7.5 cm (pathologic stage T3a, moderately differentiated HCC). There was no microvascular invasion and the preoperative positron emission tomography (PET) scan prior to liver resection showed no extrahepatic abnormal hypermetabolic lesions. Postoperatively, there was no evidence of recurrence and the serum alfa-fetoprotein (AFP) level was normal. A fine-needle aspiration (FNA) of the parotid gland performed prior to presenting to our clinic suggested the possibility of pleomorphic adenoma. A neck computed tomography (CT) scan revealed a 1.6 cm sized round and well defined enhancing nodular lesion in superficial lobe of the left parotid gland with no other remarkable lymph node enlargement and a Pleomorphic adenoma or Warthin's tumor of the parotid gland was considered (Fig. 1). The preoperative liver function and AFP level was normal. We performed superficial parotidectomy of the left parotid gland while preserving the facial nerve and great auricular nerve. There were no immediate complications after the operation and the patient was discharged 3 days after surgery. Grossly, there was a well circumscribed yellowish ovoid mass within the partotid gland measuring 2.6 cm × 2.1 cm. The cut surface of the mass showed multiple hemorrhagic and microcystic foci in the diffuse granular background (Fig. 2). On microscopic examination, a totally encapsulated multilobulating tumor with a pushing margin showed a peripheral residual lymphoid cortex. The tumor cells were polygonal cells with centrally located round nuclei containing large amount of eosinophilic cytoplasm. They were arranged in predominantly trabecular and focal pseudoglandular patterns. Occasional intracytoplasmic and canalicular bile pigmentation was also seen. The morphology of the tumor cells was identical to that of hepatocellular carcinoma and revealed moderate differentiation. In addition, immunohistochemical staining for antihepatocyte antibody showed diffuse strong positive reaction in the cytoplasm (Fig. 3). Metastatic hepatocellular carcinoma arising in the intraparotid lymph node was diagnosed. Re-examination of the FNA cytology slide obtained prior to the operation revealed high cellularity composed of many syncytial fragments of uniformly polygonal tumor cells with low nuclear–cytoplasmic ratio. Although the differential diagnoses included primary pleomorphic adenoma and oncocytoma, some endothelial cells were wrapped with tumor cells peripherally, and myxoid material, which is usually seen in pleomorphic adenoma, was not identified (Fig. 4). After diagnosis, a PET scan was performed for evaluation of other potential sites of recurrence. There was no evidence of tumor recurrence or metastasis. The patient is free of tumor recurrence for 6 months and is currently being observed at our outpatient clinic.
Fig. 1.
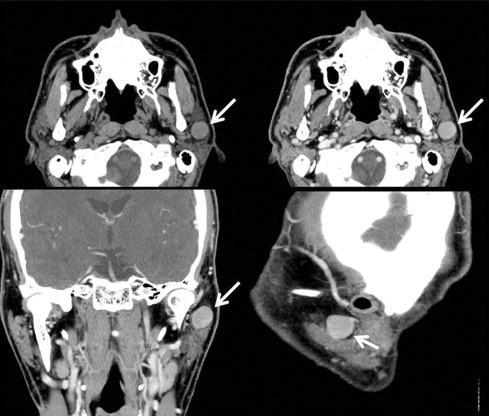
Preoperative neck computed tomography (CT) scan a 1.6 cm sized round and well defined enhancing nodular lesion in superficial lobe of the left parotid gland (white arrow).
Fig. 2.
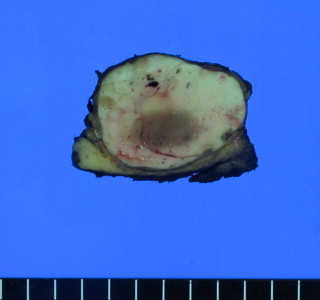
Gross findings of the parotid gland revealed a well circumscribed yellowish ovoid mass within the partotid gland measuring 2.6 cm × 2.1 cm. The cut surface of the mass showed multiple hemorrhagic and microcystic foci in the diffuse granular background.
Fig. 3.
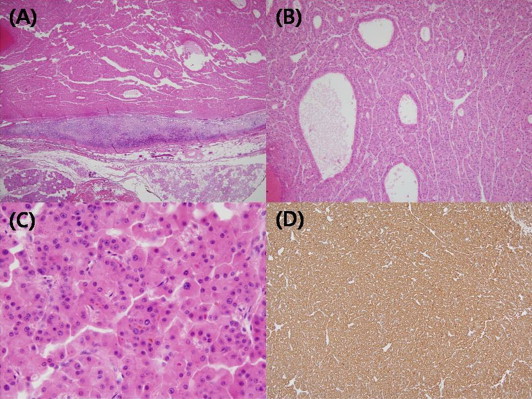
Microscopic findings of the parotid gland. (A) Totally encapsulated multilobulating tumor with a pushing margin showed peripheral residual lymphoid cortex. (B) The tumor cells were polygonal cells with centrally located round nuclei containing large amount of eosinophilic cytoplasm. They were arranged in predominantly trabecular and focal pseudoglandular patterns. (C) Occasional intracytoplasmic and canalicular bile pigmentation was also seen. (D) Immunohistochemical staining for antihepatocyte antibody showed diffuse strong positive reaction in the cytoplasm.
Fig. 4.
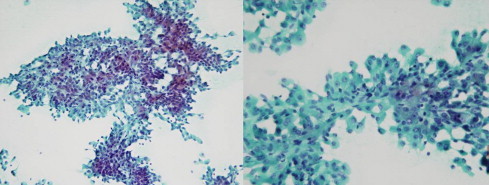
Parotid fine-needle aspiration (FNA) revealed high cellularity composed of many syncytial fragments of uniformly polygonal tumor cells with low nuclear–cytoplasmic ratio.
3. Discussion
Metastatic involvement of the salivary glands is uncommon, comprising 10–16% of all salivary gland malignancies, excluding lymphomas.14 These metastases usually originate from head and neck malignancies. Only 20% originate from infraclavicular sites, usually lung, kidney, or breast, or, more rarely, from gastrointestinal or genitourinary primary sites.14 Although HCC metastases to the oral cavity have been reported, to date, only 4 cases HCC metastasis to the parotid gland have been reported.8 To the best of our knowledge, the present case is the fifth case of metastatic HCC to the parotid gland reported in the English literature (Table 1).
Table 1.
Summary of four cases of metastatic hepatocellular carcinoma to the parotid gland previously reported and the present case.
| Case no. | Age | Sex | Metastatic site | Clinical features | Size | AFP (ng/mL) | Serology | Method of diagnosis | Initial findings of cytology | Time to recurrence | Previous treatment for HCC | Treatment | Other metastatic lesions | Disease progression | Year reported/reference no. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | Male | Left parotid gland | Fever, painful swelling of left mandible | NR | 3520 | HCV | FNAB | Typical for HCC | Liver lesion detected after parotid lesion | None | Local irradiation and tamoxifen | Bone | NR | 199813 |
| 2 | 47 | Male | Right parotid gland | Tender swollen right lower jaw and preauricular area | 4.5 cm on CT | Normal | HCV | FNAB | Typical for HCC | Liver lesion detected after parotid lesion | None | Palliative radiation and chemotherapy | None | Died after 4 months | 200414 |
| 3 | 82 | Male | Right parotid gland | Painless, palpable firm mass | 3.8 cm on US | NR | NR | Incisional biopsy | Not typical for HCC | Liver lesion detected after parotid lesion | None | No active treatment | None | Died after 6 months | 200912 |
| 4 | 75 | Male | Right parotid gland | Palpable mass | NR | NR | NR | Repeat FNAB | Not typical for HCC | Liver lesion detected after parotid lesion | None | Chemotherapy | Bone | Stable for 2 years | 201015 |
| 5 | 36 | Male | Left parotid gland | Painless, palpable firm mass | 1.6 cm on CT | Normal | HBV | Excisional biopsy | Not typical for HCC | 6 months | RL | Superfical parotidectomy | None | Stable for 1 years | Present case |
AFP, alpha-feto protein; NR, not reported; FNAB, fine-needle aspiration biopsy; RL, right lobectomy.
Establishing a diagnosis of metastatic HCC in the maxillofacial area may be difficult.12 The previous four cases of parotid metastasis from HCC and the present case were initially investigated by FNA. Despite its accuracy, specificity and sensitivity, discrepancies between the cytomorphological and histopathological examinations can occur.12 FNA of the salivary gland lesions presents one of the most challenging of diagnoses in cytopathology.15 Difficulties that arise in salivary gland FNAs not only include distinguishing benign from malignant but also the specific classification of lesions.15 Occasionally, difficulties in salivary gland FNA interpretation may also include distinguishing primary from metastatic tumors.15 In the present case, the initial FNA finding was benign. Moreover, re-evaluation of the FNA slide after parotid surgery did not show specific cytological features that allowed a diagnosis of metastatic HCC and histological and immunohistochemical examination of the surgical specimen was necessary for accurate diagnosis. Although clinicians and cytopathologists alike both agree that salivary gland FNAs are highly useful and safe diagnostic alternatives to biopsies and resections, we believe that in specific clinical situations, awareness of potential diagnostic pitfalls in salivary gland FNA is a necessary part of the microscopic interpretations of these lesions.15
HCC, primary or metastatic, often presents cytological and histopathological features sufficiently characteristic to suggest its diagnosis, as in the present case.12 A number of other metastatic tumors, notably from the breast, kidney and adrenal glands, may mimic the trabecular, liver-like pattern of HCC. Immunohistochemical analysis, including markers such as cytoplasmic alfa-fetoprotein, ‘hepatic’ cytokeratins 8 and 18, carcinoembryonic antigen, epithelial membrane antigen, CD10, CD15, K1-67 and the recently developed monoclonal antibody HepPar 1, are often required to aid in distinguishing metastatic HCC from other parotid metastatic tumors.12
The current patient, like that reported by Romanas et al., was exceptional in that he presented with metastatic disease in his left parotid gland.14 Typically, metastases from the liver to the maxillofacial area reach the lungs first, via the hepatic artery and the portal vein, and later reach the maxillofacial area.12 To explain the parotid localization without lung involvement, it has been suggested that HCC could disseminated into Batson's plexus (a connection between the azygos and hemiazygos veins and the vertebral venuous plexus) bypassing filtration through the lungs.12 However, the solitary parotid metastasis in this case might have occurred via the lymphatic route, although the exact route is not clear, since the metastatic HCC was found in the lymph node within the parotid gland.
The recurrence of HCC can be detected by an elevated serum AFP or imaging findings.16 AFP is also known as an independent prognostic factor for HCC and a markedly elevated serum AFP level may reflect advanced HCC in terms of its large size or metastasis.16 However, on reviewing the four reported cases and the current case of metastatic HCC to the parotid gland (Table 1), the AFP level was not elevated prior to parotidectomy in 2 cases including the present case and the AFP level was not checked in 2 cases. Moreover, the lesion in the liver was detected after parotid metastasis occurred in the previous four cases. Therefore, we believe that without a high suspicion for metastasis, neither the serum AFP nor imaging findings can be a useful indicator of recurrent HCC in the parotid gland.
4. Conclusion
Although rare, since HCC can metastasize to the parotid gland, high suspicion should be maintained in a patient presenting with a parotid mass with a history of HCC. In addition, since potential diagnostic pitfalls in salivary gland FNABs exist, incisional or excisional biopsy may be necessary for definite diagnosis of metastatic HCC to the parotid gland.
Conflict of interest statement
None to declare.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Dong-Sik Kim, Jeong-Hyeon Lee, and Yang-Seok Chae have made the data collection. Sung-Won Jung and Sung-Ock have performed data analysis. SuhYoung-Dong Yu and Dong-Sik Kim have designed the study and written the manuscript.
References
- 1.Hong S.S., Kim T.K., Sung K.B., Kim P.N., Ha H.K., Kim A.Y. Extrahepatic spread of hepatocellular carcinoma: a pictorial review. European Radiology. 2003;13(4):874–882. doi: 10.1007/s00330-002-1519-7. [DOI] [PubMed] [Google Scholar]
- 2.Kim T.H., Cheung D.Y., Chung W.B., Son D.K., Jo D.H., Chung J.S. A case of metastatic hepatocellular carcinoma of the ovary. Korean Journal of Gastroenterology. 2004;43(3):215–218. [PubMed] [Google Scholar]
- 3.Masci G., Magagnoli M., Grimaldi A., Covini G., Carnaghi C., Rimassa L. Metastasis of hepatocellular carcinoma to the heart: a case report and review of the literature. Tumori. 2004;90(3):345–347. doi: 10.1177/030089160409000317. [DOI] [PubMed] [Google Scholar]
- 4.Frigy A.F. Metastatic hepatocellular carcinoma of the nasal cavity. Archives of Otolaryngology. 1984;110(9):624–627. [PubMed] [Google Scholar]
- 5.Font R.L., Maturi R.K., Small R.G., Garcia-Rojas M. Hepatocellular carcinoma metastatic to the orbit. Archives of Ophthalmology. 1998;116(7):942–945. doi: 10.1001/archopht.116.7.942. [DOI] [PubMed] [Google Scholar]
- 6.Yamanishi K., Kishimoto S., Hosokawa Y., Yamada K., Yasuno H. Cutaneous metastasis from hepatocellular carcinoma resembling granuloma teleangiectaticum. Journal of Dermatology. 1989;16(6):500–504. doi: 10.1111/j.1346-8138.1989.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 7.Yasumatsu R., Okura K., Sakiyama Y., Nakamuta M., Matsumura T., Uehara S. Metastatic hepatocellular carcinoma of the external auditory canal. World Journal of Gastroenterology. 2007;13(47):6436–6438. doi: 10.3748/wjg.v13.i47.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oida Y., Ishii M., Dowaki S., Tobita K., Ohtani Y., Imaizumi T. Hepatocellular carcinoma with metastasis to the pharynx: report of a case. Tokai Journal of Experimental and Clinical Medicine. 2005;30(1):31–34. [PubMed] [Google Scholar]
- 9.Markowski J., Gierek T., Zielinska-Pajak E., Witkowska M., Wodolazski A., Pajak J. Distant metastases to the parotid gland—review of the literature and report of own two cases. Otolaryngologia Polska – The Polish Otolaryngology. 2005;59(4):547–552. [PubMed] [Google Scholar]
- 10.Borg M.F. Parotid gland as an initial site of metastasis. Australasian Radiology. 2004;48(1):88–92. doi: 10.1111/j.1440-1673.2004.01253.x. [DOI] [PubMed] [Google Scholar]
- 11.Nuyens M., Schupbach J., Stauffer E., Zbaren P. Metastatic disease to the parotid gland. Otolaryngology – Head and Neck Surgery. 2006;135(6):844–848. doi: 10.1016/j.otohns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Vitale A.R., Compilato D., Coletti G., Calvisi G., Ciuffitelli V., Barbera D. Metastatic hepatocellular carcinoma of the parotid region without lung metastasis: a case report. International Journal of Oral and Maxillofacial Surgery. 2009;38(6):696–698. doi: 10.1016/j.ijom.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Dargent J.L., Deplace J., Schneider E., Morales J., Kentos A., Willemart S. Hepatocellular carcinoma metastatic to the parotid gland: initial diagnosis by fine needle aspiration biopsy. Acta Cytologica. 1998;42(3):824–826. [PubMed] [Google Scholar]
- 14.Romanas M.M., Cherian R., McGregor D.H., Wu Y., May C.L., Baranda J.C. Hepatocellular carcinoma diagnosed by fine-needle aspiration of the parotid gland. Diagnostic Cytopathology. 2004;30(6):401–405. doi: 10.1002/dc.20040. [DOI] [PubMed] [Google Scholar]
- 15.Moore F.R., Bergman S., Geisinger K.R. Metastatic hepatocellular carcinoma mimicking acinic cell carcinoma of the parotid gland: a case report. Acta Cytologica. 2010;54(5 Suppl.):889–892. [PubMed] [Google Scholar]
- 16.Lee J.M., Park K.M., Lee S.Y., Choi J., Hwang D.W., Lee Y.J. Metastasis of hepatocellular carcinoma to the ovary: a case report and review of the literature. Gut Liver. 2011;5(4):543–547. doi: 10.5009/gnl.2011.5.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]


