Abstract
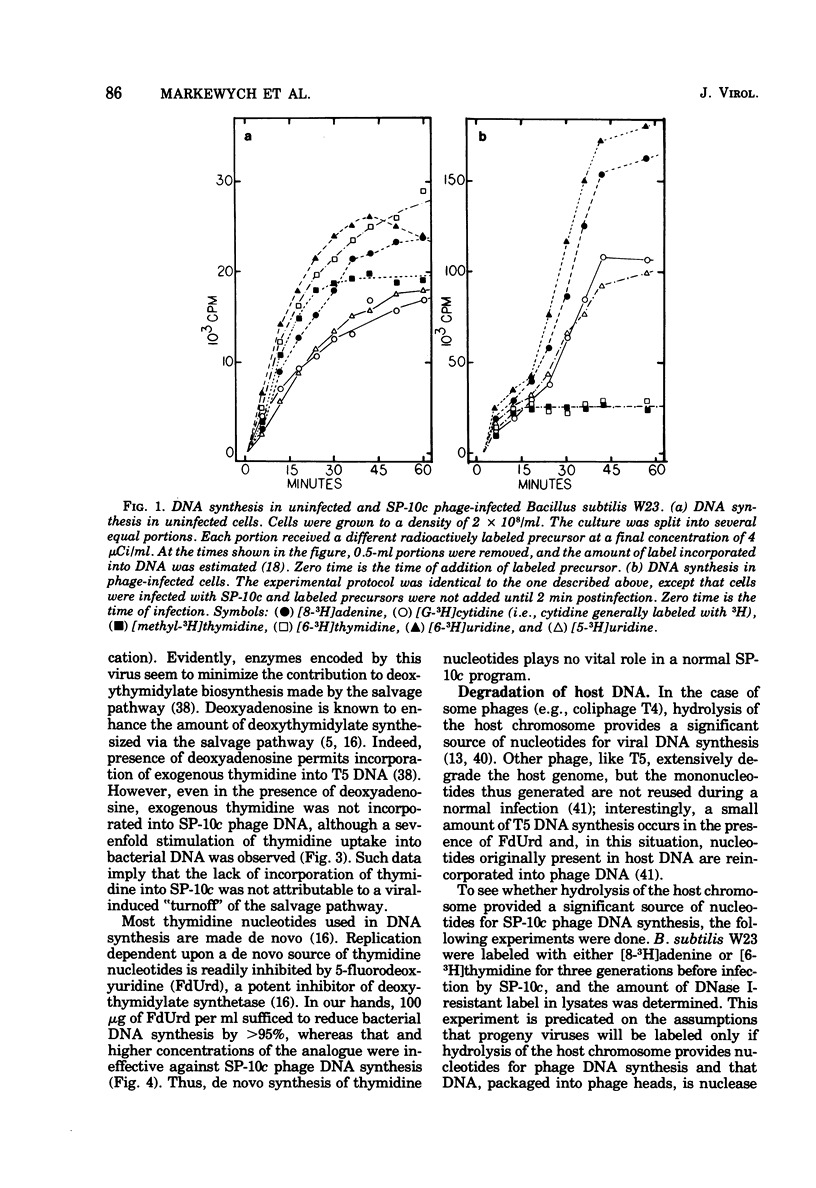
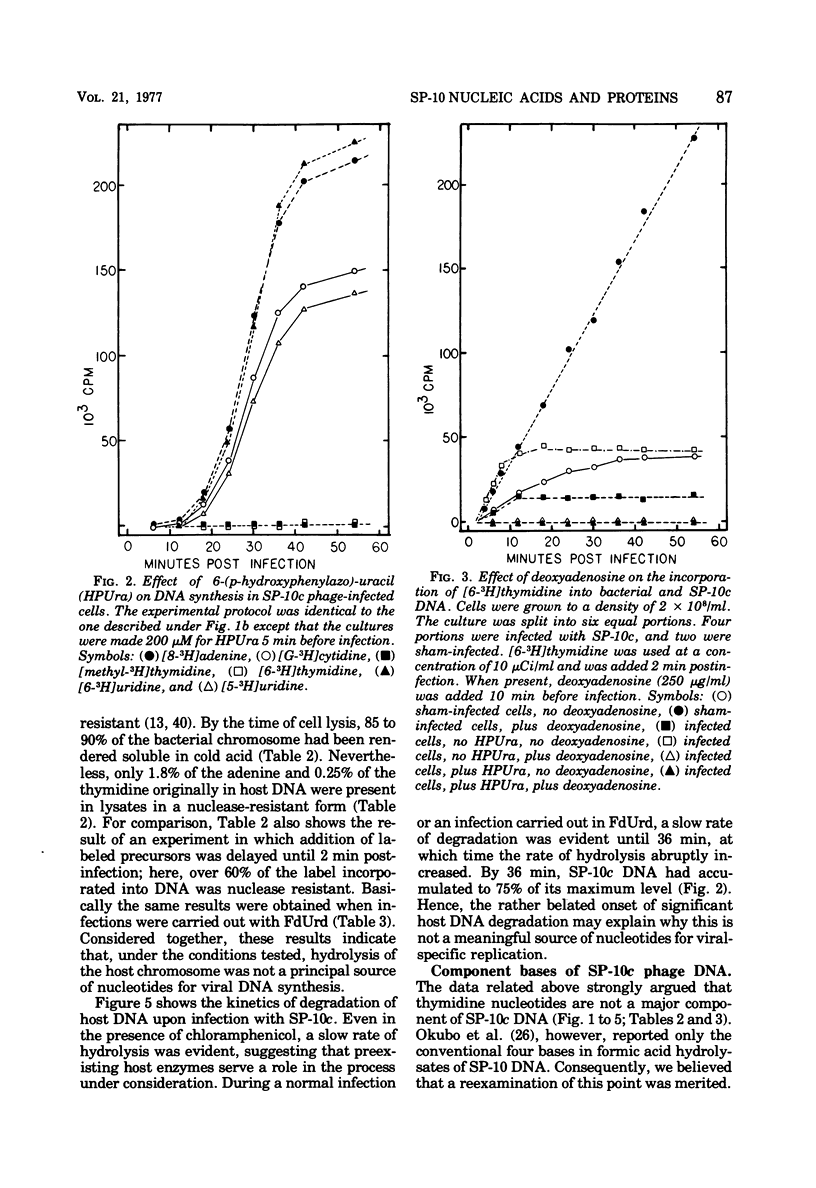
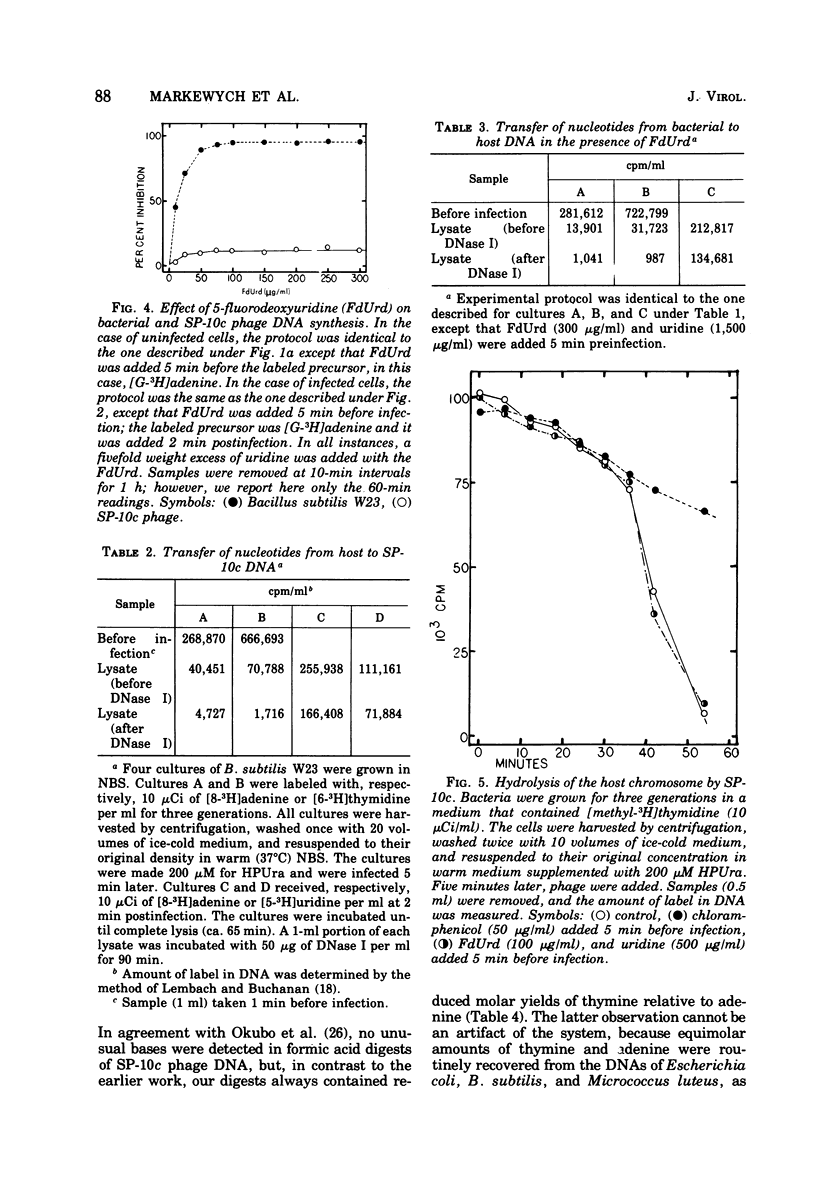
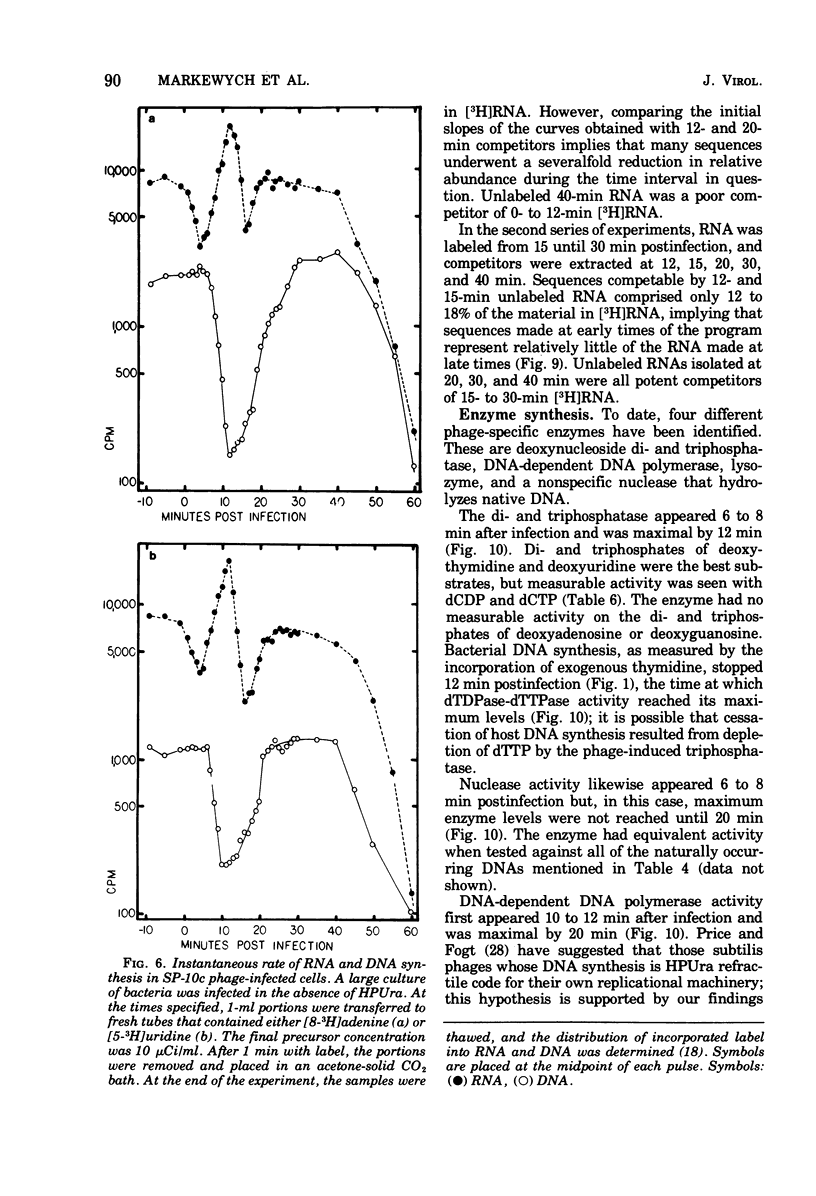
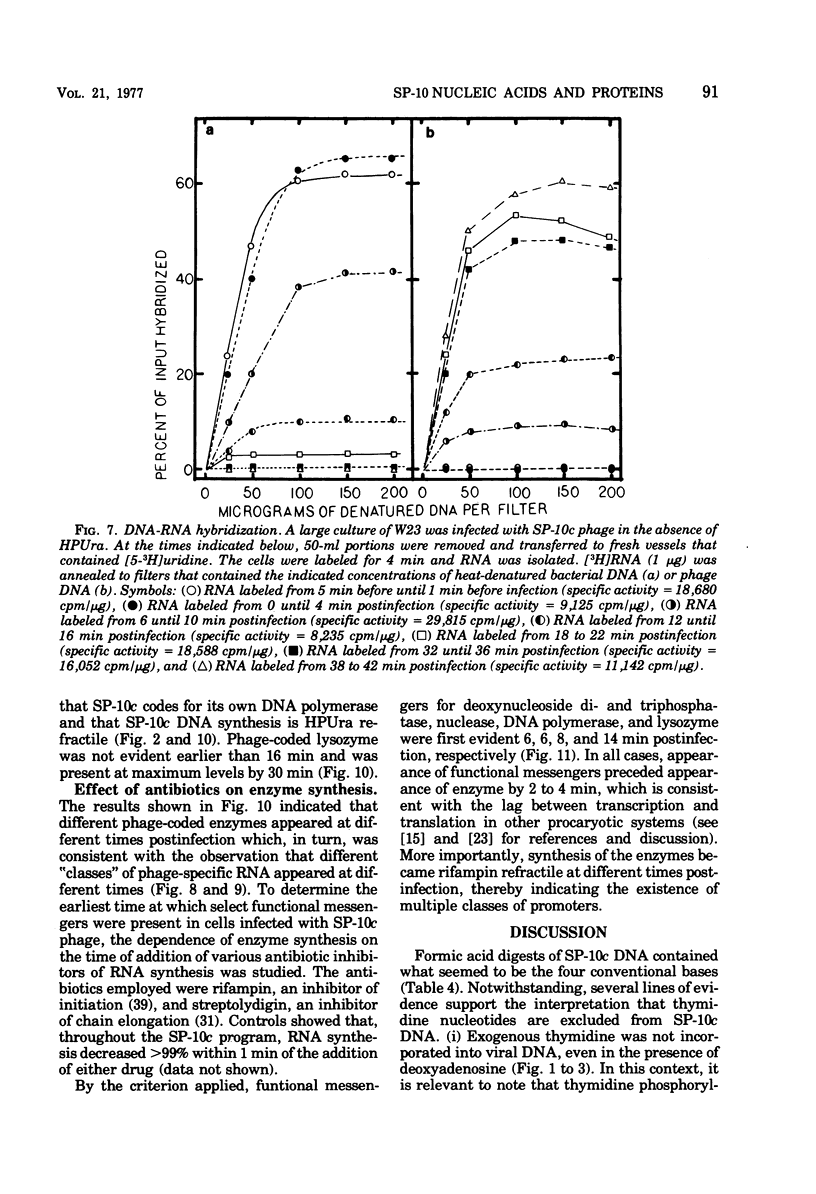
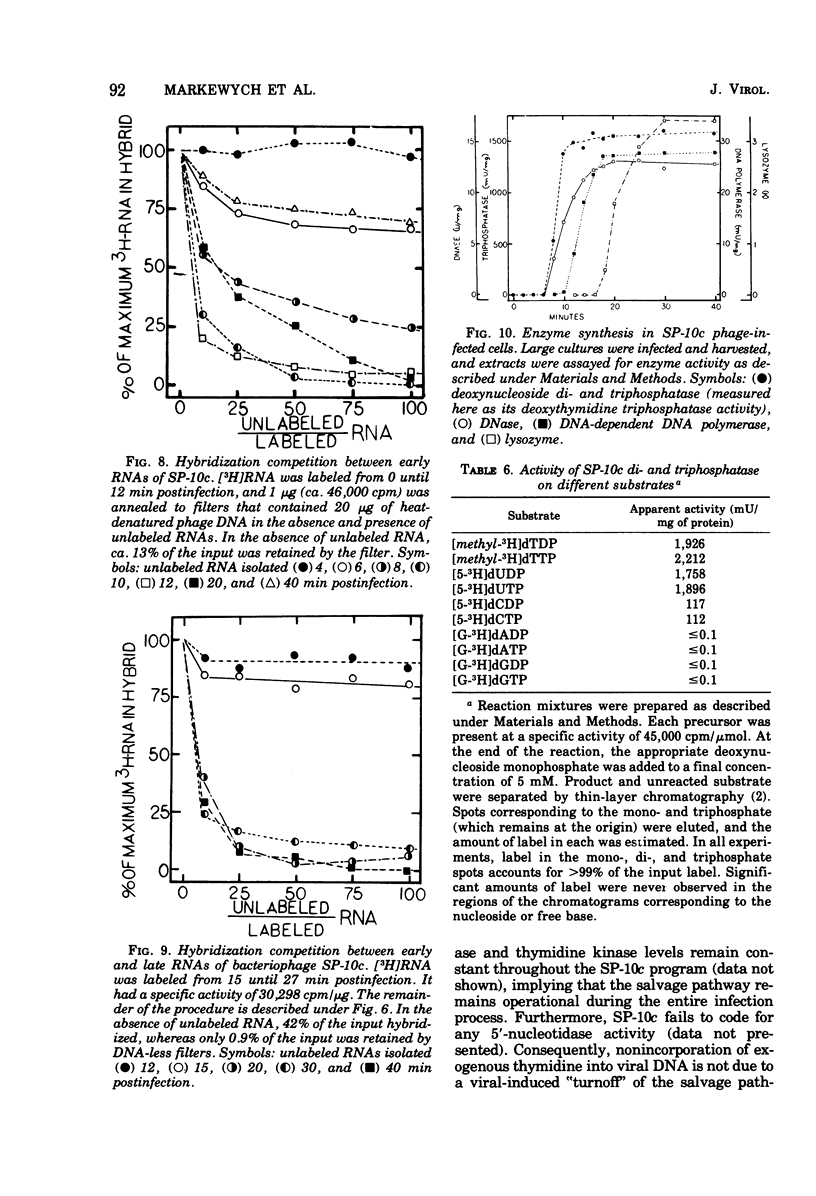
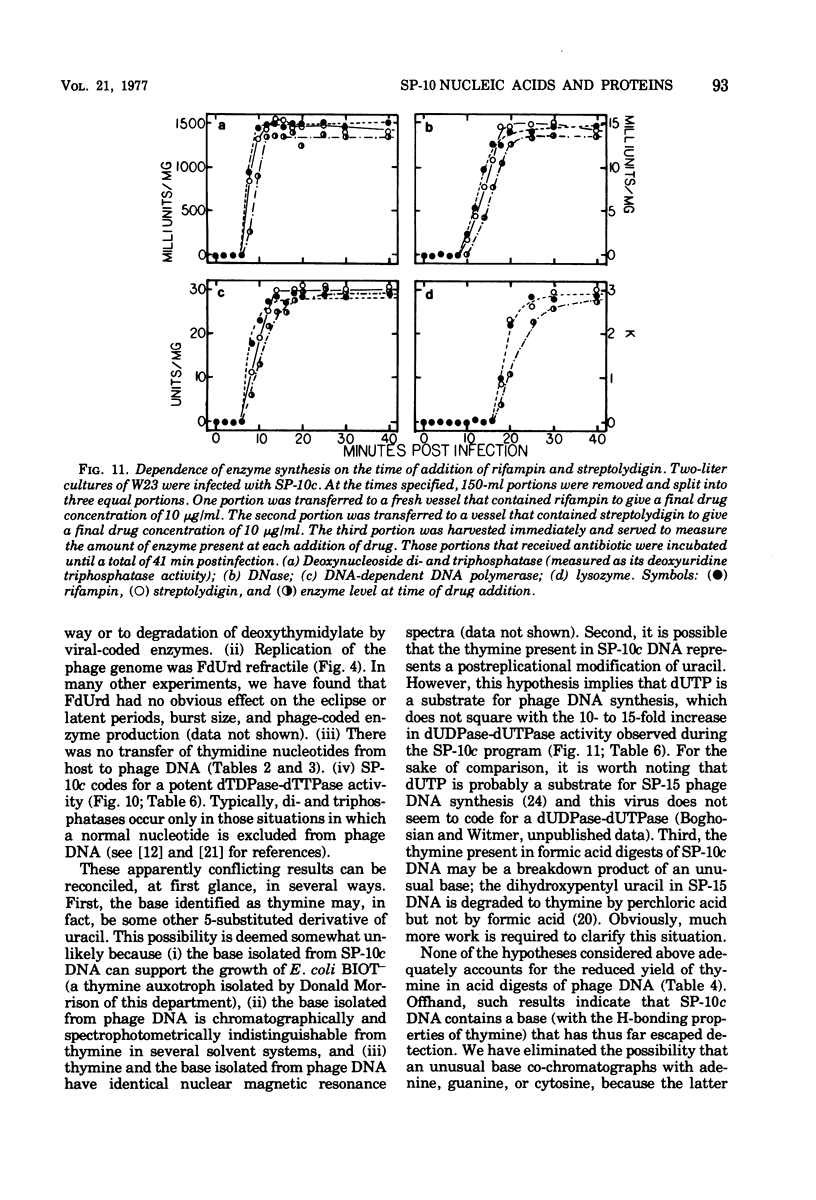
Bacillus subtilis W23 was infected with a clear-plaque variant of SP-10 phage, namely, SP-10c. Exogenous thymidine was not incorporated into phage DNA (even in the presence of deoxyadenosine), nor was there any transfer of thymidine nucleotides from bacterial to viral DNA. The lytic program was unaffected by concentrations of 5-fluorodeoxyuridine sufficient to reduce bacterial DNA synthesis by greater than 95%. Although these data are consistent with the interpretation that thymidine nucleotides are excluded from phage DNA, formic acid digests of SP-10c DNA contained what appeared to be the four conventional bases; however, adenine and thymine were not recovered in equimolar yields. DNA-RNA hybridization and hybridization competition experiments were done. Synthesis of host RNA started to wane moments postinfection and stopped completely by 36 min. SP-10c coded for discrete classes of early and late RNA. The possibility of discrete subclasses of early RNA exists. Replication of the bacterial genome appeared to terminate 12 min postinfection. Degradation of the host DNA to acid-soluble material started at 36 min and, by the end of the latent period, greater than 90% of the host chromosome was hydrolyzed. Four apparent phage-coded enzymes have been identified. A di- and triphosphatase degraded dUTP, dUDP, dTTP, and dTDP (and, to a lesser extent, dCDP and d CTP) to the corresponding monophosphates; the enzyme had no apparent activity on dATP and dGTP. SP10c also coded for a DNA-dependent DNA polymerase, lysozyme, and a nuclease that degrades native bacterial DNA. Judging from the dependence of enzyme synthesis on the time of addition of rifampin (an inhibitor of the initiation of RNA synthesis), messengers for the di- and triphosphatase, as well as the nuclease, are transcribed from promoters that start to function 6 min postinfection. Promoters for polymerase and lysozyme did not become functional until 8 and 16 min postinfection, respectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- Baros A., Witmer H. J. Effect of chloramphenicol and starvation for an essential amino acid on the synthesis and decay of T4 bacteriophage-specific messengers transcribed from early and quasi-late promoters. Arch Biochem Biophys. 1975 Aug;169(2):415–427. doi: 10.1016/0003-9861(75)90183-6. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Brody E. N., Geiduschek E. P. Transcription of the bacteriophage T4 template. Detailed comparison of in vitro and in vivo transcripts. Biochemistry. 1970 Mar 17;9(6):1300–1309. doi: 10.1021/bi00808a002. [DOI] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriphage SPO1 development. II. Some modulations and prerequisites of the transcription program. Virology. 1971 Apr;44(1):200–210. doi: 10.1016/0042-6822(71)90165-6. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. Repression of early messenger transcription in the development of a bacteriophage. J Mol Biol. 1967 Dec 14;30(2):435–440. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants in a nonessential gene of bacteriophage T4 which are defective in the degradation of Escherichia coli deoxyribonucleic acid. J Virol. 1971 Jan;7(1):95–105. doi: 10.1128/jvi.7.1.95-105.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Pène J. J. DNA replication in bacteriophage ø29: the requirement of a viral-specfic product for association of ø29 DNA with the cell membrane of Bacillus amyloliquefaciens. Virology. 1973 Apr;52(2):351–362. doi: 10.1016/0042-6822(73)90330-9. [DOI] [PubMed] [Google Scholar]
- Kennell D., Simmons C. Synthesis and decay of messenger ribonucleic acid from the lactose operon of Escherichia coli during amino-acid starvation. J Mol Biol. 1972 Oct 14;70(3):451–464. doi: 10.1016/0022-2836(72)90552-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legault-Demare L., Chambliss G. H. Natural messenger ribonucleic acid-directed cell-free protein-synthesizing system of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1300–1307. doi: 10.1128/jb.120.3.1300-1307.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembach K. J., Buchanan J. M. The relationship of protein synthesis to early transcriptive events in bacteriophage T4-infected Escherichia coli B. J Biol Chem. 1970 Apr 10;245(7):1575–1587. [PubMed] [Google Scholar]
- Marmur J., Brandon C., Neubort S., Ehrlich M., Mandel M., Konvicka J. Unique properties of nucleic acid from Bacillus subtilis phage SP-15. Nat New Biol. 1972 Sep 20;239(90):68–70. doi: 10.1038/newbio239068a0. [DOI] [PubMed] [Google Scholar]
- Mattson T., Richardson J., Goodin D. Mutant of bacteriophage T4D affecting expression of many early genes. Nature. 1974 Jul 5;250(461):48–50. doi: 10.1038/250048a0. [DOI] [PubMed] [Google Scholar]
- Natale P. J., Buchanan J. M. DNA-directed synthesis in vitro of T4 phage-specific enzymes. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2513–2517. doi: 10.1073/pnas.69.9.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubort S., Marmur J. Synthesis of the unusual DNA of Bacillus subtilis bacteriophage SP-15. J Virol. 1973 Nov;12(5):1078–1084. doi: 10.1128/jvi.12.5.1078-1084.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. M., Brown N. C. Inhibition of a discrete bacterial DNA polymerase by 6-(p-hydroxyphenylazo)-uracil and 6-(p-hydroxyphenylazo-)-isocytosine. Nat New Biol. 1972 Nov 15;240(98):80–82. doi: 10.1038/newbio240080a0. [DOI] [PubMed] [Google Scholar]
- Post L., Price A. R. Inhibition of bacteriophage PBS2 replication in Bacillus subtilis by phleomycin. J Virol. 1975 Feb;15(2):363–371. doi: 10.1128/jvi.15.2.363-371.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. R., Fogt S. M. Resistance of bacteriophage PBS2 infection to 6-(p-hydroxyphenylazo)-uracil, an inhibitor of Bacillus subtilis deoxyribonucleic acid synthesis. J Virol. 1973 Feb;11(2):338–340. doi: 10.1128/jvi.11.2.338-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W. Deoxyribonucleic acid polymerase activity in a deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis infected with temperature bacteriophage SPO2. J Virol. 1972 Oct;10(4):658–660. doi: 10.1128/jvi.10.4.658-660.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Reilly B. E., De Sain C. V., Whittington M. O., Anderson D. L. Selective replication of bacteriophage phi29 deoxyribonucleic acid in 6-(p-hydroxyphenylazo)-uracil-treated Bacillus subtilis. J Virol. 1973 Jan;11(1):153–155. doi: 10.1128/jvi.11.1.153-155.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhikol C., Erbstoeszer J. W., Weisblum B. Mode of action of streptolydigin. J Bacteriol. 1969 Jul;99(1):151–155. doi: 10.1128/jb.99.1.151-155.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR M. J., THORNE C. B. TRANSDUCTION OF BACILLUS LICHENIFORMIS AND BACILLUS SUBTILIS BY EACH OF TWO PHAGES. J Bacteriol. 1963 Sep;86:452–461. doi: 10.1128/jb.86.3.452-461.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Thorne C. B. Concurrent changes in transducing efficiency and content of transforming deoxyribonucleic acid in Bacillus subtilis bacteriophage SP-10. J Bacteriol. 1966 Jan;91(1):81–88. doi: 10.1128/jb.91.1.81-88.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Taylor M. J., Lawton W. D., Goldberg I. D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1969 Nov;100(2):1027–1036. doi: 10.1128/jb.100.2.1027-1036.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Drong R. F., Berget S. M. Early events after infection of Escherichia coli by bacteriophage T5. Induction of a 5'-nucleotidase activity and excretion of free bases. J Virol. 1975 Feb;15(2):273–280. doi: 10.1128/jvi.15.2.273-280.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Rosenkranz H. S., Morgan C. Development of coliphage T5: ultrastructural and biochemical studies. J Virol. 1972 Mar;9(3):526–543. doi: 10.1128/jvi.9.3.526-543.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



