Abstract
Eccrine sweat glands are skin-associated epithelial structures (appendages) that are unique to some primates including humans and are absent in the skin of most laboratory animals including rodents, rabbits, and pigs. On the basis of the known importance of other skin appendages (hair follicles, apocrine glands, and sebaceous glands) for wound repair in model animals, the present study was designed to assess the role of eccrine glands in the repair of wounded human skin. Partial-thickness wounds were generated on healthy human forearms, and epidermal repair was studied in skin biopsy samples obtained at precise times during the first week after wounding. Wound reepithelialization was assessed using immunohistochemistry and computer-assisted 3-dimensional reconstruction of in vivo wounded skin samples. Our data demonstrate a key role for eccrine sweat glands in reconstituting the epidermis after wounding in humans. More specifically, i) eccrine sweat glands generate keratinocyte outgrowths that ultimately form new epidermis; ii) eccrine sweat glands are the most abundant appendages in human skin, outnumbering hair follicles by a factor close to 3; and iii) the rate of expansion of keratinocyte outgrowths from eccrine sweat glands parallels the rate of reepithelialization. This novel appreciation of the unique importance of eccrine sweat glands for epidermal repair may be exploited to improve our approaches to understanding and treating human wounds.
Skin injuries are a part of everyday life, and efficient wound repair is vital to restore the protective barrier function of the skin. Defects associated with cutaneous wound repair represent a tremendous burden for patients and health systems and are estimated to cost several billion dollars a year in the United States alone.1 Despite important progress in understanding the healing process in animal models, many questions remain regarding the basic principles of cutaneous wound repair in humans. Understanding the basic mechanisms of epithelial repair in human skin after wounding is a necessary first step in the design of therapeutic strategies with the objective of restoring burdening wound-healing defects.
In rodents, elegant studies have consistently demonstrated that keratinocytes within pilosebaceous units (consisting of hair follicles and associated sebaceous glands) and at the wound edge proliferate and migrate to the center of the wound to repair the epidermis after wounding.2–6 In pigs, the sweat apparatus also participates in reepithelialization of skin wounds.7 Extrapolation of these processes to human skin, however, remains difficult for 2 main reasons. First, hair follicles in humans are of the lowest range of density among mammals, and the healing ability of individual skin sites does not correlate with their respective hair follicle density. Indeed, hairless areas of the human body such as the palms and soles have no inherent healing defects. Second, in pigs, the sweat apparatus is composed of apocrine glands,8 which in humans are restricted to the axillae and rectogenital areas.9 In contrast, in humans, the skin sweat apparatus is composed of several million eccrine glands distributed throughout the body. Body eccrine sweat glands are present only in humans and Catarrhini primates and are absent in the skin of all other primates and mammals except for footpads and the volar surface of prehensile tails, where they respond to peculiar stimuli to increase grip.9 Apocrine and eccrine glands are fully distinct and differ in their histologic features (1 versus 2 types of secretory epithelial cells), the content of their secretion (oily versus watery), their development,10 and their function and regulation.11,12 Most importantly, apocrine glands are associated with pilosebaceous units and discharge in the canal of hair follicles, whereas eccrine glands are solitary and discharge on the skin surface. In general, adult human skin contains more and denser eccrine sweat glands than pilosebaceous units (the head being an exception)13 and few and specially localized apocrine glands.9
Considering the physiologic distinctions of the skin of laboratory mammals and humans, we sought to examine the importance of human skin-associated epithelial structures (appendages) during the process of partial-thickness wound closure in humans in vivo. Partial-thickness wounds such as those triggered by a second-degree burn,14 deep abrasion, or stage II pressure ulcers15 are often left open in humans, as opposed to full-thickness wounds, which are typically closed surgically using stitches or other means. In the present study, partial-thickness wounds were generated on the forearm skin of healthy human individuals with a carbon dioxide (CO2) laser. CO2 lasers produce a beam of infrared radiation that is absorbed by water within the skin, resulting in localized production of heat and tissue vaporization.16 We have previously reported that CO2 laser wounding triggers a typical repair reaction with inflammatory, proliferative, and remodeling phases.17,18 Herein we report the key previously undocumented role of eccrine sweat glands in epidermal reepithelialization after partial-thickness wounding in humans.
Materials and Methods
Subject Recruitment and Experimental Wounding
The present study was approved by the Institutional Review Board of the University of Michigan, according to the Declaration of Helsinki protocols, and each human subject provided written informed consent before entering the study. Human subject recruitment, CO2 laser treatments, and biopsy preparation were performed as previously described.18 In brief, after administration of 1% lidocaine local anesthesia, partial-thickness wounds were generated on the volar surface of the forearm using 2 passes of a CO2 laser (Ultrapulse; Coherent, Inc., Santa Clara, CA) set at 300 mJ and 60 W and with computer pattern generator settings of 3/5/6. For palm studies, partial-thickness wounds were made on the outside edge of the palm, below the fifth finger, toward the base of the palm. For palm studies only, the lipid-rich stratum corneum was first removed using a diamond chip fraise rotated using a dermabrasion tool (Dermatologic Laboratory and Supply, Inc., Council Bluffs, IA, or Dremel 1100, Robert Bosch Tool Corp., Mount Prospect, IL) before CO2 laser treatment. The 5-mm square CO2 laser-generated wounds were gently rinsed with tap water and covered with a semipermeable dressing (Tegaderm; 3M, St. Paul, MN). Full-thickness punch biopsy samples (4 mm) were taken from the center or across the edge of the wound (see figure legends) at various times after administration of 1% lidocaine local anesthesia. Skin samples were embedded in Tissue-Tek OCT compound (Miles Scientific Laboratories, Ltd., Naperville, IL), frozen in liquid nitrogen, and stored at –80°C until processing.
Thirty-one subjects were enrolled [18 men and 13 women; age range, 18 to 63 years (mean age, 36 years)], including 14 for forearm reconstruction imaging [5 men and 9 women; age range, 18 to 34 years (mean age, 27.1 years)] and 6 for palm studies [2 men and 4 women; age range, 22 to 37 years (mean age, 29 years)].
Immunohistochemistry and Imaging
Immunohistochemistry (IHC) and imaging were performed on frozen skin sections as previously described.19 Pan-keratin antibody mix was obtained by combining anti-type II keratins (clone AE3; BioGenex, Fremont, CA), KRT5 (Biocare Medical LLC, Concord, CA), KRT15 and KRT17 (both from Chemicon, Millipore Corp., Billerica, MA), KRT16 (Novocastra, Leica Microsystems, Bannockburn, IL), KRT19 (Sigma-Aldrich Corp., St. Louis, MO), KRT20 (ARP American Research Products, Inc., Waltham, MA), and KRT8 and KRT18 (clone TS1 and Ab-4668,20 respectively; both generous gifts from Dr. M.B. Omary, University of Michigan). Digital pictures were captured using a Zeiss microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) and merged when needed using Canon Utilities Photostitch 3.1 software (Canon USA, Inc., Lake Success, NY) or captured as a whole using a Leica MXFL III Stereo Microscope (Leica Microsystems, Inc., Buffalo Grove, IL; provided by the Microscopy and Image-Analysis Laboratory, Department of Cell and Developmental Biology, University of Michigan Medical School).
3-Dimensional Reconstructions and Quantification
Consecutive skin frozen sections (7 or 10 μm thick, 96 to 135 sections for whole biopsy reconstructions) were collected and immunostained, and whole-section images were obtained as described (see Immunohistochemistry and Imaging). In some cases, sample sectioning was performed along a plane parallel to the dermal-epidermal junction. Specifics of the 3-dimensional (3D) reconstruction method for whole biopsy samples are provided in Supplemental Figure S1 and its accompanying legend. Reconstructions of defined areas within skin samples, presented in main figures, were generated according to similar protocols. In brief, computer-assisted 3D reconstructions of immunostained structures were generated using Reconstruct 1.1 software, made freely available by its inventors.21 Reconstruction was performed with minimal slanting (“rigid alignment”) and using the Boissonnat surfacing algorithm. Final renderings and animated 3D reconstructions were generated using Blender 2.62 (http://www.blender.org/development/release-logs/blender-262). Video files were compressed to MOV format using Format Factory 2.5 (freely available at http://www.pcfreetime.com). Area quantifications were performed on 3D reconstruction images using Reconstruct 1.1, and are presented as mean ± SEM.
Results
Partial-thickness Wounds Trigger a Proliferative Response in Eccrine Sweat Glands and Pilosebaceous Units Underlying the Wound
Histologic observations of wounded skin samples indicated that, as expected, the CO2 laser efficiently ablated the entire epidermis and superficial papillary dermis (Figure 1A). The amount of tissue removed was constant throughout the treated zone and was similar among individuals (data not shown). Clinically, wound closure was completed within 10 days after CO2 laser treatment. Therefore, we focused on the first week after wounding to study the role of skin appendages during wound reepithelialization in human skin.
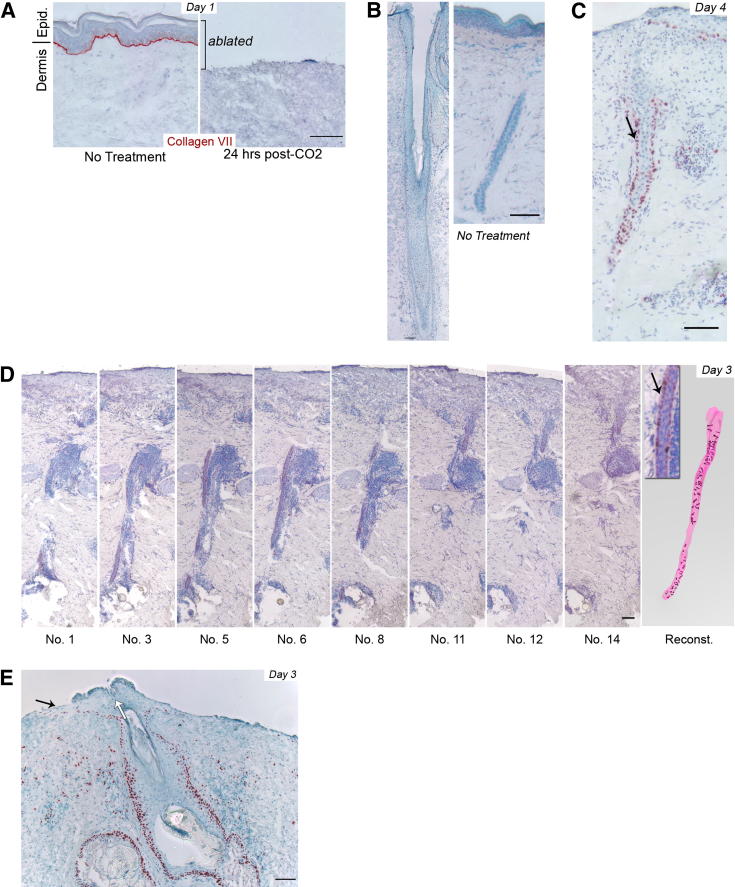
Figure 1.
Partial-thickness wounds trigger a proliferative response in eccrine sweat glands and pilosebaceous units underlying the wound. A: CO2 laser treatment efficiently ablates the entire epidermis and the superficial dermis as noted at 24 hours after wounding. Collagen VII staining (red) locates the basement membrane for reference. B: Paucity of basal Ki-67 staining in appendages of unwounded forearm skin. Hair follicle (left) and eccrine gland duct (right) with overlying epidermis, respectively. C: Ki-67 staining of an eccrine sweat gland duct in wounded forearm skin at 4 days after CO2 laser treatment. Note that almost all cells of the outermost layer of the eccrine duct are positive for Ki-67 (arrow). D: Fourteen consecutive sections of wounded human skin were taken 3 days after CO2 laser treatment, stained for Ki-67, and imaged. Eight of the 14 sections with corresponding stack number are shown. Digital images were used to generate a 3D reconstruction of the eccrine gland duct and the Ki-67–positive cells within the duct. Inset: Positive Ki-67 staining in outermost layer of the eccrine duct (arrow) as shown in C. Arbitrary colors are magenta for eccrine gland and maroon for Ki-67–positive staining. E: Ki-67 staining in a pilosebaceous unit at 3 days after CO2 laser treatment. Black arrow denotes keratinocyte outgrowth, and white arrow indicates overall direction of hair follicle. A–E: Staining patterns are representative of at least 4 subjects. Scale bars = 100 μm. Positive staining is shown in red, and hematoxylin counterstaining in blue.
In unwounded forearm skin, a small subset of keratinocytes in the lowest (basal) layer of the epidermis and in hair follicle upper segments (infundibulum) are proliferative, as revealed by selective expression of Ki-67 (a marker of proliferative cells22) (Figure 1B). In parallel, few cells are positive for Ki-67 in eccrine glands of unwounded skin (Figure 1B), consistent with a previous report that mitosis is only occasionally observed in eccrine sweat glands of intact human skin.23 In contrast, at 4 days after wounding, most of the outermost (basal) layer of the eccrine ducts underlying the wound was positive for Ki-67 (Figure 1C). Ki-67 expression was restricted to outer basal cells, and nearly no inner suprabasal (luminal) cells were positive for Ki-67 after wounding (Figure 1C). Similar results were consistently observed in each sweat gland of a given wounded skin sample and in all 8 individuals studied. Due to their relatively small size (duct ≤30 μm in diameter), eccrine glands are difficult to study in a single histologic section. Thus, to better examine eccrine sweat glands after wounding, we used computer-assisted 3D reconstructions of serial IHC images. For the images in Figure 1D, we used 14 consecutive sections (7 μm thick) to reconstitute an eccrine gland underlying the wound. These reconstructions demonstrated that after injury nearly all of the basal cells of eccrine ducts underlying the wound were positive for Ki-67.
In the same set of wounded skin biopsy samples, the rarer pilosebaceous units also exhibited increased Ki-67 staining at 3 days after wounding (Figure 1E). Ki-67 staining was predominantly observed in the outermost basal (outer root sheath) cells of the hair follicle infundibulum and in the outermost layer of the sebaceous glands. Together, these results establish that partial-thickness wounding triggers a proliferative response in eccrine gland ducts and the uppermost portion of pilosebaceous units in human skin in vivo.
Each Appendage Gives Rise to Individual Epidermal Outgrowths During Wound Repair in Human Skin
In the course of our studies, we observed that epidermal outgrowths first appeared at 3 days after wounding, above pilosebaceous units (Figure 1E), and continued to expand at the surface of wounded skin until complete reepithelialization (see below). To dissect the morphologic relationship between new epidermis and appendages in wounded human skin, consecutive skin sections corresponding to ∼1000 μm of skin sample (∼100 10-μm sections) were immunostained for epithelial markers (pan-keratin), photographed, and reassembled using computer-assisted 3D reconstructions of IHC images (procedure summarized in Supplemental Figure S1). The epithelial origin of appendageal and epidermal cells was ascertained via positive pan-keratin staining. The distinction between pilosebaceous units, eccrine sweat glands, and new epidermis structures was determined morphologically, taking into account respective singularities as follows. As previously described,13 2 or 3 pilosebaceous units are often grouped in human skin, whereas eccrine sweat glands are solitary and relatively evenly distributed (example in Supplemental Figure S1D). Eccrine sweat glands were further differentiated from pilosebaceous units on the basis of their relatively thinner diameter, the absence of associated sebaceous glands, and the presence of the coil formed by the lower duct and secretory portions in the deep dermis of the gland (not incorporated in reconstructions for clarity). In addition, epithelial outgrowths were noted at the surface of the wounded dermis, underneath the scab, and were thus distinctively localized within a subset of sections.
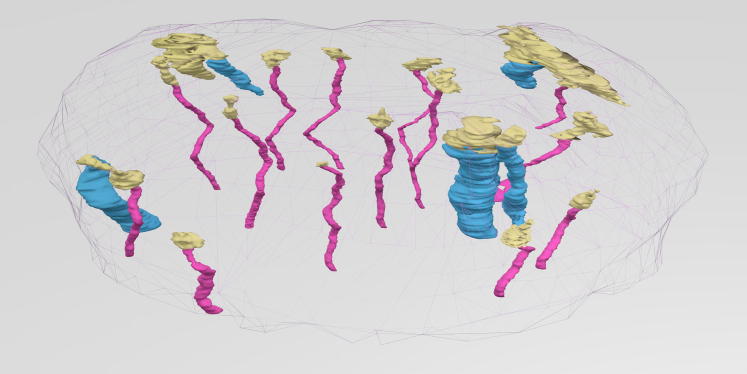
Keratinocyte outgrowths were first visible above hair follicle infundibulum at 3 days after wounding (Figure 1E). 3D reconstructions indicated that groups of keratinocytes also were noted above each eccrine sweat gland at 3 days after wounding (Figure 2; revolving 3D animation presented in Supplemental Video S1).
Figure 2.
Eccrine glands and pilosebaceous units give rise to individual keratinocyte outgrowths during wound repair in human skin. 3D reconstruction from immunohistochemistry of whole skin biopsy samples obtained 3 days after wounding. Consecutive sections were cut parallel to the skin surface, and the topmost 135 sections (7 μm thick) were used for reconstruction (representing the topmost 945 μm of skin). Arbitrary colors are cyan for pilosebaceous units, magenta for eccrine sweat glands, yellow for new epidermis, and gray mesh for sample contours.
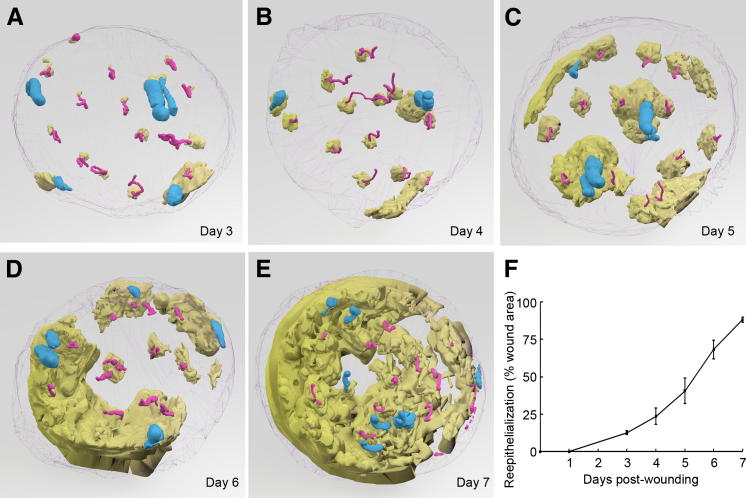
Throughout the reepithelialization process, appendageal outgrowths expanded until they merged with each other, thereby reconstituting the new interfollicular epidermis (Figure 3, A–E; see also corresponding revolving 3D animations presented in Supplemental Videos S1–S5). Quantification of epidermal coverage from 3D reconstructed images indicated that the rate of reepithelialization was sigmoidal and that epidermal coverage reached 88% ± 2% completion by day 7 (Figure 3F). Together, these results indicate that the proliferative response detected in appendages at 3 days after wounding is followed by the appearance of keratinocyte outgrowths above each epidermal appendage. Epithelial outgrowths expand laterally to regenerate interfollicular epidermis in wounded human skin.
Figure 3.
Keratinocyte outgrowths expand above appendages until they merge with each other, thereby reconstituting the new interfollicular epidermis. 3D reconstruction seen from the underside of epidermis, generated from immunohistochemistry of whole skin biopsy samples obtained at 3, 4, 5, 6, and 7 days after wounding (A–E, respectively). Sample A is identical, albeit rotated, to sample in Figure 2. Arbitrary colors are cyan for pilosebaceous units, magenta for eccrine sweat glands, yellow for new epidermis, and gray mesh for biopsy contours. Revolving animations are presented in Supplemental Videos S1–S5. F: Quantification of epidermal coverage from 3D reconstructed images. Epidermal areas (in micrometers squared) were normalized to the total biopsy area for each sample. Results represent percentage of biopsy coverage (mean ± SEM, n = 2 to 3 per time point).
Eccrine Glands Participate in Reepithelialization of Human Wounds Independently of Pilosebaceous Units
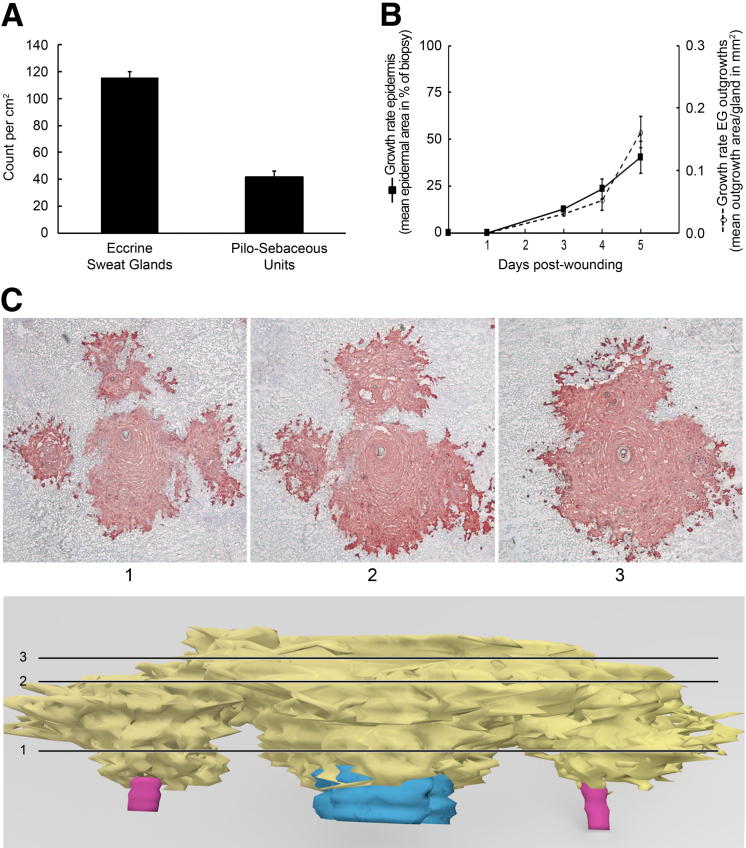
We counted 14.5 ± 0.6 eccrine sweat glands and 5.3 ± 0.6 pilosebaceous units per 4-mm diameter full-thickness biopsy sample of forearm skin (n = 12), corresponding to 115 ± 5 and 42 ± 5 average number/cm2, respectively (Figure 4A). These data indicate that eccrine glands outnumber pilosebaceous units at a ratio of 2.8:1, which is typical of human skin except for head, hands, and feet, regardless of ethnic origin.13,24 We next quantified the area of keratinocyte outgrowths located above each appendage from serial IHC sections analyzed using 3D reconstructed images at days 3, 4, and 5 after wounding. At these times, merging of keratinocyte outgrowths is minimal and appendageal origin of outgrowths is thus unambiguous. Results of these experiments indicated that the rate of expansion of eccrine gland–derived outgrowths was similar to the overall reepithelialization rate, which is the result of both pilosebaceous unit– and eccrine gland–derived cells (Figure 4B). These results indicate that the relative contribution of eccrine sweat glands remained unchanged throughout the reepithelialization process and was similar to that of pilosebaceous units.
Figure 4.
Relative contribution of eccrine glands and pilosebaceous units to the new interfollicular epidermis. A: Quantification of eccrine sweat glands and pilosebaceous units in human forearm skin. Appendages were counted in 4-mm diameter (∼0.126 cm2 area) reconstructed skin samples. Results are given as mean ± SEM, n = 12. B: Comparison of total epidermal growth rate (straight line, lefty axis) with rate of expansion of eccrine gland–derived outgrowths (dashed line, righty axis). Total epidermis growth rate was quantified as described in Figure 3F; eccrine gland growth rate represents the area of new epidermis overlying eccrine glands per eccrine gland. Total eccrine glands from 2 to 3 individuals were combined for each time point; results are given as mean ± SEM. C: 3D reconstruction of skin sample obtained at 5 days after wounding showing a close-up of an outgrowth merging area. Pan-keratin immunostaining of selected sections (top panels), according to the cutting planes represented on 3D reconstruction in the bottom panel. Arbitrary colors are cyan for pilosebaceous units, magenta for eccrine sweat glands, and yellow for new epidermis. Revolving animation is presented in Supplemental Video S6.
We also examined the morphologic details of outgrowth merging zones, focusing on the cell arrangement between new interfollicular epidermis, hair follicle infundibulum, and upper parts of sweat gland ducts. As shown in Figure 4C (for revolving 3D animation see Supplemental Video S6), we determined that keratinocyte outgrowths are morphologically distinct in their lower layers and merge with each other in the upper layers. Together, these results suggest that keratinocyte outgrowths behave separately regardless of their appendageal origin, growing independently until merging with each other. Our results also show that eccrine sweat glands outnumber pilosebaceous units by a factor close to 3 and give rise to keratinocyte outgrowths with a rate of expansion parallel to that of reepithelialization.
Eccrine Sweat Glands are Sole Appendages Involved in Reepithelialization of Palm Human Skin
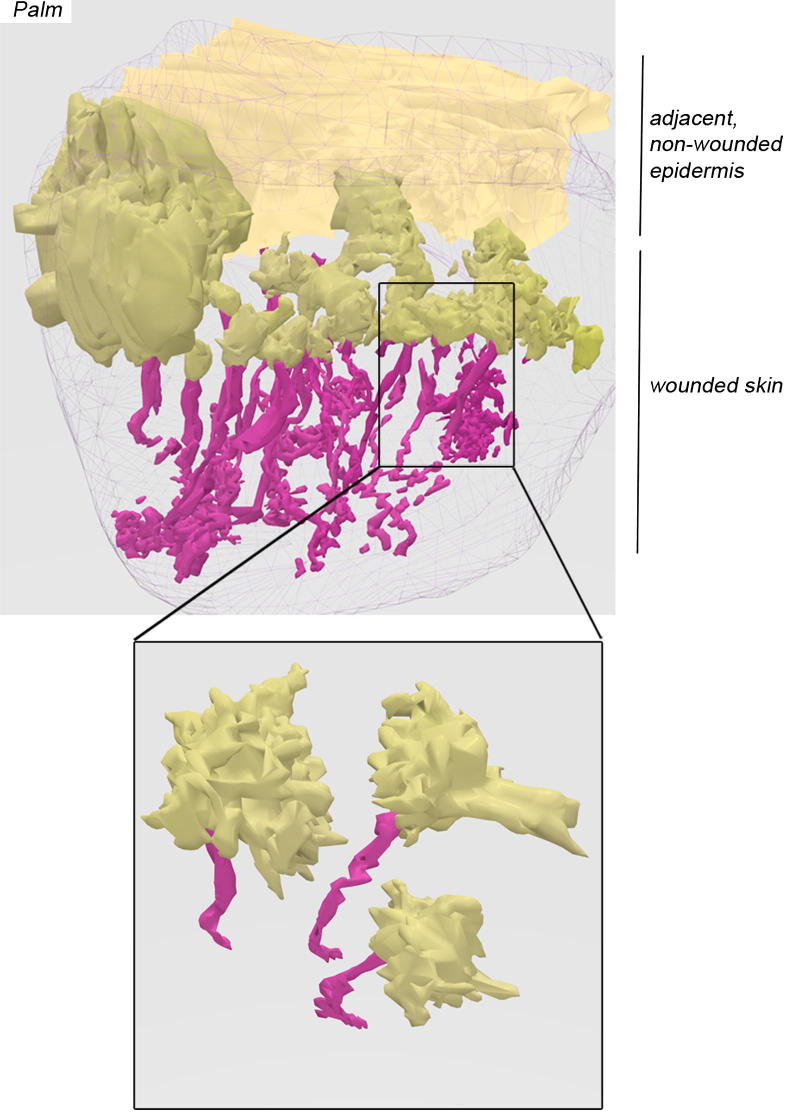
The important role of eccrine glands in reepithelialization of forearm skin strongly suggested to us that glabrous skin (devoid of hair follicles) such as on palms and soles might rely on eccrine glands for reepithelialization after wounding. Thus, we performed a series of experiments in which volunteers were wounded on the outside edge of the palm. At 4 days after wounding, biopsy samples were taken to encompass the wound and the adjacent intact skin. We observed that each eccrine gland gave rise to keratinocyte outgrowths located above the eccrine ducts (Figure 5; for revolving 3D animation, see also Supplemental Video S7). These results demonstrate that eccrine glands are involved in reepithelialization of palmar skin, as we observed in hairy skin.
Figure 5.
Eccrine sweat glands are sole appendages involved in the reepithelialization of palm human skin. Top panel: 3D reconstruction from immunohistochemistry of whole skin palm biopsy sample obtained at 4 days after wounding. Biopsy encompasses the wounded area (foreground) and adjacent nonwounded skin (far back). Arbitrary colors are cyan for pilosebaceous units, magenta for eccrine sweat glands, darker yellow for new epidermis, lighter yellow for nonwounded epidermis, and gray mesh for biopsy contours. Note lack of pilosebaceous unit in palmar skin. Bottom panel represents a close-up of 3 isolated eccrine glands located from within the wounded area, and shows that each gland gives rise to individual epidermal outgrowths. Revolving animation is presented in Supplemental Video S7.
Discussion
The present study was initiated to examine the importance of skin appendages during the process of partial-thickness wound closure in human skin in vivo. This aspect of wound healing is particularly important considering the unique appendageal makeup of human skin, ie, containing relatively sparse hair follicles and associated sebaceous glands, devoid of hair follicle–associated apocrine sweat glands except in the axillae and genital areas, and rich in eccrine sweat glands. This last characteristic is in sharp contrast with the skin of most laboratory animals that are commonly used to study wound healing and that do not have eccrine sweat glands in their body skin. Overall, our results demonstrate that i) eccrine sweat glands and pilosebaceous units contribute to the reepithelialization of human wounds by generating keratinocyte outgrowths that ultimately form new epidermis; ii) keratinocyte outgrowths expand at a similar rate whether they originate from sweat glands or pilosebaceous units; and iii) eccrine sweat glands outnumber pilosebaceous units by a factor close to 3. Together, these results indicate that eccrine sweat glands are major contributors to reepithelialization of human skin wounds.
To date, 3 functions have been attributed to eccrine sweat glands. When located in footpads in nonprimate mammals, prehensile tails in some primates, or palms and soles in primates including humans, eccrine glands produce sweat to enhance adherence and grip. These glands have little role in thermoregulation.10 In addition, humans are unique in their reliance on several million body eccrine sweat glands to regulate their temperature through evaporative heat loss (eccrine sweat glands in the body skin of nonhuman primates do not respond to heat stimulation8,25,26). In humans, eccrine sweat glands also participate in the formation of the acid mantle that protects the epidermis, regulating the growth of skin commensal organisms and preventing infection with pathogens.10,27 The demonstration of the contribution of eccrine sweat glands to epidermal repair after wounding described herein constitutes a novel function of human eccrine glands. Recent work has demonstrated that sweat glands in mouse paws remain quiescent during epidermal repair.28 These results are in sharp contrast with our findings relative to the sweat glands of the human palm described herein. Together, these observations suggest that the repair function of eccrine glands has been acquired relatively late during mammal evolution. Whether this function is unique to humans is an interesting possibility that remains to be elucidated.
The regenerative potential of sweat glands has remained underappreciated thus far, likely because of the absence of eccrine sweat glands in the skin of animals that are commonly used in wound-healing studies. In pig skin, which contains only pilosebaceous unit–associated apocrine glands,8 reepithelialization still occurs in the absence of pilosebaceous units and when lateral epidermal migration is artificially prevented, which suggests that reepithelialization can also arise from apocrine glands.7 In humans, eccrine sweat glands can self-repair after specific injury to the epidermal portion or dermal duct23,29 or in thick split-skin graft donor sites healed with thin (Thiersch) grafts.30 The hypothesis of a “reversion to surface epithelium of whatever glandular epithelial cells remain alive within the wound area” was raised by Hartwell,31 who studied human wounds more than 80 years ago. More recently, in vitro studies have shown that eccrine sweat gland–derived keratinocytes are able to stratify and form epidermis equivalents in culture.32 To our knowledge, the present study is the first to demonstrate a major contribution of eccrine sweat glands in epidermal repair of human skin in vivo. Whether eccrine sweat glands also contribute to epidermal homeostasis in the absence of wounds is an interesting possibility that should be explored.
Although not all eccrine glands are functional insofar as sweat production in humans,12,33,34 our data indicate that each sweat gland contributes to reepithelialization during wound repair. This observation has important implications in that, as opposed to a single large wound, a large wounded area should be considered as a multitude of small evenly distributed repair zones that ultimately merge with each other. The maximum distance that needs to be covered by keratinocytes is thus half the distance that separates 2 eccrine sweat glands, not half the wound diameter. This distance is variable according to body site and sweat gland density and is the shortest on palmoplantar skin, which is devoid of pilosebaceous units but has the highest density of eccrine sweat glands in the human body.10,13,24
Our data suggest that human eccrine sweat glands constitute an important reservoir of cells of high proliferative potential that can be quickly recruited on wounding. Pulse-chase BrdU experiments on human skin xenografts grown on mice revealed the presence of slow cycling cells in the coiled portion of human eccrine glands.35 In this system, label-retaining cells were distributed in a scattered pattern similar to that of stem cells in the human and mouse mammary glands.35 Recently, 4 different populations of progenitor cells have been characterized in the eccrine glands of the mouse paw.28 However, mouse paw eccrine glands remained surprisingly quiescent during repair after epidermal injury, in sharp contrast with their human counterparts, as reported by Lobitz et al23,29 and in the present report. Rather, the distinct mouse paw eccrine gland progenitor populations were elegantly found to contribute to repairing their specific compartments (epidermal, myoepithelial, and lumenal) after biochemical injury (genetically driven diphtheria toxin receptor–induced cell death).28 Unfortunately, no reliable stem cell marker has been identified in human eccrine sweat glands, whether from the body or from palmoplantar skin. In light of the recent work in mice,28 it is even plausible that there are also several distinct stem cell populations in human eccrine sweat glands. Localization, characterization, and identification of cues that trigger differentiation of eccrine sweat gland–derived cells to become a stratified epithelium will necessitate substantial additional research. Progress toward such research has exciting potential for developing targeted therapies for treating human wounds and other skin disorders such as epidermal atrophy or blistering diseases, for improving suturing and grafting techniques, and for isolating or using skin cells for therapy.
Acknowledgments
We thank Suzan Rehbine for volunteer recruitment and skin sample procurement, Dr. M. Bishr Omary for providing KRT8 and KRT18 antibodies, Laura Van Goor and Christopher Burke for helpful artwork suggestions, and Dr. Andrzej “Anj” Dlugosz for constructive comments about the manuscript.
Footnotes
The Microscopy and Image-Analysis Laboratory is a multi-user imaging facility supported by NIH-NCI, the O’Brien Renal Center, the University of Michigan Medical School, the Endowment for the Basic Sciences, the Department of Cell and Developmental Biology, and the University of Michigan. This work was funded in part by a Dermatology Foundation Research Grant (L.R.), the University of Michigan Dermatology Department Laser Research Fund, and NIH/NIAMS grant K01-AR059678 (L.R.).
Supplemental Data
Reconstruction strategy and aspect of epithelial appendages on transverse sections. A: Partial-thickness wounds (5-mm side) were performed using a CO2 laser, and 4-mm diameter full-thickness skin samples were obtained from the center of treated areas at 3 to 7 days after wounding. B: Between 96 and 135 consecutive sections (7 or 10 μm thick) were obtained from skin samples by sectioning according to a plan parallel (as shown) or perpendicular (not shown) to the skin surface (or dermal-epidermal junction). C: Consecutive skin sections were immunostained and imaged. Images were assembled and aligned, and an outline of epithelial structures was drawn on each section using Reconstruct 1.1. D: Example of pan-keratin staining of transverse sections, highlighting all epithelial structures including pilosebaceous units (blue arrows), eccrine sweat glands (magenta arrows), and new epidermis (absent on these sections). E–H: Aspect of reconstructed eccrine sweat gland (E and G) and pilosebaceous unit (F and H) before (E and F) and after (G and H) rendering using Blender 2.62. Arbitrary colors are magenta for eccrine sweat glands, blue for pilosebaceous units, and yellow for new epidermis.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 3 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2B. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 4 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2C. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 5 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2D. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 6 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2E. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 6 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2F. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 5 after wounding. Close-up of an outgrowth merging area at 5 days after wounding, corresponding to Figure 4C. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 4 after wounding. A: Palm biopsy sample encompassing the wound edge, corresponding to Figure 5. B: Close-up of 3 isolated eccrine glands located in the wounded area, as in Figure 5. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; lighter yellow, unwounded epidermis; gray, biopsy sample.
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2012.09.019.
References
- 1.Sen C.K., Gordillo G.M., Roy S., Kirsner R., Lambert L., Hunt T.K., Gottrup F., Gurtner G.C., Longaker M.T. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito M., Liu Y., Yang Z., Nguyen J., Liang F., Morris R.J., Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 3.Singer A.J., Clark R.A. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 4.Taylor G., Lehrer M.S., Jensen P.J., Sun T.T., Lavker R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 5.Levy V., Lindon C., Zheng Y., Harfe B.D., Morgan B.A. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 6.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 7.Miller S.J., Burke E.M., Rader M.D., Coulombe P.A., Lavker R.M. Re-epithelialization of porcine skin by the sweat apparatus. J Invest Dermatol. 1998;110:13–19. doi: 10.1046/j.1523-1747.1998.00087.x. [DOI] [PubMed] [Google Scholar]
- 8.Montagna W., Yun J.S. The skin of the domestic pig. J Invest Dermatol. 1964;42:11–21. [PubMed] [Google Scholar]
- 9.Montagna W. Some particularities of human skin and the skin of nonhuman primates. G Ital Dermatol Venereol. 1984;119:1–4. [PubMed] [Google Scholar]
- 10.Marples M.J. Charles C Thomas Publisher Ltd.; Springfield, IL: 1965. The Ecology of the Human Skin. [Google Scholar]
- 11.Montagna W. Academic Press; New York: 1956. The Structure and Function of Skin. [Google Scholar]
- 12.Dobson R.L., Formisano V., Lobitz W.C., Brophy D. Some histochemical observations on the human eccrine sweat glands. III: the effect of profuse sweating. J Invest Dermatol. 1958;31:147–158. discussion 158–159. [PubMed] [Google Scholar]
- 13.Szabo G. The regional anatomy of the human integument with special reference to the distribution of hair follicles, sweat glands and melanocytes. Phil Trans R Soc Lond B. 1967;252:447–485. [Google Scholar]
- 14.Hettiaratchy S., Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ. 2004;328:1427–1429. doi: 10.1136/bmj.328.7453.1427. [published correction appears in BMJ 2004, 329:148] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doughty D., Ramundo J., Bonham P., Beitz J., Erwin-Toth P., Anderson R., Rolstad B.S. Issues and challenges in staging of pressure ulcers. J Wound Ostomy Continence Nurs. 2006;33:125–130. doi: 10.1097/00152192-200603000-00004. quiz 131–132. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair R.J., Sinclair P.J. Carbon Dioxide laser in the treatment of cutaneous disorders. Australas J Dermatol. 1991;32:165–171. doi: 10.1111/j.1440-0960.1991.tb01784.x. [DOI] [PubMed] [Google Scholar]
- 17.Orringer J.S., Kang S., Johnson T.M., Karimipour D.J., Hamilton T., Hammerberg C., Voorhees J.J., Fisher G.J. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch Dermatol. 2004;140:1326–1332. doi: 10.1001/archderm.140.11.1326. [DOI] [PubMed] [Google Scholar]
- 18.Rittié L., Perbal B., Castellot J.J., Jr., Orringer J.S., Voorhees J.J., Fisher G.J. Spatial-temporal modulation of CCN proteins during wound healing in human skin in vivo. J Cell Commun Signal. 2011;5:69–80. doi: 10.1007/s12079-010-0114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rittié L., Stoll S.W., Kang S., Voorhees J.J., Fisher G.J. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738–751. doi: 10.1111/j.1474-9726.2009.00526.x. [DOI] [PubMed] [Google Scholar]
- 20.Ku N.O., Liao J., Omary M.B. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J. 1998;17:1892–1906. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiala J.C. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218(Pt 1):52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 22.Scholzen T., Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Lobitz W.C., Jr., Holyoke J.B., Montagna W. Responses of the human eccrine sweat duct to controlled injury: growth center of the epidermal sweat duct unit. J Invest Dermatol. 1954;23:329–344. doi: 10.1038/jid.1954.116. [DOI] [PubMed] [Google Scholar]
- 24.Hwang K., Baik S.H. Distribution of hairs and sweat glands on the bodies of Korean adults: a morphometric study. Acta Anat (Basel) 1997;158:112–120. doi: 10.1159/000147920. [DOI] [PubMed] [Google Scholar]
- 25.Folk G.E., Jr., Semken H.A., Jr. The evolution of sweat glands. Int J Biometeorol. 1991;35:180–186. doi: 10.1007/BF01049065. [DOI] [PubMed] [Google Scholar]
- 26.Montagna W. The skin of nonhuman primates. Am Zool. 1972;12:109–124. [Google Scholar]
- 27.Weller R., Pattullo S., Smith L., Golden M., Ormerod A., Benjamin N. Nitric oxide is generated on the skin surface by reduction of sweat nitrate. J Invest Dermatol. 1996;107:327–331. doi: 10.1111/1523-1747.ep12363167. [DOI] [PubMed] [Google Scholar]
- 28.Lu C.P., Polak L., Rocha A.S., Pasolli H.A., Chen S.C., Sharma N., Blanpain C., Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobitz W.C., Jr., Holyoke J.B., Brophy D. Response of the human eccrine sweat duct to dermal injury. J Invest Dermatol. 1956;26:247–259. doi: 10.1038/jid.1956.37. discussion 259–262. [DOI] [PubMed] [Google Scholar]
- 30.Thompson N. A clinical and histological investigation into the fate of epithelial elements buried following the grafting of “shaved” skin surfaces based on a study of the healing of split-skin graft donor sites in man. Br J Plast Surg. 1960;13:219–242. doi: 10.1016/s0007-1226(60)80040-9. [DOI] [PubMed] [Google Scholar]
- 31.Hartwell S.W. Sr: Surgical wounds in human beings: a histologic study of healing with practical applications. I: epithelial healing. Arch Surg. 1929;19:835–847. [Google Scholar]
- 32.Biedermann T., Pontiggia L., Böttcher-Haberzeth S., Tharakan S., Braziulis E., Schiestl C., Meuli M., Reichmann E. Human eccrine sweat gland cells can reconstitute a stratified epidermis. J Invest Dermatol. 2010;130:1996–2009. doi: 10.1038/jid.2010.83. [DOI] [PubMed] [Google Scholar]
- 33.Montagna W. Pergamon Press; New York, NY: 1962. Histological, histochemical, and pharmacological properties. Advances in biology of skin, vol 3. Eccrine sweat glands and eccrine sweating. pp. 6–29. [Google Scholar]
- 34.Sato K., Dobson R.L. Regional and individual variations in the function of the human eccrine sweat gland. J Invest Dermatol. 1970;54:443–449. doi: 10.1111/1523-1747.ep12259272. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura M., Tokura Y. The localization of label-retaining cells in eccrine glands. J Invest Dermatol. 2009;129:2077–2078. doi: 10.1038/jid.2008.443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reconstruction strategy and aspect of epithelial appendages on transverse sections. A: Partial-thickness wounds (5-mm side) were performed using a CO2 laser, and 4-mm diameter full-thickness skin samples were obtained from the center of treated areas at 3 to 7 days after wounding. B: Between 96 and 135 consecutive sections (7 or 10 μm thick) were obtained from skin samples by sectioning according to a plan parallel (as shown) or perpendicular (not shown) to the skin surface (or dermal-epidermal junction). C: Consecutive skin sections were immunostained and imaged. Images were assembled and aligned, and an outline of epithelial structures was drawn on each section using Reconstruct 1.1. D: Example of pan-keratin staining of transverse sections, highlighting all epithelial structures including pilosebaceous units (blue arrows), eccrine sweat glands (magenta arrows), and new epidermis (absent on these sections). E–H: Aspect of reconstructed eccrine sweat gland (E and G) and pilosebaceous unit (F and H) before (E and F) and after (G and H) rendering using Blender 2.62. Arbitrary colors are magenta for eccrine sweat glands, blue for pilosebaceous units, and yellow for new epidermis.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 3 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2B. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 4 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2C. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 5 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2D. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 6 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2E. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 6 after wounding. Skin sample, obtained from the center of the wound, corresponds to Figure 2F. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 5 after wounding. Close-up of an outgrowth merging area at 5 days after wounding, corresponding to Figure 4C. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; gray, biopsy sample.
Revolving 3D animation of wounded skin reconstruction. Skin sample obtained at day 4 after wounding. A: Palm biopsy sample encompassing the wound edge, corresponding to Figure 5. B: Close-up of 3 isolated eccrine glands located in the wounded area, as in Figure 5. Color key: blue, pilosebaceous units; magenta, eccrine sweat glands; yellow, new epidermis; lighter yellow, unwounded epidermis; gray, biopsy sample.