Abstract
Pericytes have been identified as the major source of precursors of scar-producing myofibroblasts during kidney fibrosis. The underlying mechanisms triggering pericyte-myofibroblast transition are poorly understood. Transforming growth factor β-1 (TGF-β1) is well recognized as a pluripotent cytokine that drives organ fibrosis. We investigated the role of TGF-β1 in inducing profibrotic signaling from epithelial cells to activate pericyte-myofibroblast transition. Increased expression of TGF-β1 was detected predominantly in injured epithelium after unilateral ureteral obstruction, whereas downstream signaling from the TGF-β1 receptor increased in both injured epithelium and pericytes. In mice with ureteral obstruction that were treated with the pan anti–TGF-β antibody (1D11) or TGF-β receptor type I inhibitor (SB431542), kidney pericyte-myofibroblast transition was blunted. The consequence was marked attenuation of fibrosis. In addition, epithelial cell cycle G2/M arrest and production of profibrotic cytokines were both attenuated. Although TGF-β1 alone did not trigger pericyte proliferation in vitro, it robustly induced α smooth muscle actin (α-SMA). In cultured kidney epithelial cells, TGF-β1 stimulated G2/M arrest and production of profibrotic cytokines that had the capacity to stimulate proliferation and transition of pericytes to myofibroblasts. In conclusion, this study identified a novel link between injured epithelium and pericyte-myofibroblast transition through TGF-β1 during kidney fibrosis.
Pericytes are mesenchyme-derived perivascular cells attached to the abluminal surface of capillaries.1 They share developmental origins with fibroblasts, and there may be plasticity between pericytes attached to capillaries and fibroblasts embedded in adjacent collagenous matrix; however, unlike fibroblasts, pericytes have vital functions in regulating microvascular stability, angiogenesis, capillary permeability, capillary flow, and capillary basement membrane synthesis.1 We have previously shown that pericytes are the major sources of scar-producing myofibroblasts during kidney injury, and we have identified adult kidney pericytes and perivascular fibroblasts are derived from Foxd1-expressing progenitors, positive for collagen I(α1)-GFP (Coll-GFP+), platelet-derived growth factor receptor β (PDGFR-β+), and CD73 (CD73+) and negative for α smooth muscle actin (α-SMA−) and CD45 (CD45−).2–4 Recently, spinal cord pericytes were identified as major progenitors of scar tissue in the central nervous system, intestinal pericytes as a source of myofibroblasts in models of colitis, and hepatic stellate cells, the major precursor of myofibroblasts in liver disease, have been determined to be specialized pericytes of the hepatic sinusoid,5–8 indicating that pericytes may represent myofibroblast precursors in many organs. Many independent studies support the notion of perivascular resident mesenchymal cells, not injured tubular epithelial cells, as the major source of myofibroblasts in kidneys.9–12
Prompted by the newly identified role for these perivascular cells in the pathogenesis of kidney fibrosis, we earlier investigated the cellular crosstalk that regulates pericyte detachment from capillaries and regulates the transition of pericytes to myofibroblasts.13–15 Our investigations so far have focused on pericyte-endothelial crosstalk, because pericytes form direct communications with endothelial cells of peritubular capillaries at peg and socket junctions, where direct cell-cell signaling has been thought to occur.13–20 We have recently shown that Coll-GFP+ kidney pericytes function identically to brain pericytes in migrating to and stabilizing capillary networks, functions that require expression of tissue inhibitor of metalloproteinase 3 (TIMP-3).15 These pericyte functions are lost when Coll-GFP+ pericytes transition to myofibroblasts.15 Furthermore, we reported that endothelial activation at vascular endothelial cell growth factor (VEGF) receptor 2 and PDGFR-β signaling by pericytes are two critical signaling pathways that link endothelial activation with pericyte transition to myofibroblasts.14 Our studies showed that these signaling events alone are sufficient to drive microvascular rarefaction, inflammation, and fibrosis in models of kidney disease.14 These findings are striking, because during embryonic and fetal microvascular development these same signaling pathways are critical in normal formation of the vasculature, indicating that dysregulation of signaling pathways between endothelium and pericytes is central to kidney pathogenesis.
Nonetheless, studies unequivocally show that the injured tubular epithelium can directly trigger interstitial fibrosis. For example, overexpression of VEGF-A in adult kidney epithelium is sufficient to drive fibrosis, and cell cycle arrest of the kidney proximal epithelium at the G2/M checkpoint is also sufficient to drive fibrosis.21,22 Therefore, epithelial signaling events must somehow be transmitted across the tubular basement membrane to pericytes to drive interstitial fibrosis. These obscure molecular signaling events are the focus of the studies we report here.
In previous investigations of embryonic microvascular development, endothelial cells have been shown to be a source of both PDGF and transforming growth factor β-1 (TGF-β1), cytokines that regulate pericyte attachment, differentiation, and angiogenesis.17,23,24 Moreover, genetic inactivation of either TGFB1 or of genes encoding its receptors in mice leads to vascular defects and embryonic lethality.17–19 TGF-β1 is thus a cytokine with a profound effect on microvascular development and angiogenesis.
In adult kidney injury, although endothelial cells produce PDGF and TGF-β1 in fibrosing kidneys, injured epithelial cells are a major source of these cytokines, and the TGF-β1 activator integrin αvβ6 is restricted to kidney epithelium.13,25–29 Increased TGF-β1 expression by epithelium is accompanied by activation of intracellular signaling pathways and downstream effectors in the epithelium itself.30,31 Blocking TGF-β1 and its downstream effectors can attenuate kidney injury and fibrosis,30–33 whereas transgenic overexpression of TGF-β1 in kidney epithelial cells is sufficient to trigger interstitial kidney fibrosis in the absence of migration of epithelial-derived cells into the interstitium.34,35 Therefore, epithelial transgenic overexpression of TGF-β1, which stimulates epithelial cell dedifferentiation and autophagy, must stimulate pericyte to myofibroblast transition by epithelial cell to pericyte crosstalk.34 Our aim in the present study was to identify the mechanism by which TGF-β1 signaling from injured tubular epithelial cells can activate pericytes to drive progressive kidney fibrosis.
Materials and Methods
Coll-GFP Mice
Coll-GFP transgenic mice were generated on the C57BL6 background as described previously.2 In brief, 3.2 kb of the collagen I(α1) (Col1a1) promoter and enhancer with the open reading frame of enhanced GFP yielded the highest levels of GFP expression when COL1A1 gene transcripts were generated.
Mouse Models of Kidney Fibrosis
Unilateral ureteral obstruction (UUO) was performed in adult (8 to 12 weeks) C57BL6 wild-type or Coll-GFP mice as described previously.2 Briefly, the left ureter was ligated twice using 4-0 nylon surgical sutures at the level of the lower pole of kidney. All animal studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the National Taiwan University College of Medicine.
Culture of Kidney Pericytes
Purification of kidney pericytes from normal kidney was performed as described previously.13 Kidney was diced, incubated at 37°C for 1 hour with Liberase (0.5 mg/mL; Roche Applied Science, Indianapolis, IN) and DNase (100 U/mL; Roche Applied Science) in Hank’s balanced salt solution. After centrifugation, cells were resuspended in 5 mL of PBS/1% bovine serum albumin, and filtered (40-μm mesh). Pericytes were purified by isolating GFP+PDGFR-α+ cells using a fluorescence-activated cell sorting (FACS) system (FACSAria; BD Biosciences, San Jose, CA), and then total RNA was isolated or purified cells were cultured in Dulbecco’s modified Eagle’s medium with 20% fetal bovine serum. The primary cultured cells used in the present study were between passages 4 and 8 and have been characterized previously.13
Purification and Culture of PTECs
Purification of proximal tubular epithelial cells (PTECs) from normal and day-7 UUO kidneys was performed as described previously.36 Kidney was diced, incubated at 37°C for 1 hour with collagenase (0.5 mg/mL; Worthington Biochemical, Lakewood, NJ) and soybean trypsin inhibitor (0.5 mg/mL; Gibco; Life Technologies, Carlsbad, CA) in Dulbecco’s modified Eagle’s medium/F12 basal medium. After centrifugation, cells were resuspended in 5 mL of PBS/1% bovine serum albumin, and filtered (40-μm mesh). Cells were labeled with Lotus tetragonolobus lectin (LTL)-fluorescein isothiocyanate (Vector Laboratories, Burlingame, CA), anti–CD45-PE, and anti–Kim1-biotin antibodies (RMT1-4) (1:200; eBioscience, San Diego, CA), followed by streptavidin-allophycocyanin (Jackson ImmunoResearch Laboratories, West Grove, PA). Normal and injured PTECs were sorted by FACSAria cell sorting (BD Biosciences) for LTL+CD45− cells and Kim1+CD45− cells, and then total RNA was purified using an RNeasy system (Qiagen, Valencia, CA). Day-7 UUO kidney proximal tubular cells were cultured in Dulbecco’s modified Eagle’s medium/F12 with 1× insulin-transferrin-selenium and hydrocortisone (0.5 μmol/L; Sigma-Aldrich, St. Louis, MO) using established methods that maintained tubular epithelial characteristics.37 The primary cultured tubular epithelial cells used in the present study were between passages 4 and 8. In some experiments, after 48-hour treatment of PTECs with TGF-β1 (5 ng/mL), the drug was washed out and the cells continued in culture for 24 hours. The conditioned medium was then collected and added to serum-starved kidney pericytes. Control antibody 13C4, anti–TGF-β antibody (1D11; Genzyme, Framingham, MA), and anti–PDGFR-β antibody (100 μg/mL) were added in the pericyte culture with conditioned medium. Cell cycle, cell number, and gene expression of kidney pericytes were analyzed after 24 hours.
Blocking TGF-β1 Signaling in Vivo
Mice were injected intraperitoneally with 13C4, 1D11 (5 mg/kg/every other day), or the transforming growth factor β receptor I (TGF-βRI) inhibitor SB431542 (5 mg/kg per day; Tocris Bioscience, Bristol, UK) 2 hours before surgery, and then as scheduled until sacrifice on day 4 or day 10 (n = 6 per group).
Blocking TGF-β1 Signaling in Vitro
Normal kidney pericytes were incubated with TGF-β1 (10 ng/mL; R&D Systems, Minneapolis, MN) in the presence of antibody 13C4 (100 μg/mL), 1D11 (100 μg/mL), or SB431542 (5 μg/mL). The extent of Smad2 phosphorylation was determined by Western blot analysis. In some experiments, SP600125 (10 μmol/L; Sigma-Aldrich) and SB203580 (10 μmol/L; Sigma-Aldrich) were used to block c-jun NH2-terminal kinase (JNK) and mitogen-activated protein kinase (MAPK) p38, respectively.
Tissue Preparation and Histology
Mouse tissues were prepared and stained as described previously.2 Primary antibodies against the following proteins were used for immunolabeling: α-SMA-Cy3 (clone 1A4; Sigma-Aldrich), laminin α4 (R&D Systems), Ki-67, PDGFB, TGF-βRII (Abcam, Cambridge, UK), p-Smad2, p-histone H3 (Ser10) (Cell Signaling Technology, Danvers, MA), proliferating cell nuclear antigen (PCNA) (Thermo Scientific, Fremont, CA), TGF-β1 and Nidogen (Santa Cruz Biotechnology, Santa Cruz, CA), and NG2 (gift from W. Stallcup). Fluorescent conjugated secondary antibody labeling (Jackson ImmunoResearch Laboratories), colabeled with DAPI, mounting with Vectashield medium (Vector Laboratories), and image capture and processing were performed as described previously. Quantification of specific cells in tissue sections was performed as described previously.14 In brief, sections were colabeled with DAPI, and Coll-GFP+ cells were identified by blue and green nuclear colocalization; α-SMA+ cells were identified by greater than 75% of the cell area immediately surrounding nuclei (detected by DAPI) staining positive with Cy3 fluorescence indicative of the antigen expression; Ki-67+, PCNA+, p-Smad2+, or p-histone H3+ cells were identified by positive nuclear staining for Cy3 or fluorescein fluorescence. Specific cells were counted in 10 cortical interstitial fields per mouse; the high-power fields (×400) were randomly selected. Interstitial fibrosis was quantified in Picrosirius Red-stained paraffin sections.
qPCR
cDNA was synthesized using oligo(dT) and random primers. Quantitative PCR (qPCR) was performed using methods described previously.2 The specific primer pairs used in qPCR are listed in Table 1.
Table 1.
Primer Sequences Used in qPCR
| Target | Primer | Sequence |
|---|---|---|
| PDGFB | Forward | 5′-CCCACAGTGGCTTTTCATTT-3′ |
| Reverse | 5′-GTGAACGTAGGGGAAGTGGA-3′ | |
| TGF-β1 | Forward | 5′-GGACTCTCCACCTGCAAGAC-3′ |
| Reverse | 5′-GACTGGCGAGCCTTAGTTTG-3′ | |
| Colla1 | Forward | 5′-GAGCGGAGAGTACTGGATCG-3′ |
| Reverse | 5′-GTTCGGGCTGATGTACCAGT-3′ | |
| α-SMA | Forward | 5′-CTGACAGAGGCACCACTGAA-3′ |
| Reverse | 5′-CATCTCCAGAGTCCAGCACA-3′ | |
| GAPDH | Forward | 5′-CTGGAGAAACCTGCCAAGTA-3′ |
| Reverse | 5′-AAGAGTGGGAGTTGCTGTTG-3′ |
Western Blot Analysis
Total cellular protein extracted using radioimmunoprecipitation assay buffer was subjected to Western blot analysis using methods described previously.38 The following primary antibodies were used to detect the specific protein: p-Smad2 (Ser465/467), p-JNK (Thr183/Tyr185), p-p38 MAPK (Thr180/Tyr182), phosphorylated extracellular signal-regulated kinases (p-ERK) (Thr024/Tyr206), Smad2 (Cell Signaling Technology), α-SMA (Abcam), p21, p27, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology), and GFP (Medical & Biological Laboratories, Nagoya, Japan).
FACS Analysis
PDGFR-α was exclusively expressed in interstitial Coll-GFP+ cells.13 To analyze α-SMA expression in kidney Coll-GFP+PDGFR-α+ cells, single cells were fixed in 4% paraformaldehyde/PBS for 10 minutes with shaking at 4°C, then permeabilized in 0.1% saponin/PBS for 10 minutes. After a washing, the single cells were incubated with antibodies against PDGFR-α (1:200; eBioscience), α-SMA, or isotype control (1:20; R&D Systems) for 30 minutes. Cells were then analyzed using a flow cytometer. To determine cell cycle progression, cells were first fixed with cold methanol and then stained with propidium iodide (50 μg/mL; Sigma-Aldrich) in RNase A (5 mg/mL; Invitrogen; Life Technologies, Carlsbad, CA). Analysis of DNA content was performed as described previously.39
Transfection
For transient silencing of p21, PTECs were transfected using Lipofectamine transfection reagent (Invitrogen; Life Technologies) according to the manufacturer’s protocols. siRNA sequences are listed in Table 2. ON-TARGETplus SMARTpool siRNA sequences against p21 and ON-TARGETplus nontargeting pool (Thermo Scientific) were incubated overnight at a final concentration of 50 nmol/L, and cells were then treated with TGF-β1 (5 ng/mL). Cell cycle and protein expression were analyzed after 24 hours.
Table 2.
siRNA Sequences
| ON-TARGETplus SMARTpool L-058636-00-0005, Mouse p21 | Target sequence |
|---|---|
| J-058636-05 | 5′-CGAGAACGGUGGAACUUUG-3′ |
| J-058636-06 | 5′-CAGACCAGCCUGACAGAUU-3′ |
| J-058636-07 | 5′-GAACAUCUCAGGGCCGAAA-3′ |
| J-058636-08 | 5′-GGAGCAAAGUGUGCCGUUG-3′ |
ON-TARGETplus D-001810-10-05 served as the nontargeting control pool.
Statistical Analysis
Data are expressed as means ± SEM. Statistical analyses were performed using GraphPad Prism software version 4.0 (GraphPad Software, La Jolla, CA). The statistical significance was evaluated by one-way analysis of variance.
Results
Pericyte-Myofibroblast Transition during Progressive Kidney Fibrosis
To study the response of kidney pericytes to injury, we performed UUO in Coll-GFP reporter mice. Confocal microscopy of normal kidney cortex showed the direct contact of endothelium and pericyte bodies, and showed pericyte processes passing through the capillary basement membrane (Supplemental Figure S1). In addition to its detection in pericytes, Coll-GFP was also detected in perivascular fibroblasts and glomerular podocytes of the normal kidney (Supplemental Figure S2A). Fibroblasts are spindle-shaped cells of mesenchymal origin surrounded by collagen matrix. Pericytes in the kidney were defined anatomically as extensively branched cells of mesenchymal origin that partially surrounded the endothelium of capillaries (Supplemental Figures S1 and S2A). The branched processes of the pericytes are sheathed within the capillary basement membrane, and the capillary basement membrane is often broken or incomplete between the endothelial cell and pericyte, allowing close appositions or interdigitations to occur.13,40–44 On the other hand, despite having a similar origin as that of kidney pericytes, perivascular fibroblasts surrounded arterioles within a collagenous matrix and had no close appositions with endothelial cells (Supplemental Figure S2A). After UUO injury, pericytes lost the intimate connection with endothelium and their cell population increased (Supplemental Figure S2B). α-SMA was not detected in normal kidney pericytes, but its expression markedly increased in Coll-GFP+ pericytes, indicating their transition to myofibroblasts after UUO surgery (Figure 1C). NG2 proteoglycan has been reported to be a marker of pericytes in the eye and brain, but reports also indicate that NG2 is expressed only by active pericytes.45 Our previous study showed that Coll-GFP+PDGFR-β+ pericytes express NG2 in neonatal kidney, but lose expression with maturity.2 Similar to the increase in α-SMA expression, pericytes reactivated expression of NG2 soon after UUO injury, indicating that myofibroblasts were activated pericytes during progressive kidney fibrosis (Supplemental Figure S3).
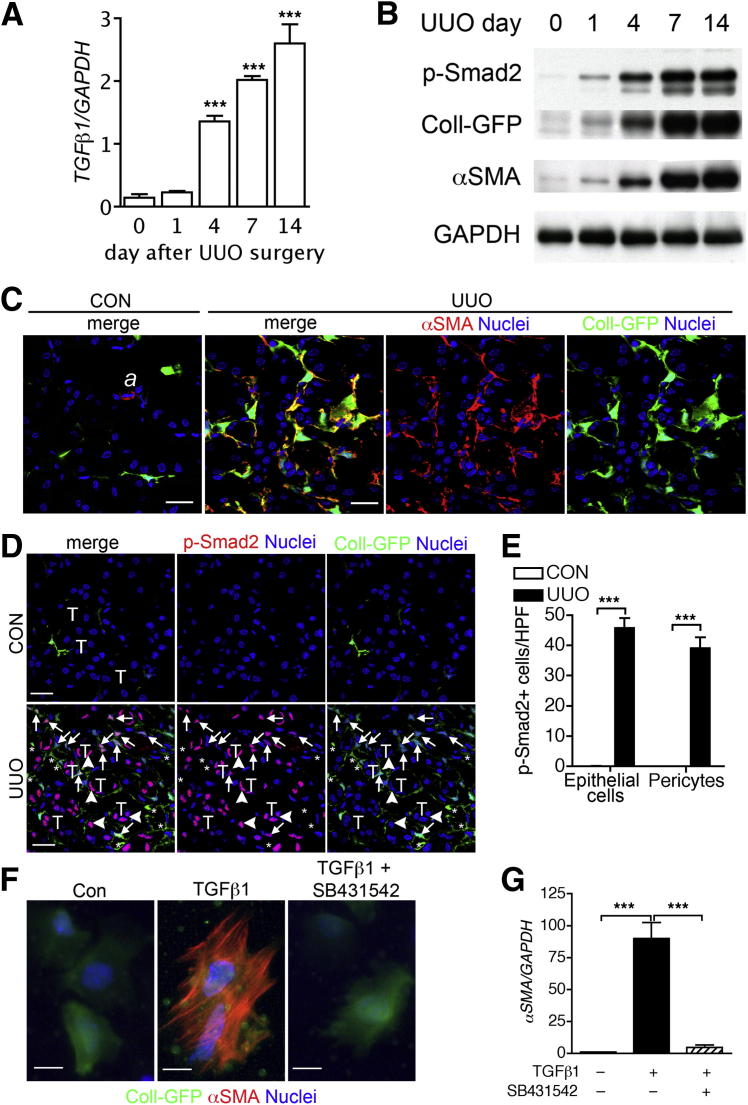
Figure 1.
Activation of TGF-β1 signaling during obstructive kidney fibrosis. A: qPCR time course of whole-kidney TGFB1 gene transcript after UUO surgery. Expression levels were normalized by GAPDH. B: Western blot of whole kidney after UUO surgery for p-Smad2, Coll-GFP, α-SMA, and GAPDH in Coll-GFP transgenic mice. C: Confocal micrographs show Coll-GFP+ pericytes in normal control kidney (CON) and Coll-GFP+ myofibroblasts with α-SMA expression. In control kidney, α-SMA is expressed only in arterial vascular smooth muscle cells (a). D: Confocal micrographs show p-Smad2 expression in both tubular epithelial cells (arrowheads) and Coll-GFP+ cells (arrows) of day-4 UUO kidney, but not in control kidney. Tubular epithelial cells are indicated by the letter T. E: Quantification of cell numbers with positive nuclear p-Smad2 staining. F: Immunofluorescence micrographs show primary cultured kidney pericytes colabeled with α-SMA. G: qPCR of gene transcripts of α-SMA of primary kidney pericyte culture in the presence and absence of TGF-β1 and SB431542. Blots are representative of three independent experiments with similar results. Data are expressed as means ± SEM. n = 5 per time point (A) or 3 per group (G). ***P < 0.001 versus normal kidney at day 0 (A) or as indicated by brackets (E and G).
TGF-β1 Signaling Responses Are Activated in Tubular Epithelial Cells and Pericytes after UUO
Whole-kidney TGFB1 gene transcripts increased after initiation of UUO injury (Figure 1A). In parallel with increased TGF-β1 expression, we detected increased phosphorylation of the canonical signaling pathway downstream effector protein Smad2 (Figure 1B). The extent of canonical TGF-β1 signaling was mirrored by expression of GFP, which reported COL1A1 gene transcripts and expression of the intermediate filament α-SMA (Figure 1, B and C). α-SMA, a robust marker of myofibroblast differentiation, was expressed in almost all Coll-GFP+ pericytes by 4 days after UUO surgery, whereas in normal kidneys α-SMA expression was restricted to vascular smooth muscle cells of the arterioles and was not expressed by Coll-GFP+ pericytes (Figure 1C). Both epithelial cells and Coll-GFP+ pericytes expressed TGF-βRII in normal kidneys and at day 4 in UUO kidneys (Supplemental Figure S4). Canonical TGF-β1 signaling, detected by nuclear p-Smad2, was seen in both tubular cells and Coll-GFP+ pericytes after UUO surgery, but not in normal adult kidneys (Figure 1, D and E). In addition to Coll-GFP+ pericytes, nuclear p-Smad2 was seen in other interstitial cells (these were probably endothelial cells or leukocytes, which were not the focus of the present study).
TGF-β1 Signaling Induces Pericyte-Myofibroblast Transition in Vivo and in Vitro
To study the role of TGF-β1 signaling in pericyte-myofibroblast transition during UUO injury, we examined the extent of Smad2 phosphorylation in primary kidney pericyte cultures triggered by TGF-β1 in the presence of the pan anti–TGF-β antibody, 1D11, or the TGF-βRI small-molecule inhibitor SB431542. TGF-β1-induced p-Smad2 in pericytes was inhibited by 1D11 antibody or SB431542 (Supplemental Figure S5). In parallel studies, we treated primary pericyte cultures with TGF-β1 alone or in the presence of SB431542 and assessed expression of the myofibroblast marker α-SMA (Figure 1, F and G). In normal culture conditions, primary pericytes weakly expressed α-SMA, but expression was markedly up-regulated by TGF-β1. This up-regulation was almost completely abrogated by the TGF-β1 inhibitor (Figure 1, F and G).
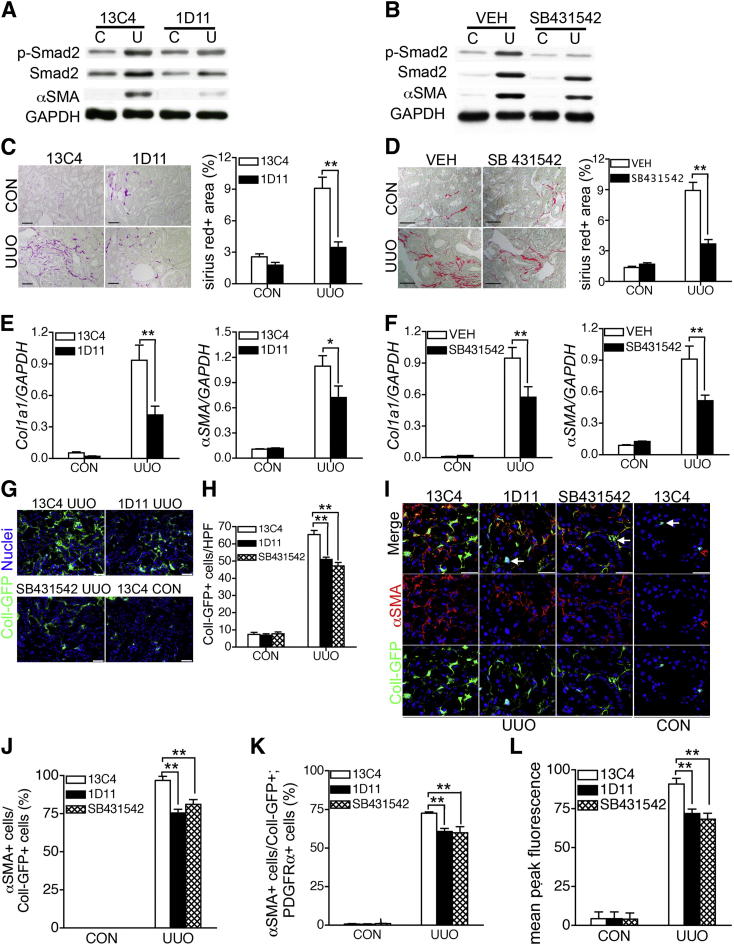
Next, we administered 1D11 antibodies or SB431542 to mice with UUO. We studied the effect of these inhibitors of TGF-β1 signaling on pericyte-myofibroblast transition and its consequences in vivo in the UUO model of kidney injury. Compared with mice treated with the isotype control antibody 13C4 or vehicle, the expected increased levels of p-Smad2 and α-SMA in UUO kidneys were attenuated on day 4 of UUO in mice treated with 1D11 antibody or SB431542 (Figure 2, A and B); by day 10, the extent of interstitial fibrosis and of gene transcripts of Col1a1 and α-SMA (encoded by ACTA1) were all markedly attenuated by TGF-β1 signaling inhibition (Figure 2, C–F). We examined the kidneys of Coll-GFP mice for pericyte expansion and found that 1D11 and SB431542 administration had decreased the expanded population of Coll-GFP+ cells in UUO kidneys by 22% and 28%, respectively (Figure 2, G and H). To determine the effect of TGF-βR blockade on α-SMA expression in Coll-GFP+ cells, regardless of the inhibitory effect on cell number, we assessed the proportion of Coll-GFP+ cells that coexpressed α-SMA at day 4 of UUO by staining or FACS analysis. In the presence of control antibodies more than 96.8% of Coll-GFP+ cells coexpressed α-SMA, whereas in the presence of 1D11 antibodies and SB431542 the proportion of Coll-GFP+ cells coexpressing α-SMA fell to 75.5% and 81.1%, respectively (Figure 2, I and J). Using a combination of Coll-GFP expression and the kidney pericyte marker PDGFR-α (which is not expressed by podocytes) to identify pericytes,13 FACS analysis also identified a significant reduction in the proportion of pericytes that expressed α-SMA and the mean peak fluorescence of α-SMA in the cells that were expressing α-SMA (Figure 2, K and L, and Supplemental Figure S6).
Figure 2.
Blocking TGF-β1 signaling inhibited pericyte-myofibroblast transition. A and B: Blocking TGF-β1 signaling by pan anti–TGF-β antibody 1D11 (5 mg/kg every other day) (A) or type I TGF-β receptor (TGF-βRI) small-molecule inhibitor SB431542 (5 mg/kg every day) (B) inhibited expression of p-Smad2, Smad2, and α-SMA expression in UUO kidneys. 13C4 was administered as isotype control antibody. Lane C, control; Lane U, UUO kidney at day 4. C and D: Picrosirius Red-stained kidney sections for interstitial fibrillar collagens (red) in mice treated with control antibody13C4 or anti–TGF-β antibody 1D11 (C) or treated with vehicle (VEH) or SB431542 (D) for 10 days after UUO surgery, with morphometric quantification of fibrillar collagen from whole sagittal kidney sections. E and F: qPCR analysis showed that increased expression of collagen I(α1) (Col1α1) and transcripts of α-SMA in UUO kidney were inhibited by either 1D11 antibody (E) or SB431542 (F). G and H: Immunofluorescence detection of Coll-GFP+ cells in control and day-4 UUO kidneys treated with 13C4, 1D11, and SB431542 and in control kidney (G), with quantification of Coll-GFP+ cells (H). I and J: Confocal micrographs show Coll-GFP+ cells colabeled with myofibroblast marker α-SMA (Coll-GFP+α-SMA–cells are indicated by arrows, I), with quantification of the percentage of Coll-GFP+ cells with α-SMA expression (J). K and L: Fluorescence-activated cell sorting quantified the percentage of α-SMA+ cells in Coll-GFP+PDGFR-α+ cells (K) and the mean peak fluorescence of α-SMA in Coll-GFP+PDGFR-α+ cells (L) of control and UUO kidneys from mice treated with 13C4, 1D11, or SB431542. Blots (A and B) are representative of six mice per group. Data are expressed as means ± SEM. n = 6 per group (C-F, H, J); n = 3 per group (K, L). *P < 0.05, **P < 0.01. Scale bars: 25 μm (C, D, G); 20 μm (I).
These different approaches, measuring either the number of positive (Coll-GFP+ or α-SMA+) cells or the relative expression of these proteins within the positive cells, indeed showed modest inhibition, compared with the inhibition of p-Smad2 and α-SMA in Western blot analyses (Figure 2, A and B), which might be due to incomplete inhibition of TGF-β1 signaling and/or the fact that pericytes might constitutively express low levels of α-SMA even without TGF-β1 signaling.
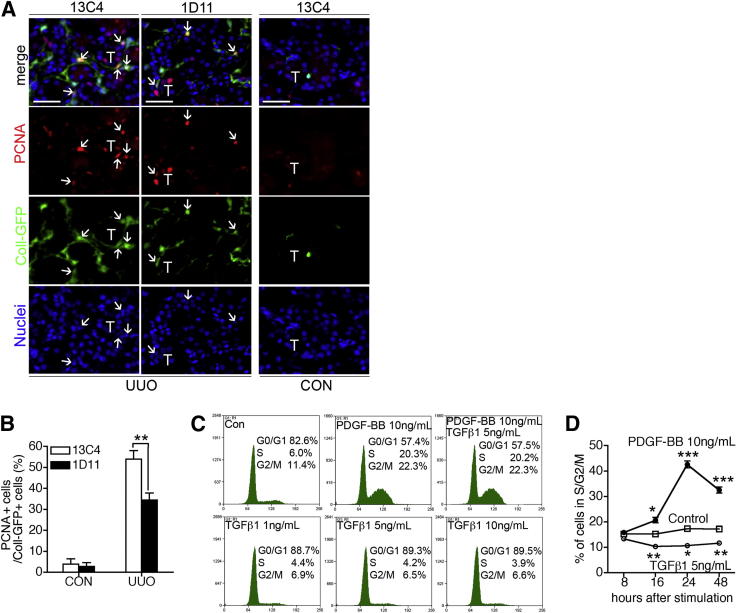
Because we had discovered that TGF-βR blockade reduces the number of Coll-GFP+ cells in the UUO kidney, in addition to reducing α-SMA expression in these cells (Figure 2), we tested whether TGF-β1 inhibition inhibited proliferation of Coll-GFP+ pericytes. In control antibody (13C4)-treated UUO kidneys at day 4, 54% of the Coll-GFP+ cells in kidneys were in cell cycle, as determined by nuclear expression of PCNA (Figure 3, A and B). In 1D11-treated kidneys, the index of proliferating Coll-GFP+ cells was only 34% (Figure 3, A and B). We therefore hypothesized that TGF-β1 would stimulate pericyte proliferation. Primary pericyte cultures were prepared in serum-free medium. DNA content analysis indicated that more than 80% of the cells were in G0/G1 phase (Figure 3, C and D). PDGF-BB stimulates cells into cell cycle (Figure 3, C and D). Surprisingly, under identical conditions, TGF-β1 did not stimulate pericytes into or through cell cycle; in fact, it tended to arrest pericytes further in G0/G1 (Figure 3, C and D). Our studies thus indicate that, although TGF-β1 signaling stimulates pericyte activation and transition to myofibroblasts both in vitro and in vivo, it stimulates pericyte proliferation only in vivo, not in vitro. These findings suggest that TGF-β1 may stimulate pericyte proliferation in vivo by an indirect mechanism.
Figure 3.
TGF-β1 signaling stimulated cell proliferation of Coll-GFP+ pericytes in vivo, but not in vitro. A and B: Blocking TGF-β1 signaling by 1D11 decreased proliferation of Coll-GFP+ myofibroblasts in UUO kidneys. Immunofluorescence micrographs show Coll-GFP+ cells (arrows) colabeled with the cell proliferation marker PCNA in control or day-4 UUO kidneys (A), with quantification of the percentage of PCNA+ cells in all Coll-GFP+ cells (B). Renal tubules are indicated by the letter T. C and D: Platelet-derived growth factor (PDGF-BB), but not TGF-β1, stimulated proliferation of primary cultured kidney pericytes. Cell cycle profiles were determined by flow cytometric analysis in serum-starved cells without (Con) or with TGF-β1, PDGF-BB stimulation for 24 hours (C) or at different time points, from 8 to 48 hours (D). Data are expressed as means ± SEM. n = 6 per group (A, B); n = 3 per group (C, D). *P < 0.05, **P < 0.01, and ***P < 0.001. Scale bar = 25 μm.
TGF-β1 Signaling Induces a Profibrotic Phenotype in Injured Kidney Epithelial Cells
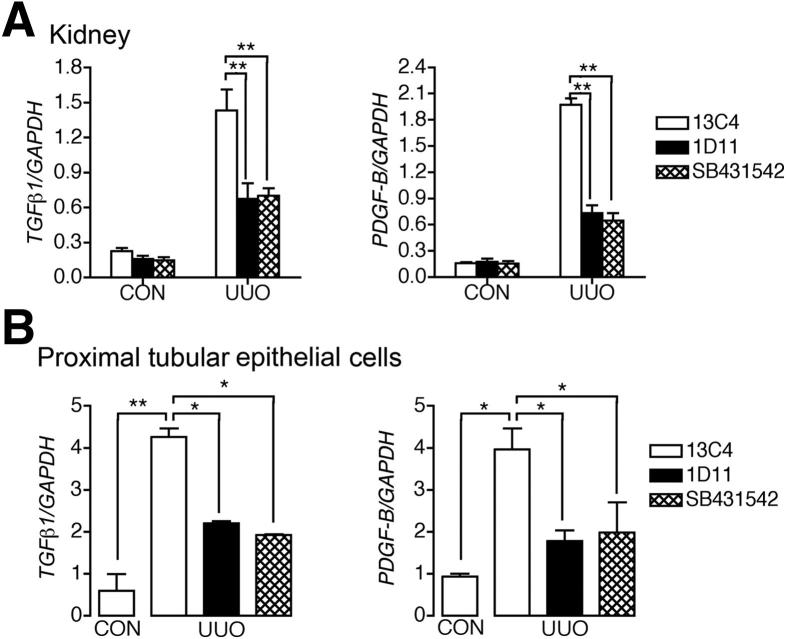
Because TGF-β1 stimulated canonical TGF-β1 signaling in epithelial cells as well as in pericytes (Figure 1), we hypothesized that TGF-β1 signaling in epithelium may be responsible for release of proproliferative factors that could contribute to pericyte proliferation in vivo. We have previously shown that PDGF signaling in pericytes is a major stimulant of pericyte detachment, migration, and transition to myofibroblasts.13 In whole kidney, TGF-βR inhibition markedly down-regulated both TGFB1 and PDGFB gene transcripts (Figure 4A). TGF-β1 and PDGFB proteins were easily identified in the cytoplasm of dilated, injured epithelium of UUO kidney at day 4, as well as in perivascular and interstitial cells (Supplemental Figure S7A). To more accurately determine the expression of PDGFB and TGF-β1 in injured PTECs compared with uninjured PTECs, we purified Kim1-expressing PTECs from day-4 UUO kidney and LTL-expressing PTECs from normal kidney by FACS of single-cell preparations (Figure 4B and Supplemental Figure S7B). Injured UUO PTECs expressed high levels of PDGFB and TGFB1 gene transcripts. Both gene transcripts were down-regulated in kidneys treated with 1D11 or SB431542 (Figure 4B). These findings suggest that TGF-βR ligation by TGF-β1 simulates both TGF-β1 and PDGFB production by epithelial cells in vivo.
Figure 4.
Blocking TGF-β1 signaling inhibits profibrotic phenotype of injured tubular epithelial cells. A: qPCR analysis showed that increased expression of TGFB1 and PDGFB gene transcripts in day-4 UUO kidney was inhibited by either 1D11 antibody or SB431542. B: qPCR analysis of PTECs purified from control and day-4 UUO kidneys using FACS showed that blocking TGF-β1 signaling inhibited the increased transcripts of TGF-β1 and PDGFB in UUO-injured PTECs. Data are expressed as means ± SEM. n = 6 per group (A); n = 3 per group (B). *P < 0.05, **P < 0.01.
TGF-β1 Signaling Blockade Limits G2/M Arrest of Kidney Epithelial Cells
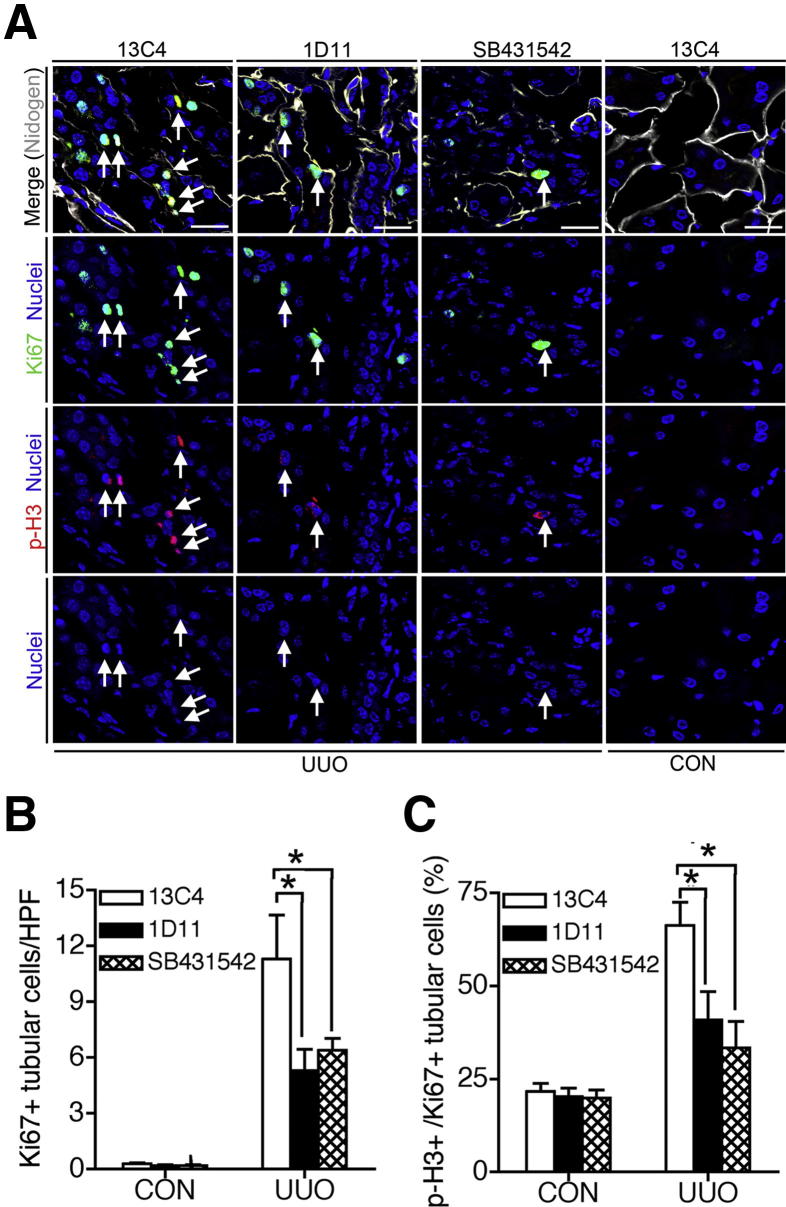
Recent investigations have shown that, during injury, kidney epithelial cells become arrested at the G2/M cell cycle checkpoint. Cell cycle arrest, of itself, endows a profibrotic phenotype on epithelial cells, and factors that drive cells through this cell cycle arrest are beneficial for kidney repair.22,36 We therefore tested whether TGF-β1 signaling in epithelium triggers a profibrotic phenotype by arresting cells in G2/M. UUO of kidneys triggered epithelial cells into cell cycle, detected by Ki-67 protein expression (Figure 5, A and B), but many (66.3%) of these were in G2/M, detected by positive nuclear staining of histone H3 with phosphorylation at Ser10 (p-H3) (Figure 5, A and C). However, in UUO kidneys of mice with blockade of TGF-βR signaling (using 1D11 antibodies or SB431542), the total number of kidney epithelial cells in cell cycle was decreased, and, in addition to those cells in cycle, many fewer were in the G2/M phase. These findings indicate that TGF-β1 may be an important factor in triggering G2/M arrest in kidney epithelium.
Figure 5.
Blocking TGF-β1 signaling prevents G2/M arrest of tubular epithelial cells. A: Confocal micrographs show tubular epithelial cells in cell cycle (staining with pan-cell cycle marker Ki-67-specific antibody) and in G2/M phase [staining with phosphorylation-specific antibody against histone H3 with Ser10 phosphorylation (p-H3)]. The p-H3 staining shows chromatin patterns depending on the cells in respective G2 and M phases of the cell cycle. Basement membrane nidogen staining was used to identify the tubules. Ki-67+p-H3+ tubular epithelial cells are indicated by arrows. B and C: Blocking TGF-β1 signaling by either 1D11 or SB431542 decreased tubular epithelial cells entering cell cycle (B) and the proportion of tubular epithelial cells in G2/M phase (C). Data are expressed as means ± SEM. n = 6 per group. *P < 0.05. Scale bar = 20 μm.
TGF-β1 Provokes Epithelial Cell Cycle G2/M Arrest and Release of Factors That Drive Pericyte to Myofibroblast Differentiation in Vitro
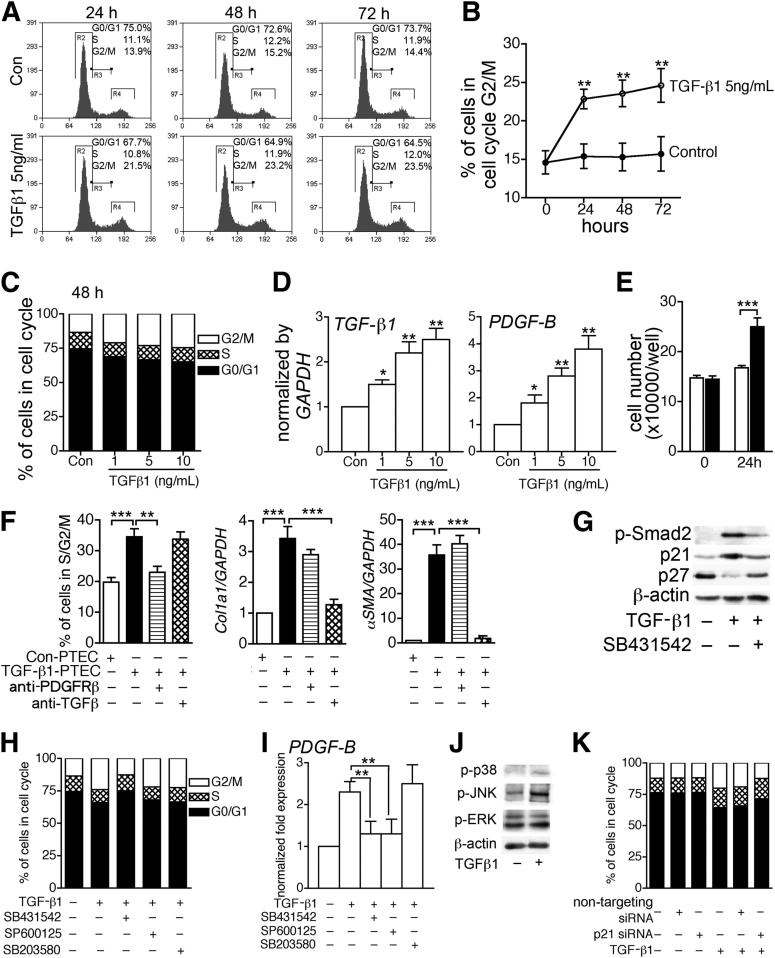
To study the effect of TGF-β1 on the phenotype of epithelial cells further, we generated PTEC cultures (Supplemental Figure S8) and stimulated these unsynchronized cultures with TGF-β1. Over a 72-hour period, TGF-β1 increased the proportion of PTECs in G2/M phase (Figure 6, A–C). TGF-β1–treated PTECs up-regulated expression of profibrotic cytokines, including TGF-β1 and PDGFB (Figure 6D). To test the importance of epithelial factors in the pericyte transition to myofibroblasts, we performed a supernatant transfer experiment by harvesting conditioned medium from TGF-β1–treated PTECs and applying it to primary kidney pericyte cultures. After 24 hours of coincubation, supernatants from TGF-β1–treated PTECs stimulated pericyte proliferation and up-regulated gene transcripts of Col1a1 and α-SMA in pericytes (Figure 6, E and F). Using specific antibody to block PDGFR-β and TGF-βR signaling, we showed that the increased cell proliferation and gene transcripts of Col1a1 and α-SMA in kidney pericytes induced by conditioned medium were PDGFB-dependent and TGF-β1-dependent, respectively (Figure 6F).
Figure 6.
TGF-β1 stimulated profibrotic epithelial signaling to pericytes. A–C: TGF-β1 arrested nonsynchronizing PTECs in cell cycle G2/M phase. D: TGF-β1 induced profibrotic phenotype of PTECs with increased transcripts of TGF-β1 and PDGFB. E: Conditioned medium from TGF-β1–treated PTECs (TGF-β1-PTEC) increased cell number in primary kidney pericyte culture. White bars, Con-PTEC; black bars, TGF-β1-PTEC. F: Conditioned medium from TGF-β1-PTEC increased cell proliferation and transcripts of Col1a1 and α-SMA in primary kidney pericyte cultures, which were blocked by anti–PDGFR-β antibody and anti–TGF-β antibody, respectively. G: TGF-β1 increased Smad2 phosphorylation and p21 expression, but decreased p27, all of which were reversed by the TGF-βRI inhibitor SB431542. H: TGF-βRI inhibitor SB431542, but not pan c-jun NH2-terminal kinase (JNK) inhibitor SP600125 and p38 inhibitor SB203580, reversed cell cycle G2/M arrest of TGF-β1–treated PTECs. I: SB431542 and SP600125, but not SB203580, decreased transcripts of PDGFB in TGF-β1–treated PTECs. J: TGF-β1 induced phosphorylation of p38 (p-p38) and JNK (p-JNK), but not extracellular regulated kinase (p-ERK). K: Silencing p21 reversed cell cycle G2/M arrest of TGF-β1–treated PTECs. The control was nontargeting siRNA. Data are expressed as means ± SEM. Quantification was from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001.
TGF-β1 increased phosphorylation of Smad2 in primary epithelial cultures, and this effect was inhibited by SB431542 (Figure 6G). TGF-βR/Smad2 signaling resulted in increased expression of the cyclin-dependent kinase inhibitor p21 and decreased expression of the cyclin-dependent kinase inhibitor p27 (Figure 6G). Inhibition of TGF-βRI signaling by SB431542 had the capacity to reverse G2/M cell cycle delay and to down-regulate transcripts of the profibrotic cytokines PDGFB and TGF-β1 in TGF-β1–treated PTECs (Figure 6, H and I, and Supplemental Figure S9). Silencing p21 reversed cell cycle G2/M arrest of TGF-β1–treated PTECs (Figure 6K and Supplemental Figure S10), but did not affect transcripts of the profibrotic cytokines PDGFB and TGF-β1.
To explore the mechanism by which TGF-βR signaling activates transcripts of the profibrotic cytokine PDGFB and TGF-β1, we dissected noncanonical downstream signaling events. TGF-β1 stimulated phosphorylation of p38 and JNK, but not ERK (Figure 6J). We next tested whether inhibiting JNK activation or p38 activation with the small-molecule inhibitor SP600125 (for JNK) or SB203580 (for p38) could reverse G2/M cell cycle delay or transcripts of profibrotic cytokines in TGF-β1–treated PTECs (Figure 6, H and I, and Supplemental Figure S9). In contrast to the inhibitory effect of SB431542, neither SP600125 nor SB203580 was capable of inhibiting cell cycle delay (Figure 6H). However, inhibition of the JNK signaling pathway specifically inhibited transcripts of the profibrotic cytokines PDGFB and TGF-β1, whereas inhibition of p38 had no effect (Figure 6I and Supplemental Figure S9).
Discussion
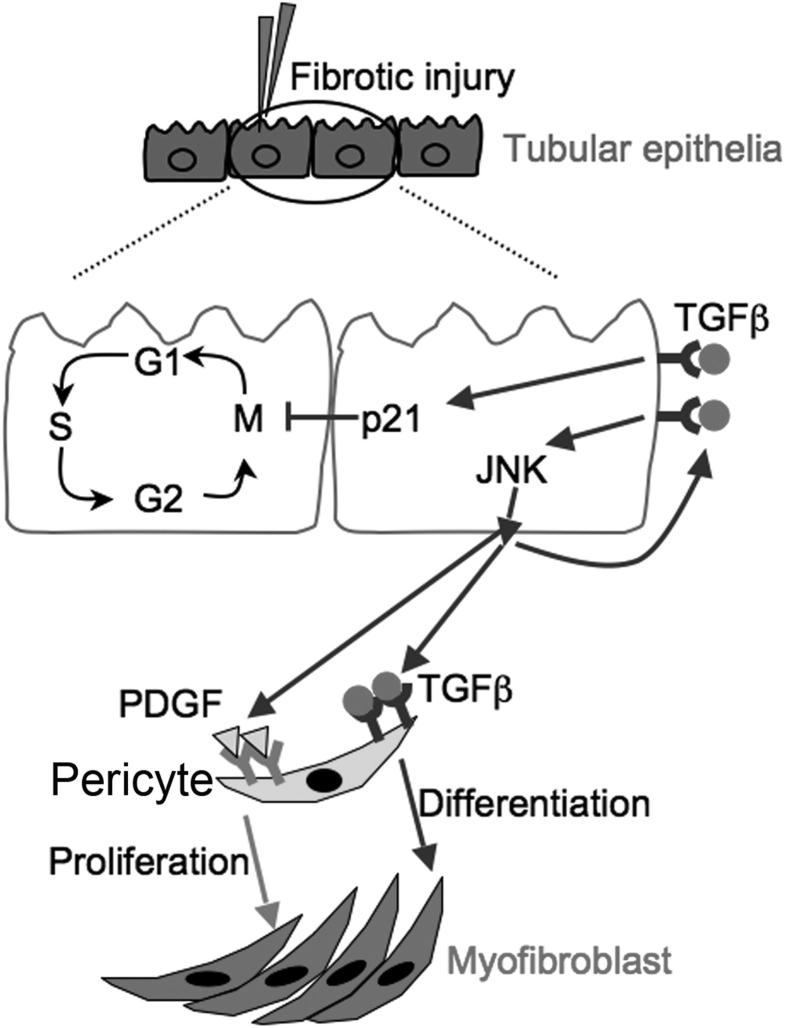
In the present study, we demonstrated that, after UUO injury, TGF-β1 promoted tubular epithelial cell cycle arrest in G2/M and stimulated profibrotic cytokine production through up-regulation of p21 and activation of the JNK pathway, respectively. Injured epithelial cells play a central role in activating pericyte-myofibroblast transition through generation of PDGF and TGF-β1, finally leading to pathological fibrosis (Figure 7).
Figure 7.
Schematic of TGF-β1 stimulated profibrotic epithelial signaling to pericytes during fibrotic kidney injury. Fibrotic injury induced TGF-β1 production of tubular epithelial cells. TGF-β1 then induced G2/M cell cycle arrest and profibrotic phenotype through up-regulation of p21 and activation of the JNK pathway, respectively. TGF-β1 and PDGF subsequently stimulated pericyte-myofibroblast transition through differentiation and proliferation, respectively.
Within 1 day after surgery, the subsequent mechanical injury to the kidney induced both epithelial cells and pericytes to phosphorylate Smad2, indicating that activation of TGF-β1 signaling is a very early event, much earlier than the activation of PDGFR signaling.13 In normal and diseased kidney, the TGF-βR is widely expressed, including kidney epithelium and pericytes, whereas synthesis of the ligand, TGF-β, is most up-regulated in injured tubular epithelium; inflammatory macrophages and endothelial cells of the peritubular capillaries in UUO kidney express lower levels of TGF-β.14,46 Previous studies have identified TGF-β as an important cytokine in myofibroblast expansion and progressive fibrosis not only in chronic kidney disease, but also in injury and in the loss of epithelial cells known as tubular atrophy.25,27,30,31,47,48
A common feature of kidney injury models that result in interstitial fibrosis induced by ureteral obstruction, ischemia-reperfusion, or aristolochic acid is epithelial G2/M arrest, which contributes directly to a profibrotic phenotype of the epithelial cell.22 A presumed central role of G2/M arrest in regulating the epithelial profibrotic phenotype was demonstrated by administration of an inhibitor of the nuclear factor p53, which attenuates fibrosis in the unilateral postischemic kidney. Although the causal association between G2/M arrest and a fibrotic outcome is further supported by reinterpretation of many previous studies,49–52 the common pathway leading to tubular G2/M arrest in different animal models is unclear. In the present study, blocking TGF-β1 signaling attenuated epithelial G2/M arrest, which supports a role for TGF-β1 signaling in both cell cycle regulation and profibrotic dedifferentiation of injured epithelial cells. In support of the in vivo findings, our in vitro epithelial cell culture confirmed that TGF-β1 arrested cells in G2/M phase, but at the same time increased expression of profibrotic factors TGF-β1 and PDGFB. In accord with our findings in tubular epithelial cells, previous studies have shown that TGF-β1 induces cell cycle G2/M arrest in cultured podocytes.53 Cell cycle arrest and profibrotic cytokine production was reversed by TGF-βRI kinase inhibitor SB431542 in TGF-β1–treated tubular epithelial cells, which confirms the role of TGF-βR signaling in the cell cycle regulation and profibrotic dedifferentiation. In the present study, TGF-β1 released tubular epithelial cells from G0/G1 phase by decreased p27 levels, but further arrested cells in G2/M phase by increased p21 through a TGF-βR-dependent pathway. In accord with our data, in other studies TGF-β1 decreased p27 in primary epithelial cultures, which typically provokes cell cycle arrest in G1 phase, but increased p21, which regulates progression through S phase and also the G2 DNA checkpoint.54–57
Although the present study is the first to report this important connection, previous reports can be reinterpreted as supportive of the involvement of p21 in TGF-β-induced kidney epithelial G2/M arrest.55,58 However, our data did not support the role of up-regulated p21 in the profibrotic cytokine production of TGF-β1–treated tubular epithelial cells. TGF-β1 itself activated many signaling pathways, including Smad, JNK, and p38, through TGF-βR in cultured tubular epithelial cells. Among these activated signaling pathways, inhibitor studies using SP600125 indicated that JNK signaling was responsible for mediating the TGF-βR downstream signaling that resulted in expression of profibrotic cytokines. Specific JNK inhibition by SP600125 was previously shown to attenuate fibrosis in a unilateral postischemic kidney model.22 Thus, TGF-β1 can induce cell cycle arrest and profibrotic cytokine production of injured tubular epithelial cells through disparate intracellular signaling pathways, further supporting the important role of TGF-β1 and tubular epithelial cells in kidney fibrosis.
The early response to kidney injury, irrespective of underlying mechanisms, consists of an expanding population of interstitial cells and deposition of collagen.2,3 The expanding interstitial cells comprise collagen-producing myofibroblasts and inflammatory leukocytes.2,3,36,46,59 A large population of endogenous cells derived from Foxd1-expressing stromal precursors overlying cap mesenchyme during embryogenesis is the source of myofibroblast precursors.3,4,10 In the adult kidney, the branched processes of these cells are embedded in the capillary basement membrane of peritubular capillaries and are therefore considered pericytes that support microvascular stability.2–4,14,15 Although kidney pericytes are directly apposed to the abluminal surface of endothelial cells, they are also in close proximity to the tubular basement membrane.4,14 We have previously shown by electron microscopy that some pericytes have processes that abut directly on the tubular basement membrane.4,14 Moreover, there is normally a molecular and solute flux from the tubular compartment to the peritubular capillary.14,25,26,30 It makes sense, therefore, that epithelial cell signaling (either via the interstitial space or via direct receptor engagement on pericyte processes on the tubular basement membrane) can regulate pericyte functions in the kidney. Recent studies have indicated that injured tubular epithelial cells either die through programmed cell death (including apoptosis and autophagy) or remain in a state of G2/M arrest with characteristic phenotypic changes, including flattened morphology and loss of polarity.22,34,47 This injured phenotype is associated with up-regulated TGF-β1 signaling and, as we have shown here, a profibrotic phenotype.3,34,47 Our experiments indicate that supernatants generated by primary epithelial cultures can transfer factors sufficient to stimulate pericyte-myofibroblast transition in vitro, suggesting that soluble factors rather than matrix-bound or membrane-tethered factors, are the major mechanism of epithelial signaling to pericytes.
In the present study, pericytes responded to TGF-β1 differently than did kidney epithelial cells. Blocking TGF-β1 signaling decreased both pericyte proliferation and pericyte-myofibroblast transition in the UUO kidney in vivo. However, TGF-β1 did not directly stimulate pericyte proliferation, but it did stimulate transition to myofibroblasts. The mechanism by which TGF-β1 stimulates pericyte proliferation in vivo has been shown to be indirect, through activation of local epithelium to generate pericyte growth factors, including PDGF.13,26 In contrast, in the present study TGF-β1 induced pericyte-myofibroblast transition in vitro, but PDGF did not. Supernatant transfer from TGF-β1-activated epithelial cells stimulated both pericyte proliferation and myofibroblast transition, suggesting that the activated epithelial cells can produce factors sufficient for pericyte transition and expansion. Thus, PDGF and TGF-β1 exert distinct effects on kidney pericytes, both of which are necessary for the population expansion of myofibroblasts.
It is likely that PDGF and TGF-β1 form a positive feedback network in vivo by up-regulating one another in injured epithelial cells, in interstitial cells (including macrophages), and in endothelial cells.13,14 Injured tubular epithelium apparently plays a central role in activating pericyte-myofibroblast transition and renal fibrosis through responding to the injuries, sensing the injury-stimulated cytokine (TGF-β1 in the present study), and amplifying the profibrotic cytokines. Injury-induced or TGF-β1-induced cell death of tubular epithelial cells further contributes to the attrition of nephrons and loss of renal function.34 Further studies are required to define other critical factors released by injured epithelium that can promote pericyte detachment from the capillaries and sustain myofibroblast expansion. In addition, further studies will be required to understand the underlying signaling cascades that explain the distinct cellular responses of kidney pericytes to TGF-β1, compared with kidney epithelial cells.
In conclusion, TGF-β1 induces tubular epithelial cell cycle arrest in G2/M through up-regulation of p21 and stimulates profibrotic cytokine production in a TGF-βR/Smad-dependent pathway, thereby stimulating pericyte proliferation and transition to myofibroblasts by effector cytokines PDGF and TGF-β1, respectively (Figure 7). By blocking TGF-βR signaling, we can promote normal cell cycle progression in injured tubular epithelial cells and prevent pericyte-myofibroblast transition by both direct and indirect mechanisms. TGF-βR/Smad and p21 signaling effectors are important therapeutic targets for attenuating interstitial fibrosis and chronic kidney disease progression.
Acknowledgments
We thank Genzyme Corporation for antibodies 13C4 and 1D11, Dr. William Stallcup (Sanford-Burnham Institute, La Jolla, CA) for anti-NG2 and anti–PDGFR-β antibody, Dr. David A. Brenner (University of California, San Diego, CA) for Coll-GFP mice, Dr. Christine Abrass (University of Washington, Seattle, WA) for valuable discussion, the Department of Medical Research of National Taiwan University Hospital for equipment support, and the Cell Sorting Core Facility of the First Core Laboratory and the Transgenic Mouse Core Facility in the Center for Genomic Medicine, National Taiwan University College of Medicine.
Footnotes
Supported by grants from the National Science Council (99-2628-B-002-013, 101-2321-B-002-060 to S.L.L.; 99-2628-B-002-011 to Y.M.C.), National Taiwan University Hospital (99-S1302 to S.L.L), Ta-Tung Kidney Foundation, Mrs. Hsiu-Chin Lee Kidney Research Foundation, and the NIH (DK87389 to J.S.D.).
Disclosures: H.L. is an employee of the Sanofi-Genzyme R&D Center. J.S.D. is on the scientific advisory boards for Promedior, Inc., and Regulus Therapeutics, has stock options with Promedior, Inc., and has consulted for the following pharmaceutical companies: Gilead, Abbott, Takeda, Bristol-Myers Squibb, GlaxoSmithKline, Amira, and Boehringer Ingelheim. J.S.D. is the cofounder of Muregen, LLC, and holds patents related to the use of inhibitors and potentiators of the WNT pathway in kidney disease. Genzyme Corporation supplied antibodies 13C4 and 1D11.
Supplemental Data
Characterization of microvascular pericytes in normal adult kidney. Four-color confocal micrographs of normal kidney cortex show pericytes (Coll-GFP), endothelium (CD31), and capillary basement membranes (laminin α4), with pericyte processes (arrowheads) passing through capillary basement membrane and the splits in the capillary basement membrane. Endothelium and pericyte bodies in direct contact are indicated by arrows. Scale bar = 20 μm.
Characterization of microvascular pericytes and perivascular fibroblasts in normal adult kidney. A: Four-color confocal micrographs of normal kidney cortex show pericytes (top row), perivascular fibroblasts (middle row), and glomerular podocytes (bottom row) and their relationship to endothelium (CD31) and capillary basement membranes (laminin α4). Examples of areas of intimate connection between either pericyte bodies or pericyte processes and endothelium are indicated by arrowheads (top row). Some of the pericyte bodies and pericyte processes are embedded in basement membrane. Perivascular fibroblasts (green) surround arterioles (a) within a collagenous matrix and have no close appositions with arterial endothelial cells (middle). Glomerular podocytes are Coll-GFP+; perivascular fibroblasts (green) (arrows) surround an arteriole (a) (bottom). B: Microvascular Coll-GFP+ pericytes lose the intimate connection with endothelium (asterisk) and the cell population increases in kidneys at day 2 after UUO. Scale bar = 20 μm.
Microvascular pericytes increased expression of NG2 proteoglycan after unilateral ureteral obstruction. A: NG2 proteoglycan was expressed in glomerular mesangium (M) and vascular smooth muscle cells (V), but no expression was detected in microvascular Coll-GFP+ pericytes in normal kidneys. B: NG2 expression increased markedly in Coll-GFP+ pericytes in day-2 UUO kidneys. Scale bar = 20 μm.
Immunofluorescence micrographs of type II TGF-β receptor (TGF-βRII) in kidneys. Ubiquitous expression of TGF-βRII was found in tubular epithelial cells (indicated by the letter T) and Coll-GFP+ cells (arrowheads) in control (CON) and UUO kidneys. Isotype IgG control antibody staining is shown (far right column). Scale bar = 25 μm.
Blocking TGF-β1 signaling primary kidney pericyte culture. Smad2 phosphorylation induced by TGF-β1 (5 ng/mL) in primary kidney pericyte culture was inhibited by anti–TGF-β antibody 1D11 (100 μg/mL) and type I TGF-β receptor inhibitor SB431542 (5 μg/mL). Blots are representative of three independent experiments.
Fluorescence-activated cell sorting analysis of α-SMA expression in kidney pericytes. Single-cell preparations of control and UUO kidneys from Coll-GFP mice were subjected to FACS analysis. Kidney pericytes were identified by expression of Coll-GFP and PDGFR-α and then were subjected to analysis of α-SMA. Representative plots show α-SMA expression (solid line) in Coll-GFP+PDGFR-α+ pericytes of UUO kidneys from mice treated with antibodies 13C4, 1D11, or the inhibitor SB431542. The isotype control is shown as a dashed line. Control 13C4 data are from a 13C4-treated mouse. Positive α-SMA expression (R4 region) was determined by comparison with the isotype control.
Immunofluorescence images of injured tubular epithelial cells in UUO kidneys. A: Immunofluorescence images show expression of TGF-β1 and PDGFB mainly in dilated tubular epithelial cells (indicated by the letter T) of day-4 UUO kidney. Note the very weak expression in interstitial cells (arrows). B: Immunofluorescence images show Kim1 expression in injured tubular epithelial cells. Some Kim1+ tubular epithelial cells retain a low level of Lotus tetragonolobus lectin (LTL) staining (arrowhead), but no Dolichos biflorus agglutinin (DBA) staining can be detected. Scale bar = 25 μm.
Culture of mouse PTECs. Bright-field and immunofluorescence images show the epithelial origin of primary cultured tubular cells, with positive staining of γ-glutamyl transpeptidase and aquaporin-1 and with negative staining of aquaporin-2, Tamm-Horsfall protein, and vimentin. Scale bar = 25 μm.
SB431542 and SP600125, but not SB203580, decreased the transcripts of TGF-β1 in TGF-β1–treated PTECs. Data were quantified from three independent experiments. ∗∗P < 0.01.
Silencing p21 reversed cell cycle G2/M arrest of TGF-β1–treated proximal tubular epithelial cells. A: Transfection of p21 siRNA decreased TGF-β1 induction of p21 in PTECs, without effect on p27 expression. B: Silencing p21 reversed cell cycle G2/M arrest of TGF-β1–treated PTECs. Western blots and FACS analysis profiles are representative of three independent experiments.
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2012.09.009.
References
- 1.Allt G., Lawrenson J.G. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 2.Lin S.L., Kisseleva T., Brenner D.A., Duffield J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphreys B.D., Lin S.L., Kobayashi A., Hudson T.E., Nowlin B.T., Bonventre J.V., Valerius M.T., McMahon A.P., Duffield J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang F.C., Chou Y.H., Chen Y.T., Lin S.L. Novel insights into pericyte-myofibroblast transition and therapeutic targets in renal fibrosis. J Formos Med Assoc. 2012 doi: 10.1016/j.jfma.2012.09.008. http://dx.doi.org/10.1016/j.jfma.2012.09.008 DOI: [DOI] [PubMed] [Google Scholar]
- 5.Kisseleva T., Cong M., Paik Y., Scholten D., Jiang C., Benner C., Iwaisako K., Moore-Morris T., Scott B., Tsukamoto H., Evans S.M., Dillmann W., Glass C.K., Brenner D.A. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Göritz C., Dias D.O., Tomilin N., Barbacid M., Shupliakov O., Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 7.Suematsu M., Aiso S. Professor Toshio Ito: a clairvoyant in pericyte biology. Keio J Med. 2001;50:66–71. doi: 10.2302/kjm.50.66. [DOI] [PubMed] [Google Scholar]
- 8.Sponheim J., Pollheimer J., Olsen T., Balogh J., Hammarström C., Loos T., Kasprzycka M., Sørensen D.R., Nilsen H.R., Küchler A.M., Vatn M.H., Haraldsen G. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177:2804–2815. doi: 10.2353/ajpath.2010.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picard N., Baum O., Vogetseder A., Kaissling B., Le Hir M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem Cell Biol. 2008;130:141–155. doi: 10.1007/s00418-008-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asada N., Takase M., Nakamura J., Oguchi A., Asada M., Suzuki N., Yamamura K., Nagoshi N., Shibata S., Rao T.N., Fehling H.J., Fukatsu A., Minegishi N., Kita T., Kimura T., Okano H., Yamamoto M., Yanagita M. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121:3981–3990. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faulkner J.L., Szcykalski L.M., Springer F., Barnes J.L. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol. 2005;167:1193–1205. doi: 10.1016/S0002-9440(10)61208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bielesz B., Sirin Y., Si H., Niranjan T., Gruenwald A., Ahn S., Kato H., Pullman J., Gessler M., Haase V.H., Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y.T., Chang F.C., Wu C.F., Chou Y.H., Hsu H.L., Chiang W.C., Shen J., Chen Y.M., Wu K.D., Tsai T.J., Duffield J.S., Lin S.L. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 14.Lin S.L., Chang F.C., Schrimpf C., Chen Y.T., Wu C.F., Wu V.C., Chiang W.C., Kuhnert F., Kuo C.J., Chen Y.M., Wu K.D., Tsai T.J., Duffield J.S. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrimpf C., Xin C., Campanholle G., Gill S.E., Stallcup W., Lin S.L., Davis G.E., Gharib S.A., Humphreys B.D., Duffield J.S. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visconti R.P., Richardson C.D., Sato T.N. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci USA. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson M.C., Martin J.S., Cousins F.M., Kulkarni A.B., Karlsson S., Akhurst R.J. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 18.Larsson J., Goumans M.J., Sjöstrand L.J., van Rooijen M.A., Ward D., Levéen P., Xu X., ten Dijke P., Mummery C.L., Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGFbeta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshima M., Oshima H., Taketo M.M. TGFbeta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 20.Darland D.C., Massingham L.J., Smith S.R., Piek E., Saint-Geniez M., D’Amore P.A. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Hakroush S., Moeller M.J., Theilig F., Kaissling B., Sijmonsma T.P., Jugold M., Akeson A.L., Traykova-Brauch M., Hosser H., Hähnel B., Gröne H.J., Koesters R., Kriz W. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am J Pathol. 2009;175:1883–1895. doi: 10.2353/ajpath.2009.080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L., Besschetnova T.Y., Brooks C.R., Shah J.V., Bonventre J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J., Cao R., Zhang Y., Jia T., Cao Y., Wahlberg E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009;23:153–163. doi: 10.1096/fj.08-113860. [DOI] [PubMed] [Google Scholar]
- 24.Lindahl P., Johansson B.R., Levéen P., Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda K., Yoshitomi K., Yanagida T., Tokumoto M., Hirakata H. Quantification of TGFbeta1 mRNA along rat nephron in obstructive nephropathy. Am J Physiol Renal Physiol. 2001;281:F513–F521. doi: 10.1152/ajprenal.2001.281.3.F513. [DOI] [PubMed] [Google Scholar]
- 26.Floege J., Eitner F., Alpers C.E. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 27.Ma L.J., Yang H., Gaspert A., Carlesso G., Barty M.M., Davidson J.M., Sheppard D., Fogo A.B. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyano J.V., Greciano P.G., Buschmann M.M., Koch M., Matlin K.S. Autocrine transforming growth factor-β1 activation mediated by integrin αVβ3 regulates transcriptional expression of laminin-332 in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2010;21:3654–3668. doi: 10.1091/mbc.E10-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahm K., Lukashev M.E., Luo Y., Yang W.J., Dolinski B.M., Weinreb P.H., Simon K.J., Chun Wang L., Leone D.R., Lobb R.R., McCrann D.J., Allaire N.E., Horan G.S., Fogo A., Kalluri R., Shield C.F., 3rd, Sheppard D., Gardner H.A., Violette S.M. αv β6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato M., Muragaki Y., Saika S., Roberts A.B., Ooshima A. Targeted disruption of TGFbeta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Wilkes M.C., Leof E.B., Hirschberg R. Noncanonical TGFbeta pathways, mTORC1 and Abl, in renal interstitial fibrogenesis. Am J Physiol Renal Physiol. 2010;298:F142–F149. doi: 10.1152/ajprenal.00320.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Chaar M., Chen J., Seshan S.V., Jha S., Richardson I., Ledbetter S.R., Vaughan E.D., Jr., Poppas D.P., Felsen D. Effect of combination therapy with enalapril and the TGFbeta antagonist 1D11 in unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2007;292:F1291–F1301. doi: 10.1152/ajprenal.00327.2005. [DOI] [PubMed] [Google Scholar]
- 33.Wang S., Wilkes M.C., Leof E.B., Hirschberg R. Imatinib mesylate blocks a non-Smad TGFbeta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 34.Koesters R., Kaissling B., Lehir M., Picard N., Theilig F., Gebhardt R., Glick A.B., Hähnel B., Hosser H., Gröne H.J., Kriz W. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traykova-Brauch M., Schönig K., Greiner O., Miloud T., Jauch A., Bode M., Felsher D.W., Glick A.B., Kwiatkowski D.J., Bujard H., Horst J., von Knebel Doeberitz M., Niggli F.K., Kriz W., Grone H.J., Koesters R. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med. 2008;14:979–984. doi: 10.1038/nm.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin S.L., Li B., Rao S., Yeo E.J., Hudson T.E., Nowlin B.T., Pei H., Chen L., Zheng J.J., Carroll T.J., Pollard J.W., McMahon A.P., Lang R.A., Duffield J.S. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheridan A.M., Schwartz J.H., Kroshian V.M., Tercyak A.M., Laraia J., Masino S., Lieberthal W. Renal mouse proximal tubular cells are more susceptible than MDCK cells to chemical anoxia. Am J Physiol. 1993;265:F342–F350. doi: 10.1152/ajprenal.1993.265.3.F342. [DOI] [PubMed] [Google Scholar]
- 38.Lin S.L., Chen R.H., Chen Y.M., Chiang W.C., Lai C.F., Wu K.D., Tsai T.J. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol. 2005;16:2702–2713. doi: 10.1681/ASN.2005040435. [DOI] [PubMed] [Google Scholar]
- 39.Lin S.L., Chen R.H., Chen Y.M., Chiang W.C., Tsai T.J., Hsieh B.S. Pentoxifylline inhibits platelet-derived growth factor-stimulated cyclin D1 expression in mesangial cells by blocking Akt membrane translocation. Mol Pharmacol. 2003;64:811–822. doi: 10.1124/mol.64.4.811. [DOI] [PubMed] [Google Scholar]
- 40.Rhodin J.A. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968;25:452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- 41.Bruns R.R., Palade G.E. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968;37:244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouget C. Memoire sur le developpement, la structure et les proprietes physiologiques des capillaries sanguins et lymphatiques [Treatise on the development, structure and properties of physiological blood and lymphatic capillaries] Arch Physiol Norm Pathol. 1873;5:603–663. [Google Scholar]
- 43.Courtoy P.J., Boyles J. Fibronectin in the microvasculature: localization in the pericyte-endothelial interstitium. J Ultrastruct Res. 1983;83:258–273. doi: 10.1016/s0022-5320(83)90133-8. [DOI] [PubMed] [Google Scholar]
- 44.Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 45.Ozerdem U., Monosov E., Stallcup W.B. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63:129–134. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- 46.Lin S.L., Castaño A.P., Nowlin B.T., Lupher M.L., Jr., Duffield J.S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 47.Miyajima A., Chen J., Lawrence C., Ledbetter S., Soslow R.A., Stern J., Jha S., Pigato J., Lemer M.L., Poppas D.P., Vaughan E.D., Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58:2301–2313. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 48.Ling H., Li X., Jha S., Wang W., Karetskaya L., Pratt B., Ledbetter S. Therapeutic role of TGFbeta-neutralizing antibody in mouse cyclosporin A nephropathy: morphologic improvement associated with functional preservation. J Am Soc Nephrol. 2003;14:377–388. doi: 10.1097/01.asn.0000042168.43665.9b. [DOI] [PubMed] [Google Scholar]
- 49.Kailong L., Du X., Yani H., Lin Z., Jvrong Y., Ruihua S., Lin C. P53-Rb signaling pathway is involved in tubular cell senescence in renal ischemia/reperfusion injury. Biocell. 2007;31:213–223. [PubMed] [Google Scholar]
- 50.Megyesi J., Price P.M., Tamayo E., Safirstein R.L. The lack of a functional p21(WAF1/CIP1) gene ameliorates progression to chronic renal failure. Proc Natl Acad Sci USA. 1999;96:10830–10835. doi: 10.1073/pnas.96.19.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zahedi K., Revelo M.P., Barone S., Wang Z., Tehrani K., Citron D.P., Bissler J.J., Rabb H., Soleimani M. Stathmin-deficient mice develop fibrosis and show delayed recovery from ischemic-reperfusion injury. Am J Physiol Renal Physiol. 2006;290:F1559–F1567. doi: 10.1152/ajprenal.00424.2005. [DOI] [PubMed] [Google Scholar]
- 52.Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J.P., Sedivy J.M., Kinzler K.W., Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 53.Wu D.T., Bitzer M., Ju W., Mundel P., Böttinger E.P. TGFbeta concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol. 2005;16:3211–3221. doi: 10.1681/ASN.2004121055. [DOI] [PubMed] [Google Scholar]
- 54.Xie J., Shaikh Z.A. Cadmium induces cell cycle arrest in rat kidney epithelial cells in G2/M phase. Toxicology. 2006;224:56–65. doi: 10.1016/j.tox.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 55.Fenouille N., Robert G., Tichet M., Puissant A., Dufies M., Rocchi S., Ortonne J.P., Deckert M., Ballotti R., Tartare-Deckert S. The p53/p21Cip1/Waf1 pathway mediates the effects of SPARC on melanoma cell cycle progression. Pigment Cell Melanoma Res. 2011;24:219–232. doi: 10.1111/j.1755-148X.2010.00790.x. [DOI] [PubMed] [Google Scholar]
- 56.Wolf G., Jablonski K., Schroeder R., Reinking R., Shankland S.J., Stahl R.A. Angiotensin II-induced hypertrophy of proximal tubular cells requires p27Kip1. Kidney Int. 2003;64:71–81. doi: 10.1046/j.1523-1755.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 57.Grande J.P., Warner G.M., Walker H.J., Yusufi A.N., Cheng J., Gray C.E., Kopp J.B., Nath K.A. TGFbeta1 is an autocrine mediator of renal tubular epithelial cell growth and collagen IV production. Exp Biol Med (Maywood) 2002;227:171–181. doi: 10.1177/153537020222700304. [DOI] [PubMed] [Google Scholar]
- 58.Chou W.W., Guh J.Y., Tsai J.F., Hwang C.C., Chen H.C., Huang J.S., Yang Y.L., Hung W.C., Chuang L.Y. Arecoline-induced growth arrest and p21WAF1 expression are dependent on p53 in rat hepatocytes. Toxicology. 2008;243:1–10. doi: 10.1016/j.tox.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Castaño A.P., Lin S.L., Surowy T., Nowlin B.T., Turlapati S.A., Patel T., Singh A., Li S., Lupher M.L., Duffield J.S. Serum amyloid P inhibits fibrosis through FcγR-dependent monocyte-macrophage regulation in vivo. Sci Transl Med. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [Erratum appeared in Sci Transl Med 2009, 1:5ra13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of microvascular pericytes in normal adult kidney. Four-color confocal micrographs of normal kidney cortex show pericytes (Coll-GFP), endothelium (CD31), and capillary basement membranes (laminin α4), with pericyte processes (arrowheads) passing through capillary basement membrane and the splits in the capillary basement membrane. Endothelium and pericyte bodies in direct contact are indicated by arrows. Scale bar = 20 μm.
Characterization of microvascular pericytes and perivascular fibroblasts in normal adult kidney. A: Four-color confocal micrographs of normal kidney cortex show pericytes (top row), perivascular fibroblasts (middle row), and glomerular podocytes (bottom row) and their relationship to endothelium (CD31) and capillary basement membranes (laminin α4). Examples of areas of intimate connection between either pericyte bodies or pericyte processes and endothelium are indicated by arrowheads (top row). Some of the pericyte bodies and pericyte processes are embedded in basement membrane. Perivascular fibroblasts (green) surround arterioles (a) within a collagenous matrix and have no close appositions with arterial endothelial cells (middle). Glomerular podocytes are Coll-GFP+; perivascular fibroblasts (green) (arrows) surround an arteriole (a) (bottom). B: Microvascular Coll-GFP+ pericytes lose the intimate connection with endothelium (asterisk) and the cell population increases in kidneys at day 2 after UUO. Scale bar = 20 μm.
Microvascular pericytes increased expression of NG2 proteoglycan after unilateral ureteral obstruction. A: NG2 proteoglycan was expressed in glomerular mesangium (M) and vascular smooth muscle cells (V), but no expression was detected in microvascular Coll-GFP+ pericytes in normal kidneys. B: NG2 expression increased markedly in Coll-GFP+ pericytes in day-2 UUO kidneys. Scale bar = 20 μm.
Immunofluorescence micrographs of type II TGF-β receptor (TGF-βRII) in kidneys. Ubiquitous expression of TGF-βRII was found in tubular epithelial cells (indicated by the letter T) and Coll-GFP+ cells (arrowheads) in control (CON) and UUO kidneys. Isotype IgG control antibody staining is shown (far right column). Scale bar = 25 μm.
Blocking TGF-β1 signaling primary kidney pericyte culture. Smad2 phosphorylation induced by TGF-β1 (5 ng/mL) in primary kidney pericyte culture was inhibited by anti–TGF-β antibody 1D11 (100 μg/mL) and type I TGF-β receptor inhibitor SB431542 (5 μg/mL). Blots are representative of three independent experiments.
Fluorescence-activated cell sorting analysis of α-SMA expression in kidney pericytes. Single-cell preparations of control and UUO kidneys from Coll-GFP mice were subjected to FACS analysis. Kidney pericytes were identified by expression of Coll-GFP and PDGFR-α and then were subjected to analysis of α-SMA. Representative plots show α-SMA expression (solid line) in Coll-GFP+PDGFR-α+ pericytes of UUO kidneys from mice treated with antibodies 13C4, 1D11, or the inhibitor SB431542. The isotype control is shown as a dashed line. Control 13C4 data are from a 13C4-treated mouse. Positive α-SMA expression (R4 region) was determined by comparison with the isotype control.
Immunofluorescence images of injured tubular epithelial cells in UUO kidneys. A: Immunofluorescence images show expression of TGF-β1 and PDGFB mainly in dilated tubular epithelial cells (indicated by the letter T) of day-4 UUO kidney. Note the very weak expression in interstitial cells (arrows). B: Immunofluorescence images show Kim1 expression in injured tubular epithelial cells. Some Kim1+ tubular epithelial cells retain a low level of Lotus tetragonolobus lectin (LTL) staining (arrowhead), but no Dolichos biflorus agglutinin (DBA) staining can be detected. Scale bar = 25 μm.
Culture of mouse PTECs. Bright-field and immunofluorescence images show the epithelial origin of primary cultured tubular cells, with positive staining of γ-glutamyl transpeptidase and aquaporin-1 and with negative staining of aquaporin-2, Tamm-Horsfall protein, and vimentin. Scale bar = 25 μm.
SB431542 and SP600125, but not SB203580, decreased the transcripts of TGF-β1 in TGF-β1–treated PTECs. Data were quantified from three independent experiments. ∗∗P < 0.01.
Silencing p21 reversed cell cycle G2/M arrest of TGF-β1–treated proximal tubular epithelial cells. A: Transfection of p21 siRNA decreased TGF-β1 induction of p21 in PTECs, without effect on p27 expression. B: Silencing p21 reversed cell cycle G2/M arrest of TGF-β1–treated PTECs. Western blots and FACS analysis profiles are representative of three independent experiments.