Abstract
Because recent studies implicate Toll-like receptors (TLRs) in the pathogenesis of fibrosis, we sought to investigate the in vitro and in vivo role and mechanism of TLR4-mediated fibroblast responses in fibrogenesis. We found that TLR4 was constitutively expressed, and accumulation of endogenous TLR4 ligands significantly elevated, in lesional skin and lung tissues from patients with scleroderma. Activation of TLR4 signaling in explanted fibroblasts resulted in enhanced collagen synthesis and increased expression of multiple genes involved in tissue remodeling and extracellular matrix homeostasis. Moreover, TLR4 dramatically enhanced the sensitivity of fibroblasts to the stimulatory effect of transforming growth factor-β1. These profibrotic responses were abrogated by both genetic and pharmacological disruption of TLR4 signaling in vitro, and skin fibrosis induced by bleomycin in vivo was attenuated in mice harboring a mutated TLR4. Activation of TLR4 in fibroblasts augmented the intensity of canonical Smad signaling, and was accompanied by suppression of anti-fibrotic microRNA expression. Together, these results suggest a novel model to account for persistent fibrogenesis in scleroderma, in which activation of fibroblast TLR4 signaling, triggered by damage-associated endogenous TLR4 ligands, results in augmented transforming growth factor-β1 sensitivity with increased matrix production and progressive connective tissue remodeling. Under these conditions, fibroblast TLR4 serves as the switch for converting self-limited tissue repair into intractable fibrosis.
Fibrosis, the hallmark of scleroderma or systemic sclerosis, is a complex and dynamic pathophysiological process in which inflammatory cell infiltration and the release of cytokines and growth factors results in fibroblast activation and enhanced extracellular matrix (ECM) synthesis and deposition.1 In contrast to normal wound healing, which is characterized by tightly regulated and self-limited ECM remodeling, leading to tissue regeneration, pathological fibrosis is associated with unrestrained ECM deposition, culminating in the formation of an intractable scar. Transforming growth factor (TGF)-β induces the full repertoire of fibrotic responses and plays a key role in both physiological tissue repair and in pathogenesis of scleroderma and related fibrotic disorders.2 Although multiple intracellular signaling pathways, DNA-binding factors, and transcriptional cofactors are implicated in TGF-β–mediated fibrotic responses, the cross talk among these complex networks and the nature of their persistent deregulation in pathological inflammation and fibrosis remain poorly understood. Elucidation of how the factors mediating the switch from self-limited repair to intractable scar accumulation is essential for the design of rational therapies to interrupt the process in scleroderma.
Toll-like receptors (TLRs) are evolutionarily conserved pattern recognition receptors that play key roles in host defenses from microbial pathogens.3 Some TLRs (TLR2, TLR4, TLR5, and TLR6) are expressed at the cell surface, whereas others (TLR3, TLR7, TLR8, and TLR9) are normally found in intracellular vesicles.4 TLR signaling triggered by pathogen-associated molecular patterns is mediated by the cytoplasmic adaptor molecule, MyD88, and serine/threonine kinases of the IL-1R–associated kinase family, resulting in translocation of NF-κB to the nucleus and production of type I interferons and inflammatory cytokines.4 Aberrant TLR activation and signaling are increasingly linked to a variety of inflammatory and autoimmune diseases.5,6
Toll-like receptor 4 was originally identified as the receptor for lipopolysaccharide (LPS).7 On LPS binding, TLR4 forms a complex with myeloid differentiation factor-2, resulting in the recruitment of adaptor proteins to the intracellular Toll/IL-1 receptor domains.4 In addition to microbial pathogens, TLR4 can also be activated by endogenous ligands. One class of endogenous ligands for TLR4 includes damage-associated molecular patterns that are generated in situ as a consequence of injury resulting in cell damage, ECM remodeling, autoimmunity, and oxidative stress.8,9 Connective tissue molecules [eg, hyaluronan, alternatively spliced fibronectin extradomain (Fn-EDA), tenascin C, and biglycan], cellular stress proteins (eg, HMGB1 and gp96), and nucleic acids released from necrotic cells can all bind to and activate TLR4. Endogenous TLR4 ligands are implicated in a variety of diseases, and in experimental fibrosis in the kidney and liver.10–12 Moreover, mice with genetic ablation of TLR4 are protected from experimentally induced fibrosis in these organs.13–15 However, the role of TLR4 in fibrosis is controversial, with some studies demonstrating that TLR4 contributes to the resolution of postinflammatory fibrogenesis.16
The present studies were undertaken to elucidate the expression, mechanism of action, and potential involvement of TLR4 in fibrogenesis. The results reveal markedly elevated expression of TLR4, together with increased accumulation of endogenous TLR4 ligands, in lesional skin and lung tissues from patients with scleroderma, and in mice with bleomycin-induced experimental scleroderma. The classic TLR4 ligand, LPS, induced stimulation of ECM gene expression in explanted skin fibroblasts in vitro, and dramatically enhanced their ability to mount a profibrotic response when challenged with TGF-β1. These responses were associated with augmented intensity of canonical Smad signaling and suppression of anti-fibrotic microRNAs (miRNAs). Mice with a mutated TLR4 showed attenuated skin fibrosis in vivo when challenged with s.c. bleomycin. Together, these results implicate innate immune signaling via TLR4 in fibroblast activation in scleroderma; they lead us to propose a model for fibrogenesis in which TLR4 signaling elicited by damage-associated endogenous TLR4 ligands within the fibrotic milieu sensitizes stromal cells to the profibrotic activities of TGF-β1, resulting in enhanced production and accumulation of ECM molecules. This, in turn, establishes a positive feedback loop contributing to the persistence and progression of fibrosis.
Materials and Methods
Cell Culture and Reagents
Primary cultures of human skin fibroblasts were established by explantation from neonatal foreskin or from skin biopsy specimens of the affected forearms of patients with scleroderma and the forearm of healthy adult volunteers and studied at early passage.17 Human studies were performed in accordance with protocols approved by the Institutional Review Board for Human Studies at Northwestern University (Chicago, IL).
Mouse fibroblast cultures were established from skin biopsy specimens of 8-week-old female C3H/HeJ and C3H/HeOuJ mice (Jackson Laboratories, Sacramento, CA) and studied in parallel. Cultures were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Gibco BRL, Grand Island, NY), 1% vitamin solutions, and 2 mmol/L l-glutamine. All other tissue culture reagents were from Lonza (Basel, Switzerland). For experiments, cultures were placed in serum-free media containing 0.1% bovine serum albumin for 24 hours before the addition of highly purified LPS (Sigma-Aldrich, St. Louis, MO) and TGF-β1 (PeproTech, Rocky Hill, NJ). In selected experiments, indicated concentrations of the selective TLR4 inhibitor, CLI-095 (InvivoGen, San Diego, CA), was added to the cultures 30 minutes before LPS or TGF-β1.
qPCR
Levels of mRNA were determined by real-time quantitative PCR (qPCR), as previously described.18 Briefly, at the end of each experiment, total RNA was isolated from skin fibroblasts and reverse transcribed to cDNA using supermix (cDNA Synthesis Supermix; Quanta Biosciences, Gaithersburg, MD). The products (50 ng) were amplified using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) with primers shown in Table 1 on the Applied Biosystems 7500 Prism Sequence Detection System. Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA, and fold change is represented as follows: 2-ΔΔCT (2-[(CTtarget - C TTGAPDH) treatment - (C target - C TGAPDH) non-treatment]).
Table 1.
PCR Primers
| Target gene | Primer sequence |
|---|---|
| Human | |
| αSMA | |
| Forward | 5′-CAGGGCTGTTTTCCCATCCAT-3′ |
| Reverse | 5′-GCCATGTTCTATCGGGTACTTC-3′ |
| COL1A1 | |
| Forward | 5′-CCAGAAGAACTGGTACATCAGCA-3′ |
| Reverse | 5′-CGCCATACTCGAACTGGGAAT-3′ |
| COL1A2 | |
| Forward | 5′-GATGTTGAACTTGTTGCTGAGG-3′ |
| Reverse | 5′-TCTTTCCCCATTCATTTGTCTT-3′ |
| IL-6 | |
| Forward | 5′-AAATTCGGTACATCCTCGACGG-3′ |
| Reverse | 5′-GGAAGGTTCAGGTTGTTTTCTGC-3′ |
| IL-8 | |
| Forward | 5′-ACTGAGAGTGATTGAGAGTGGAC-3′ |
| Reverse | 5′-AACCCTCTGCACCCAGTTTTC-3′ |
| MCP-1 | |
| Forward | 5′-ACTGAAGCTCGTACTCTC-3′ |
| Reverse | 5′-CTTGGGTTGTGGAGTGAG-3′ |
| Mouse | |
| αSMA | |
| Forward | 5′-ATGCAGAAGGAGATCACAGC-3′ |
| Reverse | 5′-GTATTCCTGTTTGCTGATCCAC-3′ |
| COL1A1 | |
| Forward | 5′-AGCCGCAAAGAGTCTACATG-3′ |
| Reverse | 5′-CTTAGGCCATTGTGTATGCAG-3′ |
| COL1A2 | |
| Forward | 5′-CCGTGCTTCTCAGAACATCA-3′ |
| Reverse | 5′-CTTGCCCCATTCATTTGTCT-3′ |
| 36B4 | |
| Forward | 5′-AGATGCAGCAGATCCGCAT-3′ |
| Reverse | 5′-GTTCTTGCCCATCAGCACC-3′ |
miRNA Isolation and Quantitation by qPCR
miRNA was isolated from confluent fibroblasts using the mirVana miRNA Isolation kit (Ambion/Applied Biosystems, Foster City, CA). Specific TaqMan probes for hsa-miR-29a, hsa-miR-29b, hsa-miR-29c, and RNU24 (Applied Biosystems) were used to measure miRNA expression levels using the Applied Biosystems 7500 Prism Sequence Detection System.18
Genome-Wide Expression Profiling and Data Analysis
To examine responses induced by TLR4 activation at the genome-wide level, confluent fibroblasts in serum-free media were incubated with LPS for 6 hours, and RNA was isolated using RNeasy mini kits (Qiagen, Valencia, CA). The integrity of RNA was ascertained using an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA), and cDNA was labeled using an Ambion labeling kit (Ambion) and hybridized to Illumina human HT-12 version 4 Expression Microarray Chips (Illumina, San Diego, CA), as previously described.19 Raw signal intensities for each probe were obtained using Illumina Bead version 2 studio data analysis software and imported to the Bioconductor lumi package for data transformation and normalization.19–22 Probes with all samples absent (near or lower than background levels) were filtered. To control for multiple testing and reduce the false-positive rate (FDR), stringent statistical criteria were used to identify differentially expressed genes with raw P < 0.01 and FDR-adjusted P < 0.05.19
Western Blot Analysis
Whole cell lysates were prepared, and equal amounts of proteins (20 to 50 μg per lane) were subjected to Western blot analysis using primary antibodies specific for type I collagen (Southern Biotechnology, Birmingham, AL), phosphorylated Smad2 or Smad3 (both from Cell Signaling, Beverly, MA), Smad1/2/3 (Santa-Cruz Biotechnology, Santa Cruz, CA), tubulin (Sigma-Aldrich), and GAPDH (Invitrogen, Carlsbad, CA), as previously described.19 Membranes were then incubated with appropriate secondary antibodies and subjected to enhanced chemiluminescence detection using an electrochemiluminescent reagent (Pierce, Rockford, IL).
Transient Transfection Assays
The reporter constructs 772COL1A2-luc, harboring the 2772/58-bp fragment of the human proα1(I) collagen, [SBE]4-luc harboring four tandem copies of the minimal Smad-binding element,23 and NF-κB-luc were used in transient transfection assays. Subconfluent cultures of skin fibroblasts in serum-free media were transfected using Superfect reagent (Qiagen, Valencia, CA). After 24 hours of incubation with TGF-β1 (5 or 10 ng/mL), cultures were harvested and whole cell lysates were assayed for their luciferase activities.24 In each experiment, fibroblasts were cotransfected with Renilla luciferase pRL-TK plasmids (Promega, Madison, WI) as a control for transfection efficiency. Experiments were performed in triplicate and repeated at least twice, with consistent results.
Immunofluorescence Microscopy of Explanted Fibroblasts
Fibroblasts were incubated on eight-well Lab-Tek II chamber glass slides (Nalgene Nunc International, Naperville, IL) in serum-free Dulbecco’s modified Eagle’s medium supplemented with 0.1% bovine serum albumin in the presence of LPS (1 μg/mL) and TGF-β (1 or 10 ng/mL) for 24 hours. Cells were fixed and permeabilized, and slides were incubated with antibodies to type I collagen (Southern Biotechnology) or α-smooth muscle actin (α-SMA; Sigma-Aldrich), at 1:100 or 1:1000 dilution, followed by Alexa Fluor secondary antibodies (Invitrogen). Nuclei were identified using DAPI. Subcellular distribution of immunofluorescence was evaluated under an immunofluorescence microscope.24
IHC and Immunofluorescence Analysis of Skin and Lung Biopsy Specimens
Skin biopsy specimens from 20 patients with scleroderma and nine healthy volunteers, and lung biopsy specimens from four patients with scleroderma with end-stage lung disease and four normal lungs were obtained in accordance with protocols approved by the Institutional Review Boards for Human Studies at Boston University (Boston, MA) and the University of Pittsburgh (Pittsburgh, PA). Immunohistochemistry (IHC) was performed on paraffin-embedded sections, as previously described.17 Briefly, after incubation of sections (4 μm thick) with primary rabbit antibodies against TLR4 (1:100 dilution; Abcam, Cambridge, MA), appropriate anti-rabbit or anti-mouse IgG was applied as a secondary antibody. Bound antibodies were detected using the Dako Envision + System (Santa Clara, CA), according to the manufacturer’s instructions. To quantify tissue levels of TLR4, immunopositive cells in four randomly selected microscopic fields were counted by two blinded examiners. To identify TLR4-positive cells in skin, serial sections (4 μm thick) were incubated with primary antibodies against TLR4 (1:50; Abcam), CD31 (1:50; Cell Signaling, Beverly, MA), CD11B (1:100; BD Pharmingen, San Jose, CA), and α-SMA (1:200; Sigma-Aldrich). Endogenous TLR4 ligands were identified in the skin using antibodies against Fn-EDA (1:50; Sigma-Aldrich) or tenascin C (1:50; Abcam), followed by mouse Alexa Fluor or fluorescein isothiocyanate–labeled secondary antibodies (1:100; Invitrogen). Hyaluronic acid (HA) in the tissue was identified using biotinylated HA-binding protein (1:100; Calbiochem, San Diego, CA). Nuclei were identified using DAPI. Slides were mounted, evaluated, and photographed under a Zeiss UV Meta 510 confocal microscope (Carl Zeiss Inc., Jena, Germany). For lung immunofluorescence double labeling, paraffin-embedded sections (6 μm thick) were incubated with primary antibodies against TLR4 (1:50; Abcam) or α-SMA (1:200; Sigma-Aldrich), followed by biotinylated rabbit or mouse secondary antibodies (GE Healthcare Ltd, Little Chalfont, Buckinghamshire, UK), as previously described.25 After washing with PBS, sections were incubated with avidin-conjugated Texas Red and Fluorescein DCS (20 μg/mL) (A-2006 and A-1100; Vector Laboratories). Nuclei were detected using a Hoechst nuclear stain (Sigma-Aldrich). Images were captured using an Olympus Provis Fluorescence microscope (Olympus America Inc., Melville, NY). Each section was examined independently by two investigators in a blinded manner.
TLR4 Expression in a Mouse Model of Scleroderma
To evaluate the role of TLR4 in skin fibrosis in vivo, C3H/HeJ mutant mice and wild-type (WT) C3H/HeOuJ control mice (Jackson Laboratories, Sacramento, CA) were studied. The C3H/HeJ strain of mice harbor a missense mutation in the intracellular signaling domain of TLR4 that replaces proline with histidine at position 712, resulting in hyporesponsiveness to LPS.26 Eight-week-old female C3H/HeJ mice and WT C3H/HeOuJ mice were given bleomycin (10 mg/kg per day) or PBS by daily s.c. injections.27 At 3 and 21 days after the initiation of the injections, mice were sacrificed, skin and lungs were harvested, and sections of paraffin-embedded tissues (4 μm thick) were stained with H&E, Masson’s trichrome, or Picrosirius red.27 For immunofluorescence, sections (4 μm thick) of skin were incubated with mouse antibodies against Fn-EDA (1:100; Sigma-Aldrich) or tenascin C (1:50; Abcam), followed by Alexa Fluor–labeled mouse secondary antibodies. Nuclei were detected using DAPI. Slides were mounted, and immunofluorescence was evaluated under a Zeiss UV Meta 510 confocal microscope. Each experimental group consisted of at least three to five mice. Studies were performed according to institutionally approved protocols and were in compliance with the animal welfare guidelines of the Northwestern University Animal Care and Use Committee.
Quantification of Tissue Collagen
Lesional skin biopsy samples were homogenized in 0.5 mol/L glacial acetic acid. After centrifugation at 8944 × g for 15 minutes, supernatants were assayed in triplicate for soluble collagen using Sircol Collagen Assay kits (Biocolor, Newtown Abbey, Ireland).27
Statistical Analysis
Data are presented as mean ± SD. A Student’s t-test was used for comparisons between two groups. P < 0.05 was considered significant.
Results
Elevated Expression of TLR4 and Endogenous TLR4 Ligands in Scleroderma Skin and Lung
To characterize the expression of TLR4 in scleroderma, skin biopsy specimens from 20 patients with scleroderma (19 with diffuse and 1 with limited cutaneous systemic sclerosis) and nine healthy controls matched for sex, age, and biopsy site were studied in parallel. The clinical and demographic features of the study subjects are shown in Table 2. Fourteen of the patients had early disease. The scleroderma skin biopsy specimens showed a variable degree of dermal thickening and sclerosis, with a paucity of inflammatory cell infiltrates (data not shown). IHC demonstrated that, in contrast to healthy control biopsy specimens that revealed little detectable TLR4, scleroderma biopsy specimens showed TLR4 immunostaining in a significant proportion of fibroblasts, and vascular cells, within the lesional dermis (Figure 1A). Semiquantitative analysis of dermal TLR4 expression confirmed a significant increase in scleroderma biopsy specimens compared with healthy controls (P < 0.05). Our data also showed an increased expression of TLR4 in keratinocytes in scleroderma biopsy specimens, suggesting that keratinocytes might be activated via TLR4 and play a role in fibroblast activation via epidermal-dermal cross talk. Western blot analysis against a TLR4 antibody demonstrated one single band showing antibody specificity (data not shown).
Table 2.
Clinical Characteristics of Patients
| Patient no. | Age (years) | Sex | SSc type | Early/late∗ | MRSS |
|---|---|---|---|---|---|
| Skin Immunostaining | |||||
| SSc1 | 65 | F | dcSSC | Early | 12 |
| SSc2 | 48 | F | dcSSc | Early | 21 |
| SSc3 | 52 | F | dcSSc | Early | 13 |
| SSc4 | 45 | F | dcSSc | Early | 9 |
| SSc5 | 40 | F | dcSSc | Early | 32 |
| SSc6 | 35 | F | dcSSc | Early | 10 |
| SSc7 | 44 | F | dcSSc | Early | 12 |
| SSc8 | 34 | F | dcSSc | Late | 32 |
| SSc9 | 46 | F | dcSSc | Late | 35 |
| SSc10 | 30 | F | dcSSc | Late | 4 |
| SSc11 | 60 | F | lcSSc | Late | 4 |
| SSc12 | 54 | F | dcSSc | Late | 13 |
| SSc13 | 43 | F | dcSSc | Early | 9 |
| SSc14 | 30 | F | dcSSc | Late | 18 |
| SSc15 | 43 | M | dcSSc | Early | 34 |
| SSc16 | 32 | F | dcSSc | Early | 31 |
| SSc17 | 56 | F | dcSSc | Early | 13 |
| SSc18 | 51 | F | dcSSc | Early | 11 |
| SSc19 | 44 | F | dcSSc | Early | 20 |
| SSc20 | 33 | M | dcSSc | Early | 13 |
| Lung Immunostaining | |||||
| SSc1 | 59 | F | NA | Late | |
| SSc2 | 46 | M | NA | Late | |
| SSc3 | 60 | M | lcSSc | Late | |
| SSc4 | 50 | F | dcSSc | Late | |
F, female; M, male; dcSSc, diffuse cutaneous SSc; lcSSc, limited cutaneous SSc; MRSS, modified Rodnan skin score; NA, not available.
Early indicates <2 years from the first non-Raynaud’s disease manifestation.
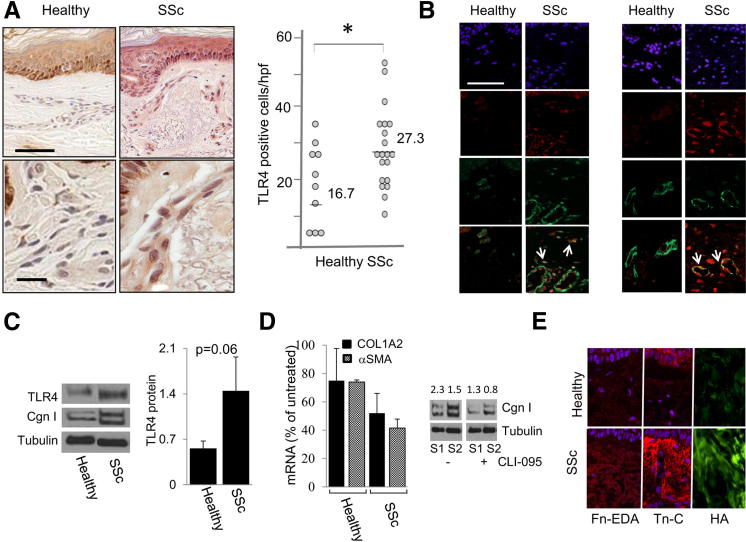
Figure 1.
Elevated TLR4 expression in scleroderma skin. A: Skin biopsy specimens from the lesional forearm of patients with scleroderma (n = 19) and forearms of healthy controls (n = 8) were examined by IHC. Left panels: Representative images. Brown staining indicates TLR4-positive cells. Scale bars: 200 μm (top panels); 50 μm (bottom panels). Blue, hematoxylin counterstain. Right panel: Quantitation of dermal cell TLR4 staining. Each dot, number (mean) of immune-positive spindle-shaped interstitial cells (fibroblast-like) from four separate microscopic fields per biopsy. B: Double-immunofluorescence labeling using antibodies to TLR4 and CD31 or α-SMA, or stained with DAPI. Yellow, colocalization of two antibodies. Arrows, colocalization. Scale bar = 50 μm. C: Cell lysates from explanted skin fibroblasts from healthy controls (n = 3) and patients with scleroderma (n = 4) were subjected to Western blot analysis using antibodies against TLR4 and type I collagen (Cgn I). Left panel: Representative immunoblots. Right panel: Quantitation of TLR4 levels. Values represent the mean ± SD, corrected for tubulin in each lane. D: Explanted healthy control (n = 3) and scleroderma (n = 3) fibroblasts were incubated with CLI-095 (3 μmol/L) for 24 hours. RNA and protein were isolated and subjected to qPCR. Left panel: Mean ± SD of triplicate determinations relative to levels in untreated fibroblasts. Right panel: Western blot analysis. Values shown indicate relative levels corrected for tubulin in each lane. E: Immunofluorescence. Antibodies to Fn-EDA or tenascin-C, or HA-binding protein, were used on skin biopsy specimens from healthy controls (n = 3) and patients with scleroderma (n = 5). Scale bar = 50 μm. *P < 0.05.
To determine the identity of TLR4-immunopositive cells, double-immunofluorescence labeling was performed on five scleroderma and five healthy control skin biopsy specimens in parallel. A substantial increase in the number of α-SMA–positive interstitial cells throughout the dermis was noted in each of the scleroderma skin biopsy specimens compared with the control biopsy specimens, which showed α-SMA immunoreactivity prominent in the perivascular regions. Moreover, approximately 50% of the TLR4-positive interstitial cells in the scleroderma dermis were also positive for α-SMA. Modest TLR4 colocalization (approximately 25%) with the prototype microvascular endothelial cell marker, CD31, was detected (Figure 1B). Only scant CD11b immunoreactivity was found in the dermis from either control or scleroderma skin biopsy specimens. However, macrophage contribution cannot be excluded, based on our results, because not all macrophages expressed CD11b and, therefore, the overall identity of the TLR4+ cells was not well established.
We next investigated TLR4 expression in explanted early-passage scleroderma and healthy control skin fibroblasts in parallel. Western blot analysis showed a 2.3-fold (mean) increase in TLR4 protein expression in vitro that was associated with elevated type I collagen levels in all four scleroderma fibroblast lines compared with control fibroblasts (Figure 1C and data not shown). To examine the functional role of TLR4 in the ex vivo–activated phenotype of scleroderma fibroblasts, cultures were incubated with the potent TLR4 inhibitor, CLI-095, which blocked TLR4 signaling by binding to the intracellular signaling domain. The results demonstrated that, although there was no significant decrease in COL1A2 and α-SMA mRNA levels in healthy control fibroblasts, an approximately 50% reduction was seen in mRNA level in scleroderma fibroblasts treated with CLI-095 (P < 0.05). Similarly, Western blot analysis showed a comparable approximately 55% decrease in type I collagen levels, with no significant change in the healthy control fibroblasts (Figure 1D and data not shown).
Molecules generated or released in situ on chronic tissue injury could be recognized as damage-associated molecular patterns that serve as endogenous TLR4 ligands. We examined the expression of the ECM components Fn-EDA, tenascin C, and HA, each of which is a potential TLR4 ligand, in scleroderma skin biopsy specimens. Immunofluorescence and histochemistry results demonstrated that, in contrast to healthy control biopsy specimens that had little detectable Fn-EDA, tenascin C, or HA in the dermis, a dramatic increase in the accumulation of each of these three endogenous TLR4 ligands was noted in all five scleroderma biopsy specimens examined (Figure 1E).
Pulmonary fibrosis associated with myofibroblast accumulation was a common and devastating complication of scleroderma. Normal donor lungs showed only a low level of TLR4 expression by IHC (Figure 2A). In these biopsy specimens, TLR4 was largely restricted to alveolar epithelial lining cells and scattered intra-alveolar macrophages. In marked contrast, lung biopsy specimens from all four patients with scleroderma and pulmonary fibrosis showed strong intracellular TLR4 expression. Immunostaining in these lungs was most prominent in parenchymal fibroblasts (Figure 2A) and infiltrating cells (Figure 2A) located at, or adjacent to, fibrotic loci. Semiquantitative analysis of TLR4 expression confirmed a significant increase in scleroderma lung biopsy specimens compared with healthy controls (P < 0.05). Numerous TLR4-positive interstitial cells in the fibrotic stroma showed strong α-SMA staining, identifying them as myofibroblasts (Figure 2B). In contrast, only scant TLR4 immunostaining was noted around vascular structures.
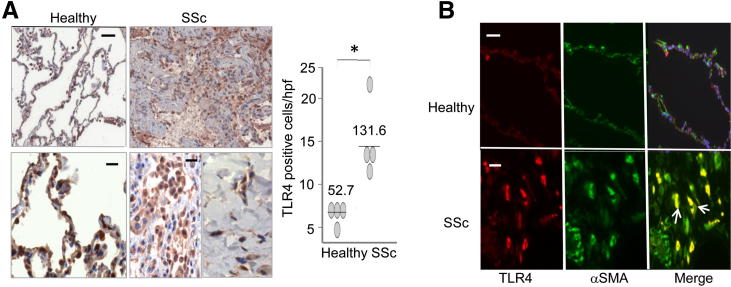
Figure 2.
Elevated TLR4 expression in scleroderma lungs. A: IHC of lung biopsy specimens from patients with scleroderma and pulmonary fibrosis (n = 4) and normal donor lungs (n = 4). Left panels: Brown staining indicates TLR4-positive cells. Blue, hematoxylin counterstain. Scale bars: 200 μm (top panels); 50 μm (bottom panels). Right panel: Quantitation of TLR4 staining. Each dot, number (mean) of immunopositive fibroblasts from four separate microscopic fields per biopsy. B: Red, immunofluorescence using antibodies to TLR4; green, α-SMA; yellow, colocalization of the two antibodies (white arrows). Mouse isotype antibodies were used as a negative control, and a Hoechst stain was used to detect nuclei. Scale bars: 200 μm (top panels); 50 μm (bottom panels). *P < 0.05.
TLR4 Signaling Induces Global Gene Expression Changes in Skin Fibroblasts
Innate immune signaling via TLR4 was extensively characterized in cells of the immune system.28 In contrast, the expression, regulation, and function of TLR4 in mesenchymal cells have received scant attention. The presence of an intact TLR4 signaling axis in skin fibroblasts was first confirmed by demonstrating potent stimulation of classic TLR4-dependent inflammatory genes by the TLR4 ligand, LPS (Supplemental Figure S1A and data not shown). Moreover, transient transfection assays demonstrated significant LPS stimulation of NF-κB-luc activity in fibroblasts, which was abrogated in the presence of the TLR4 inhibitor, CLI-095 (Supplemental Figure S1B and data not shown). Endotoxin contamination in these experiments was excluded as the cause of these TLR4 responses using the natural LPS antagonist, polymyxin B (data not shown).
To assess the pattern of TLR4-induced gene expression changes at the genome-wide level, confluent healthy skin fibroblasts were incubated with the classic TLR4 ligand, LPS. After 6 hours of incubation, total RNA was harvested and processed for hybridizations to Illumina Human HT-12 microarray chips containing >48,000 probes. Analysis of the data demonstrated that LPS induced a broad pattern of changes in gene expression in healthy fibroblasts, with a greater than twofold increase or decrease compared with vehicle-treated cultures (P < 0.01; FDR, 0.05) in the expression of 342 and 271 genes up- and down-regulated, respectively (Supplemental Table S1). Gene Ontology analysis revealed significant enrichment with LPS-regulated genes in functional groups related to ECM synthesis and wound healing (Table 3). Representative of this group were genes encoding multiple collagens (COL1A1, COL3A1, COL5A1, COL8A1, COL11A1, and COL12A1), mediators of fibrogenesis (plasminogen activator inhibitor-1, Wnt2 and Wnt5A, insulin-like growth factor binding protein 3, and connective tissue growth factor), and the matricellular proteins SPARC and periostin (Supplemental Figure S2). Changes in the expression of multiple LPS-regulated genes were confirmed by qPCR (data not shown).
Table 3.
Biological Processes with Differentially Expressed Genes Belonging to Individual GO Categories Significantly Enriched with LPS-Regulated Genes
| GO terms | LPS/control (P values) |
|---|---|
| Response to wounding | 137 (3.63 × 10−6) |
| Response to organic substance | 199 (2.89 × 10−13) |
| Cell migration | 111 (1.89 × 10−12) |
| Defense response | 133 (3.27 × 10−6) |
| Extracellular matrix organization | 28 (2.19 × 10−7) |
| System development | 387 (2.03 × 10−13) |
| Programmed cell death | 190 (4.21 × 10−9) |
| Response to cytokine stimulus | 65 (7.25 × 10−9) |
| Inflammatory response | 55 (P > 0.01) |
| Vasculature development | 67 (2.98 × 10−7) |
| Cardiovascular system development | 92 (5.5 × 10−8) |
GO, gene ontology.
TLR4 Sensitizes Fibroblasts to Profibrotic Stimulation by TGF-β1
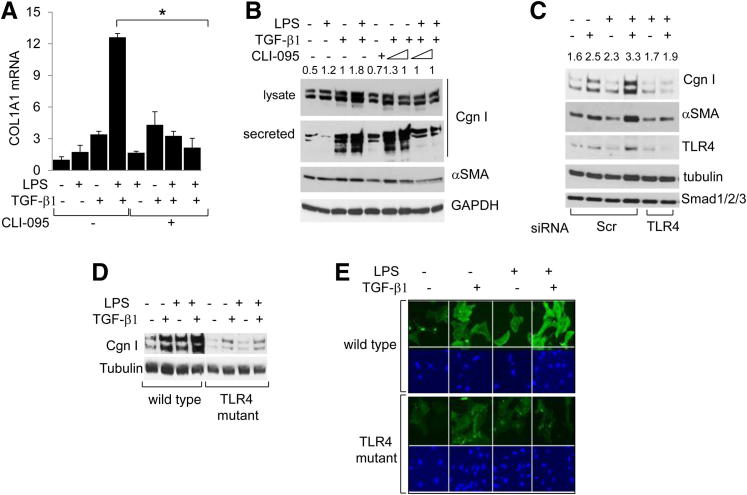
The analysis of the microarray data indicated that in fibroblasts, LPS prominently enhanced the expression of genes related to ECM remodeling and fibrogenesis. To examine how TLR4 signaling modulated collagen gene expression in stimulated skin fibroblasts, cultures were incubated with LPS in the presence or absence of TGF-β1. qPCR confirmed that LPS treatment by itself resulted in increased COL1A1 mRNA expression and type I collagen levels, which was relatively modest compared with the increase induced by TGF-β1 (Figure 3). However, LPS sensitized fibroblasts to TGF-β1 stimulation of collagen gene expression. Furthermore, although LPS alone had no significant effect on α-SMA expression, it markedly enhanced the stimulation induced by TGF-β1 (Figure 3, C and D, and data not shown).
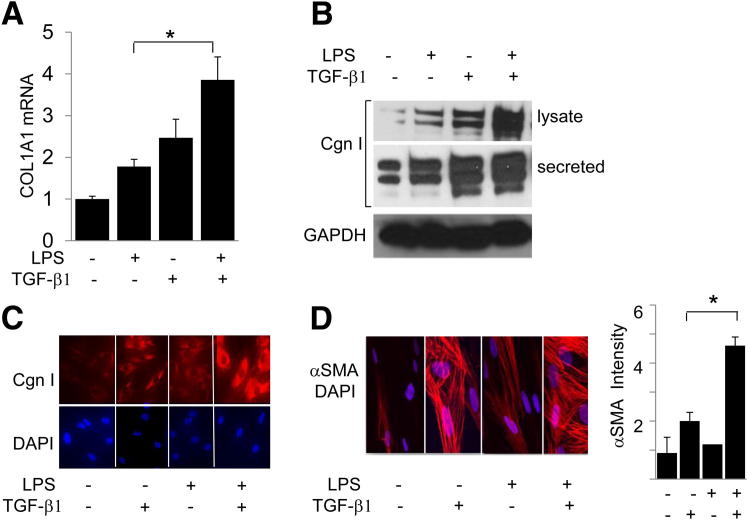
Figure 3.
LPS sensitizes fibroblasts to TGF-β1 stimulation of profibrotic gene expression. Fibroblasts were incubated with TGF-β1 (10 ng/mL) in the presence or absence of LPS for 24 hours. A: Total RNA was examined by qPCR. Results, normalized with GAPDH mRNA, are shown as the mean ± SD of triplicate determinations. B: Whole cell lysates and culture supernatants were subjected to Western blot analysis. Representative immunoblots are shown. C: Fibroblasts were immunostained with antibodies to type I collagen (Cgn I) or α-SMA or stained with DAPI, and viewed by immunofluorescence microscopy (top panels) or laser-scanning confocal microscopy (bottom panels). Original magnification, ×400. D: Right panel: Mean immunofluorescence intensity of individual fibroblasts stained with α-SMA was determined in five different microscopic fields. Results are given as the mean ± SD. *P < 0.05.
To examine the effect of activated TLR4 on collagen gene expression directly, fibroblasts were transfected with constitutively active TLR4. Ectopic expression of constitutively active TLR4 was by itself sufficient to induce a significant increase in COL1A1 mRNA and type I collagen expression, as well as COL1A1-luc activity (Figure 4). Remarkably, stimulation by TLR4 was further enhanced in the presence of TGF-β1. As expected, ectopic TLR4 induced a dose-dependent stimulation of NF-κB-luc activity (data not shown). In contrast, transfection of fibroblasts with a TLR4 mutant harboring a proline-to-histidine substitution within the intracellular domain failed to stimulate COL1A2-luc activity or type I collagen synthesis (Figure 4, B and C). Moreover, the stimulatory effects of LPS on type I collagen gene expression in TGF-β1–activated fibroblasts were completely abrogated by pretreatment of the cultures with the TLR4 inhibitor, CLI-095 (Figure 5, A and B).
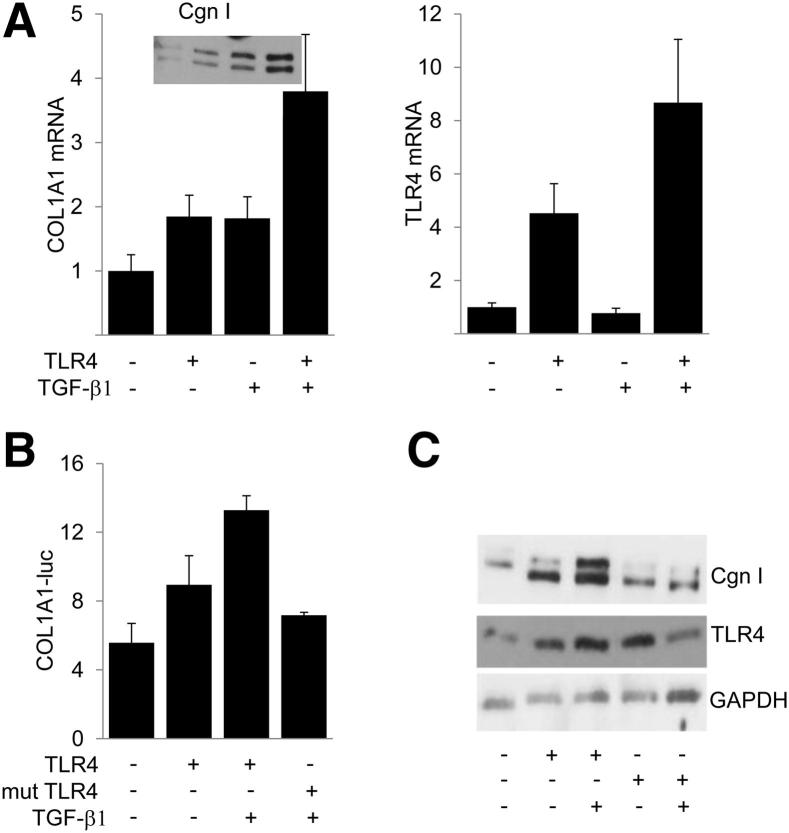
Figure 4.
TLR4 stimulates collagen gene expression. Fibroblasts were transiently transfected with constitutively active TLR4 (CD4TLR4) or inactive TLR4 (P-H CD4TLR4), 772COL1A2-luc, or empty vector and incubated with TGF-β1 for 24 hours. A: Total RNA was examined by qPCR. Results, normalized with GAPDH, are the mean ± SD of triplicate determinations. Inset: Secreted type I collagen (Cgn I). Whole cell lysates were assayed for their luciferase activities (B) or examined by using Western blot analysis (C). The results of luciferase assays are normalized with Renilla luciferase. mut, mutant.
Figure 5.
LPS synergy with TGF-β1 is TLR4 dependent. A: Normal skin fibroblasts were incubated with LPS and TGF-β1 in the absence or presence of TLR4 inhibitors. Total RNA was examined by qPCR. B: Whole cell lysates and secreted proteins in the culture media were subjected to Western blot analysis. Type I collagen (Cgn I) protein levels were quantitated. Values shown indicate relative levels corrected for GAPDH in each lane. C: Normal skin fibroblasts were transfected with TLR4-specific siRNA or scrambled (Scr) control siRNA and incubated with LPS and TGF-β1 for 24 hours. Whole cell lysates were examined by using Western blot analysis. Representative immunoblots. Cgn I levels were quantitated. Values shown indicate relative levels corrected for tubulin in each lane. D and E: Skin fibroblasts from WT mice and TLR4-mutant (C3H/HeJ) mice were incubated in parallel with LPS and TGF-β1 for 24 hours. Whole cell lysates were subjected to Western blot analysis (D). Fibroblasts were fixed, incubated with antibodies to α-SMA, and examined by immunofluorescence microscopy (E). Blue, nuclei were identified by DAPI. Original magnification, ×100. *P < 0.05.
LPS Fails to Synergize with TGF-β1 in the Absence of Functional TLR4
The role of TLR4 in mediating LPS-induced fibrotic responses was further characterized using complementary loss-of-function approaches. First, an RNA interference approach was taken. Efficient knockdown of TLR4 in human skin fibroblasts transfected with TLR4 small-interfering RNA (siRNA) was accompanied by complete loss of the synergistic effect of LPS on TGF-β1–stimulated collagen gene expression (Figure 5C). No change in Smad1/2/3 was observed as an off-target effect. Next, primary skin fibroblasts explanted from TLR4-mutant C3H/HeJ mice and WT control C3H/HeOuJ mice were incubated with LPS and TGF-β1. Western blot and qPCR analysis showed that, in contrast to WT control fibroblasts, in TLR4-mutant fibroblasts, LPS failed to synergize with TGF-β1 to stimulate collagen or α-SMA gene expression or stress fiber incorporation (Figure 5, D and E, and data not shown). As expected, LPS also failed to stimulate IL-6 mRNA expression, confirming the loss of functional TLR4 signaling in these mutant fibroblasts (data not shown). These complementary genetic and pharmacological gain-of-function and loss-of- function experiments collectively implicated TLR4 as both sufficient and necessary for synergistic stimulation of collagen gene expression in fibroblasts, establishing a crucial functional role for TLR4 signaling in augmenting the TGF-β1–dependent profibrotic response.
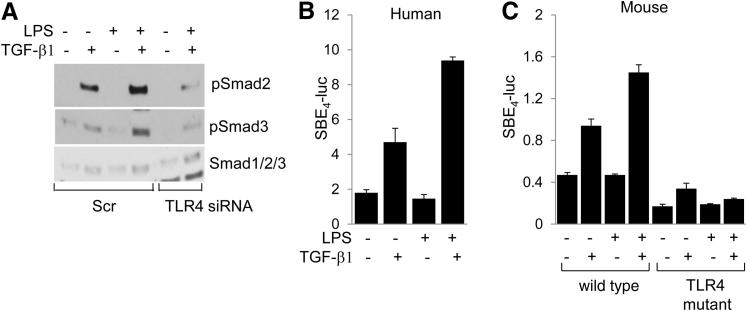
TLR4 Enhances Smad Signaling Intensity
We next focused on the effect of TLR4 on canonical Smad signaling, which was recognized as a fundamental mechanism underlying profibrotic TGF-β1 responses.29 Western blot analysis indicated that, although LPS on its own had no significant effect on Smad2/3 phosphorylation, it enhanced the stimulation induced by TGF-β1, whereas this effect was substantially attenuated by TLR4-specific siRNA, or by CLI-095 pretreatment (Figure 6A and data not shown). To investigate the effect of LPS on Smad-dependent transcription, fibroblasts were transiently transfected with [SBE]4-luc. Incubation of transfected fibroblasts with TGF-β1 in the presence of LPS resulted in significantly enhanced stimulation of Smad-responsive reporter activity compared with TGF-β1 alone (Figure 6B). Moreover, in contrast to WT fibroblasts, in TLR4-mutant fibroblasts, LPS failed to further enhance the stimulatory effect of TGF-β1 (Figure 6C). Collectively, these results indicated that TLR4 signaling in fibroblasts directly affected the canonical Smad pathway of TGF-β1 signaling.
Figure 6.
TLR4 mediates fibrotic responses via enhanced Smad signaling. A: Normal skin fibroblasts transfected with TLR4-specific siRNA or scrambled (Scr) control siRNA were incubated in media with LPS and TGF-β1 for 24 hours. Smad2/3 expression and phosphorylation were examined by using Western blot analysis. Representative immunoblots are shown. Human (B) or WT (C3H/HeOuJ) and TLR4-mutant (C3H/HeJ) mouse skin fibroblasts (C) were transfected with [SBE]4-luc in the presence of LPS and TGF-β1 for 24 hours, and cell lysates were assayed for their luciferase activities, normalized with Renilla luciferase. Results, normalized with GAPDH, are shown as the mean ± SD from triplicate determinations.
In search of mechanisms underlying the TLR4-mediated amplification of Smad signaling, we initially focused on the TGF-β1 pseudoreceptor, bone morphogenetic protein, and activin membrane-bound inhibitor (BAMBI), which negatively modulated TGF-β1 responses and were previously implicated in TLR4 signaling.13 In normal skin fibroblasts, LPS caused a time-dependent decrease in BAMBI that was abrogated by CLI-095, or by knockdown or genetic loss of TLR4 (data not shown). Because loss of BAMBI amplified the intensity of Smad-mediated TGF-β1 responses,30 down-regulation of BAMBI levels in response to TLR4 activation observed in these experiments might at least, in part, be responsible for LPS sensitizing fibroblasts to TGF-β1.
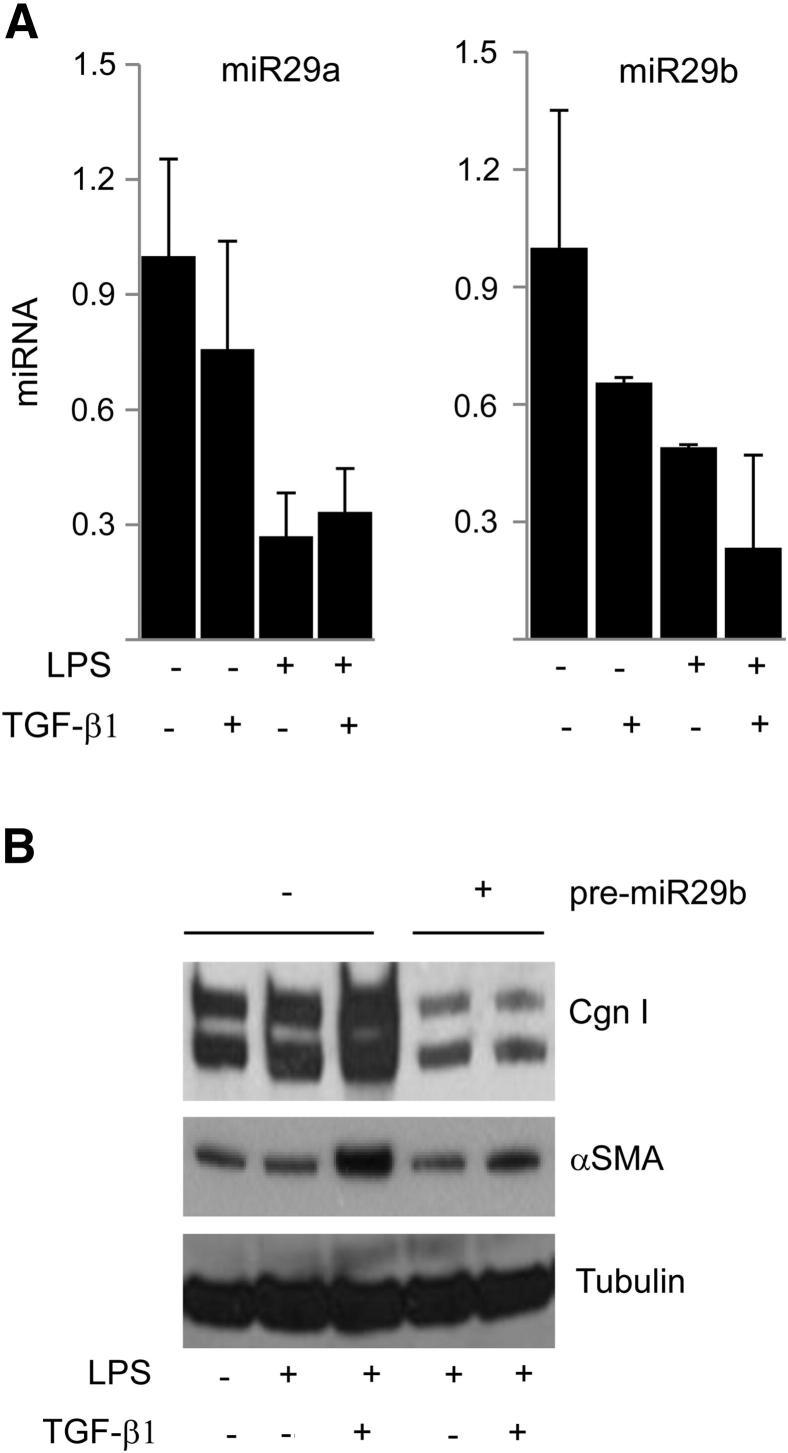
TLR4 Suppresses Anti-Fibrotic miR-29 Expression
Noncoding miRNAs regulated gene expression by binding to the 3′-untranslated region of target mRNAs and were implicated in a variety of cellular responses. In light of their emerging importance in collagen regulation, ECM homeostasis, and fibrogenesis,31,32 we investigated the effect of LPS on miRNA levels, focusing on miR-29, which was implicated in multiple fibrosing conditions. The results of qPCR analysis showed that all three miR-29 species (a, b, c) were detected in unstimulated normal skin fibroblasts. Incubation with LPS resulted in a marked reduction in miR-29 levels in both the presence or absence of TGF-β1 (Figure 7A). Most important, overexpression of pre-miR-29a and pre-miR-29b in these fibroblasts abrogated the synergistic stimulation of collagen gene expression induced by LPS in the presence of TGF-β1, functionally implicating miR-29 in sensitizing fibroblasts to TGF-β1 in the presence of LPS (Figure 7B and data not shown).
Figure 7.
TLR4 down-regulates antifibrotic miR-29. A: miRNA was isolated and subjected to qPCR analysis. Results, expressed relative to RNU24, are the mean ± SD of triplicate determinations. B: Normal skin fibroblasts were transfected with pre-miR-29b and incubated with LPS and TGF-β1 for 24 hours. Cell lysates were examined by using Western blot analysis. Representative immunoblots are shown. Cgn I, type I collagen.
Attenuated Dermal Fibrosis in TLR4-Mutant Mice
To investigate the biological role of TLR4 in the pathogenesis of fibrosis in vivo, scleroderma was induced in TLR4-mutant C3H/HeJ mice and C3H/HeOuJ WT mice by daily s.c. injections of bleomycin.27 At early time points, a comparable increase in the numbers of skin-infiltrating inflammatory cells, accompanied by increased inflammatory cytokine production, was observed in both control C3H/HeOuJ mice and TLR4-mutant mice.
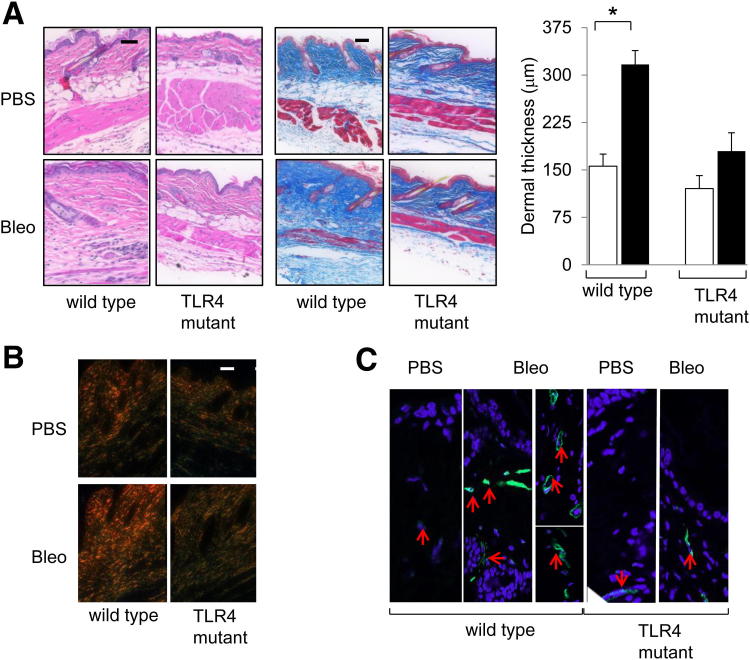
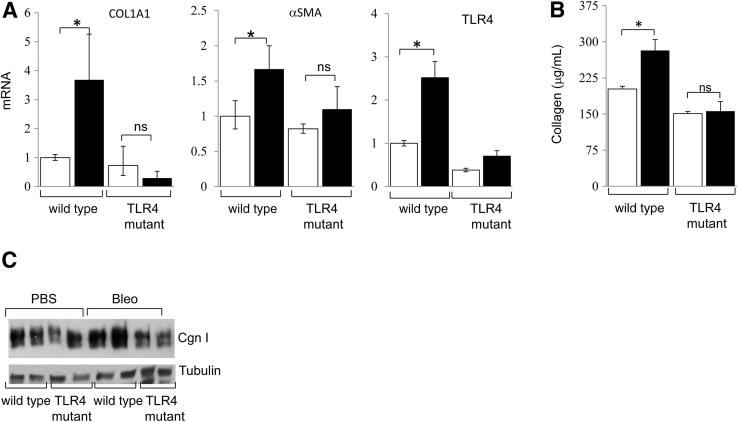
At late time points (day 21) of bleomycin injections, a considerable increase in dermal thickness (greater than twofold; P < 0.05) and accumulation of densely packed collagen bundles in the skin were evident in WT mice, compared with only a modest (approximately 30%, P = 0.11) increase in dermal thickness in C3H/HeJ mice (Figure 8A). Moreover, red birefringent collagen fiber accumulation and α-SMA expression in the lesional dermis and s.c. layers were notably attenuated in TLR4-mutant C3H/HeJ mice (Figure 8, B and C). qPCR and Sircol assays showed that a bleomycin-induced increase in collagen and α-SMA mRNA levels and collagen accumulation in the skin were virtually absent in C3H/HeJ mice (Figure 9). Examination of lungs showed no significant fibrosis or interstitial collagen accumulation in either WT C3H/OuJ or C3H/HeJ mutant mice. Interestingly, however, emphysematous changes with enlarged alveoli were noted in C3H/HeJ mice, as previously described (Supplemental Figure S3).33
Figure 8.
Attenuated skin fibrosis in TLR4-mutant mice. C3H/HeJ and WT mice, in parallel, received daily s.c. injections of PBS or bleomycin (Bleo) for up to 21 days. Mice were sacrificed, and lesional skin was examined. A: Left panel: H&E stain. Scale bar = 200 μm. Middle panel: Masson’s Trichrome stain. Scale bar = 200 μm. Right panel: Dermal thickness. Results are the mean ± SD of triplicate determination from five mice per group. Open bars, PBS; closed bars, Bleo. B: Picrosirius red stain, viewed under polarized light. Original magnification, ×100. C: Immunofluorescence images are shown. Red arrows, α-SMA–positive cells. *P < 0.05. Original magnification, ×400.
Figure 9.
Reduced fibrotic gene expression in TLR4-mutant mice. C3H/HeJ and WT mice received daily s.c. injections of PBS or bleomycin (Bleo) for 21 days. Lesional skin was harvested and analyzed. A: RNA was quantitated by qPCR. The results, normalized for 36B4, represent the mean ± SD of triplicate determinations from four mice per group. Open bars, PBS; closed bars, Bleo. B: Collagen content determined by colorimetric assays. Results represent the mean ± SD of triplicate determinations from at least three mice per group. Open bars, PBS; closed bars, Bleo. C: Protein was examined by using Western blot analysis. Cgn I, type I collagen. *P < 0.05.
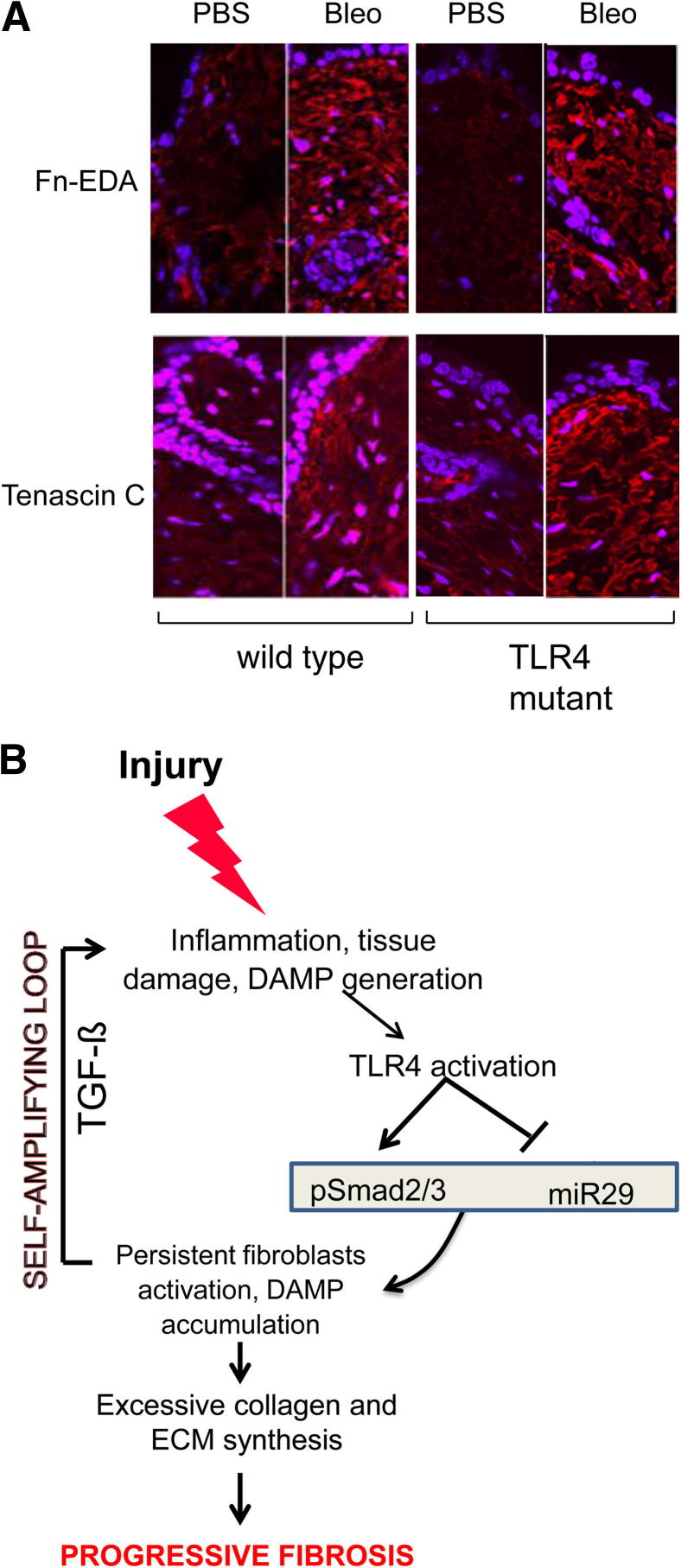
Immunofluorescence demonstrated a dramatic increase in the accumulation of the endogenous TLR4 ligands Fn-EDA and tenascin C in the skin in both WT and TLR4-mutant C3H/HeJ mice injected with bleomycin (Figure 10A). These results demonstrated that mice with defective TLR4 signaling were protected from skin fibrosis despite the robust accumulation of damage-associated endogenous TLR4 ligands, suggesting an important functional role of TLR4 in sustaining pathological fibrogenesis. The schematic depicted in Figure 10B illustrated this proposed model for a self-sustaining cycle of fibrogenesis driven by endogenous TLR4 ligand stimulation of fibroblasts.
Figure 10.
A: Bleomycin (Bleo)-induced skin fibrosis is associated with accumulation of endogenous TLR4 ligands. TLR4-mutant mice and WT mice received daily s.c. injections of PBS or Bleo for 21 days. Lesional skin was harvested and examined by immunofluorescence. Representative images are shown. Original magnification, ×400. B: Cycle of fibrogenesis. Innate immune signaling via TLR4 switches self-limited repair into sustained fibrogenesis. Prolonged injury leads to tissue damage, and generation of endogenous TLR4 ligands, with activation of TLR4 and sustained fibroblast activation.
Discussion
The mechanisms underlying the persistence and progression of fibrosis in scleroderma remain poorly understood. Emerging evidence suggests a role for innate immune recognition signaling in wound healing and fibrogenesis.34 Our present results show that levels of both TLR4 and damage-associated endogenous TLR4 ligands were elevated in skin and lung tissues from patients with scleroderma, and fibroblasts explanted from lesional skin showed elevated TLR4 expression in vitro. The results provide evidence supporting a role for TLR4 signaling induced by endogenous TLR4 ligands in fibroblast activation and persistent fibrogenesis. Activated TLR4 signaling in fibroblasts induced a genome-wide cellular response, with up-regulation of genes involved in ECM synthesis, connective tissue remodeling, and fibrogenesis. Moreover, the TLR4 ligand, LPS, sensitized fibroblasts to the profibrotic effects of TGF-β1, resulting in a substantial synergistic stimulation of fibrogenic response. These LPS-induced stimulatory responses appeared to be partially dependent on TLR4-mediated down-regulation of the endogenous TGF-β1 antagonist, BAMBI, resulting in unopposed Smad signaling, and suppression of the anti-fibrotic miR-29, causing exaggerated TGF-β1 responses and progressive fibrogenesis. Mutant mice with nonfunctional TLR4 signaling failed to develop bleomycin-induced skin fibrosis. These findings, therefore, implicate TLR4 as a novel fibrotic mediator with a fundamental role in the persistence and progression of pathological fibrosis.
Toll-like receptors are expressed on both inflammatory and parenchymal cells, and initiate immune responses when activated by pathogen-associated molecular patterns.4 We found constitutive TLR4 expression associated with fibroblasts and vascular cells in the lesional dermis in scleroderma skin biopsy specimens, and parenchymal fibroblasts and infiltrating inflammatory cells at or adjacent to fibrotic loci in lung biopsy specimens. Elevated expression of TLR4 has been noted in diabetic nephropathy, chronic periodontitis, and inflammatory bowel disease.35–37 In chronic inflammatory conditions, TLR4 may be activated by damage-associated endogenous ligands, such as Fn-EDA, HA, tenascin C, and biglycan, that are generated in situ as a consequence of chronic injury. TLR4 is also implicated in fibrogenesis, and mice lacking Fn-EDA and tenascin-C fail to develop fibrosis in the lung.38,39 We observed elevated TLR4 expression in scleroderma skin, lungs, and explanted fibroblasts. Moreover, our results suggest that signaling through elevated TLR4 may contribute to the activated phenotype of explanted scleroderma fibroblasts. The underlying mechanism is not known and might be due, in part, to stimulation by endogenous TLR4 ligands forming a self-amplifying positive feedback loop, resulting in persistent progression of fibrosis.
We demonstrated that activation of TLR4 signaling by LPS in fibroblasts elicited significant global changes in gene expression, with modulation of many genes involved in ECM remodeling, tissue repair, and wound healing. In contrast, LPS induced only a relatively modest effect on inflammation-associated genes in fibroblasts. In inflammatory cells, LPS induces many genes associated with innate immunity and inflammation40,41; thus, our results suggest distinct roles for TLR4 signaling in inflammatory cells, where it serves primarily to elicit a powerful non-specific inflammatory response, versus parenchymal cells, where it might serve primarily to promote robust tissue repair.
We found that LPS enhanced Smad2/3-dependent TGF-β1 transcriptional responses in normal fibroblasts through TLR4. These findings are consistent with other reports delineating a profibrotic TLR4 response and enhanced Smad activation.13
In contrast to our results, Kim and Kim42 showed that LPS blocked TGF-β–Smad signaling in RAW264.7 cells by inducing Smad3 phosphorylation in the linker region. This divergence of LPS responses might be related to the cell specificity, with mesenchymal cells and fibroblasts showing positive cross talk between TLR4 and TGF-β, whereas inhibitory cross talk is observed in inflammatory cells. LPS induced down-regulation of BAMBI, a member of the TGF-β type I receptor family that lacks the intracellular kinase domain.30 BAMBI inhibits TGF-β signaling by forming a ternary complex with Smad7 and the type I receptor, resulting in blockade of Smad3 activation.43 Down-regulation of BAMBI sensitized LPS-treated fibroblasts to TGF-β signaling, resulting in increased Smad2/3 phosphorylation and Smad-driven transcriptional responses. Our findings are consistent with those in LPS-treated hepatic stellate cells.13
miRNAs are involved in the regulation of a broad range of physiological and pathological processes.31,44 Recent evidence suggests that miRNAs play important roles in connective tissue regulation, wound healing, and pathological fibrosis.31 In particular, miR-29 has potent effects on collagen gene expression in normal and scleroderma fibroblasts,32 and its expression is reduced in mouse models of renal, pulmonary, and liver fibrosis.45 Patients with advanced liver cirrhosis showed significantly reduced serum and tissue levels of miR-29.46 In the present studies, we found that LPS caused a potent decrease in miR-29 expression in normal fibroblasts that was TLR4 dependent. More important, rescuing miR-29 in LPS-treated fibroblasts abrogated the stimulation of collagen gene expression in the presence of TGF-β1, directly implicating repression of miR-29 as a contributor to exaggerated response to TGF-β1.
A crucial role of TLR4 in fibrogenesis was highlighted in experiments with TLR4-mutant C3H/HeJ mice.7 In the absence of functional TLR4 signaling, bleomycin-induced fibrosis was attenuated. Previous studies13–15 have implicated TLR4 in hepatic fibrosis after bile duct ligation and renal fibrosis after ureteral ligation. We also found that bleomycin caused a dramatic accumulation of the endogenous ligands Fn-EDA and tenascin-C in the dermis. Endogenous TLR4 ligands have been previously implicated in fibrogenesis, and mice lacking Fn-EDA or tenascin-C fail to develop fibrosis when challenged with bleomycin.38,39
In summary, the present results indicate that the expression of both TLR4 and of its endogenous ligands is markedly elevated in lesional skin and lungs from patients with scleroderma. Stimulation of TLR4 in explanted normal fibroblasts was associated with the induction of many genes involved in ECM remodeling and tissue repair, and synergistic enhancement of TGF-β1–mediated fibrotic responses. Although multiple mechanisms underlie these profibrotic effects of TLR4 in fibroblasts, enhanced Smad signaling with down-regulation of BAMBI and suppression of miR-29, which normally limits the intensity and duration of fibrotic responses, appear to be prominent. The relative deficiency of these physiological restraints on fibroblast activity associated with sustained TLR4 signaling might be expected to result in exaggerated and persistent collagen synthesis and myofibroblast transition due to unopposed TGF-β signaling. Thus, in a fibrogenic milieu enriched with TGF-β and endogenous TLR4 ligands, fibroblasts expressing elevated TLR4 are likely to engage in unrestrained collagen synthesis, myofibroblast transformation, and related fibrogenic responses contributing to progression of fibrosis. Because the fibrogenic response itself contributes to further accumulation of endogenous TLR4 ligands in the cellular environment, a self-sustaining feed-forward loop might develop that underlies maintenance and progression of fibrosis. Disrupting persistent TLR4 signaling by targeting endogenous ligand accumulation or its interaction with TLR4, or by blocking intracellular TLR4 signaling, represents a potential novel strategy for breaking the cycle of progressive fibrosis in scleroderma.
Acknowledgments
We thank Ruslan Medzhitov (Howard Hughes Medical Institute, Yale School of Medicine), Andrei E. Medvedev (University of Maryland School of Medicine), and Robert P. Schleimer (Northwestern University) for valuable reagents, the Northwestern University Microarray Core and confocal imaging facility core for technical assistance, and members of the Varga laboratory for helpful discussions.
Footnotes
Supported by NIH grants AR-42309 and AR055240.
Contributor Information
Swati Bhattacharyya, Email: s-bhattacharyya@northwestern.edu.
John Varga, Email: j-varga@northwestern.edu.
Supplemental Data
TLR4 is functional in normal skin fibroblasts. A: Confluent skin fibroblasts were incubated with LPS for 24 hours, and total RNA was examined by qPCR. Results, normalized with GAPDH mRNA, are shown as the mean ± SD from triplicate determinations. B: Fibroblasts transiently transfected with NF-κB-luc were incubated with LPS in the presence or absence of CLI-095 for 24 hours. Whole cell lysates were assayed for their luciferase activities. The results are given as the mean ± SD of triplicate determinations.
Heat map of LPS-induced transcriptional responses in skin fibroblasts (FDR, 0.05, and twofold change). Dermal fibroblasts were incubated with LPS for 6 hours, and RNA was isolated and hybridized to Illumina human HT-12 version 4 Expression Microarray Chips, as previously described.19 The heat map shows the fold change of gene expression compared with the average in control samples. Red, increased; green, decreased.
Lungs from 12-week-old TLR4-mutant mice and WT controls. Trichrome stain. Scale bars: upper panels, 50 mm; lower panels, 25 mm.
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2012.09.007.
References
- 1.Bhattacharyya S., Wei J., Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol. 2011;8:42–54. doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varga J., Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann J.A., Kafatos F.C., Janeway C.A., Ezekowitz R.A. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 5.Huang Q.Q., Pope R.M. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landreth G.E., Reed-Geaghan E.G. Toll-like receptors in Alzheimer’s disease. Curr Top Microbiol Immunol. 2009;336:137–153. doi: 10.1007/978-3-642-00549-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poltorak A., He X., Smirnova I., Liu M.Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 8.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Piccinini A.M., Midwood K.S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/672395. pii: 672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H., Chen G., Wyburn K.R., Yin J., Bertolino P., Eris J.M., Alexander S.I., Sharland A.F., Chadban S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gondokaryono S.P., Ushio H., Niyonsaba F., Hara M., Takenaka H., Jayawardana S.T., Ikeda S., Okumura K., Ogawa H. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J Leukoc Biol. 2007;82:657–665. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 12.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 13.Seki E., De Minicis S., Osterreicher C.H., Kluwe J., Osawa Y., Brenner D.A., Schwabe R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 14.Pulskens W.P., Rampanelli E., Teske G.J., Butter L.M., Claessen N., Luirink I.K., van der Poll T., Florquin S., Leemans J.C. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol. 2010;21:1299–1308. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell M.T., Hile K.L., Zhang H., Asanuma H., Vanderbrink B.A., Rink R.R., Meldrum K.K. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res. 2011;168:e61–e69. doi: 10.1016/j.jss.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H.Z., Wang J.P., Mi S., Liu H.Z., Cui B., Yan H.M., Yan J., Li Z., Liu H., Hua F., Lu W., Hu Z.W. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol. 2012;180:275–292. doi: 10.1016/j.ajpath.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya S., Chen S.J., Wu M., Warner-Blankenship M., Ning H., Lakos G., Mori Y., Chang E., Nihijima C., Takehara K., Feghali-Bostwick C., Varga J. Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma. Am J Pathol. 2008;173:1085–1099. doi: 10.2353/ajpath.2008.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang F., Ooka K., Bhattacharyya S., Wei J., Wu M., Du P., Lin S., Del Galdo F., Feghali-Bostwick C.A., Varga J. The early growth response gene Egr2 (Alias Krox20) is a novel transcriptional target of transforming growth factor-β that is up-regulated in systemic sclerosis and mediates profibrotic responses. Am J Pathol. 2011;178:2077–2090. doi: 10.1016/j.ajpath.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharyya S., Sargent J.L., Du P., Lin S., Tourtellotte W.G., Takehara K., Whitfield M.L., Varga J. Egr-1 induces a profibrotic injury/repair gene program associated with systemic sclerosis. PLoS One. 2011;6:e23082. doi: 10.1371/journal.pone.0023082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du P., Kibbe W.A., Lin S.M. nuID: a universal naming scheme of oligonucleotides for Illumina, Affymetrix, and other microarrays. Biol Direct. 2007;2:16. doi: 10.1186/1745-6150-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du P., Kibbe W.A., Lin S.M. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 22.Lin S.M., Du P., Huber W., Kibbe W.A. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 2008;36:e11. doi: 10.1093/nar/gkm1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharyya S., Ghosh A.K., Pannu J., Mori Y., Takagawa S., Chen G., Trojanowska M., Gilliam A.C., Varga J. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor beta. Arthritis Rheum. 2005;52:1248–1258. doi: 10.1002/art.20996. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya S., Wei J., Melichian D.S., Milbrandt J., Takehara K., Varga J. The transcriptional cofactor nab2 is induced by TGF-β and suppresses fibroblast activation: physiological roles and impaired expression in scleroderma. PLoS One. 2009;4:e7620. doi: 10.1371/journal.pone.0007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brissett M., Veraldi K.L., Pilewski J.M., Medsger T.A., Jr., Feghali-Bostwick C.A. Localized expression of tenascin in systemic sclerosis-associated lung fibrosis and its regulation by insulin-like growth factor binding protein 3. Arthritis Rheum. 2012;64:272–280. doi: 10.1002/art.30647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 27.Wu M., Melichian D.S., de la Garza M., Gruner K., Bhattacharyya S., Barr L., Nair A., Shahrara S., Sporn P.H., Mustoe T.A., Tourtellotte W.G., Varga J. Essential roles for early growth response transcription factor Egr-1 in tissue fibrosis and wound healing. Am J Pathol. 2009;175:1041–1055. doi: 10.2353/ajpath.2009.090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontana M.F., Vance R.E. Two signal models in innate immunity. Immunol Rev. 2011;243:26–39. doi: 10.1111/j.1600-065X.2011.01037.x. [DOI] [PubMed] [Google Scholar]
- 29.Varga J. Scleroderma and Smads: dysfunctional Smad family dynamics culminating in fibrosis. Arthritis Rheum. 2002;46:1703–1713. doi: 10.1002/art.10413. [DOI] [PubMed] [Google Scholar]
- 30.Onichtchouk D., Chen Y.G., Dosch R., Gawantka V., Delius H., Massague J., Niehrs C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 31.Pandit K.V., Milosevic J., Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Maurer B., Stanczyk J., Jungel A., Akhmetshina A., Trenkmann M., Brock M., Kowal-Bielecka O., Gay R.E., Michel B.A., Distler J.H., Gay S., Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X., Shan P., Jiang G., Cohn L., Lee P.J. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meneghin A., Hogaboam C.M. Infectious disease, the innate immune response, and fibrosis. J Clin Invest. 2007;117:530–538. doi: 10.1172/JCI30595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin M., Yiu W.H., Wu H.J., Chan L.Y., Leung J.C., Au W.S., Chan K.W., Lai K.N., Tang S.C. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23:86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojo-Botello N.R., García-Hernández A.L., Moreno-Fierros L. Expression of toll-like receptors 2, 4 and 9 is increased in gingival tissue from patients with type 2 diabetes and chronic periodontitis. J Periodontal Res. 2012;47:62–73. doi: 10.1111/j.1600-0765.2011.01405.x. [DOI] [PubMed] [Google Scholar]
- 37.Brint E.K., MacSharry J., Fanning A., Shanahan F., Quigley E.M. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106:329–336. doi: 10.1038/ajg.2010.438. [DOI] [PubMed] [Google Scholar]
- 38.Muro A.F., Moretti F.A., Moore B.B., Yan M., Atrasz R.G., Wilke C.A., Flaherty K.R., Martinez F.J., Tsui J.L., Sheppard D., Baralle F.E., Toews G.B., White E.S. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carey W.A., Taylor G.D., Dean W.B., Bristow J.D. Tenascin-C deficiency attenuates TGF-ss-mediated fibrosis following murine lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L785–L793. doi: 10.1152/ajplung.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin P., Han T.H., Ren J., Saunders S., Wang E., Marincola F.M., Stroncek D.F. Molecular signatures of maturing dendritic cells: implications for testing the quality of dendritic cell therapies. J Transl Med. 2010;8:4. doi: 10.1186/1479-5876-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ban J.Y., Kim B.S., Kim S.C., Kim D.H., Chung J.H. Microarray analysis of gene expression profiles in response to treatment with melatonin in lipopolysaccharide activated RAW 264.7 cells. Korean J Physiol Pharmacol. 2011;15:23–29. doi: 10.4196/kjpp.2011.15.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim E.Y., Kim B.C. Lipopolysaccharide inhibits transforming growth factor-beta1-stimulated Smad6 expression by inducing phosphorylation of the linker region of Smad3 through a TLR4-IRAK1-ERK1/2 pathway. FEBS Lett. 2011;585:779–785. doi: 10.1016/j.febslet.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 43.Yan X., Lin Z., Chen F., Zhao X., Chen H., Ning Y., Chen Y.G. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem. 2009;284:30097–30104. doi: 10.1074/jbc.M109.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jinek M., Doudna J.A. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–412. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 45.Zhong X., Chung A.C., Chen H.Y., Meng X.M., Lan H.Y. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol. 2011;22:1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roderburg C., Urban G.W., Bettermann K., Vucur M., Zimmermann H., Schmidt S., Janssen J., Koppe C., Knolle P., Castoldi M., Tacke F., Trautwein C., Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TLR4 is functional in normal skin fibroblasts. A: Confluent skin fibroblasts were incubated with LPS for 24 hours, and total RNA was examined by qPCR. Results, normalized with GAPDH mRNA, are shown as the mean ± SD from triplicate determinations. B: Fibroblasts transiently transfected with NF-κB-luc were incubated with LPS in the presence or absence of CLI-095 for 24 hours. Whole cell lysates were assayed for their luciferase activities. The results are given as the mean ± SD of triplicate determinations.
Heat map of LPS-induced transcriptional responses in skin fibroblasts (FDR, 0.05, and twofold change). Dermal fibroblasts were incubated with LPS for 6 hours, and RNA was isolated and hybridized to Illumina human HT-12 version 4 Expression Microarray Chips, as previously described.19 The heat map shows the fold change of gene expression compared with the average in control samples. Red, increased; green, decreased.
Lungs from 12-week-old TLR4-mutant mice and WT controls. Trichrome stain. Scale bars: upper panels, 50 mm; lower panels, 25 mm.