Summary
Given the "inborn" nature of the innate immune system, it is surprising to find that innate immune function does in fact change with age. Similar patterns of distinct Toll-like receptor (TLR)-mediated immune responses come to light when one contrasts innate immune development at the beginning of life with that toward the end of life. Importantly, these developmental patterns of innate cytokine responses correlate with clinical patterns of susceptibility to disease: A heightened risk of suffering from excessive inflammation is often detected in prematurely born infants, disappears over the first few months of life, and reappears toward the end of life. In addition, risk periods for particular infections in early life reemerge in older adults. The near-mirror-image patterns that emerge in contrasts of early versus late innate immune ontogeny emphasize changes in host-environment interactions as the underlying molecular and teleologic drivers.
Introduction
Susceptibility to infection is greatest early and late in life. Although the mechanistic basis for these clinical observations might differ for different age groups, the functional parallels are striking. For example, the risk for infection rapidly decreases from pre-term to term newborns and decreases even further in infancy (Goldenberg et al., 2010; Lawn et al., 2005). However, after ~65 years of age, the risk of suffering severe infections increases again with advancing age (Ongradi and Kovesdi, 2010; Yoshikawa, 1981). Severe infections early in life often have lasting impact even on those who survive, resulting in alteration of the immune response to subsequent infections (Strunk et al., 2012a; Strunk et al., 2012c) as well as affecting the risk for autoimmune disease (Lisciandro and van den Biggelaar, 2010; Shanks et al., 2000) and malignancy (Goldin et al., 2011). Infections late in life not only accelerate the general aging process but also often represent "catastrophic events" from which seniors do not fully recover (McElhaney and Effros, 2009). Furthermore, both of these age-defined high-risk groups also display a suboptimal response to many vaccines, impairing our ability to protect these large segments of the population (Mortellaro and Ricciardi-Castagnoli, 2011).
Innate immune function can broadly be categorized into sensor, effector, and regulatory functions; sensor activation of innate immune cells triggers the downstream effector and regulatory function (Mantovani et al., 2011; Netea et al., 2011a). The best-known innate sensors are pattern-recognition receptors (PRRs), of which the Toll-like receptors (TLRs) have received substantial attention (Travis, 2011); however, other PRRs (e.g., integrins, C-type lectin receptors, NOD-like receptors, and inflammasomes) are equally important sensor pathways, both on their own and via crosstalk with TLRs (Kawai and Akira, 2011; Negishi et al., 2012). Here, we discuss how age-dependent changes in innate TLR responses correlate with specific periods of susceptibility to infection, inflammatory diseases, and responses to vaccination. These correlations suggest that TLR-mediated innate immune responses are most critically important at the extreme ends of life.
Early Life
TLR Expression, Signaling, and Function
TLR sensor function is well developed in newborns (Strunk et al., 2011). The expression of TLR, as well as downstream signaling molecules, in healthy infants over the first 5 years of life appears to be stable and to occur at adult-like levels (Danis et al., 2008; Dasari et al., 2011; Reece et al., 2011; Tulic et al., 2011). Furthermore, human newborns with bacterial sepsis are able to appropriately upregulate TLR expression on peripheral blood mononuclear cells (Zhang et al., 2010). Thus, differential TLR expression appears unlikely to be a main cause for altered susceptibility to infection, inflammation, or vaccine response in early versus adult life.
Although, TLR expression and signaling in early life appear similar to expression and signaling in adults, TLR-mediated production of innate immune effector molecules such as oxygen radicals is strikingly reduced in early life (Chang et al., 2011; De Paepe et al., 2011; Lavoie et al., 2010; Vento and Tanko, 2009). The response of preterm infants to oxygen radicals, on the other hand, is exaggerated; lower superoxide dismutase expression results in decreased clearance of oxygen radicals (Nassi et al., 2009). Further compounding this tissue-damaging response in premature infants, oxygen radicals themselves signal directly via TLR2 (Paul-Clark et al., 2009) and increase the sensitivity of TLR8 (Yanagisawa et al., 2009), whereas membrane phospholipids oxidized by oxygen radicals directly signal via TLR4 (Imai et al., 2008). These mechanisms further enhance the already tissue-damaging inflammation (Rubartelli et al., 2011; Zhou et al., 2010).
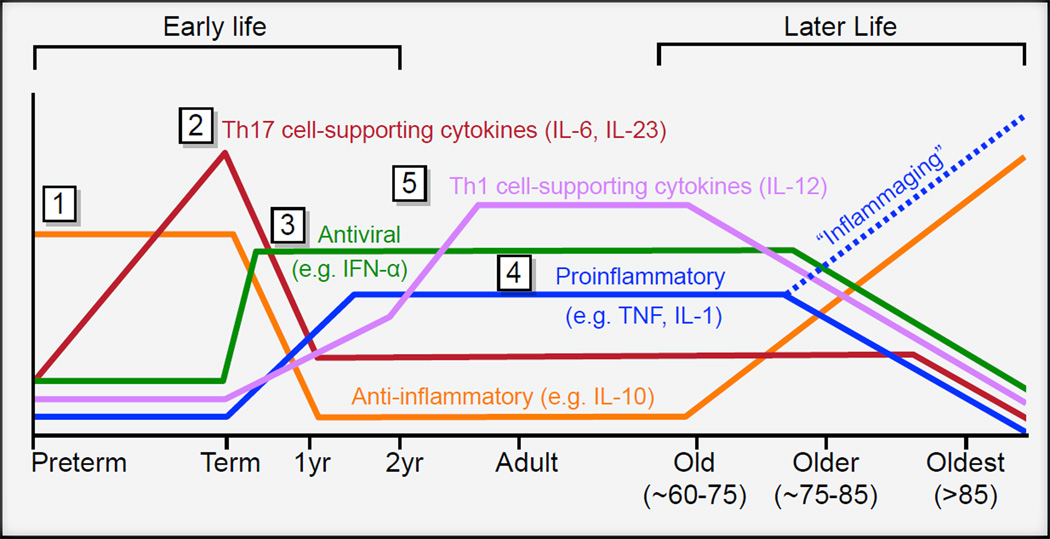
The developmental pattern of cytokine production after TLR stimulation has been characterized in detail from premature infants to adults (Figure 1). These cytokines released upon TLR stimulation have potent regulatory effect, on both innate and adaptive immune cells (Buonaguro and Pulendran, 2011; Kasturi et al., 2011; Pulendran and Ahmed, 2006). For example, after TLR stimulation of whole blood, production of anti-inflammatory innate cytokines (IL-10) dominates in preterm infants, whereas production of Th17 cell-promoting cytokines IL-6 and IL-23 dominates in term infants (Belderbos et al., 2009; Burl et al., 2011; Corbett et al., 2010; Kollmann et al., 2009; Lavoie et al., 2010; Lisciandro et al., 2012; Nguyen et al., 2010). As a result, compared to adults, term infants have elevated numbers and increased function of Th17 cells (Black et al., 2012). Interestingly, production of IL-10, IL-6, and IL-23 declines over the first few years of life; this decline is paralleled by a steady increase in production of the proinflammatory cytokines TNFα, IL-1β in whole blood, monocytes, and conventional dendritic cells (cDCs) (Belderbos et al., 2009; Burl et al., 2011; Corbett et al., 2010; Kollmann et al., 2009; Lavoie et al., 2010; Lisciandro et al., 2012; Nguyen et al., 2010). TLR-induced antiviral and Th1 cell-supporting type 1 IFNs in plasmacytoid dendritic cells (pDCs), although substantially reduced at birth, rapidly reach adult-level function within a few weeks after birth (Nguyen et al., 2010). One of the last cytokines to reach adult-level production in cDCs after TLR stimulation is IL-12p70, which promotes the development of Th1 cell immune responses (Corbett et al., 2010).
Figure 1. Age-Dependent Changes in TLR-Induced Immune Regulatory Function.
Early Life: (1) Cord blood of pre-term infants produces large amounts of the anti-inflammatory cytokine IL-10 but low amounts of proinflammatory cytokines. (2) Whole blood of term newborns produces large quantities of IL-6 and IL-23 after TLR stimulation; these cytokines are known to support Th17 cell differentiation. (3) TLR-mediated induction of type 1 IFN in pDCs is markedly reduced at birth but rapidly reaches adult-level function within a few weeks after birth. (4) Over the first few years of life, TLR-induced generation of proinflammatory cytokines such as TNF-a and IL-1b steadily increases in monocytes and cDCs. The gradual postnatal increase in the ability to produce TNF-a and IL-1b is paralleled by a slow decline of IL-10, IL-6, and IL-23. (5) TLR-mediated production of Th1-cell-supporting cytokines such as IL-12p70 reaches adult levels only after 2 years of age. Later Life: Chronic low-grade systemicinflammation (inflammaging) reflected by higher plasmatic levels of IL-6, TNF-a, and other innate cytokines; “unimpaired” older people instead express higher levels of IL-10 and lower levels of most other innate cytokines in response to whole-blood TLR stimulation.
Molecular Mechanisms
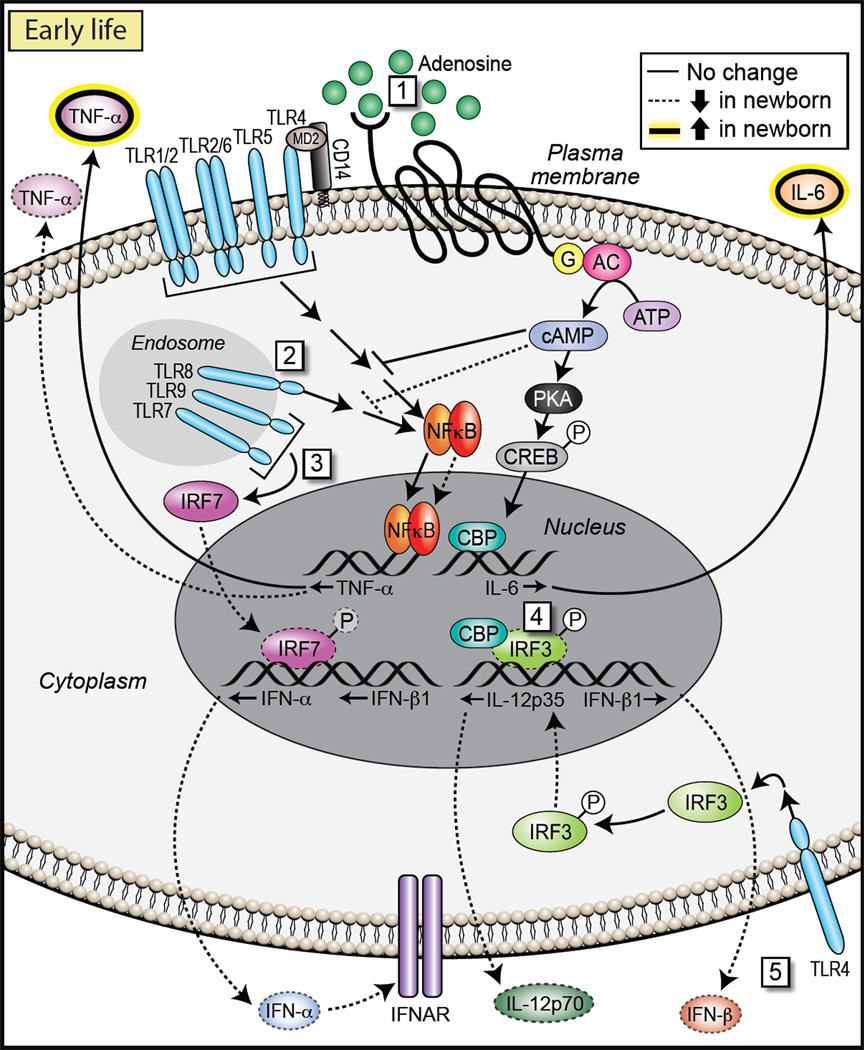
Very little is known about the underlying molecular mechanism(s) responsible for developmental change of TLR-mediated innate function early in life (Figure 2). Among the best-studied differences in transcriptional regulation of innate immune gene expression between newborns and adults are the interferon response factor (IRF) family of transcription factors, namely IRF3, IRF5, and IRF7 (Goriely and Goldman, 2008; Goriely et al., 2008). Production of type 1 IFN after TLR stimulation has been found to be strikingly reduced in newborn as compared to adult blood (Danis et al., 2008). TLR7- and TLR9-mediated production of IFN-α and -β is regulated by assembly of a multi-component complex comprised of MyD88, IRAK1, IRAK4, TRAF3, and TRAF6, which allows direct recruitment and activation of IRF7. All of the molecules regulating IRF7 function, as well as IRF7 itself, are expressed at similar amounts in purified neonatal and adult pDCs. Phosphorylation by IRAK1 and subsequent activation via TRAF6 allow IRF7 to translocate to the nucleus and initiate IFN-α and -β transcription. This nuclear translocation of IRF7 appears to be impaired in the newborn, leading to markedly reduced type I IFN production in purified cord blood pDCs as compared to adult peripheral blood pDCs (Danis et al., 2008). It is currently not clear which of the specific aspects involved in this complex interaction are responsible for the reduced nuclear translocation. The rapid maturation of type 1 IFN production to adult levels within weeks of birth suggests that underlying regulatory mechanisms affecting nuclear translocation of IRF7 change over a relatively short time period.
Figure 2. Molecular Mechanisms Active Early in Life.
NF-κB activation downstream of cell-surface TLRs in newborn monocytes occurs at least at adult levels, suggesting that age-dependent differences in NF-κB-dependent cytokine production must be the result of other pathways. For example, (1) higher levels of extracellular adenosine early in life, coupled with an elevated sensitivity of G protein-coupled adenosine receptors, leads to higher cytosolic concentrations of cAMP, which might produce a bias toward Th2 and against Th1 cell responses. (2) For endosomal TLRs, TLR8 signaling in newborn cells occurs at least at adult levels, leading to robust TNFα production. However, in cord-blood plasmacytoid DCs, (3) signals downstream of TLR7 or TLR9 leading to IRF7 phosphorylation and nuclear translocation are reduced, and production of type 1 IFN is thus impaired. Furthermore, although TRIF-dependent activation and nuclear translocation of IRF3 downstream of TLR3 or TLR4 occurs at adult levels in cord-blood-derived myeloid DCs, (4) IRF3 DNA-binding activity and association with CBP is decreased in newborn DCs as compared to adult DCs, providing a basis for (5) impaired neonatal IL-12p35 and IFN-β production.
IRF5 nuclear translocation, together with activation of the transcription factors NF-κB and MAPK, is crucial for the expression of IL-12 p40 and IL-23 p19. TLR agonist stimulation of newborn blood induces coordinated expression of p19 and p40, resulting in IL-23 secretion at amounts markedly higher than those of adults (Corbett et al., 2010; Kollmann et al., 2009; Vanden Eijnden et al., 2006). These observations suggest that IRF5-, NF-κB-, and MAPK-mediated responses are functioning at least at adult levels in newborn cells. In contrast, TRIF-dependent activation of IRF3 downstream of TLR3 or TLR4 is compromised in neonatal DCs. This alteration leads to impaired production of type I IFNs and interferon-dependent genes (Aksoy et al., 2007). Induction of IL-12p35, which together with IL-12p40 is necessary for production of IL-12p70, requires direct recruitment of IRF3 and activation of the autocrine feedback loop triggered by type I IFNs. Interestingly, early steps of IRF3 activation—phosphorylation, dimerization, and nuclear translocation—occur at similar levels in adult and neonatal DCs after TLR stimulation. However, IRF3 DNA-binding activity and association with the coactivator CREB-binding protein (CBP) appear to be decreased in the human neonate as compared to the adult (Aksoy et al., 2007). The precise molecular events underlying the decreased stability of the IRF3-CBP-DNA complex formation are currently not known. They might reflect differential regulation of the two-step activation process of IRF3; phosphorylation of Ser396 via the TBK1 pathway, which is sufficient for IRF3 nuclear translocation, occurs at comparable levels in neonatal and adult cells (Aksoy et al., 2007). This suggests that phosphorylation of other Ser or Thr residues required for CBP recruitment and IRF3 binding to promoter regions might be reduced in newborn DCs. A more global age-dependent perturbation in chromatin remodeling could also be involved through an impact on the stability of transcriptional complexes for IRF3-dependent genes. Support for the latter idea comes from observations indicating impaired nucleosome remodeling in neonatal DCs at the IRF3-dependent IL12A locus (Goriely et al., 2004). IRF3 is required for initiation of SWI-SNF-mediated nucleosome remodeling for several innate cytokine genes (Ramirez-Carrozzi et al., 2009). Chromatin remodeling early in life is known to progress through developmentally regulated stages (Martino et al., 2011). For example, epigenetic regulation is centrally involved in the ontogeny of T cell IFN-γ production (Balasubramani et al., 2010; Vuillermin et al., 2009). Although it is currently unclear how expression of IFN-γ is regulated in innate immune cells, IFN-γ and SWI-SNF functionally coregulate immune gene expression at multiple levels (Pattenden et al., 2002). Thus, SWI-SNF activity, similar to IFN-γ expression, might also undergo developmentally regulated changes.
Among the mechanisms that serve to polarize neonatal immune responses, the effects of soluble plasma factors feature prominently (Levy et al., 2006). Studies of human cord blood plasma and leukocytes demonstrate that soluble factors in human newborn cord-blood plasma differentially modulate TLR-mediated cytokine production, and that they have a relatively greater impact on TLR2 agonists (bacterial lipopeptides), whereas TLR7 and TLR8 agonists such as imidazoquinolines and single-stranded RNAs appear to be relatively refractory to this inhibition (Levy et al., 2004; Philbin et al., 2012). Compared to adult blood plasma, human cord-blood plasma contains ~4-fold-higher concentrations of adenosine, an endogenous purine metabolite that acts via seven-transmembrane adenosine receptors to enhance intracellular concentrations of the second messenger cyclic adenosine monophosphate (cAMP). In turn, cAMP inhibits production of Th1-cell-polarizing cytokines by both protein-kinase-A-dependent and - independent mechanisms. In addition, neonatal mononuclear cells are more sensitive to the inhibitory effects of adenosine. The adenosine-cAMP signaling pathway generally appears to suppress production of Th1-cell-polarizing cytokines such as IL-12p70 and IFN-γ while enhancing production of anti-inflammatory (IL-10) and Th17 cell (IL-6, IL-23)-supporting innate cytokines. This pattern matches neonatal immune polarization (Drygiannakis et al., 2011; Levy, 2007; Levy et al., 2006; Levy et al., 2004; Philbin et al., 2012; Power Coombs et al., 2011). Of note, expression of soluble factors that impair TLR4-mediated IL-12p70 production and enhance TLR4-mediated IL-10 production evolve distinctly over the first weeks of life, raising the possibility that additional soluble factors contribute to immune polarization (Belderbos et al., 2012). Overall, age-dependent expression of plasma-modulating factors appears to be an important mechanism that regulates ontogeny of responses to TLR agonists.
Innate immune ontogeny is further impacted by early exposure to environmental triggers that alter molecular mechanisms of TLR-mediated cytokine production. For example, upon an infant's initial exposure to LPS shortly after birth, intestinal epithelial cells become hypo-responsive to subsequent TLR stimulation, presumably to facilitate microbial colonization and host-microbe homeostasis. The mechanisms underlying this transition include micro-RNA-mediated downregulation of IL-1 receptor-associated kinase-1 (IRAK-1)(Lotz et al., 2006) and increased expression of the negative regulator IRAK-M (Leavy, 2010; Nanthakumar et al., 2011). Lastly, it is possible that innate immune cells develop from different stem cell lineages at different times during life, as has been shown for human T cells (Mold et al., 2010); a different origin might support different regulatory mechanisms in the same innate cell lineages.
Impact on Host Immune Response in Early Life
The TLR-induced innate cytokine response pattern in early life correlates with patterns of age-specific susceptibility to several of the most common serious systemic infections of early childhood (Table 1) (Kenzel and Henneke, 2006).
Table 1.
Age-Associated Patterns of Innate TLR Response and Risk for Infection
| Age and Innate Immune Status | Associations with Infectious Disease |
|---|---|
| Premature newborn; mostly anti-inflammatory (high IL-10); all other innate responses low | Invasive infections due to extracellular pathogens such as E. coli, coagulase-negative staphylococcus (CoNS), and Candida spp. are largely restricted to premature infants. Optimal defense against all of these depends on Th-17-type adaptive immunity. This pattern of susceptibility correlates with the respective low-TLR-induced production of Th17-supporting cytokines (Brereton et al., 2011; Cheung and Otto, 2010; Gaffen et al., 2011; Gow et al., 2011; Smeekens et al., 2011; Strunk et al., 2009; Strunk et al., 2010; Strunk et al., 2012b; Strunk et al., 2007; van de Veerdonk et al., 2011; Venkatesh et al., 2006). |
| Term newborn and up to ~2-month-old infant; strong innate Th17 cell support; low innate antiviral type 1 IFN response; low innate Th1 cell support | Host defense against herpes simplex virus (HSV), Listeria monocytogenes, and group B streptococci (GBS) is profoundly impacted by type 1 IFN (Charrel-Dennis et al., 2008; Currie et al., 2011; Mancuso et al., 2009; Melchjorsen, 2012; Posfay-Barbe and Wald, 2009; Rayamajhi et al., 2010; Xiao et al., 2009; Zhang et al., 2008). The highest risk period for severe HSV, listeriosis, and GBS infection is restricted to infants less than 2 months of age, correlating with the period of low TLR-mediated type 1 IFN production (Charrel-Dennis et al., 2008; Currie et al., 2011; Mancuso et al., 2009; Melchjorsen, 2012; Posfay-Barbe and Wald, 2009; Rayamajhi et al., 2010; Xiao et al., 2009; Zhang et al., 2008). |
| Child up to ~2–5 years of age; low innate Th1 cell support | Defense against Mycobacterium tuberculosis (Mtb), Burkholderia pseudomallei, and Salmonella spp. is largely dependent on Th1-celltype immunity (Ottenhoff et al., 2005; Simpson et al., 2003; White, 2003). Outside of immunocompromised adults, disseminated infections with Mtb, Burkholderia pseudomallei, and Salmonella spp. are largely restricted to children under 5 years of age (Cheng and Currie, 2005; Donald et al., 2010; Gan, 2005; Wiersinga et al., 2006). This pattern closely corresponds to reduced childhood expression of the innate cytokines that support optimal Th1 defense against these intracellular pathogens, namely IFN-γ and IL-12p70 (Ottenhoff et al., 2005; Simpson et al., 2003; White, 2003). |
| Older adult; decrease in nearly all innate TLR-induced responses; higher basal level of many proinflammatory innate cytokines in “inflammaging" | (1) Decreased TLR1 expression on monocytes correlates with high risk of reactivation of Mtb in the elderly; Mtb reactivation is a common cause of geriatric pulmonary infections (Uciechowski et al., 2011). (2) Impaired pDC function in the elderly, along with impaired type 1 IFN production, probably contributes to increased susceptibility to herpes viruses, in particular VZV reactivation and associated complications (Goldstein, 2012; Kittan et al., 2007; Leng and Goldstein, 2010; Mueller et al., 2008) as well as influenza (Canaday et al., 2010; Ferrucci et al., 1997; Fleming and Elliot, 2005; Reichert et al., 2004; Thompson et al., 2003) and listeriosis (Mook et al., 2012). (3) Older subjects fail to downregulate TLR3 in response to WNV infection; this leads to prolonged proinflammatory cytokine production, which in turn may contribute to increased permeability of the blood-brain barrier and more severe WNV infection in older individuals (Kong et al., 2008). (4) Elevated production of cytokines at baseline (i.e., inflammaging), but reduced response to stimulation, correlates with increased severity of sepsis after community-acquired pneumonia in the elderly (Mira et al., 2008). |
The TLR-mediated innate cytokine response pattern described above for early life also correlates with patterns of vaccine responsiveness. Consistent with the mostly anti-inflammatory response to innate immune stimulation in premature infants, impaired responses to vaccines given around birth have been noted (Baxter, 2010; D’Angio, 2007; Tsuda et al., 2011). However, TLR-mediated cytokine responses of preterm infants change over the first year of life. Accordingly, vaccines administered to premature infants later during the first year of life induce a response similar to that of full-term infants of the same postnatal age (D’Angio, 2007; Esposito et al., 2009). Furthermore, in term infants, vaccines, such as hepatitis B virus and Bordetella pertussis vaccines induce weak Th1-cell-type responses if administered at birth. Consistent with the change in TLR-mediated cytokine response patterns described above, the immune response to BCG immunization changes from a Th17-cell-dominated response when given at birth to a more Th1-cell-dominated response when BCG immunization is delayed for several months (Burl et al., 2010; Ota et al., 2002). These findings show that the strong influence of postnatal age on the quality of adaptive vaccine responses is mediated by changes in innate immunity.
Lastly, developmental changes in the TLR response in early life also correlate with periods of high vulnerability to organ injury from innate immune hyperactivity (Fleer and Krediet, 2007). For example, necrotizing enterocolitis (NEC) is characterized by dysregulated innate inflammatory responses in the premature infant’s intestine (Afrazi et al., 2011; Gourlay, 2012; Lin and Stoll, 2006; Martin and Walker, 2006; Nanthakumar et al., 2011; Sampath et al., 2011). Excessive reactivity of the TLR innate response also contributes to periventricular white-matter injury (PWMI) (Volpe, 2008) and neonatal chronic lung disease (CLD), both affecting extremely premature infants (De Paepe et al., 2011; Kramer et al., 2009; Nathe et al., 2009; Petrikin et al., 2010). Although this heightened risk of inflammation at first appears contrary to the aforementioned elevated amount of immune suppressive IL-10 production in preterm cord blood, regulation of innate immune responses is specific to each anatomic site, and the elevated cord blood IL-10 response is found around the time of preterm birth but not beyond that (Lavoie et al., 2010). Overall, existing data suggest that a dysregulated TLR-mediated tissue-damaging innate inflammatory response in premature infants contributes to NEC, CLD, and PWMI.
Later Life
TLR Expression, Signaling, and Function
Despite the challenge of defining "normal" aging (Ligthart et al., 1984), there is strong evidence that innate immunity and, in particular, certain TLR-mediated responses change dramatically with advancing age (McElhaney and Effros, 2009; Panda et al., 2009; Panda et al., 2010; Shaw et al., 2010). For example, whereas TLR2 expression in monocytes and cDCs (Panda et al., 2010; van Duin et al., 2007b) and TLR9 expression in pDCs (Panda et al., 2010) appears to be unchanged as age increases, TLR1 and TLR4 in monocytes and TLR1, TLR7, and TLR8 in cDCs and pDCs are generally expressed at lower levels in older individuals (Jing et al., 2009; Panda et al., 2010). In contrast, TLR5 expression in monocytes increases as age increases (Qian et al., 2012). Basal levels of TLR3 in macrophages are lower in older donors. However, the virus-induced downregulation of TLR3 expression noted in young adults fails to occur in older subjects (Kong et al., 2008). Overall, distinct, but not uniformly lower, TLR expression in later life (Panda et al., 2009; Panda et al., 2010; Shaw et al., 2010; Shaw et al., 2011) might contribute to altered innate immune function as individuals age.
Little is known regarding TLR-mediated innate effector responses late in life. Multiple macrophage and neutrophil effector functions, including priming, activation, phagocytosis of microbes, and TLR-induced superoxide generation, are reduced in later life (Fortin et al., 2008; Plackett et al., 2004). Innate TLR-mediated regulation of adaptive immune function appears to be generally diminished in old versus young adults (Panda et al., 2010; Shaw et al., 2011; van Duin et al., 2007b). Healthy, "unimpaired" older adults express increasingly higher amounts of IL-10 with advancing age (Shurin et al., 2007; Vallejo et al., 2011), in pattern that is the reverse of that found in early life (Figure 1). However, the innate immune system of the frail elderly is often found in a state of heightened inflammation, called "inflammaging" (Figure 1)(Franceschi et al., 2007; Panda et al., 2010; Qian et al., 2012). This term most often refers to increases in circulating IL-6, C-reactive protein (CRP), and TNF-α concentrations in older individuals, but it also includes higher basal concentrations of IFN-γ, IL-12p70, IP-10, and CXCL9. However, this increased basal production of proinflammatory cytokines in the frail elderly does not necessarily translate to a generally elevated response after stimulation of TLRs (Bruunsgaard et al., 1999). For example, whereas TLR5- or TLR9-induced cytokine responses are often elevated in the elderly (Agrawal et al., 2007a; Qian et al., 2012), age-associated impairments of TLR1-, TLR2-, or TLR7-induced production of IL-6 and TNF-α are frequently observed (Nyugen et al., 2010; van Duin et al., 2007b). pDCs of older donors also produce markedly lower type I IFN than do those of younger adult donors (Jing et al., 2009; Qian et al., 2011; Shaw et al., 2011). Further underscoring the complexity of changes in the TLR system with advancing age, differences in in vitro TLR4-mediated cytokine responses between the elderly and younger adults depend on the cell type investigated (Ciaramella et al., 2011; Della Bella et al., 2007; Shaw et al., 2011; van Duin et al., 2007a).
Molecular Mechanisms
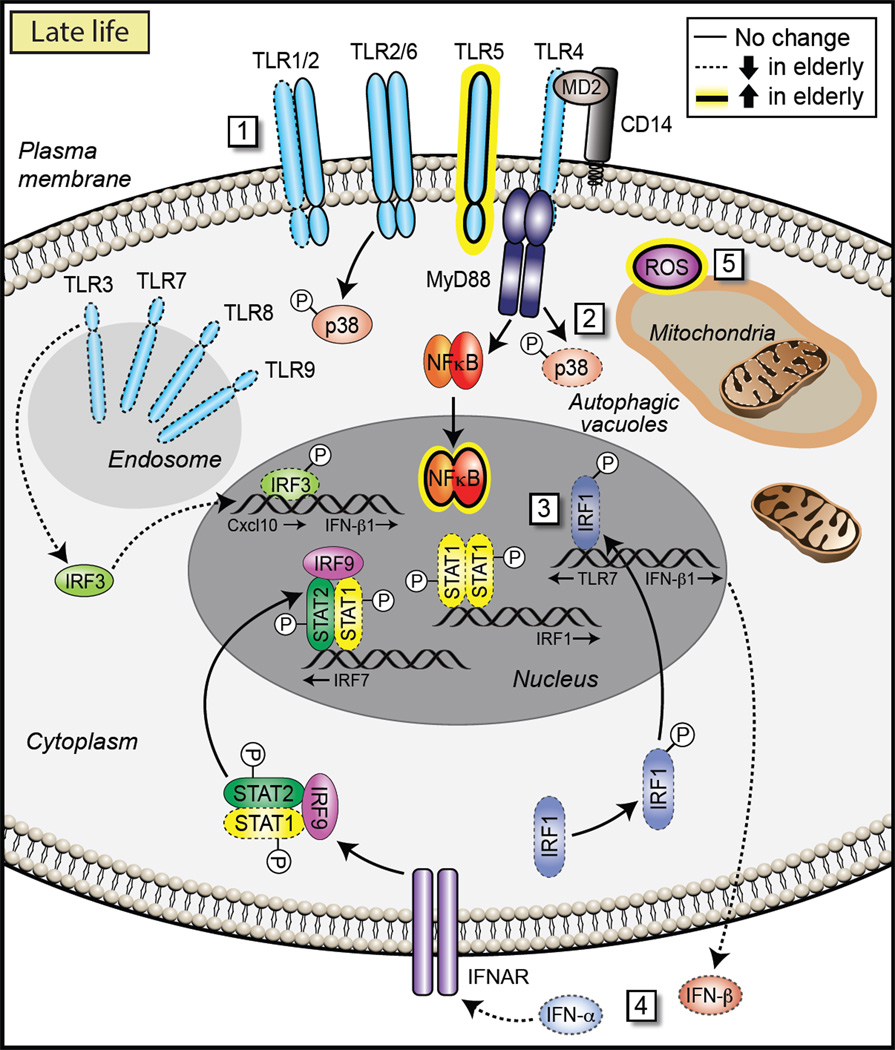
Our understanding of the cellular and molecular mechanisms underlying age-dependent changes in TLR function and age-associated chronic inflammation is only now emerging (Figure 3). Because chronic metabolic disorders (e.g., atherosclerosis, hyperlipidemia, and type 2 diabetes) also contribute to age-related inflammation, multiple signaling pathways can in fact directly or indirectly be implicated in the maintenance of this inflammatory state. It has been proposed that with aging, local accumulation of DNA damage within stromal and stem cells leads to cytokine release, an event further amplified by tissue-resident macrophages responding the these cytokines (Bonafe et al., 2012). Moreover, increased TLR-mediated IL-6 and TNF-α production by monocyte-derived DCs appears to be related to decreased activity of phospho-inositol 3(PI3) kinase, in a mechanism relevant to the inflammaging processes mentioned above (Agrawal et al., 2007a; Agrawal et al., 2007b; Shaw et al., 2011).
Figure 3. Molecular Mechanisms Active Late in Life.
(1) Reduced expression of most TLRs, except TLR5, leads to lower TLR responses in older adults. Older adults also display altered signaling downstream of TLRs. For example, they display (2) lower p38 signaling via TLR4 and (3) diminished induction of late-phase responses mediated by STAT1, IRF7, and IRF1. This, in turn, leads to (4) defective regulation of the type I IFN axis in DCs. Some differences in older adults are lineage specific: Monocytes demonstrate greater TLR5 expression and increased p38 signaling, whereas macrophages demonstrate impaired downregulation of TLR3 and elevated cytokine production. Furthermore, (5) an age-associated decrease of autophagy reduces clearance of damaged mitochondria, which elevates cellular ROS production and associated RLR signaling and inflammation, setting into motion a vicious cycle.
The molecular mechanisms underlying the decrease that occurs in innate immune function as people age have been delineated in detail for only some of these observations (Figure 3). The striking dysregulation of West-Nile-virus-induced downregulation of TLR3 apparently relates to impairment of the signal transducer and activator of transcription 1 (STAT1) pathway (Kong et al., 2008). Purified cDCs from older donors display diminished induction of late-phase responses that are mediated by STAT1, IRF7, and IRF1, suggesting defective regulation of the type I IFN axis (Qian et al., 2011); this defective regulation is also noted in TLR9-mediated antiviral responses (Stout-Delgado et al., 2008). A further insult to antiviral capacity is the age-related increase in expression of Axl, a member of the TAM (Tyro3, Axl, and Mer) family of receptor tyrosine kinases, which broadly inhibit both TLR and TLR-induced cytokine receptor cascades (Lemke and Rothlin, 2008). Higher Axl activity in the DCs of older donors is believed to negatively regulate TLR signaling and contribute to a reduced efficiency of antiviral responses (Qian et al., 2011).
Dysregulation of innate immune responses in the elderly is also influenced by intracellular crosstalk of TLRs with other innate mechanisms. One of the leading theories of age-induced cellular damage, the “free radical theory of aging,” posits that the accumulation of reactive oxygen species (ROS) in older subjects leads to damage of biomolecules (Finkel and Holbrook, 2000). According to this theory, oxidative stress inhibits cellular pathways directly, e.g., ROS inhibits IFN-α-induced antiviral gene expression by blocking the JAK-STAT pathway (Di Bona et al., 2006). Intricately linked with this is autophagy, the cellular process for appropriate clearance of dysfunctional proteins and organelles within the cell. It has been shown that autophagy declines with aging, and recent work suggests that this event can lead to amplification of antiviral signaling through the RIG-I-like pathway (Tal et al., 2009). Damaged mitochondria that are not removed by autophagy provide an ongoing source of ROS that can enhance RLR signaling and inflammation, setting up a vicious cycle (Cuervo et al., 2005; Tal et al., 2009; Vellai, 2009). Further, accumulation of damaged mitochondria leads to release of mitochondrial DNA, which contributes to activation of the NLRP3 inflammasome (Hipkiss, 2010; Tal and Iwasaki, 2011; Terman et al., 2010; Vandanmagsar et al., 2011). The scenario in the older adult overall appears to be similar to that found in the premature infant, where oxygen radicals themselves activate TLRs, either directly or indirectly via damaged biomolecules, and further amplify destructive inflammation (Paul-Clark et al., 2009; Yanagisawa et al., 2009; Imai et al., 2008).
Impact on Host Immune Response in Later Life
The aforementioned pattern of TLR-mediated innate responses correlates with pathogen-specific clinical patterns of increased susceptibility to infection in the older adult (Table 1).
TLR-mediated cytokine responses late in life also correlate with reduced vaccine responses (McElhaney et al., 2012). Although influenza vaccination in the elderly is effective at inducing protection (Bourée, 2003; Gruver et al., 2007; Nichol et al., 2003; Ongradi and Kovesdi, 2010), decreased TLR responsiveness is associated with the inability to mount protective antibody responses to the trivalent inactivated influenza vaccine (Panda et al., 2010; van Duin et al., 2007a; van Duin et al., 2007b). In addition, the age-associated elevation of serum IL-6 and TNF-α concentrations in inflammaging are associated with dampened responses to influenza vaccine (Panda et al., 2010; Trzonkowski et al., 2003) and pneumococcal vaccine (Ridda et al., 2009).
Although functional proinflammatory responses are beneficial for survival in older adults, (Wijsman et al., 2011), innate response patterns late in life strongly and consistently correlate with patterns of pathological inflammation and associated complications. For example, the elevated concentration of circulating IL-6 in inflammaging on its own is a strong predictor of thromboembolic complications and cardiovascular diseases (Ferrucci et al., 2005), whereas elevated TNF-α concentrations correlate with frailty (Bruunsgaard et al., 2003; Forsey et al., 2003; Krabbe et al., 2004; Myśliwska et al., 1998). Elevated TNF-α serum concentrations in the elderly also associate with increased risk of malignancies (Chen et al., 2007; Fulop et al., 2010; Malaguarnera et al., 2010) and neurodegeneration (Carty and Bowie, 2011; Krabbe et al., 2004; Ravaglia et al., 2007). Even depression in older adults is associated with an increased serum proinflammatory cytokine profile, and antidepressant therapy decreases IL-1β and IL-6 amounts in depressed patients (Bouhuys et al., 2004; Irwin and Miller, 2007; Penninx et al., 2003; Trzonkowski et al., 2004). Overall, elevated proinflammatory levels in the aged—perhaps under the influence of elevated levels of TLR5—predict a more than 6-fold increased risk of three-year mortality and a more than 3-fold higher risk for seven-year mortality independently of other measures of health status (Krabbe et al., 2004; Reuben et al., 2002; van den Biggelaar et al., 2004; Wikby et al., 2006). TLR pathways in older individuals with marked atherosclerosis are activated even at baseline (Huang et al., 2011); obesity further enhances this basal activation of the TLR pathway (Scholtes et al., 2011). Specific TLR4 allelic variants associate with increased body fat and decreased insulin sensitivity, suggesting that TLR4 might link obesity and insulin resistance and thereby contribute to diabetes (Weyrich et al., 2010). Age-dependent changes in TLR responses thus feature prominently in several aspects of inflammaging.
Perspective
Deciphering the clinical role of innate immune function might be most readily accomplished during periods of reduced or absent adaptive immune function, i.e., early and late in life. Recent studies indicate that in addition to age-dependent changes of adaptive immunity, innate immune function changes dramatically with age. For adaptive immunity, these changes with age are inherent in its characteristic function, namely antigen specificity and memory formation (Lewis et al., 2006; Wilson and Kollmann, 2008). It is possible that such changes in adaptive immunity contribute to changes in TLR-mediated innate immune responses. However, the above-summarized changes that occur in the TLR response as individuals age are found in purified innate cells, suggesting that at least some of these age-specific changes in innate immune function are cell autonomous. This suggestion points to the existence of innate-specific mechanisms directing innate immune ontogeny. Are the mechanisms that drive changes in TLR-induced innate responses over life related to genetically encoded developmental programs, or are they due to particular age-dependent changes of environmental exposures, or both (Netea et al., 2012)? The answer to this question not only is of great academic interest but also holds the key to identifying possible interventions aimed at reducing the risk for infectious and inflammatory diseases early and late in life.
Genetics influences innate immune ontogeny early (Netea and van der Meer, 2011) as well as late in life (Weyrich et al., 2010). Genetic defects of pattern recognition probably manifest themselves throughout life as limited, focused immunodeficiencies (Casanova and Abel, 2007; Netea et al., 2011b). On the basis of the paradigm of evolutionary purifying selection (Casals et al., 2011), one would predict that genetic programs of innate immune ontogeny more likely direct developmental patterns in early rather than late life. Indeed, the influence of genetic programs in early life can readily be identified in the strong influence of family history of atopy on lower IL-12p70 responses in infants suffering from allergy and asthma (Gabrielsson et al., 2001; Nilsson et al., 2004; van den Biggelaar et al., 2009). Moreover, TLR polymorphisms associate with an altered immune response to BCG administered to infants at birth (Randhawa et al., 2011), and genetic defects, such as IRAK-4 and MyD88 deficiency, that impair signaling downstream from TLRs demonstrate the greatest clinical impact early in life, and those surviving through the teenage years go on to an apparently normal lifespan (Ku et al., 2007). These observations strongly suggest that the TLR system is most important for optimal host defense early in life.
Environmental exposure, however, has been found to be a major modulator of innate immune ontogeny (Belderbos et al., 2012; Reikie et al. 2012; Renz et al., 2012; Taylor et al., 2006). For example, innate immune development in the young and very old is modulated by changes in the composition of the intestinal microbiome (Biagi et al., 2010; Renz et al., 2012), a relationship most apparent in situations of a compromised intestinal barrier (Figueiredo et al., 2009; Schiffrin et al., 2010). And recent studies in murine models identify a role for interplay of TLRs and inflammasome components in influencing cytokine production and obesity through modulation of the gut microflora (Elinav et al., 2011; Henao-Mejia et al., 2012; Vandanmagsar et al., 2011). Furthermore, nutrition directly impacts innate immune function (LeBouder et al., 2006; Taylor et al., 2006). Mode and season of birth also impact postnatal innate immune ontogeny long into adulthood (Belderbos et al., 2012; Blimkie et al., 2011; Malamitsi-Puchner et al., 2005; Moore et al., 2006a; Moore et al., 2006b). Even prenatal environmental stimuli potentially have long-lasting effects on postnatal innate immune development (Graham et al., 2006), a paradigm furthered in the "developmental origin of health and disease" (Barker, 2007). An emerging concept, age-specific windows of vulnerability to external influences that modulate long-term immune development have also been observed in the field of developmental immunotoxicology (Dietert, 2011).
Teleologically, the innate immune system has to be particularly sensitive to being molded by external environmental stimuli because its sentinel function is meant to detect changes in the internal and external environment (Graham et al., 2006). A recently described concept points to the memory-like innate immune function after microbial encounters of plants, invertebrates, and mammals and is labeled "trained immunity" (Netea et al., 2011a). This concept is supported by the correlations of laboratory to clinical findings regarding innate immune ontogeny we describe here, in that specific stages of innate immune function correlate with specific windows of vulnerability to particular infections. Recent clinical evidence also supports this hypothesis: even in utero, TLR stimulation impacts outcomes of subsequent episodes of postnatal sepsis (Azizia et al., 2012; Strunk et al., 2012a; Wynn et al., 2008). Taken together, these observations coalesce to form a testable hypothesis for interventions. Namely, this hypothesis is that exposure to specific environmental stimuli triggers long-lasting, clinically relevant changes in innate immunity.
Interventions aimed at altering the innate immune status of the young or old thus appear to be rational pharmaceutical targets (Hedayat et al., 2011; Melvan et al., 2010; Romagne, 2007). However, many questions need to be addressed before we can confidently chart the course for therapeutic or preventive manipulation of innate ontogeny early and late in life. For example, what precise role does prenatal exposure play for postnatal innate development (Barker, 2007)? What are the implications of altered TLR function during pregnancy? How does the innate immune response to TLR stimulation change when a child is weaned from breast to bottle feeding (Belderbos et al., 2012)? How does it change around adolescence (puberty) and menopause or andropause? What impact does altering innate immune ontogeny have on vaccine responses? Should we try to boost the budding or the aging innate immune system, or would that augment a possibly negative impact, e.g., increase asthma and allergies in the young (Lisciandro and van den Biggelaar, 2010) or inflammaging in the elderly? The first tests have been promising: vaccination with TLR5-targeted adjuvants in elderly populations that express elevated levels of TLR5 enhanced vaccine responsiveness without overtly increasing inflammation (Taylor et al., 2011).
The essential first step toward finding answers to these important questions is an increased awareness of the age-dependent changes of innate immune ontogeny and their clinically relevant implications for infectious risk, vaccine response, and inflammatory diseases, both at the beginning and toward the end of life. On the basis of our discussion of the ontogeny of TLR responses, we suggest that innate responses are not static but change with age and that they do so in ways that appear to be highly relevant to clinical practice. Delineating the underlying molecular and cellular mechanisms is likely to provide important insight of substantial clinical importance for the prevention and treatment of diseases early and later in life.
Acknowledgments
T.R.K. is supported in part by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, a Michael Smith Foundation for Health Research Career Investigator Award, an educational grant from GlaxoSmithKline, and a research grant from Advaxis. O.L. is supported by U.S. National Institutes of Health (NIH) RO1 AI067353-01A1, American Recovery and Reinvestment Act Administrative Supplement 3R01AI067353-05S1, Global Health Grant OPPGH5284 from The Bill & Melinda Gates Foundation, and sponsored support from VentiRx Pharmaceuticals. R.R.M. is supported by awards from the NIH (HHSN272201100019C and U19 AI 089992). S.G. is a research associate from le Fonds de la Recherche Scientifique-FNRS, Belgium; the Institute for Medical Immunology is sponsored by the government of the Walloon Region and GlaxoSmithKline Biologicals. The authors are grateful to Pascal Lavoie, Albert Shaw, Nathalie Compté, and Arnaud Marchant for their helpful contributions and comments and to Kristin Johnson for graphic artwork.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afrazi A, Sodhi CP, Richardson W, Neal M, Good M, Siggers R, Hackam DJ. New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr. Res. 2011;69:183–188. doi: 10.1203/PDR.0b013e3182093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 2007a;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Agrawal S, Gupta S. Dendritic cells in human aging. Exp. Gerontol. 2007b;42:421–426. doi: 10.1016/j.exger.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy E, Albarani V, Nguyen M, Laes JF, Ruelle JL, De Wit D, Willems F, Goldman M, Goriely S. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109:2887–2893. doi: 10.1182/blood-2006-06-027862. [DOI] [PubMed] [Google Scholar]
- Azizia M, Lloyd J, Allen M, Klein N, Peebles D. Immune status in very preterm neonates. Pediatrics. 2012;129:e967–e974. doi: 10.1542/peds.2011-1579. [DOI] [PubMed] [Google Scholar]
- Balasubramani A, Mukasa R, Hatton RD, Weaver CT. Regulation of the Ifng locus in the context of T-lineage specification and plasticity. Immunol. Rev. 2010;238:216–232. doi: 10.1111/j.1600-065X.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Baxter D. Impaired functioning of immune defenses to infection in premature and term infants and their implications for vaccination. Hum. Vaccin. 2010;6:494–505. doi: 10.4161/hv.6.6.12008. [DOI] [PubMed] [Google Scholar]
- Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JLL, Bont L. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin. Immunol. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belderbos ME, Houben ML, van Bleek GM, Schuijff L, van Uden NO, Bloemen-Carlier EM, Kimpen JL, Eijkemans MJ, Rovers M, Bont LJ. Breastfeeding modulates neonatal innate immune responses: A prospective birth cohort study. Pediatr. Allergy Immunol. 2012;23:65–74. doi: 10.1111/j.1399-3038.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur. J. Immunol. 2012;42:311–319. doi: 10.1002/eji.201141847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blimkie D, Fortuno ES, 3rd, Yan H, Cho P, Ho K, Turvey SE, Marchant A, Goriely S, Kollmann TR. Variables to be controlled in the assessment of blood innate immune responses to Toll-like receptor stimulation. J. Immunol. Methods. 2011;366:89–99. doi: 10.1016/j.jim.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafe M, Storci G, Franceschi C. Inflamm-aging of the stem cell niche: breast cancer as a paradigmatic example: breakdown of the multi-shell cytokine network fuels cancer in aged people. Bioessays. 2012;34:40–49. doi: 10.1002/bies.201100104. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Flentge F, Oldehinkel AJ, van den Berg MD. Potential psychosocial mechanisms linking depression to immune function in elderly subjects. Psychiatry Res. 2004;127:237–245. doi: 10.1016/j.psychres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Bourée P. Immunity and immunization in elderly. Pathol. Biol. (Paris) 2003;51:581–585. doi: 10.1016/j.patbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Brereton CF, Sutton CE, Ross PJ, Iwakura Y, Pizza M, Rappuoli R, Lavelle EC, Mills KH. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. J. Immunol. 2011;186:5896–5906. doi: 10.4049/jimmunol.1003789. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin. Exp. Immunol. 1999;118:235–241. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Pulendran B. Immunogenomics and systems biology of vaccines. Immunol. Rev. 2011;239:197–208. doi: 10.1111/j.1600-065X.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burl S, Adetifa UJ, Cox M, Touray E, Ota MO, Marchant A, Whittle H, McShane H, Rowland-Jones SL, Flanagan KL. Delaying bacillus Calmette-Guérin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J. Immunol. 2010;185:2620–2628. doi: 10.4049/jimmunol.1000552. [DOI] [PubMed] [Google Scholar]
- Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, Philbin VJ, Mancuso C, Kampmann B, Whittle H, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS ONE. 2011;6:e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday DH, Amponsah NA, Jones L, Tisch DJ, Hornick TR, Ramachandra L. Influenza-induced production of interferon-alpha is defective in geriatric individuals. J. Clin. Immunol. 2010;30:373–383. doi: 10.1007/s10875-010-9374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem. Pharmacol. 2011;81:825–837. doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Casals F, Sikora M, Laayouni H, Montanucci L, Muntasell A, Lazarus R, Calafell F, Awadalla P, Netea MG, Bertranpetit J. Genetic adaptation of the antibacterial human innate immunity network. BMC Evol. Biol. 2011;11:202. doi: 10.1186/1471-2148-11-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317:617–619. doi: 10.1126/science.1142963. [DOI] [PubMed] [Google Scholar]
- Chang BA, Huang Q, Quan J, Chau V, Ladd M, Kwan E, McFadden DE, Lacaze-Masmonteil T, Miller SP, Lavoie PM. Early inflammation in the absence of overt infection in preterm neonates exposed to intensive care. Cytokine. 2011;56:621–626. doi: 10.1016/j.cyto.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel-Dennis M, Latz E, Halmen KA, Trieu-Cuot P, Fitzgerald KA, Kasper DL, Golenbock DT. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe. 2008;4:543–554. doi: 10.1016/j.chom.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int. Immunopharmacol. 2007;7:1271–1285. doi: 10.1016/j.intimp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Currie BJ. Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Otto M. Understanding the significance of Staphylococcus epidermidis bacteremia in babies and children. Curr. Opin. Infect. Dis. 2010;23:208–216. doi: 10.1097/QCO.0b013e328337fecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramella A, Spalletta G, Bizzoni F, Salani F, Caltagirone C, Bossù P. Effect of age on surface molecules and cytokine expression in human dendritic cells. Cell. Immunol. 2011;269:82–89. doi: 10.1016/j.cellimm.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, Crabtree J, Rein-Weston A, Lavoie PM, Turvey SE, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS ONE. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Currie AJ, Curtis S, Strunk T, Riley K, Liyanage K, Prescott S, Doherty D, Simmer K, Richmond P, Burgner D. Preterm infants have deficient monocyte and lymphocyte cytokine responses to group B streptococcus. Infect. Immun. 2011;79:1588–1596. doi: 10.1128/IAI.00535-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angio CT. Active immunization of premature and low birth-weight infants: a review of immunogenicity, efficacy, and tolerability. Paediatr. Drugs. 2007;9:17–32. doi: 10.2165/00148581-200709010-00003. [DOI] [PubMed] [Google Scholar]
- Danis B, George TC, Goriely S, Dutta B, Renneson J, Gatto L, Fitzgerald-Bocarsly P, Marchant A, Goldman M, Willems F, De Wit D. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur. J. Immunol. 2008;38:507–517. doi: 10.1002/eji.200737760. [DOI] [PubMed] [Google Scholar]
- Dasari P, Zola H, Nicholson IC. Expression of Toll-like receptors by neonatal leukocytes. Pediatr. Allergy Immunol. 2011;22:221–228. doi: 10.1111/j.1399-3038.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- De Paepe ME, Hanley LC, Lacourse Z, Pasquariello T, Mao Q. Pulmonary dendritic cells in lungs of preterm infants: neglected participants in bronchopulmonary dysplasia? Pediatr. Dev. Pathol. 2011;14:20–27. doi: 10.2350/09-09-0709-OA.1. [DOI] [PubMed] [Google Scholar]
- Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Di Bona D, Cippitelli M, Fionda C, Cammà C, Licata A, Santoni A, Craxì A. Oxidative stress inhibits IFN-alpha-induced antiviral gene expression by blocking the JAK-STAT pathway. J. Hepatol. 2006;45:271–279. doi: 10.1016/j.jhep.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Dietert RR. Role of developmental immunotoxicity and immune dysfunction in chronic disease and cancer. Reprod. Toxicol. 2011;31:319–326. doi: 10.1016/j.reprotox.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Donald PR, Marais BJ, Barry CE., 3rd Age and the epidemiology and pathogenesis of tuberculosis. Lancet. 2010;375:1852–1854. doi: 10.1016/S0140-6736(10)60580-6. [DOI] [PubMed] [Google Scholar]
- Drygiannakis I, Ernst PB, Lowe D, Glomski IJ. Immunological alterations mediated by adenosine during host-microbial interactions. Immunol. Res. 2011;50:69–77. doi: 10.1007/s12026-011-8207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, Serra D, Gualtieri L, Cesati L, Principi N. Vaccines and preterm neonates: why, when, and with what. Early Hum. Dev. 2009;85(10, Suppl):S43–S45. doi: 10.1016/j.earlhumdev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA. 1997;277:728–734. [PubMed] [Google Scholar]
- Figueiredo CA, Alcântara-Neves NM, Veiga R, Amorim LD, Dattoli V, Mendonça LR, Junqueira S, Genser B, Santos M, de Carvalho LC, et al. Spontaneous cytokine production in children according to biological characteristics and environmental exposures. Environ. Health Perspect. 2009;117:845–849. doi: 10.1289/ehp.0800366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fleer A, Krediet TG. Innate immunity: Toll-like receptors and some more. A brief history, basic organization and relevance for the human newborn. Neonatology. 2007;92:145–157. doi: 10.1159/000102054. [DOI] [PubMed] [Google Scholar]
- Fleming DM, Elliot AJ. The impact of influenza on the health and health care utilisation of elderly people. Vaccine. 2005;23(Suppl 1):S1–S9. doi: 10.1016/j.vaccine.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Forsey RJ, Thompson JM, Ernerudh J, Hurst TL, Strindhall J, Johansson B, Nilsson BO, Wikby A. Plasma cytokine profiles in elderly humans. Mech. Ageing Dev. 2003;124:487–493. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Fortin CF, McDonald PP, Lesur O, Fülöp T., Jr Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11:873–882. doi: 10.1089/rej.2008.0750. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Fulop T, Kotb R, Fortin CF, Pawelec G, de Angelis F, Larbi A. Potential role of immunosenescence in cancer development. Ann. N Y Acad. Sci. 2010;1197:158–165. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- Gabrielsson S, Söderlund A, Nilsson C, Lilja G, Nordlund M, Troye-Blomberg M. Influence of atopic heredity on IL-4-, IL-12- and IFN-gamma-producing cells in in vitro activated cord blood mononuclear cells. Clin. Exp. Immunol. 2001;126:390–396. doi: 10.1046/j.1365-2249.2001.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Hernández-Santos N, Peterson AC. IL-17 signaling in host defense against Candida albicans. Immunol. Res. 2011;50:181–187. doi: 10.1007/s12026-011-8226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan YH. Interaction between Burkholderia pseudomallei and the host immune response: sleeping with the enemy? J. Infect. Dis. 2005;192:1845–1850. doi: 10.1086/497382. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet. 2010;375:1482–1490. doi: 10.1016/S0140-6736(09)61712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin LR, Landgren O, Kristinsson SY, Björkholm M, Paltiel O. Infection in infancy and subsequent risk of developing lymphoma in children and young adults. Blood. 2011;117:1670–1672. doi: 10.1182/blood-2010-09-306274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DR. Role of aging on innate responses to viral infections. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:242–246. doi: 10.1093/gerona/glr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonté D, Willems F, Goldman M. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J. Exp. Med. 2004;199:1011–1016. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely S, Goldman M. Interleukin-12 family members and the balance between rejection and tolerance. Curr. Opin. Organ Transplant. 2008;13:4–9. doi: 10.1097/MOT.0b013e3282f406c4. [DOI] [PubMed] [Google Scholar]
- Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Natl. Rev. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- Gourlay DM. The good and the bad of the innate immune response in necrotizing enterocolitis. J. Surg. Res. 2012;175:51–53. doi: 10.1016/j.jss.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 2011;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Christian LM, Kiecolt-Glaser JK. Stress, age, and immune function: Toward a lifespan approach. J. Behav. Med. 2006;29:389–400. doi: 10.1007/s10865-006-9057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J. Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayat M, Netea MG, Rezaei N. Targeting of Toll-like receptors: a decade of progress in combating infectious diseases. Lancet Infect. Dis. 2011;11:702–712. doi: 10.1016/S1473-3099(11)70099-8. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss AR. Mitochondrial dysfunction, proteotoxicity, and aging: causes or effects, and the possible impact of NAD+-controlled protein glycation. Adv. Clin. Chem. 2010;50:123–150. [PubMed] [Google Scholar]
- Huang CC, Liu K, Pope RM, Du P, Lin S, Rajamannan NM, Huang QQ, Jafari N, Burke GL, Post W, et al. Activated TLR signaling in atherosclerosis among women with lower Framingham risk score: the multi-ethnic study of atherosclerosis. PLoS ONE. 2011;6:e21067. doi: 10.1371/journal.pone.0021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009;70:777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kenzel S, Henneke P. The innate immune system and its relevance to neonatal sepsis. Curr. Opin. Infect. Dis. 2006;19:264–270. doi: 10.1097/01.qco.0000224821.27482.bd. [DOI] [PubMed] [Google Scholar]
- Kittan NA, Bergua A, Haupt S, Donhauser N, Schuster P, Korn K, Harrer T, Schmidt B. Impaired plasmacytoid dendritic cell innate immune responses in patients with herpes virus-associated acute retinal necrosis. J. Immunol. 2007;179:4219–4230. doi: 10.4049/jimmunol.179.6.4219. [DOI] [PubMed] [Google Scholar]
- Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K-F, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, Fikrig E, Montgomery RR. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J. Virol. 2008;82:7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin. Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, Chrabieh M, Issekutz AC, Cunningham CK, Gallin J, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie PM, Huang Q, Jolette E, Whalen M, Nuyt AM, Audibert F, Speert DP, Lacaze-Masmonteil T, Soudeyns H, Kollmann TR. Profound lack of interleukin (IL)-12/IL-23p40 in neonates born early in gestation is associated with an increased risk of sepsis. J. Infect. Dis. 2010;202:1754–1763. doi: 10.1086/657143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn JE, Cousens S, Zupan J Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- Leavy O. (micro)Tolerance in the gut. Natl. Rev. 2010;10:810. doi: 10.1038/nri2894. [DOI] [PubMed] [Google Scholar]
- LeBouder E, Rey-Nores JE, Raby AC, Affolter M, Vidal K, Thornton CA, Labéta MO. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. J. Immunol. 2006;176:3742–3752. doi: 10.4049/jimmunol.176.6.3742. [DOI] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Natl. Rev. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng J, Goldstein DR. Impact of aging on viral infections. Microbes Infect. 2010;12:1120–1124. doi: 10.1016/j.micinf.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Natl. Rev. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- Lewis DB, Gern JE, Hill HR, Friedlander SL, La Pine TR, Lemanske RF, Jr, Stiehm ER. Newborn immunology: relevance to the clinician. Curr. Probl. Pediatr. Adolesc. Health Care. 2006;36:189–204. doi: 10.1016/j.cppeds.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, Kennes B, Müller-Hermelink HK, Steinmann GG. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech. Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- Lisciandro JG, Prescott SL, Nadal-Sims MG, Devitt CJ, Pomat W, Siba PM, Tulic MC, Holt PG, Strickland D, van den Biggelaar AH. Ontogeny of Toll-like and NOD-like receptor-mediated innate immune responses in Papua New Guinean infants. PLoS ONE. 2012;7:e36793. doi: 10.1371/journal.pone.0036793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisciandro JG, van den Biggelaar AH. Neonatal immune function and inflammatory illnesses in later life: lessons to be learnt from the developing world? Clin. Exp. Allergy. 2010;40:1719–1731. doi: 10.1111/j.1365-2222.2010.03629.x. [DOI] [PubMed] [Google Scholar]
- Lotz M, Gütle D, Walther S, Ménard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera L, Cristaldi E, Malaguarnera M. The role of immunity in elderly cancer. Crit. Rev. Oncol. Hematol. 2010;74:40–60. doi: 10.1016/j.critrevonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Malamitsi-Puchner A, Protonotariou E, Boutsikou T, Makrakis E, Sarandakou A, Creatsas G. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum. Dev. 2005;81:387–392. doi: 10.1016/j.earlhumdev.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat. Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Natl. Rev. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin. Fetal Neonatal Med. 2006;11:369–377. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Tulic MK, Gordon L, Hodder M, Richman T, Metcalfe J, Prescott SL, Saffery R. Evidence for age-related and individual-specific changes in DNA methylation profile of mononuclear cells during early immune development in humans. Epigenetics. 2011;6:1085–1094. doi: 10.4161/epi.6.9.16401. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Effros RB. Immunosenescence: What does it mean to health outcomes in older adults? Curr. Opin. Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, Pawelec G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–2067. doi: 10.1016/j.vaccine.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchjorsen J. Sensing herpes: More than toll. Rev. Med. Virol. 2012;22:106–121. doi: 10.1002/rmv.716. [DOI] [PubMed] [Google Scholar]
- Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int. Rev. Immunol. 2010;29:315–348. doi: 10.3109/08830181003792803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira JP, Max A, Burgel PR. The role of biomarkers in community-acquired pneumonia: predicting mortality and response to adjunctive therapy. Crit. Care. 2008;12(Suppl 6):S5. doi: 10.1186/cc7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook P, Patel B, Gillespie IA. Risk factors for mortality in non-pregnancy-related listeriosis. Epidemiol. Infect. 2012;140:706–715. doi: 10.1017/S0950268811001051. [DOI] [PubMed] [Google Scholar]
- Moore SE, Collinson AC, Fulford AJ, Jalil F, Siegrist CA, Goldblatt D, Hanson LA, Prentice AM. Effect of month of vaccine administration on antibody responses in The Gambia and Pakistan. Trop. Med. Int. Health. 2006a;11:1529–1541. doi: 10.1111/j.1365-3156.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- Moore SE, Collinson AC, Tamba N’Gom P, Aspinall R, Prentice AM. Early immunological development and mortality from infectious disease in later life. Proc. Nutr. Soc. 2006b;65:311–318. doi: 10.1079/pns2006503. [DOI] [PubMed] [Google Scholar]
- Mortellaro A, Ricciardi-Castagnoli P. From vaccine practice to vaccine science: The contribution of human immunology to the prevention of infectious disease. Immunol. Cell Biol. 2011;89:332–339. doi: 10.1038/icb.2010.152. [DOI] [PubMed] [Google Scholar]
- Mueller NH, Gilden DH, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection: Clinical features, molecular pathogenesis of disease, and latency. Neurol. Clin. 2008;26:675–697. doi: 10.1016/j.ncl.2008.03.011. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myśliwska J, Bryl E, Foerster J, Myśliwski A. Increase of interleukin 6 and decrease of interleukin 2 production during the ageing process are influenced by the health status. Mech. Ageing Dev. 1998;100:313–328. doi: 10.1016/s0047-6374(97)00154-1. [DOI] [PubMed] [Google Scholar]
- Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. PLoS ONE. 2011;6:e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi N, Ponziani V, Becatti M, Galvan P, Donzelli G. Anti-oxidant enzymes and related elements in term and preterm newborns. Pediatr. Int. 2009;51:183–187. doi: 10.1111/j.1442-200X.2008.02662.x. [DOI] [PubMed] [Google Scholar]
- Nathe KE, Parad R, Van Marter LJ, Lund CA, Suter EE, Hernandez-diaz S, Boush EB, Ikonomu E, Gallington L, Morey JA, et al. Endotoxin-directed innate immunity in tracheal aspirates of mechanically ventilated human neonates. Pediatr. Res. 2009;66:191–196. doi: 10.1203/PDR.0b013e3181aa33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi H, Yanai H, Nakajima A, Koshiba R, Atarashi K, Matsuda A, Matsuki K, Miki S, Doi T, Aderem A, et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat. Immunol. 2012;13:659–666. doi: 10.1038/ni.2307. [DOI] [PubMed] [Google Scholar]
- Netea MG, Quintin J, van der Meer JW. Trained immunity: A memory for innate host defense. Cell Host Microbe. 2011a;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Netea MG, van de Veerdonk FL, van Deuren M, van der Meer JW. Defects of pattern recognition: primary immunodeficiencies of the innate immune system. Curr. Opin. Pharmacol. 2011b;11:412–422. doi: 10.1016/j.coph.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Netea MG, van der Meer JW. Immunodeficiency and genetic defects of pattern-recognition receptors. N. Engl. J. Med. 2011;364:60–70. doi: 10.1056/NEJMra1001976. [DOI] [PubMed] [Google Scholar]
- Netea MG, Wijmenga C, O’Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nat. Immunol. 2012;13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS ONE. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N. Engl. J. Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Larsson AK, Höglind A, Gabrielsson S, Troye Blomberg M, Lilja G. Low numbers of interleukin-12-producing cord blood mononuclear cells and immunoglobulin E sensitization in early childhood. Clin. Exp. Allergy. 2004;34:373–380. doi: 10.1111/j.1365-2222.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 2010;30:806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongradi J, Kovesdi V. Factors that may impact on immunosenescence: An appraisal. Immun. Ageing. 2010;7:7. doi: 10.1186/1742-4933-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Sanneh M, Kidd M, Newport MJ, Aaby P, Whittle H, Lambert PH, et al. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 2002;168:919–925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- Ottenhoff TH, Verreck FA, Hoeve MA, van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis (Edinb.) 2005;85:53–64. doi: 10.1016/j.tube.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: Causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattenden SG, Klose R, Karaskov E, Bremner R. Interferon-gamma-induced chromatin remodeling at the CIITA locus is BRG1 dependent. EMBO J. 2002;21:1978–1986. doi: 10.1093/emboj/21.8.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Clark MJ, McMaster SK, Sorrentino R, Sriskandan S, Bailey LK, Moreno L, Ryffel B, Quesniaux VF, Mitchell JA. Toll-like receptor 2 is essential for the sensing of oxidants during inflammation. Am. J. Respir. Crit. Care Med. 2009;179:299–306. doi: 10.1164/rccm.200707-1019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: Results from the Health, Aging and Body Composition study. Biol. Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Petrikin JE, Gaedigk R, Leeder JS, Truog WE. Selective Toll—like receptor expression in human fetal lung. Pediatr. Res. 2010;68:335–338. doi: 10.1203/PDR.0b013e3181ed1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbin VJ, Dowling DJ, Gallington LC, Cortes G, Tan Z, Suter EE, Chi KW, Shuckett A, Stoler-Barak L, Tomai M, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J. Allergy Clin. Immunol. 2012;130:195 e9–204 e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J. Leukoc. Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- Posfay-Barbe KM, Wald ER. Listeriosis. Semin. Fetal Neonatal Med. 2009;14:228–233. doi: 10.1016/j.siny.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Power Coombs MR, Belderbos ME, Gallington LC, Bont L, Levy O. Adenosine modulates Toll-like receptor function: Basic mechanisms and translational opportunities. Expert Rev. Anti Infect. Ther. 2011;9:261–269. doi: 10.1586/eri.10.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: Implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Qian F, Wang X, Zhang L, Chen S, Piecychna M, Allore H, Bockenstedt L, Malawista S, Bucala R, Shaw AC, et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell. 2012;11:104–110. doi: 10.1111/j.1474-9726.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J. Infect. Dis. 2011;203:1415–1424. doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi VR, Braas D, Bhatt DM, Cheng CS, Hong C, Doty KR, Black JC, Hoffmann A, Carey M, Smale ST. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell. 2009;138:114–128. doi: 10.1016/j.cell.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa AK, Shey MS, Keyser A, Peixoto B, Wells RD, de Kock M, Lerumo L, Hughes J, Hussey G, Hawkridge A, et al. South African Tuberculosis Vaccine Initiative Team. Association of human TLR1 and TLR6 deficiency with altered immune responses to BCG vaccination in South African infants. PLoS Pathog. 2011;7:e1002174. doi: 10.1371/journal.ppat.1002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, Mariani E, Licastro F, Patterson C. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol. Aging. 2007;28:1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Rayamajhi M, Humann J, Kearney S, Hill KK, Lenz LL. Antagonistic crosstalk between type I and II interferons and increased host susceptibility to bacterial infections. Virulence. 2010;1:418–422. doi: 10.4161/viru.1.5.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece P, Thanendran A, Crawford L, Tulic MK, Thabane L, Prescott SL, Sehmi R, Denburg JA. Maternal allergy modulates cord blood hematopoietic progenitor Toll-like receptor expression and function. J. Allergy Clin. Immunol. 2011;127:447–453. doi: 10.1016/j.jaci.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am. J. Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- Reikie BA, Adams RC, Ruck CE, Ho K, Leligdowicz A, Pillay S, Naidoo S, Fortuno ES, 3rd, de Beer C, Preiser W, et al. Ontogeny of Toll-like receptor mediated cytokine responses of South African infants throughout the first year of life. PLoS ONE. 2012;7:e44763. doi: 10.1371/journal.pone.0044763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Natl. Rev. 2012;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J. Am. Geriatr. Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, McIntyre PB. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27:1628–1636. doi: 10.1016/j.vaccine.2008.11.098. [DOI] [PubMed] [Google Scholar]
- Romagne F. Current and future drugs targeting one class of innate immunity receptors: The Toll-like receptors. Drug Discov. Today. 2007;12:80–87. doi: 10.1016/j.drudis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Gattorno M, Netea MG, Dinarello CA. Interplay between redox status and inflammasome activation. Trends Immunol. 2011;32:559–566. doi: 10.1016/j.it.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Sampath V, Le M, Lane L, Patel AL, Cohen JD, Simpson PM, Garland JS, Hines RN. The NFKB1 (g.-24519delATTG) variant is associated with necrotizing enterocolitis (NEC) in premature infants. J. Surg. Res. 2011;169:e51–e57. doi: 10.1016/j.jss.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Schiffrin EJ, Morley JE, Donnet-Hughes A, Guigoz Y. The inflammatory status of the elderly: The intestinal contribution. Mutat. Res. 2010;690:50–56. doi: 10.1016/j.mrfmmm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Scholtes VP, Versteeg D, de Vries JP, Hoefer IE, Schoneveld AH, Stella PR, Doevendans PA, van Keulen KJ, de Kleijn DP, Moll FL, Pasterkamp G. Toll-like receptor 2 and 4 stimulation elicits an enhanced inflammatory response in human obese patients with atherosclerosis. Clin. Sci. 2011;121:205–214. doi: 10.1042/CS20100601. [DOI] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc. Natl. Acad. Sci. USA. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr. Opin. Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR. Dysregulation of human Toll-like receptor function in aging. Ageing Res. Rev. 2011;10:346–353. doi: 10.1016/j.arr.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007;39:123–129. doi: 10.1016/j.cyto.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Newton PN, Chierakul W, Chaowagul W, White NJ. Diabetes mellitus, insulin, and melioidosis in Thailand. Clin. Infect. Dis. 2003;36:e71–e72. doi: 10.1086/367861. [DOI] [PubMed] [Google Scholar]
- Smeekens SP, Plantinga TS, van de Veerdonk FL, Heinhuis B, Hoischen A, Joosten LA, Arkwright PD, Gennery A, Kullberg BJ, Veltman JA, et al. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS ONE. 2011;6:e29248. doi: 10.1371/journal.pone.0029248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J. Matern. Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- Strunk T, Doherty D, Jacques A, Simmer K, Richmond P, Kohan R, Charles A, Burgner D. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. 2012a;129:e134–e141. doi: 10.1542/peds.2010-3493. [DOI] [PubMed] [Google Scholar]
- Strunk T, Doherty D, Richmond P, Simmer K, Charles A, Levy O, Liyanage K, Smith T, Currie A, Burgner D. Reduced levels of antimicrobial proteins and peptides in human cord blood plasma. Arch. Dis. Child. Fetal Neonatal Ed. 2009;94:F230–F231. doi: 10.1136/adc.2008.143438. [DOI] [PubMed] [Google Scholar]
- Strunk T, Power Coombs MR, Currie AJ, Richmond P, Golenbock DT, Stoler-Barak L, Gallington LC, Otto M, Burgner D, Levy O. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS ONE. 2010;5:e10111. doi: 10.1371/journal.pone.0010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk T, Prosser A, Levy O, Philbin V, Simmer K, Doherty D, Charles A, Richmond P, Burgner D, Currie A. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr. Res. 2012b;72:10–18. doi: 10.1038/pr.2012.48. [DOI] [PubMed] [Google Scholar]
- Strunk T, Richmond P, Simmer K, Currie A, Levy O, Burgner D. Neonatal immune responses to coagulase-negative staphylococci. Curr. Opin. Infect. Dis. 2007;20:370–375. doi: 10.1097/QCO.0b013e3281a7ec98. [DOI] [PubMed] [Google Scholar]
- Strunk T, Simmer K, Burgner D. Prematurity and mortality in childhood and early adulthood. JAMA. 2012c;307:32. doi: 10.1001/jama.2011.1952. author reply 32-33. [DOI] [PubMed] [Google Scholar]
- Tal MC, Iwasaki A. Mitoxosome: A mitochondrial platform for cross-talk between cellular stress and antiviral signaling. Immunol. Rev. 2011;243:215–234. doi: 10.1111/j.1600-065X.2011.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. USA. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Hale J, Wiltschut J, Lehmann H, Dunstan JA, Prescott SL. Evaluation of the effects of probiotic supplementation from the neonatal period on innate immune development in infancy. Clin. Exp. Allergy. 2006;36:1218–1226. doi: 10.1111/j.1365-2222.2006.02552.x. [DOI] [PubMed] [Google Scholar]
- Taylor DN, Treanor JJ, Strout C, Johnson C, Fitzgerald T, Kavita U, Ozer K, Tussey L, Shaw A. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29:4897–4902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid. Redox Signal. 2010;12:503–535. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Travis J. Nobel Prize in physiology or medicine. Immunology prize overshadowed by untimely death of awardee. Science. 2011;334:31. doi: 10.1126/science.334.6052.31. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Myśliwska J, Godlewska B, Szmit E, Łukaszuk K, Wieckiewicz J, Brydak L, Machała M, Landowski J, Myśliwski A. Immune consequences of the spontaneous pro-inflammatory status in depressed elderly patients. Brain Behav. Immun. 2004;18:135–148. doi: 10.1016/S0889-1591(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Myśliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, Machała M, Myśliwski A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—An impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Iwasaki S, Horiguchi H, Mori M, Nishimaki S, Seki K, Taguri M, Yokota S, Ishiwada N. Immune response to Haemophilus influenzae type b conjugate vaccine in preterm infants. Pediatr. Int. 2011;54:64–67. doi: 10.1111/j.1442-200X.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- Tulic MK, Hodder M, Forsberg A, McCarthy S, Richman T, D’Vaz N, van den Biggelaar AH, Thornton CA, Prescott SL. Differences in innate immune function between allergic and nonallergic children: New insights into immune ontogeny. J. Allergy Clin. Immunol. 2011;127:470–478. doi: 10.1016/j.jaci.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Uciechowski P, Imhoff H, Lange C, Meyer CG, Browne EN, Kirsten DK, Schröder AK, Schaaf B, Al-Lahham A, Reinert RR, et al. Susceptibility to tuberculosis is associated with TLR1 polymorphisms resulting in a lack of TLR1 cell surface expression. J. Leukoc. Biol. 2011;90:377–388. doi: 10.1189/jlb.0409233. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Hamel DL, Jr, Mueller RG, Ives DG, Michel JJ, Boudreau RM, Newman AB. NK-like T cells and plasma cytokines, but not anti-viral serology, define immune fingerprints of resilience and mild disability in exceptional aging. PLoS ONE. 2011;6:e26558. doi: 10.1371/journal.pone.0026558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Huizinga TW, de Craen AJ, Gussekloo J, Heijmans BT, Frölich M, Westendorp RG. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp. Gerontol. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Prescott SL, Roponen M, Nadal-Sims MA, Devitt CJ, Phuanukoonnon S, Pomat W, Tulic MK, Lehmann D, Siba PM, et al. Neonatal innate cytokine responses to BCG controlling T-cell development vary between populations. J. Allergy Clin. Immunol. 2009;124:544–550. doi: 10.1016/j.jaci.2009.03.040. [DOI] [PubMed] [Google Scholar]
- van Duin D, Allore HG, Mohanty S, Ginter S, Newman FK, Belshe RB, Medzhitov R, Shaw AC. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J. Infect. Dis. 2007a;195:1590–1597. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated defect in human TLR-1/2 function. J. Immunol. 2007b;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur. J. Immunol. 2006;36:21–26. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- Vellai T. Autophagy genes and ageing. Cell Death Differ. 2009;16:94–102. doi: 10.1038/cdd.2008.126. [DOI] [PubMed] [Google Scholar]
- Venkatesh MP, Placencia F, Weisman LE. Coagulase-negative staphylococcal infections in the neonate and child: An update. Semin. Pediatr. Infect. Dis. 2006;17:120–127. doi: 10.1053/j.spid.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Vento S, Tanko MN. The bacterium that could cause cancer. Lancet Oncol. 2009;10:528. doi: 10.1016/S1470-2045(09)70038-5. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J. Pediatr. 2008;153:160–163. doi: 10.1016/j.jpeds.2008.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermin PJ, Ponsonby AL, Saffery R, Tang ML, Ellis JA, Sly P, Holt P. Microbial exposure, interferon gamma gene demethylation in naïve T-cells, and the risk of allergic disease. Allergy. 2009;64:348–353. doi: 10.1111/j.1398-9995.2009.01970.x. [DOI] [PubMed] [Google Scholar]
- Weyrich P, Staiger H, Stančáková A, Machicao F, Machann J, Schick F, Stefan N, Kuusisto J, Laakso M, Schäfer S, et al. The D299G/T399I Toll-like receptor 4 variant associates with body and liver fat: Results from the TULIP and METSIM Studies. PLoS ONE. 2010;5:e13980. doi: 10.1371/journal.pone.0013980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: Insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 2006;4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- Wijsman CA, Maier AB, de Craen AJ, van den Biggelaar AH, Westendorp RG. An unopposed proinflammatory response is beneficial for survival in the oldest old. Results of the Leiden 85-plus Study. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:393–399. doi: 10.1093/gerona/glq212. [DOI] [PubMed] [Google Scholar]
- Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Löfgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech. Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wilson CB, Kollmann TR. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr. Workshop Ser. Pediatr. Program. 2008;61:183–195. doi: 10.1159/000113493. [DOI] [PubMed] [Google Scholar]
- Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Eidenschenk C, Krebs P, Brandl K, Blasius AL, Xia Y, Khovananth K, Smart NG, Beutler B. The Tpl2 mutation Sluggish impairs type I IFN production and increases susceptibility to group B streptococcal disease. J. Immunol. 2009;183:7975–7983. doi: 10.4049/jimmunol.0902718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Koarai A, Sugiura H, Ichikawa T, Kanda M, Tanaka R, Akamatsu K, Hirano T, Matsunaga K, Minakata Y, Ichinose M. Oxidative stress augments toll-like receptor 8 mediated neutrophilic responses in healthy subjects. Respir. Res. 2009;10:50. doi: 10.1186/1465-9921-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa TT. Important infections in elderly persons. West. J. Med. 1981;135:441–445. [PMC free article] [PubMed] [Google Scholar]
- Zhang JP, Yang Y, Levy O, Chen C. Human neonatal peripheral blood leukocytes demonstrate pathogen-specific coordinate expression of TLR2, TLR4/MD2, and MyD88 during bacterial infection in vivo. Pediatr. Res. 2010;68:479–483. doi: 10.1203/PDR.0b013e3181f90810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, Picard C, Abel L, Jouanguy E, Casanova JL. Inborn errors of interferon (IFN)-mediated immunity in humans: Insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol. Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]