Abstract
Objectives
Available D-dimer assays have low specificity and may increase radiographic testing for pulmonary embolism (PE). To help clinicians better target testing, this study sought to quantify the effect of risk factors for a positive quantitative D-dimer in patients evaluated for PE.
Methods
This was a prospective, multicenter, observational study. Emergency department (ED) patients evaluated for PE with a quantitative D-dimer were eligible for inclusion. The main outcome of interest was a positive D-dimer. Odds ratio (ORs) and 95% confidence intervals (CIs) were determined by multivariable logistic regression. Adjusted estimates of relative risk were also calculated.
Results
A total of 4,346 patients had D-dimer testing, of whom 2,930 (67%) were women. A total of 2,500 (57%) were white, 1,474 (34%) were black or African American, 238 (6%) were Hispanic, and 144 (3%) were of other race or ethnicity. The mean (±SD) age was 48 (±17) years. Overall, 1,903 (44%) D-dimers were positive. Model fit was adequate (c-statistic = 0.739, Hosmer and Lemeshow p-value = 0.13). Significant positive predictors of D-dimer positive included female sex; increasing age; black (vs. white) race; cocaine use; general, limb, or neurologic immobility; hemoptysis; hemodialysis; active malignancy; rheumatoid arthritis; lupus; sickle cell disease; prior venous thromboembolism (VTE; not under treatment); pregnancy and postpartum state; and abdominal, chest, orthopedic, or other surgery. Warfarin use was protective. In contrast, several variables known to be associated with PE were not associated with positive D-dimer results: body mass index (BMI), estrogen use, family history of PE, (inactive) malignancy, thrombophilia, trauma within 4 weeks, travel, and prior VTE (under treatment).
Conclusions
Many factors are associated with a positive D-dimer test. The effect of these factors on the usefulness of the test should be considered prior to ordering a D-dimer.
Keywords: D-dimer, pulmonary embolism, venous thromboembolism, testing
Plasma D-dimer measurement is commonly used as the first test in patients suspected of having acute pulmonary embolism (PE). D-dimer testing is noninvasive and rapid, so it is not surprising that the availability of these tests can increase the number of patients evaluated for possible PE.1 However, low specificity limits the usefulness of D-dimer testing. Specificity is typically between 40% and 60%, leading to a high rate of false-positive results.2
Several factors, other than PE or deep vein thrombosis (DVT), are associated with positive D-dimer results. Some, such as advanced age, malignancy, and pregnancy, have been described in the medical literature.3–9 However, most prior studies have evaluated D-dimer testing in a select population of patients with a particular risk factor, rather than in an undifferentiated population of patients evaluated for PE.10–12 As a result, the ability to adjust results for the large variety of conditions that may elevate the D-dimer has been limited. In addition, risk factors have generally been studied as broad categories (e.g., recent surgery, history of cancer), but whether the described effects are consistent across more detailed subcategories (e.g., type of surgery, active vs. inactive malignancy) is not well known. We used data obtained from a large multicenter study of emergency department (ED) patients evaluated for PE to identify factors associated with a positive D-dimer result and quantify the effect of each factor in a multivariable analysis.
Methods
Study Design
This was a prospective, multicenter, observational study of ED patients undergoing testing for possible PE. The institutional review board of each participating institution approved the protocol.
Study Setting and Population
Data were collected from May 1, 2003, to March 31, 2007. This study analyzed data from 10 academic medical centers and two community hospitals in the United States.
Patients were eligible for enrollment if the treating clinician ordered a D-dimer to rule out PE. All D-dimer tests were ordered as part of an evaluation for acute PE, and studies ordered to evaluate DVT without PE, or other diagnoses, did not trigger enrollment.
Study Protocol
Details of study enrollment are described elsewhere.10 After a diagnostic test for PE was ordered, but before results were known, we prospectively collected data describing patient demographics, presenting signs and symptoms, and comorbid illnesses. A study investigator uploaded data into a Web-based, secure, electronic data collection form.13 The Web-based data collection instrument was programmed with logic that did not permit missing or nonsense data.
Potential predictors were collected prospectively at the time of the PE evaluation. Predictor variables described a variety of conditions, including demographics, past medical history and comorbidities, and medications (Table 1). Surgery and trauma were considered positive if they occurred in the past 4 weeks and if the trauma was significant enough to require hospitalization. Travel was defined as any travel lasting greater than 6 hours, occurring within the past 4 weeks.
Table 1. Predictors Included in Final Multivariable Model.
| Predictor | Categories |
|---|---|
| Age (yr)* | <30, 30–39, 40–49, 50–59, 60–69, 70–79, ≥80 |
| Antiplatelet drug use† | Yes/no |
| Anxiety | Yes/no |
| BMI | <25.0, 25.0–29.9, 30.0–34.9, ≥35.0 |
| CAD | Yes/no |
| Cerebrovascular disease‡ | Yes/no |
| CHF | Yes/no |
| Cocaine use | Yes/no |
| Connective tissue disease | Systemic lupus erythematosis, rheumatoid arthritis, other |
| Diabetes mellitus | Yes/no |
| D-dimer assay | Advanced, Biopool, Hemosil, Liatest, MDA, Vidas |
| Estrogen replacement therapy | Yes/no |
| Family history of VTE | Yes/no |
| Sex | Female, male |
| Hemodialysis | Yes/no |
| Hemoptysis | Yes/no |
| Hypertension | Yes/no |
| Lung disease | Asthma, COPD, other |
| Malignancy | Active, inactive |
| Pregnancy | First trimester, second trimester, third trimester, ≤4 weeks postpartum |
| Sickle cell disease or trait | Yes/no |
| Smoking | Yes/no |
| Thrombophilia | Yes/no |
| Immobility | Generalized, limb, neurologic |
| Race | Asian, Black or African American, Hispanic, white, other |
| Surgery | Abdominal, chest, orthopedic, other |
| Trauma | Yes/no |
| Travel | Yes/no |
| Warfarin use | Yes/no |
| VTE history | None, under treatment, not under treatment |
BMI = body mass index; CAD = coronary artery disease (a history of angina, myocardial infarction, or coronary artery revascularization procedure); CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; VTE = venous thromboembolism (deep vein thrombosis or PE).
Age was considered categorical, although a separate analysis was also performed where age was included in the model as an ordinal variable.
Antiplatelet drugs include aspirin, ticlopidine, and clopidogrel.
Cerebrovascular disease includes any history of stroke or transient ischemic attack.
The outcome of interest was a positive D-dimer result. The decision to order a D-dimer was at the discretion of the evaluating physician. D-dimer assays were those used for routine clinical care at participating institutions. We analyzed patients from participating institutions where a quantitative D-dimer assay was available, including Advanced (Dade Behring, Marburg, Germany), Biopool Minutex (diaPharma, West Chester, OH), Hemosil (Dade Behring), Liatest (Diagnostica Stago, Asnières sur Seine, France), MDA (bioMérieux SA, Marcy-l'Etoile, France, formerly Organon Teknika Corporation, Durham, NC), or VIDAS (bioMérieux SA). Tests were performed as a part of routine clinical care by personnel working in each hospital's laboratory, blinded to the goals of the study. The positive and negative cutoff for each assay was defined by the institution where the test was used and was generally consistent with manufacturers' recommendations. Liatest, VIDAS, and MDA D-dimers were considered positive at concentrations of ≥500 ng/mL, Biopool Minutex at ≥250 ng/mL, Hemosil at ≥244 ng/mL, and the Advanced D-dimer at ≥1.6 μg/mL.
Data Analysis
The purpose of our study was to provide guidance to physicians prior to ordering a D-dimer, so we included both true positive (positive D-dimer results in patients diagnosed with PE) and false positive (positive D-dimer results in patients not diagnosed with PE) in our analysis. We also performed a sensitivity analysis excluding patients diagnosed with PE (i.e., only including false-positive D-dimers).
We used SAS v 9.l (SAS Institute, Cary, NC) for all of our statistical calculations. Baseline characteristics are reported as simple proportions, means, and medians. In our primary analysis we calculated odds ratios (ORs) and 95% confidence intervals (95% CIs) using a multi-variable logistic regression model, which in addition to potential predictors of positive D-dimers, also included the D-dimer assay used. To adjust for the high frequency of our outcome, and better estimate the risk ratio (RR), we then transformed the OR using the method described by Zhang and Yu.14 Predictor variables were included in the model first as general predictor categories (e.g., recent surgery). In the event that a predictor was found to be significantly associated with positive D-dimer results, data describing subcategories of the predictor (e.g., different types of surgery) were available, and if there were greater than 20 subjects in each subcategory, those subcategories of the predictor were included in the model. If a general category was found not to be associated with positive D-dimer results, subcategories of the predictor were not analyzed, but the predictor was kept in the model as a potential confounder. Age and body mass index (BMI) were considered categorical variables. However, a separate analysis was performed with these variables considered ordinal in the model. White race, age <30 years, and BMI <25 were reference groups. For subcategories of diseases (e.g., active and inactive malignancy), lack of the disease (e.g., no cancer) was the reference group. Significant predictors were defined as those where the 95% CI did not cross unity. Table 1 lists the predictors included in the final multivariable model.
Results
In the original study, 7,940 patients were prospectively enrolled and 6,175 patients were enrolled in an institution where a quantitative D-dimer was available. Of these patients, 4,356 (71%) had a quantitative D-dimer performed and were therefore included in the current analysis (Figure 1). The Liatest was the most commonly performed assay (1,466, 34%), followed by the VIDAS (1,262, 29%). A total of 1,003 patients (44%) had a positive D-dimer. The overall mean age was 47.7(±16.7) years. Among patients with a negative D-dimer the mean age was 44.2 (±14.1) years, but among those with a positive D-dimer the mean age was 52.3 (±18.6) years. The majority of patients (2930, 67%) were female. Table 2 describes the baseline characteristics of enrolled patients, stratified by whether D-dimer was positive or negative.
Figure 1.
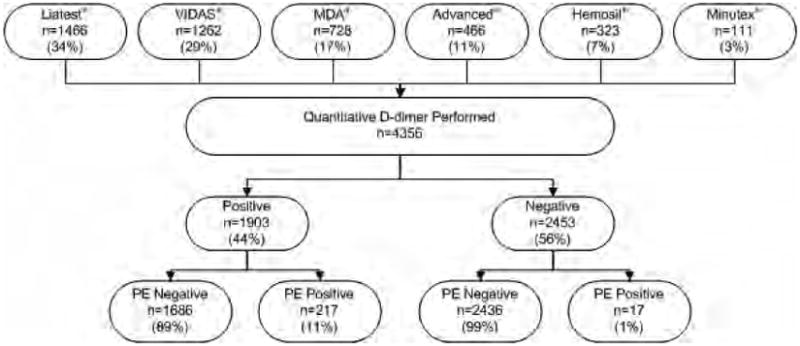
Study enrollment. PE = pulmonary embolism.
Table 2. Characteristics of Enrolled Patients.
| Characteristic | Total, n (%) | D-dimer Negative, n (%) | D-dimer Positive, n (%) | Ratio of False- to True-positive D-dimers |
|---|---|---|---|---|
| Enrolled | 4,356 (100) | 2,453 (56) | 1,903 (44) | 7.8 |
| Female | 2,930 (67) | 1,320 (45) | 1,610 (55) | 9.5 |
| Age (yr) | ||||
| <30 | 605 (14) | 373 (62) | 232 (38) | 14.5 |
| 30–39 | 961 (22) | 642 (67) | 319 (33) | 8.4 |
| 40–49 | 954 (22) | 619 (65) | 335 (35) | 8.6 |
| 50–59 | 798 (18) | 468 (59) | 330 (41) | 6.3 |
| 60–69 | 507 (12) | 217 (43) | 290 (57) | 8.1 |
| 70–79 | 328 (8) | 100 (30) | 228 (70) | 6.4 |
| ≥80 | 203 (5) | 34 (17) | 169 (83) | 5.8 |
| Race/ethnicity | ||||
| Black or African American | 1,474 (34) | 753 (51) | 721 (49) | 8.2 |
| Hispanic | 238 (5) | 155 (65) | 83 (35) | 40.5 |
| White | 2,500 (57) | 1,454 (58) | 1,046 (42) | 6.9 |
| Other | 144 (3) | 91 (63) | 53 (37) | 9.6 |
| Pretest probability of PE* | ||||
| Low (<15%) | 3,224 (74) | 1,206 (37) | 2,018 (63) | 14.1 |
| Intermediate (15%–40%) | 933 (21) | 561 (60) | 372 (40) | 5.6 |
| High (>40%) | 191 (4) | 132 (69) | 59 (31) | 1.5 |
| BMI | ||||
| <25 | 1,517 (35) | 871 (57) | 646 (43) | 9.4 |
| 25–29.9 | 1,240 (28) | 695 (56) | 545 (44) | 7.5 |
| 30–34.9 | 790 (18) | 447 (57) | 343 (43) | 7.4 |
| ≥35 | 809 (19) | 440 (54) | 369 (46) | 6.4 |
| Cocaine use | 68 (2) | 31 (46) | 37 (54) | 17.5 |
| Family history of PE | 538 (12) | 331 (62) | 207 (38) | 4.9 |
| Immobility | ||||
| General | 243 (6) | 71 (29) | 172 (71) | 4.9 |
| Limb | 73 (2) | 21 (29) | 52 (71) | 3.3 |
| Neurologic | 26 (1) | 8 (31) | 18 (69) | 8.0 |
| Hemoptysis | 116 (3) | 45 (39) | 71 (61) | 10.8 |
| Medication use | ||||
| Antiplatelet | 652 (15) | 290 (44) | 362 (56) | 6.9 |
| Estrogen | 400 (9) | 249 (62) | 151 (38) | 5.3 |
| Warfarin | 178 (4) | 84 (47) | 94 (53) | 5.7 |
| Past medical history | ||||
| Anxiety | 548 (13) | 335 (61) | 213 (39) | 10.2 |
| CAD | 416 (10) | 152 (37) | 264 (63) | 10.5 |
| Cerebrovascular disease | 151 (3) | 67 (44) | 84 (56) | 15.8 |
| CHF | 287 (7) | 99 (34) | 188 (66) | 8.0 |
| Connective tissue disease | ||||
| Rheumatoid arthritis | 76 (2) | 30 (39) | 46 (61) | 22.0 |
| Systemic lupus erythematosis | 53 (1) | 22 (42) | 31 (58) | 9.3 |
| Other connective tissue disease | 26 (1) | 11 (42) | 15 (58) | 4.0 |
| Diabetes | 556 (13) | 243 (44) | 313 (56) | 9.4 |
| Hemodialysis | 38 (1) | 12 (32) | 26 (68) | 25 |
| Hypertension | 1,503 (35) | 696 (46) | 807 (54) | 7.9 |
| Lung disease | ||||
| Asthma | 478 (11) | 292 (61) | 186 (39) | 15.9 |
| COPD | 273 (6) | 107 (39) | 166 (61) | 10.1 |
| Malignancy | ||||
| Active | 203 (5) | 59 (29) | 144 (71) | 4.5 |
| Inactive | 211 (5) | 95 (45) | 116 (55) | 7.3 |
| Sickle cell disease or trait | 15 (0) | 1 (7) | 14 (93) | 13.0 |
| Thrombophilia | 45 (1) | 24 (53) | 21 (47) | 4.3 |
| VTE | ||||
| Under treatment | 118 (3) | 56 (47) | 62 (53) | 2.9 |
| Not under treatment | 258 (6) | 118 (46) | 140 (54) | 4.6 |
| Pregnancy | ||||
| First trimester | 18 (0) | 8 (44) | 10 (56) | —† |
| Second trimester | 31 (1) | 7 (23) | 24 (77) | — |
| Third trimester | 23 (1) | 1 (4) | 22 (96) | — |
| Postpartum | 65 (1) | 19 (29) | — | — |
| Smoking | 1,635 (38) | 905 (55) | 730 (45) | 11.0 |
| Surgery | ||||
| Abdominal | 72 (2) | 18 (25) | 54 (75) | 5.8 |
| Chest | 34 (1) | 10 (29) | 24 (71) | 11.0 |
| Orthopedic | 49 (1) | 15 (31) | 34 (69) | 2.8 |
| Other | 37 (1) | 11 (30) | 26 (70) | — |
| Trauma | 36 (1) | 17 (47) | 19 (53) | 8.5 |
| Travel | 398 (9) | 261 (66) | 137 (34) | 6.2 |
BMI = body mass index; CAD = coronary artery disease (a history of angina, myocardial infarction, or coronary artery revascularization procedure); CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; PE = pulmonary embolism; VTE = venous thromboembolism (deep vein thrombosis or PE).
Pretest probability determined by treating physician.
No patients who had D-dimer performed were diagnosed with PE.
Results of the multivariable model are presented in Table 3. Model fit was adequate (c-statistic = 0.739, Hosmer and Lemeshow test p = 0.13). Women were more likely to have a positive D-dimer than were men. Older age was highly associated with positive D-dimer (OR = 1.40 [95% CI = 1.32 to 1.47], adjusted RR = 1.19 [95% CI = 1.16 to 1.22] per 10 years after age 30, p < 0.0001). The association with age became statistically significant in the fourth to fifth decades of life. Patients of African American or black race were more likely to have a positive D-dimer compared to whites, but there was no observed association for other races. All categories of surgery were associated with positive D-dimer results. Active cancer was associated with positive D-dimer results (OR = 2.58 [95% CI = 1.84 to 3.63], adjusted RR = 1.55 [95% CI = 1.36 to 1.72], p < 0.0001), whereas inactive cancer was not. Immobility was associated with positive D-dimer results, with a 50% to 60% increase across all categories of immobility, although there was no association with travel. There was an increasingly positive association between trimester of pregnancy and positive D-dimers. The association in the first trimester of pregnancy was borderline, but nearly all (96%) women in the third trimester had a positive D-dimer. In terms of past medical history, connective tissue disease was associated with a roughly one-third higher risk of a positive D-dimer and end-stage renal disease with a 45% higher risk, but the strongest disease association was seen with sickle cell disease/trait (OR = 24.17 [95% CI = 3.08 to 189.53], adjusted RR = 2.18 [95% CI = 1.62 to 2.28], p = 0.002). Prior venous thromboembolism (VTE) was associated with a small increase in the odds of D-dimer positivity (OR = 1.41 [95% CI = 1.07 to 1.87], adjusted RR = 1.20 [95% CI = 1.04 to 1.36], p = 0.016), only if the patient was not actively being treated. The only factor with a significant “ protective effect” was warfarin use; anti-platelet drugs had no such effect. Overall, variables not associated with positive D-dimer included antiplatelet drugs, anxiety, asthma, BMI, coronary artery disease (CAD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), diabetes, estrogen use, family history of PE, hypertension, (inactive) malignancy, stroke, thrombophilia, trauma within 4 weeks, travel, or VTE (under treatment).
Table 3. Multivariable Analysis of Factors Associated With Positive D-dimer Results.
| OR | 95% CI | Adjusted Risk Ratio* | 95% CI | p-value | |
|---|---|---|---|---|---|
| Female | 1.22 | 1.04–1.42 | 1.12 | 1.03–1.21 | 0.01 |
| Age (yr) | |||||
| 30–39 | 0.93 | 0.73–1.18 | 0.96 | 0.84–1.09 | 0.54 |
| 40–49 | 1.09 | 0.86–1.39 | 1.05 | 0.92–1.18 | 0.48 |
| 50–59 | 1.33 | 1.03–1.71 | 1.16 | 1.01–1.30 | 0.03 |
| 60–69 | 2.58 | 1.92–3.47 | 1.55 | 1.39–1.70 | <0.0001 |
| 70–79 | 4.54 | 3.20–6.46 | 1.84 | 1.67–1.98 | <0.0001 |
| ≥80 | 10.49 | 6.63–16.61 | 2.11 | 1.98–2.21 | <0.0001 |
| Race/ethnicity | |||||
| Black or African American | 1.48 | 1.26–1.73 | 1.23 | 1.14–1.33 | <0.0001 |
| Hispanic | 1.02 | 0.75–1.40 | 1.01 | 0.84–1.19 | 0.88 |
| Other | 1.09 | 0.74–1.60 | 1.05 | 0.84–1.27 | 0.67 |
| BMI | |||||
| 25–29.9 | 1.10 | 0.92–1.31 | 1.05 | 0.96–1.15 | 0.29 |
| 30–34.9 | 1.07 | 0.88–1.31 | 1.04 | 0.93–1.15 | 0.50 |
| ≥35 | 1.16 | 0.95–1.43 | 1.09 | 0.97–1.20 | 0.15 |
| Cocaine use | 2.02 | 1.20–3.38 | 1.40 | 1.11–1.66 | 0.008 |
| Family history of PE | 0.96 | 0.78–1.18 | 0.98 | 0.86–1.09 | 0.70 |
| Immobility | |||||
| General | 2.34 | 1.70–3.22 | 1.50 | 1.31–1.66 | <0.0001 |
| Limb | 2.76 | 1.56–4.89 | 1.57 | 1.26–1.82 | 0.001 |
| Neurologic | 3.05 | 1.24–7.51 | 1.61 | 1.12–1.96 | 0.02 |
| Hemoptysis | 2.01 | 1.33–3.04 | 1.40 | 1.16–1.62 | 0.001 |
| Medication use | |||||
| Antiplatelet drug | 1.09 | 0.88–1.35 | 1.05 | 0.93–1.18 | 0.41 |
| Estrogen | 1.22 | 0.96–1.55 | 1.12 | 0.98–1.25 | 0.10 |
| Warfarin | 0.57 | 0.38–0.85 | 0.70 | 0.52–0.91 | 0.006 |
| Past medical history | |||||
| Anxiety | 0.82 | 0.67–1.01 | 0.89 | 0.78–1.00 | 0.06 |
| CAD | 1.22 | 0.93–1.59 | 1.12 | 0.96–1.28 | 0.15 |
| Cerebrovascular disease | 0.71 | 0.48–1.06 | 0.81 | 0.62–1.03 | 0.09 |
| CHF | 1.23 | 0.91–1.65 | 1.12 | 0.95–1.30 | 0.18 |
| Connective tissue disease | |||||
| Rheumatoid arthritis | 1.80 | 1.07–3.00 | 1.33 | 1.04–1.61 | 0.03 |
| Systemic lupus erythematosis | 2.09 | 1.15–3.80 | 1.42 | 1.08–1.71 | 0.02 |
| Other connective tissue disease | 1.50 | 0.63–3.57 | 1.23 | 0.75–1.68 | 0.37 |
| Diabetes mellitus | 1.05 | 0.84–1.31 | 1.03 | 0.90–1.16 | 0.66 |
| Hemodialysis | 2.24 | 1.06–4.72 | 1.45 | 1.03–1.80 | 0.03 |
| Hypertension | 1.02 | 0.87–1.21 | 1.01 | 0.91–1.12 | 0.80 |
| Lung disease | |||||
| Asthma | 0.83 | 0.67–1.03 | 0.90 | 0.78–1.02 | 0.09 |
| COPD | 1.02 | 0.75–1.37 | 1.01 | 0.84–1.18 | 0.92 |
| Malignancy | |||||
| Active | 2.58 | 1.84–3.63 | 1.55 | 1.36–1.72 | <0.0001 |
| Inactive | 1.09 | 0.79–1.50 | 1.05 | 0.87–1.23 | 0.60 |
| Sickle cell disease or trait | 24.17 | 3.08–189 | 2.18 | 1.62–2.28 | 0.002 |
| Thrombophilia | 1.20 | 0.61–2.36 | 1.11 | 0.74–1.48 | 0.59 |
| VTE | |||||
| Under treatment | 1.60 | 0.99–2.60 | 1.27 | 0.99–1.53 | 0.06 |
| Not under treatment | 1.41 | 1.07–1.87 | 1.20 | 1.04–1.36 | 0.02 |
| Pregnancy | |||||
| First trimester | 2.65 | 0.99–7.05 | 1.54 | 1.00–1.94 | 0.05 |
| Second trimester | 7.31 | 3.04–17.61 | 1.95 | 1.61–2.14 | <0.0001 |
| Third trimester | 51.25 | 6.79–386 | 2.25 | 1.93–2.30 | 0.0001 |
| Postpartum | 4.18 | 2.33–7.47 | 1.76 | 1.48–1.97 | <0.0001 |
| Smoking | 1.13 | 0.98–1.30 | 1.07 | 0.99–1.15 | 0.10 |
| Surgery | |||||
| Abdominal | 3.47 | 1.93–6.25 | 1.68 | 1.38–1.91 | <0.0001 |
| Chest | 2.69 | 1.21–5.98 | 1.55 | 1.11–1.89 | 0.02 |
| Orthopedic | 2.20 | 1.12–4.33 | 1.45 | 1.06–1.77 | 0.02 |
| Other | 3.24 | 1.00–10.44 | 1.64 | 1.00–2.05 | 0.05 |
| Trauma | 0.71 | 0.33–1.53 | 0.81 | 0.46–1.24 | 0.38 |
| Travel | 0.85 | 0.67–1.08 | 0.91 | 0.78–1.04 | 0.19 |
Reference groups: age = <30 years; race = white; BMI = <25.
BMI = body mass index; CAD = coronary artery disease (a history of angina, myocardial infarction, or coronary artery revascularization procedure); CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; PE = pulmonary embolism; VTE = venous thromboembolism (deep vein thrombosis or PE).
Adjusted according to method of Zhang and Yu.14 RR = OR/(1 – P0) + (P0 × OR), where RR = risk ratio, OR = odds ratio, and P0 = the incidence of the outcome of interest in nonexposed subjects.
Sensitivity Analysis
When we restricted our multivariable analysis to the 4,122 patients not diagnosed with PE (i.e., false-positive D-dimers), results were similar (Table 4). The only variables to change statistical significance were CAD, VTE not under treatment, and smoking, although point estimates were similar and the lower confidence bounds for these variables were close to unity in both analyses. Estimated ORs were also similar when we reran our model only including significant predictors.
Table 4. Multivariable Analysis of Factors Associated With False-positive D-dimer Results.
| OR | 95% CI | Adjusted Risk Ratio* | 95% CI | p-value | |
|---|---|---|---|---|---|
| Female | 1.36 | 1.15–1.60 | 1.20 | 1.09–1.31 | 0.002 |
| Age (yr) | |||||
| 30–39 | 0.92 | 0.72–1.17 | 0.96 | 0.83–1.08 | 0.50 |
| 40–49 | 1.06 | 0.83–1.36 | 1.03 | 0.90–1.17 | 0.64 |
| 50–59 | 1.26 | 0.97–1.65 | 1.13 | 0.98–1.28 | 0.08 |
| 60–69 | 2.46 | 1.82–3.34 | 1.53 | 1.35–1.69 | <0.0001 |
| 70–79 | 4.27 | 2.97–6.14 | 1.81 | 1.63–1.96 | <0.0001 |
| ≥80 | 9.54 | 5.97–15.26 | 2.09 | 1.94–2.19 | <0.0001 |
| Race/ethnicity | |||||
| Black or African American | 1.46 | 1.24–1.71 | 1.23 | 1.13–1.32 | <0.0001 |
| Hispanic | 1.11 | 0.81–1.52 | 1.06 | 0.88–1.24 | 0.53 |
| Other | 1.12 | 0.75–1.66 | 1.06 | 0.84–1.29 | 0.57 |
| BMI | |||||
| 25–29.9 | 1.09 | 0.91–1.30 | 1.05 | 0.95–1.15 | 0.36 |
| 30–34.9 | 1.04 | 0.85–1.28 | 1.02 | 0.91–1.14 | 0.72 |
| ≥35 | 1.09 | 0.88–1.35 | 1.05 | 0.93–1.17 | 0.44 |
| Cocaine use | 2.14 | 1.26–3.60 | 1.46 | 1.14–1.75 | 0.005 |
| Family history of PE | 0.91 | 0.73–1.13 | 0.95 | 0.82–1.07 | 0.38 |
| Immobility | |||||
| General | 2.18 | 1.57–3.05 | 1.49 | 1.28–1.69 | <0.0001 |
| Limb | 2.38 | 1.31–4.34 | 1.53 | 1.16–1.84 | 0.004 |
| Neurologic | 2.89 | 1.16–7.25 | 1.63 | 1.09–2.04 | 0.02 |
| Hemoptysis | 2.07 | 1.35–3.16 | 1.45 | 1.18–1.69 | 0.008 |
| Medication use | |||||
| Antiplatelet drug | 1.07 | 0.86–1.33 | 1.04 | 0.91–1.18 | 0.57 |
| Estrogen | 1.13 | 0.88–1.45 | 1.07 | 0.93–1.22 | 0.35 |
| Warfarin | 0.63 | 0.41–0.97 | 0.74 | 0.54–0.98 | 0.04 |
| Past medical history | |||||
| Anxiety | 0.82 | 0.67–1.03 | 0.89 | 0.78–1.02 | 0.08 |
| CAD | 1.31 | 1.0–1.72 | 1.17 | 1.00–1.34 | 0.05 |
| Cerebrovascular disease | 0.75 | 0.51–1.12 | 0.83 | 0.64–1.07 | 0.16 |
| CHF | 1.21 | 0.89–1.64 | 1.12 | 0.93–1.31 | 0.22 |
| Connective tissue disease | |||||
| Rheumatoid arthritis | 1.87 | 1.12–3.15 | 1.38 | 1.07–1.68 | 0.02 |
| Systemic lupus erythematosis | 2.06 | 1.12–3.79 | 1.44 | 1.07–1.77 | 0.02 |
| Other connective tissue disease | 1.56 | 0.60–4.04 | 1.27 | 0.72–1.80 | 0.36 |
| Diabetes mellitus | 1.10 | 0.88–1.37 | 1.06 | 0.92–1.20 | 0.42 |
| Hemodialysis | 2.41 | 1.14–5.12 | 1.53 | 1.08–1.91 | 0.02 |
| Hypertension | 1.07 | 0.90–1.27 | 1.04 | 0.93–1.16 | 0.45 |
| Lung disease | |||||
| Asthma | 0.87 | 0.69–1.08 | 0.92 | 0.79–1.05 | 0.20 |
| COPD | 1.02 | 0.76–1.39 | 1.01 | 0.84–1.20 | 0.87 |
| Malignancy | |||||
| Active | 2.50 | 1.75–3.57 | 1.57 | 1.35–1.77 | <0.0001 |
| Inactive | 1.07 | 0.77–1.49 | 1.04 | 0.85–1.24 | 0.70 |
| Sickle cell disease or trait | 25.78 | 3.26–204 | 2.32 | 1.70–2.44 | 0.002 |
| Thrombophilia | 1.30 | 0.62–2.71 | 1.16 | 0.73–1.60 | 0.48 |
| VTE | |||||
| Under treatment | 1.21 | 0.71–2.06 | 1.11 | 0.81–1.41 | 0.49 |
| Not under treatment | 1.32 | 0.98–1.78 | 1.16 | 0.99–1.33 | 0.07 |
| Pregnancy | |||||
| First trimester | 2.89 | 1.08–7.69 | 1.63 | 1.05–2.06 | 0.04 |
| Second trimester | 7.71 | 3.20–18.55 | 2.07 | 1.69–2.28 | <0.0001 |
| Third trimester | 55.38 | 7.34–417 | 2.40 | 2.05–2.45 | <0.0001 |
| Postpartum | 4.57 | 2.57–8.19 | 1.87 | 1.57–2.10 | <0.0001 |
| Smoking | 1.20 | 1.03–1.39 | 1.11 | 1.02–1.20 | 0.004 |
| Surgery | |||||
| Abdominal | 3.27 | 1.79–5.99 | 1.71 | 1.36–1.99 | <0.0001 |
| Chest | 3.09 | 1.35–7.08 | 1.67 | 1.18–2.04 | 0.01 |
| Orthopedic | 2.08 | 1.03–4.23 | 1.44 | 1.02–1.83 | 0.04 |
| Other | 3.80 | 1.18–12.31 | 1.78 | 1.10–2.20 | 0.03 |
| Trauma | 0.80 | 0.36–1.75 | 0.87 | 0.49–1.34 | 0.57 |
| Travel | 0.81 | 0.63–1.05 | 0.88 | 0.75–1.03 | 0.11 |
Reference groups: age = <30 years; race = white; BMI = <25.
BMI = body mass index; CAD = coronary artery disease (a history of angina, myocardial infarction, or coronary artery revascularization procedure); CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; PE = pulmonary embolism; VTE = venous thromboembolism (deep vein thrombosis or PE).
Adjusted according to method of Zhang and Yu.14 RR = OR/(1 – P0) + (P0 × OR), where RR = risk ratio, OR = odds ratio, and P0 = the incidence of the outcome of interest in non-exposed subjects.
Discussion
To our knowledge, this is the largest and most detailed study yet performed quantifying the association between clinical factors and positive D-dimer results. We found several factors to be highly associated with positive D-dimer results. This study has several advantages over previous studies; with our large sample size, we were able to adjust for a large number of covariates. This provides a more accurate estimate of the risk of a positive D-dimer, even for factors previously known to be associated with positive results. In addition, we were able to identify several factors not previously known to be associated with positive D-dimer results and describe the association of subcategories of malignancy, surgery, connective tissue disease, lung disease, and pregnancy—describing the relationship between these factors and D-dimer results in more detail than was previously available.
We found several findings of particular interest. We confirmed strong associations with age, surgery, and malignancy, previously described. We also found associations with immobility and sickle cell disease that were not well known. The adjusted relative risks for these associations are generally between 1.5 and 2. While this may seem low, it is important to consider that the baseline risk of a positive D-dimer was 44% in our study, so increasing the risk by 50% to 100% represents a significant change. We believe that identifying factors associated with positive D-dimer results, and understanding the strength of the associations, will help clinicians target their use of D-dimer testing to those patients where the result is likely to be the most useful. These results may also aid the development of decision instruments that guide the use of D-dimer testing and the evaluation of patients suspected of having PE.
Our data support the association between older age and positive D-dimer results that has been described previously.8,9 There does not seem to be a great effect below the age of 50 years, with patients younger than 50 years having a negative D-dimer approximately 60% of the time. In contrast, there is a drop to 42% in patients 60–69 years old, to 31% in patients 70–79 years old, and only 17% in patients older than 80 years. Clinicians should consider the low likelihood of a negative D-dimer before ordering the test in patients more than 60 years old.
We also found that recent surgery increases the odds of a positive D-dimer by approximately 60%. More than two-thirds of our patients who had undergone recent surgery had a positive D-dimer. While elevated D-dimer values after surgery are understandable, most previous reports have studied enrolled patients at the time of surgery, showing that postoperative patients have a high rate of D-dimer positivity.10–12 Our study is the most detailed exploration to date of the association between recent surgery and D-dimer results in patients being evaluated for PE. By studying an undifferentiated population of patients, and comparing those who had recent surgery to those who had not, we were also able to determine the relative effect of surgery on the odds of a positive D-dimer result.
We also studied the relationship between malignancy and D-dimer results. Previous authors have shown that patients with cancer were much more likely to have a positive D-dimer, with a number needed to rule out equal to 8.6.6 However, our study has the advantage of being able to distinguish between active and inactive malignancy. Our results show a strong association for patients with malignancy that is actively being treated or palliated. However, we found no association in patients whose cancer is inactive or has been completely treated. Clinicians should distinguish between active and inactive cancer when assessing the likelihood of a positive D-dimer.
The association we found for immobility seems to vary little depending on the type of immobility. This is in contrast to a recent study from our database demonstrating that the risk of PE is different depending on the type of immobility.15 In our study, regardless of whether a patient's immobility was described as generalized, limb, or neurologic, the risk of a positive D-dimer increased by 50% to 60%. While immobility is known to be related to PE risk,15 to the authors' knowledge, this is the first study to demonstrate an independent association between immobility and D-dimer positivity. Two prior studies investigated the issue of hypercoagulability during a simulated airplane flight in 10 healthy subjects and one subject who was heterozygous for factor V Leiden and found no association.16,17 These results are consistent with our finding of no association between recent travel and D-dimer results. However, our definition of immobility included patients with prolonged or permanent immobility and complete or near complete inability to mobilize one or more limbs. This likely explains the significant association we found. We believe that the pathophysiology of hypercoagulability associated with immobility should be investigated further.
Our data support a strong association between sickle cell disease/trait and D-dimer positivity, such that D-dimer testing is likely to be of limited utility in ruling out PE in these patients. Prior studies have demonstrated elevated D-dimer levels in sickle cell patients, particularly during vaso-occlusive crises.18,19 While we did not distinguish between patients having a painful crisis, it is reasonable to conclude that the high frequency of D-dimer positivity may be related to concomitant vaso-occlusive crises in patients with sickle cell disease. Clinicians should be aware of this possibility and treat their patients accordingly.
Finally, it is worth noting that many of the above conditions (e.g., age, immobility, malignancy, pregnancy) both increase the risk of PE and elevate the D-dimer. This is demonstrated in our data as well. We found that factors known to increase the risk of PE, such as immobility (except neurologic), active malignancy, thrombophilia, a history of VTE, and recent surgery (except chest surgery) all had ratios of false to true D-dimer results that were lower than that of the study population as a whole. For ordinal factors, such as increasing age and BMI, the ratio decreased linearly. Increasing physician estimate of pretest probability of PE was also inversely proportional to the ratio of false to true D-dimer results. These findings are consistent with known associations between these factors and the risk of PE and with our prior research.20 Conversely, certain inflammatory conditions without well-established associations with the risk of PE (rheumatoid arthritis, sickle cell disease, asthma) had high ratios of false to true D-dimer results. D-dimer use in patients with these conditions is likely to yield a large number of false positives for each subject ruled out for PE. Interestingly, we found that some of the above conditions known to increase the risk of PE were not associated with increased D-dimer values. We found no association between BMI or smoking and D-dimer positivity, although large epidemiologic studies have demonstrated their association with PE.20–24 Similarly, we found no association between known thrombophilia or family history of VTE and positive D-dimers. Thus, even in the presence of strong family history or known thrombophilia, D-dimer testing may be useful. We found no association with recent trauma, although trauma is a heterogeneous disease, and we did not attempt to quantify the amount of bleeding or clotting that might have occurred in each patient. Last, we found a relatively weak association between prior VTE and positive D-dimers, but only in patients who were not being actively treated.
Limitations
This was a large multicenter study with an observational design. The selection of patients appropriate for D-dimer testing was left to the discretion of the treating physician, which may have biased the results. It is possible that, because patients with conditions (e.g., malignancy) known to elevate the D-dimer may not have been selected for testing, we have underestimated the association of these factors. However, many of the factors we describe, including subcategories of known associations, were not previously so well known that they would likely have altered physician test ordering. Nonetheless, our results probably should not be compared to studies that purported to follow a rigid study protocol. We enrolled patients in academic and community centers, resulting in a heterogeneous population with a variety of D-dimer assays used. We adjusted our analysis for the D-dimer assay used, to account for differences in test characteristics across assays, but we did not specifically explore differences between assays. We included patients diagnosed with PE in our primary analysis. We did so to provide guidance to clinicians trying to determine whether a D-dimer is likely to be positive and therefore less useful in ruling out PE. It is likely that a homogenous population of patients with true-positive D-dimers would be different than one with false-positive D-dimers. However, we found no major differences in our associations when we performed a sensitivity analysis excluding PE-positive patients. Finally, our analysis was meant to be exploratory and was designed to identify as many predictors of positive D-dimers as possible. We did not seek to create the most efficient model, but rather to include a broad array of potential confounders. However, when we excluded nonsignificant predictors from our model, results were similar.
Conclusions
Many factors are associated with a positive D-dimer test. Age, surgery, immobility, and pregnancy are all strongly associated with D-dimer positivity. Active malignancy is associated, but inactive malignancy is not. Several factors that are known to be associated with pulmonary embolism are not associated with a positive D-dimer. Clinicians should consider these associations when they weigh the usefulness of D-dimer testing for patients with suspected pulmonary embolism.
Acknowledgments
Supported by NIH-2R42 HL074415–02A1, 2004–2006.
Footnotes
Presented at the Society of Academic Emergency Medicine annual meeting, New Orleans, LA, 2009.
Contributor Information
Christopher Kabrhel, Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
D. Mark Courtney, Department of Emergency Medicine, Northwestern University Medical Center, Chicago, IL;.
Carlos A. Camargo, Jr, Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Michael C. Plewa, Department of Emergency Medicine, St. Vincent Mercy Medical Center, Toledo, OH.
Kristen E. Nordenholz, Division of Emergency Medicine, Department of Surgery, University of Colorado, Denver, CO.
Christopher L. Moore, Department of Emergency Medicine, Yale University Medical Center, New Haven, CT.
Peter B. Richman, Department of Emergency Medicine, Mayo Clinic Arizona, Scottsdale, AZ.
Howard A. Smithline, Department of Emergency Medicine, Baystate Medical Center, Springfield, MA.
Daren M. Beam, East Carolina University School of Medicine, Greenville, NC
Jeffrey A. Kline, Department of Emergency Medicine, Carolinas Medical Center, Charlotte, NC.
References
- 1.Kabrhel C, Matts C, McNamara M, Katz J, Ptak T. A highly sensitive ELISA D-dimer increases testing but not diagnosis of pulmonary embolism. Acad Emerg Med. 2006;13:519–24. doi: 10.1197/j.aem.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 3.Epiney M, Boehlen F, Boulvain M, et al. D-dimer levels during delivery and the postpartum. J Thromb Haemost. 2005;3:268–71. doi: 10.1111/j.1538-7836.2004.01108.x. [DOI] [PubMed] [Google Scholar]
- 4.Francalanci I, Comeglio P, Alessandrello Liotta A, et al. D-dimer plasma levels during normal pregnancy measured by specific ELISA. Int J Clin Lab Res. 1997;27:65–7. doi: 10.1007/BF02827245. [DOI] [PubMed] [Google Scholar]
- 5.Kline JA, Williams GW, Hernandez-Nino J. D-dimer concentrations in normal pregnancy: new diagnostic thresholds are needed. Clin Chem. 2005;51:825–9. doi: 10.1373/clinchem.2004.044883. [DOI] [PubMed] [Google Scholar]
- 6.Righini M, Le Gal G, De Lucia S, et al. Clinical usefulness of D-dimer testing in cancer patients with suspected pulmonary embolism. Thromb Haemost. 2006;95:715–9. [PubMed] [Google Scholar]
- 7.King V, Vaze AA, Moskowitz CS, Smith LJ, Ginsberg MS. D-dimer assay to exclude pulmonary embolism in high-risk oncologic population: correlation with CT pulmonary angiography in an urgent care setting. Radiology. 2008;247:854–61. doi: 10.1148/radiol.2473070939. [DOI] [PubMed] [Google Scholar]
- 8.Righini M, Nendaz M, Le Gal G, Bounameaux H, Perrier A. Influence of age on the cost-effectiveness of diagnostic strategies for suspected pulmonary embolism. J Thromb Haemost. 2007;5:1869–77. doi: 10.1111/j.1538-7836.2007.02667.x. [DOI] [PubMed] [Google Scholar]
- 9.Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000;109:357–61. doi: 10.1016/s0002-9343(00)00493-9. [DOI] [PubMed] [Google Scholar]
- 10.Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772–80. doi: 10.1111/j.1538-7836.2008.02944.x. [DOI] [PubMed] [Google Scholar]
- 11.Misaki T, Kitajima I, Kabata T, et al. Changes of the soluble fibrin monomer complex level during the perioperative period of hip replacement surgery. J Orthop Sci. 2008;13:419–24. doi: 10.1007/s00776-008-1266-y. [DOI] [PubMed] [Google Scholar]
- 12.Richman PB, Dominguez S, Kasper D, et al. Interob-server agreement for the diagnosis of venous thromboembolism on computed tomography chest angiography and indirect venography of the lower extremities in emergency department patients. Acad Emerg Med. 2006;13:295–301. doi: 10.1197/j.aem.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Kline JA, Johnson CL, Webb WB, Runyon MS. Prospective study of clinician-entered research data in the emergency department using an Internet-based system after the HIPAA Privacy Rule. BMC Med Inform Decis Mak. 2004;4:17. doi: 10.1186/1472-6947-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 15.Beam DM, Courtney DM, Kabrhel C, Moore CL, Richman PB, Kline JA. Risk of thromboembolism varies, depending on category of immobility in outpatients. Ann Emerg Med. 2009;54:147–52. doi: 10.1016/j.annemergmed.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Ansari MT, Mahmood MT, Karlberg JP. The association between seated immobility and local lower-limb venous coagulability in healthy adult volunteers: a simulation of prolonged travel immobility. Blood Coagul Fibrinolysis. 2006;17:335–41. doi: 10.1097/01.mbc.0000233362.80486.6c. [DOI] [PubMed] [Google Scholar]
- 17.Ansari MT, Cheung BM, Karlberg JP. Prolonged seated immobility-associated venous coagulability in a factor V Leiden heterozygote: a case-comparative study. Blood Coagul Fibrinolysis. 2006;17:187–91. doi: 10.1097/01.mbc.0000220240.45585.5e. [DOI] [PubMed] [Google Scholar]
- 18.Devine DV, Kinney TR, Thomas PF, Rosse WF, Greenberg CS. Fragment D-dimer levels: an objective marker of vaso-occlusive crisis and other complications of sickle cell disease. Blood. 1986;68:317–9. [PubMed] [Google Scholar]
- 19.Fiessinger JN, Huisman MV, Davidson BL, et al. Ximelagatran vs low-molecular-weight heparin and warfarin for the treatment of deep vein thrombosis: a randomized trial. JAMA. 2005;293:681–9. doi: 10.1001/jama.293.6.681. [DOI] [PubMed] [Google Scholar]
- 20.Kabrhel C, Varraso R, Goldhaber SZ, Rimm EB, Camargo CA. Prospective study of BMI and the risk of pulmonary embolism in women. Obesity (Silver Spring) 2009;17:2040–6. doi: 10.1038/oby.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canonico M, Oger E, Conard J, et al. Obesity and risk of venous thromboembolism among postmenopausal women: differential impact of hormone therapy by route of estrogen administration. The ESTHER Study. J Thromb Haemost. 2006;4:1259–65. doi: 10.1111/j.1538-7836.2006.01933.x. [DOI] [PubMed] [Google Scholar]
- 22.Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. JAMA. 1997;277:642–5. [PubMed] [Google Scholar]
- 23.Goldhaber SZ, Savage DD, Garrison RJ, et al. Risk factors for pulmonary embolism. The Framingham Study. Am J Med. 1983;74:1023–8. doi: 10.1016/0002-9343(83)90805-7. [DOI] [PubMed] [Google Scholar]
- 24.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–80. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]


