Abstract
Objective
Despite the recognized risk of accelerated atherosclerosis in patients with rheumatoid arthritis (RA), little is known about cardiovascular risk management in contemporary cohorts of these patients. We tested the hypotheses that major modifiable cardiovascular risk factors were more frequent and rates of treatment, detection, and control were lower in patients with RA than in non-RA controls.
Methods
The prevalence of hypertension, diabetes, elevated low-density lipoprotein (LDL) cholesterol, elevated body mass index, smoking, moderate-high 10-year cardiovascular risk and the rates of underdiagnosis, therapeutic treatment, and recommended management were compared in 197 RA patients and 274 frequency-matched control subjects, and their associations with clinical characteristics were examined.
Results
Eighty percent of RA patients and 81% of control subjects had at least 1 modifiable traditional cardiovascular risk factor. Hypertension was more prevalent in the RA group (57%) than in controls [42%, P =0.001]. There were no statistically significant differences in the frequency of diabetes, elevated body mass index, smoking, intermediate-high 10-year coronary heart disease risk, or elevated LDL in patients with RA versus controls. Rates of newly identified diabetes, hypertension, and hyperlipidemia were similar in RA patients versus controls. Rates of therapeutic interventions were low in both groups but their use was associated with well-controlled blood pressure (OR = 4.55, 95% CI: 1.70, 12.19) and lipid levels (OR = 9.90, 95% CI: 3.30, 29.67).
Conclusions
Hypertension is more common in RA than in controls. Other traditional cardiovascular risk factors are highly prevalent, underdiagnosed, and poorly controlled in patients with RA, as well as controls.
Keywords: rheumatoid arthritis, cardiovascular risk, epidemiology
Patients with rheumatoid arthritis (RA) have higher mortality (1) than the general population and the leading cause of death is cardiovascular disease (2–7). Atherosclerosis is a low-grade inflammatory disease with macrophages, T cells, and pro-inflammatory cytokines playing a key role in its pathogenesis (8). RA is a high-grade inflammatory disease with an overlapping cellular and cytokine profile; thus, it has been hypothesized that rheumatoid inflammation is a major cause of the accelerated atherosclerosis observed in this patient population.
Nonetheless, there is increasing evidence that traditional nonmodifiable and modifiable cardiovascular risk factors also play an important role in the higher risk of coronary atherosclerosis and coronary heart disease (CHD) events (fatal myocardial infarction, CHD deaths) in patients with RA (9–11). Recently, a population-based cohort study evaluating patients with RA showed that the absolute risk of a cardiovascular event rose dramatically if traditional cardiovascular risk factors such as hypertension, dyslipidemia, smoking, diabetes and obesity were present (10). For example, patients who were 60 to 69 years old and did not have risk factors had a 10-year cardiovascular event risk of 17%. The risk increased to 60% in patients with multiple modifiable cardiovascular risk factors (10). However, some patients in this study presented their symptoms more than 50 years ago; thus, rates from contemporary cohorts are of interest.
The recognized increase in atherosclerosis in patients with RA has led to attempts to estimate prevalence of conventional CV risk factors in this patient population (12,13) and to recommend strategies to prevent cardiovascular risk (14). However, the adequacy of cardiovascular risk management in RA patients and how this compares to the general population remains to be clarified.
Thus, we set out to test the hypotheses that major modifiable cardiovascular risk factor rates of detection, treatment, and adequate control were lower in patients with RA than in a contemporary, geographically comparable, community-based control cohort. Both groups were evaluated using the same rigorous protocols.
METHODS
We cross sectionally studied 197 patients with RA enrolled in the Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis (ESCAPE RA) study (11) and 274 non-RA control subjects enrolled at the Baltimore site of the Multi-Ethnic Study of Atherosclerosis (MESA) study (15).
ESCAPE RA is a cohort assembled to evaluate the prevalence, progression, and risk factors for subclinical atherosclerosis in patients with RA. Inclusion criteria for this study were as follows: (1) fulfillment of the American College of Rheumatology classification criteria for RA (16), (2) age 45 to 84 years. Exclusion criteria were as follows: (1) self-reported or physician-diagnosed history of myocardial infarction, heart failure, coronary artery revascularization, peripheral vascular disease, implanted pacemaker or defibrillator devices, and current atrial fibrillation; (2) weight exceeding 300 pounds; and (3) computerized tomographic scan of the chest within 6 months before study enrollment.
The MESA study is a population-based cohort study of subclinical cardiovascular disease. Using 16 substrata defined by gender (male/female), age (9 year increments), and ethnicity (black/white), control subjects were frequency matched to the RA group on age, sex, and ethnicity.
Both cohorts excluded individuals with prior clinically evident cardiovascular (CV) disease. This study, therefore, focused on adequacy of risk factor management for primary prevention of CV disease.
Both groups were evaluated following MESA protocols (15) using the same definitions, questionnaires, imaging equipment and technicians, laboratory methods, and quality control procedures.
Demographic and socioeconomic variables, clinical characteristics, and cardiovascular risk factors were collected, and Framingham risk scores (FRS) calculated (17). In RA patients, measures of disease activity (number of tender and swollen joints, high-sensitivity C-reactive protein, and the calculation of the 28-joint based disease activity score (DAS28)) (18) and functional capacity (with the health assessment questionnaire (HAQ)) (19) were computed. Fasting blood was collected to measure glucose and lipids (20). All participants gave written informed consent and the study was approved by the Institutional Review Board of The Johns Hopkins University School of Medicine.
Study Outcomes
The study evaluated the prevalence of hypertension, elevated low-density lipoprotein (LDL) cholesterol, diabetes, smoking, and elevated body mass index (BMI).
Hypertension
Resting blood pressure was measured 3 times in the sitting position and the mean of the 2 last measurements was used in the analysis (15). Hypertension was defined by systolic BP ≥140 mm Hg, diastolic BP ≥90, self-reported previous diagnosis, or antihypertensive medication use.
Controlled Blood Pressure
Concordant with current guidelines from the American Heart Association (21), participants with low 10-year FRS (<10%) who met a target blood pressure of <140/90 mm of Hg were classified as well controlled. In participants with coronary heart disease equivalent (diabetes mellitus) or with a 10-year FRS ≥10%, a target blood pressure of 130/80 mm Hg was required to be classified as well controlled.
Elevated LDL Cholesterol
Total and high-density lipoprotein cholesterol for both groups were measured at the MESA core laboratory, the Laboratory for Clinical Biochemistry Research (University of Vermont). LDL was calculated using the Friedewald equation (22). Patients and control subjects were classified as having elevated LDL cholesterol if they self-reported a previous diagnosis of hypercholesterolemia, and/or use of lipid lowering drugs, and/or had a high LDL cholesterol according to their 10-year FRS: (1) ≥100 mg/dL for individuals with 10-year FRS >20% or a CHD equivalent disease; (2) ≥130 mg/dL for those with 2+ risk factors and 10-year FRS of 10 to 20%; and (3) ≥160 mg/dL for those with 0 to 1 risk factor and 10-year FRS <10%) (23).
Controlled Lipid Levels
Good control was defined using the following target LDL cholesterol values based on current guidelines: <100 mg/dL for individuals at high risk (presence of a CHD equivalent diagnosis or 10-year risk >20%, <130 mg/dL for those with moderate cardiovascular risk (+2 risk factors and 10 year-FRS <20%), and <160 mg/dL for those in the lowest CV risk category (0–1 risk factor) (23).
Diabetes
Diabetes mellitus was defined as the presence of any of the following: (1) fasting glucose ≥126 mg/dL, (2) self-reported previous diagnosis of diabetes mellitus, (3) current use of insulin, and/or (4) current use of oral hypoglycemic agents. Controlled diabetes was defined as normal fasting glucose (<110 mg/dL) (24).
Moderate to High Framingham Risk
The composite prediction Framingham model includes age, LDL, total and high-density lipoprotein cholesterol, blood pressure, antihypertensive medications, and smoking. Based on these variables, the 10-year CHD risk was calculated (25). Moderate-high cardiovascular risk was defined as an estimated 10-year CHD risk higher than 10% or self-reported previous diagnosis of diabetes (a coronary artery disease equivalent). These individuals are eligible for aspirin use (24).
Smoking
Smoking was defined as self-reported current smoking with a cumulative history of more than 100 cigarettes.
Elevated BMI
Height and weight were measured. The BMI was calculated by dividing weight (in kilograms) by the square of height (in meters). Elevated BMI was defined as BMI equal or higher than 30 kg/m2.
Statistical Analysis
We explored the association between RA and the presence of 2 main sets of outcomes: (1) the prevalence of modifiable traditional cardiovascular risk factors, and (2) the achievement of cardiovascular risk management goals.
We calculated the prevalence of each individual modifiable cardiovascular risk factor and compared its prevalence in RA patients and control subjects using Fisher’s exact test. Then, ordinary logistic regression modeling was used to calculate odds ratios and corresponding P values and 95% confidence intervals (CI) and adjustment was performed for demographic and socioeconomic variables.
We evaluated cardiovascular risk management, focusing on the percentage of patients in whom previously underdiagnosed risk factors were newly found, use of pharmacologic interventions, and percentage with good control in those with either previously diagnosed or newly diagnosed elevated risk factors.
Univariate analyses were performed comparing the percentages of RA patients and non-RA controls using Fisher’s exact test. Logistic regression modeling was used to calculate odds ratios and adjust for prespecified potential confounders including age, ethnicity, and sex as demographics, and education and Medicare enrollment as socioeconomic variables. All statistical tests were calculated assuming a 5% 2-sided significant level using STATA/IC 11.0 (StataCorp, College Station, TX).
RESULTS
Demographics
Frequency matching was successful with RA and control groups having similar age distributions (mean ages, 59.4 ±8.7 and 58.1 ± 8.2, respectively, = 0.25), proportion of females (60% and 58%, P = 0.64), and white participants (86% and 85%). Seventy-six percent of patients with RA had a level of education of at least completing the 12th grade as compared with 81% in control subjects (P = 0.21). The proportion of Medicare enrollees was 28% in the RA and 25% in the control groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients with RA and Control Subjects
| RA Patients (n = 197) | Control Subjects (N = 274) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age (yr), mean ± SD | 59.4 ± 8.7 | 58.1 ± 8.2 | 0.25 |
| Female sex, n (%) | 118 (60%) | 158 (58%) | 0.64 |
| Caucasians, n (%) | 169 (86%) | 233 (85%) | 0.90 |
| Education greater than 12th grade, n (%) | 148 (76%) | 219 (81%) | 0.21 |
| Medicare enrollment, n (%) | 54 (28%) | 69 (25%) | 0.60 |
| Cardiovascular risk factors | |||
| Hypertension, n (%) | 113 (57%) | 115 (42%) | 0.001 |
| Systolic blood pressure (mm Hg) mean ± SD | 128.1 ± 18.6 | 122.1 ± 19.2 | <0.001 |
| Diastolic blood pressure (mm Hg) mean ± SD | 75.7 ± 8.9 | 70.9 ± 9.7 | <0.001 |
| Diabetes mellitus, n (%) | 14 (7%) | 26 (9%) | 0.41 |
| High LDL cholesterol, n (%) | 88 (45%) | 139 (51%) | 0.22 |
| Elevated BMI, n (%) | 68 (35%) | 97 (35%) | 0.92 |
| Current smoking, n (%) | 23 (12%) | 38 (14%) | 0.58 |
| Moderate to high cardiovascular riska (%) | 72 (37%) | 93 (34%) | 0.62 |
>10% 10-year risk of cardiovascular events.
Prevalence of Cardiovascular Risk Factors in RA and Matched Control Subjects
A total of 158 (80%) RA patients and 221 (81%) controls (P = 0.91) had at least 1 of the 5 traditional modifiable cardiovascular risk factors evaluated.
Table 1 shows the frequency of individual modifiable cardiovascular risk factors. Of note, 113 (57%) patients with RA and 115 control subjects (42%) had hypertension (P = 0.001). Both systolic and diastolic blood pressure contributed to this difference, with both mean measures being higher in patients with RA than controls.
A higher proportion of control individuals had elevated LDL cholesterol (51%) than patients with RA (45%), but this difference was not statistically significant. The proportions of patients with BMI ≥30, current smoking, and a moderate-to-high 10-year cardiovascular risk were similar in the RA and control groups.
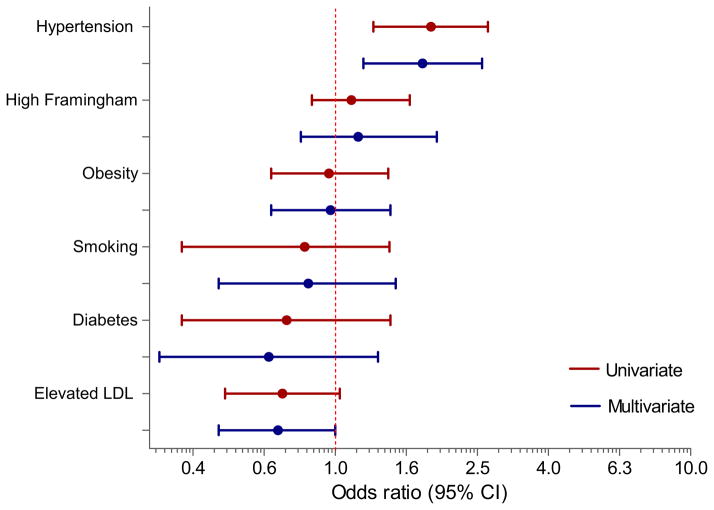
Figure 1 shows the results of multivariate models for the association between RA and individual modifiable traditional cardiovascular risk factors after adjustment for age, gender, ethnicity, level of education, and Medicare enrollment. The association between RA and hypertension was independent of these potential confounders [OR = 1.77 (95% CI: 1.19, 2.59)]. No other CV risk factors were independently associated with RA.
Figure 1.
Relationship between CV risk factors and RA multivariate models include age, gender, race, education, and Medicare enrollment as covariates. (Color version of figure is available online.)
Previously Underdiagnosed Cardiovascular Risk Factors
Figure 2 shows the rates of newly identified cardiovascular risk factors—ie, those diagnosed for the first time as a result of participation in this study. Twenty-four of 113 (21%) hypertensive RA patients and 18 of 115 (16%) hypertensive non-RA control subjects were found to have previously undiagnosed hypertension. Twenty-four (27%) RA patients and 40 (29%) non-RA control subjects were found to have previously undiagnosed hypercholesterolemia. One of 14 patients with RA (7%) and 8 of 26 non-RA control subjects (31%) with diabetes mellitus were previously undiagnosed.
Figure 2.
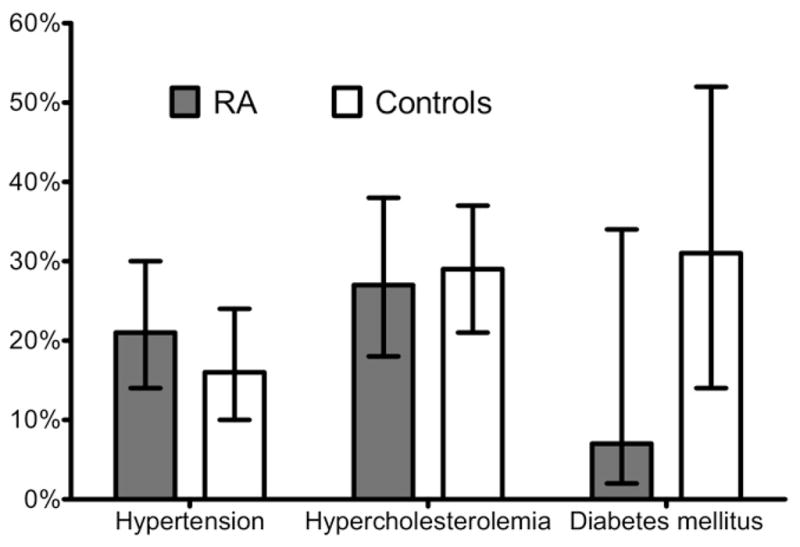
Cardiovascular risk factor awareness: proportion of patients who were newly identified with hypertension, dyslipidemia, and diabetes.
Clinical Characteristics Associated with Cardiovascular Risk Factors in Patients with RA
In the RA group, factors significantly associated with hypertension were older age (OR = 1.07, 95% CI: 1.03, 1.11) and higher HAQ scores (OR: 1.62, 95% CI: 1.0, 2.63). Other demographic characteristics (sex, ethnicity, education), disease characteristics (disease duration, DAS28, radiographic damage, or CRP concentrations), or RA therapeutic interventions (current use of methotrexate, corticosteroids, biologic agents, or any nonaspirin nonsteroidal anti-inflammatory drugs (NSAIDs)) were not associated with hypertension.
Elevated BMI was associated with CRP (logarithmic transformed) (OR = 1.30, 95% CI: 1.03, 1.64). RA patients who were taking corticosteroids had a higher odds of high LDL cholesterol compared with controls [multivariable-adjusted OR = 2.12 (1.14, 3.95)]. RA patients with estimated moderate to high cardiovascular risk were older[OR = 1.10, 95% CI(1.06,1.15) per year of life] and were more likely male (OR =14.47, 95% CI: 7.13–29.33). (Appendix 1).
Appendix 1.
Association of Clinical Characteristics and Medications with Cardiovascular Risk Factors in Patients with RA
| Hypertension
|
Diabetes Mellitus
|
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | |
| Demographics | ||||
| Age (yr) | 1.07 (1.03–1.11) | NA | 1.04 (0.98–1.11) | NA |
| Male sex | 1.06 (0.60–1.89) | NA | 2.10 (0.70–6.32) | NA |
| Caucasians | 1.67 (0.75–3.73) | NA | 0.38 (0.11–1.30) | NA |
| Educationa | 0.59 (0.30–1.16) | NA | 1.85 (0.39–8.64) | NA |
| Disease characteristics | ||||
| Disease duration (yr) | 1.02 (1.0–1.05) | 1.01 (0.98–1.04) | 1.0 (0.95–1.05) | 0.99 (0.93–1.05) |
| DAS28 | 1.18 (0.91–1.55) | 1.25 (0.93–1.68) | 0.81 (0.48–1.36) | 0.87 (0.50–1.49) |
| HAQ | 1.60 (1.03–2.49) | 1.62 (1.00–2.63) | 0.97 (0.43–2.17) | 0.77 (0.30–1.98) |
| Sharp score | 1.0 (1.0–1.0) | 1.0 (1.0–1.01) | 1.0 (1.0–1.01) | 1.0 (0.99–1.01) |
| CRP (log) | 1.05 (0.85–1.30) | 1.01 (0.81–1.27) | 0.93 (0.63–1.40) | 0.85 (0.55–1.31) |
| Current use of medications | ||||
| MTX | 0.93 (0.52–1.69) | 0.92 (0.49–1.73) | 0.75 (0.25–2.26) | 0.78 (0.24–2.50) |
| Corticosteroids | 0.87 (0.49–1.55) | 0.84 (0.45–1.56) | 0.41 (0.11–1.52) | 0.29 (0.06–1.39) |
| Biologic agents | 0.72 (0.41–1.28) | 0.77 (0.42–1.42) | 0.89 (0.30–2.68) | 1.27 (0.39–4.17) |
| NSAIDs | 0.78 (0.43–1.40) | 0.92 (0.49–1.75) | 0.53 (0.18–1.56) | 0.55 (0.17–1.80) |
| Antihypertensives | NA | NA | 1.13 (0.38–3.39) | 1.22 (0.38–3.91) |
| Antidiabetics | 1.51 (0.38–6.24) | 1.17 (0.25–5.44) | NA | NA |
| Lipid lowering agents | 2.10 (0.95–4.66) | 1.77 (0.75–4.17) | 3.98 (1.29–12.33) | 3.41 (0.94–12.42) |
| High LDL Cholesterol
|
Elevated BMI
|
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | Unadjusted OR (95% CI) | Adjusted OR (95% CI)β | |
| Demographics | ||||
| Age (yr) | 1.04 (1.01–1.08) | NA | 0.99 (0.95–1.02) | NA |
| Male sex | 2.22 (1.24–3.97) | NA | 1.29 (0.71–2.34) | NA |
| Caucasians | 0.64 (0.29–1.44) | NA | 1.13 (0.48–2.66) | NA |
| Educationa | 1.30 (0.67–2.52) | NA | 0.82 (0.42–1.62) | NA |
| Disease characteristics | ||||
| Disease duration (yr) | 0.99 (0.96–1.01) | 0.98 (0.95–1.01) | 1.0 (0.97–1.02) | 1.0 (0.97–1.02) |
| DAS28 | 0.86 (0.66–1.12) | 0.94 (0.71–1.25) | 1.20 (0.92–1.58) | 1.27 (0.96–1.70) |
| HAQ | 0.95 (0.63–1.44) | 1.01 (0.64–1.59) | 1.40 (0.92–2.14) | 1.58 (0.99–2.51) |
| Sharp score | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| CRP (log) | 0.89 (0.72–1.09) | 0.84 (0.67–1.05) | 1.26 (1.01–1.57) | 1.30 (1.03–1.64) |
| Current use of medications | ||||
| MTX | 0.60 (0.33–1.07) | 0.69 (0.37–1.28) | 1.20 (0.65–2.22) | 1.16 (0.61–2.20) |
| Corticosteroids | 1.84 (1.03–3.30) | 2.12 (1.14–3.95) | 1.15 (0.61–2.16) | 1.97 (0.70–5.51) |
| Biologic agents | 1.04 (0.59–1.84) | 1.32 (0.72–2.42) | 1.45 (0.80–2.62) | 1.59 (0.86–2.94) |
| NSAIDs | 0.94 (0.52–1.68) | 1.02 (0.55–1.91) | 1.24 (0.67–2.31) | 1.36 (0.71–2.59) |
| Antihypertensives | 1.94 (1.09–3.45) | 1.78 (0.97–3.26) | 1.70 (0.94–3.09) | 1.96 (1.05–3.66) |
| Antidiabetics | 4.62 (0.94–22.85) | 2.93 (0.56–15.44) | 4.06 (0.98–16.80) | 3.95 (0.86–18.10) |
| Lipid lowering agents | NA | NA | 2.06 (0.98–4.31) | 2.07 (0.94–4.57) |
| High 10-year Risk Framingham
|
Smoking
|
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | |
| Demographics | ||||
| Age (yr) | 1.10 (1.06–1.15) | NA | 0.97 (0.92–1.02) | NA |
| Male sex | 14.47 (7.13–29.33) | NA | 2.16 (0.90–5.21) | NA |
| Caucasians | 1.07 (0.46–2.46) | NA | 1.07 (0.30–3.90) | NA |
| Educationa | 0.76 (0.39–1.48) | NA | 0.45 (0.18–1.12) | NA |
| Disease characteristics | ||||
| Disease duration (yr) | 1.01 (0.98–1.04) | 0.99 (0.95–1.03) | 0.97 (0.92–1.01) | 0.97 (0.92–1.02) |
| DAS28 | 0.62 (0.46–0.83) | 0.81 (0.56–1.17) | 0.93 (0.62–1.39) | 1.0 (0.66–1.51) |
| HAQ | 0.73 (0.47–1.14) | 1.01 (0.53–1.92) | 0.83 (0.42–1.64) | 0.92 (0.45–1.90) |
| Sharp score | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (0.99–1.01) |
| CRP (log) | 1.12 (0.91–1.39) | 1.04 (0.76–1.43) | 1.0 (0.72–1.37) | 1.0 (0.72–1.39) |
| Current use of medications | ||||
| MTX | 0.73 (0.40–1.33) | 1.26 (0.53–3.02) | 1.38 (0.54–3.52) | 1.53 (0.57–4.12) |
| Corticosteroids | 0.99 (0.54–1.78) | 1.26 (0.54–2.96) | 1.56 (0.65–3.74) | 1.69 (0.69–4.19) |
| Biologic agents | 0.48 (0.26–0.87) | 0.80 (0.35–1.82) | 1.10 (0.46–2.64) | 1.27 (0.51–3.18) |
| NSAIDs | 0.82 (0.45–1.50) | 1.07 (0.45–1.54) | 1.62 (0.61–4.33) | 1.79 (0.65–4.92) |
| Antihypertensives | NA | NA | 0.61 (0.24–1.57) | 0.62 (0.23–1.64) |
| Antidiabetics | NA | NA | NA | NA |
| Lipid lowering agents | NA | NA | 1.38 (0.47–4.01) | 1.42 (0.46–4.39) |
>12th grade vs ≦12th grade.
Adjusted OR are calculated in a multivariate model including age, sex, race, education, and Medicare enrollment.
Treatment of Cardiovascular Risk Factors and Use of Aspirin
Seventy-nine (70%) patients with either new or previously diagnosed hypertension in the RA group and 84 (73%) in the control group were receiving at least 1 anti-hypertensive medication, (P = 0.66, OR = 0.93 (95% CI: 0.52, 1.69)) after adjusting for age, sex, ethnicity, education, and Medicare enrollment (Table 2).
Table 2.
Association Between RA and the Treatment of Modifiable Cardiovascular Risk Factors
| Univariate Model, OR (95% CI) | P Value | Multivariate Model, OR (95% CI) | P Value | |
|---|---|---|---|---|
| Antihypertensives | 0.86 (0.48–1.52) | 0.60 | 0.93 (0.52–1.69) | 0.82 |
| Lipid lowering agents | 1.18 (0.68–2.04) | 0.56 | 1.08 (0.61–1.93) | 0.79 |
| Hypoglycemic agents | 7.71 (0.85–69.98) | 0.07 | 6.79 (0.66–70.14) | 0.11 |
| Aspirin among patients with high CV risk | 2.93 (1.28–6.67) | 0.01 | 2.25 (0.93–5.41) | 0.07 |
| Controlled HTN | 0.72 (0.42–1.22) | 0.22 | 0.73 (0.42–1.27) | 0.27 |
| Controlled LDL | 1.30 (0.76–2.22) | 0.34 | 1.34 (0.76–2.39) | 0.32 |
| Controlled DM | 16.0 (2.67–95.75) | 0.002 | 125.97 (3.01–5279.66) | 0.01 |
Rates of use of cholesterol-lowering medications among those with new or previous diagnosis of elevated LDL cholesterol were also similar in patients and controls (40% versus 36%, P = 0.58).
Although the number of participants with diabetes mellitus was small, there was a trend toward higher rates of treatment among patients with RA (90%) than control subjects (54%) (P = 0.06).
Among individuals with moderate to high cardiovascular risk, the use of aspirin was similar in RA patients and controls (25% versus 22%, P = 0.71).
Achievement of Target Goals
Forty-five of 113 hypertensive RA patients (40%) and 55 (48%) hypertensive non-RA subjects had blood pressures in the targeted range (P = 0.23) (Fig 3). A sensitivity analysis restricted to patients with previously diagnosed hypertension showed achievement of target blood pressures in only 51% RA and 57% non-RA control subjects, respectively (P = 0.46). An exploratory unadjusted analysis suggested that younger age (OR = 0.94, 95% CI: 0.90, 0.99 per year of life), higher education (OR 4.12, 95% CI: 1.53, 11.09, for those patients with at least 12th grade versus those with less than 12th grade of education), and current use of any antihypertensive agent (OR = 4.55, 95% CI: 1.70, 12.19) were factors significantly associated with achieving adequate control of hypertension in patients with RA. (Appendix 2).
Figure 3.
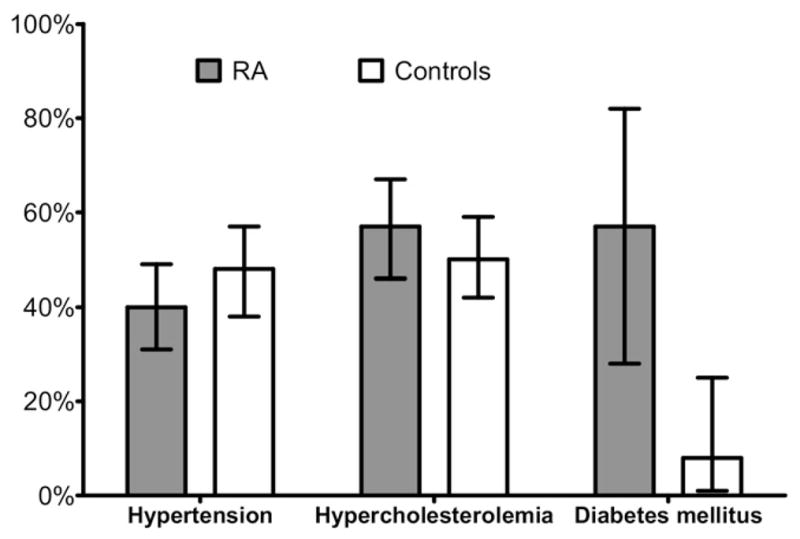
Control of cardiovascular risk factors: proportion of patients in whom therapeutic goals were met for treatment of hypertension, dyslipidemia, and diabetes.
Appendix 2.
Characteristics of Patients Achieving Therapeutic Goals in Patients with RA
| Hypertension
|
Diabetes Mellitus
|
||
|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | Unadjusted OR (95% CI) | |
| Demographics | |||
| Age (yr) | 0.94 (0.90–0.99) | NA | 1.07 (0.95–1.19) |
| Male sex | 0.51 (0.23–1.12) | NA | 0.50 (0.06–4.47) |
| Caucasians | 0.53 (0.16–1.68) | NA | 1.50 (0.15–15.46) |
| Educationa | 4.12 (1.53–11.09) | NA | 1.20 (0.06–4.47) |
| Disease characteristics | |||
| Disease duration (yr) | 0.98 (0.94–1.01) | 1.0 (0.95–1.04) | 1.21 (0.92–1.58) |
| DAS28 | 1.37 (0.94–2.0) | 1.19 (0.77–1.84) | 1.23 (0.38–4.02) |
| HAQ | 1.07 (0.62–1.86) | 1.06 (0.54–2.08) | 4.21 (0.50–35.69) |
| Sharp score | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.02 (0.99–1.04) |
| CRP (log) | 1.08 (0.81–1.43) | 1.15 (0.83–1.60) | 1.25 (0.40–3.92) |
| Current use of medications | |||
| MTX | 0.96 (0.44–2.09) | 0.97 (0.40–2.34) | 6.0 (0.58–61.84) |
| Corticosteroids | 0.65 (0.29–1.43) | 0.61 (0.25–1.50) | NA |
| Biologic agents | 1.47 (0.68–3.18) | 1.08 (0.45–2.55) | 0.60 (0.07–5.14) |
| NSAIDs | 1.02 (0.47–2.22) | 0.64 (0.26–1.57) | 0.07 (0.0–0.97) |
| Antihypertensives | 4.55 (1.70–12.19) | 5.50 (1.80–16.77) | 0.60 (0.07–5.14) |
| Antidiabetics | 1.55 (0.30–8.03) | 2.91 (0.39–21.53) | 0.83 (0.09–7.68) |
| Lipid lowering agents | 1.01 (0.41–2.50) | 1.31 (0.46–3.69) | 0.17 (0.02–1.72) |
| Adjusted OR (95% CI)b | Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | |
|---|---|---|---|
| Demographics | |||
| Age (yr) | NA | 0.99 (0.94–1.04) | NA |
| Male sex | NA | 0.62 (0.26–1.45) | NA |
| Caucasians | NA | 2.40 (0.72–8.04) | NA |
| Educationa | NA | 1.69 (0.61–4.69) | NA |
| Disease characteristics | |||
| Disease duration (yr) | NA | 0.99 (0.95–1.03) | 1.01 (0.96–1.05) |
| DAS28 | NA | 1.21 (0.82–1.80) | 1.19 (0.76–1.87) |
| HAQ | NA | 1.11 (0.58–2.13) | 1.38 (0.62–3.10) |
| Sharp score | NA | 1.0 (0.99–1.0) | 1.0 (0.99–1.0) |
| CRP (log) | NA | 0.91 (0.65–1.27) | 0.99 (0.69–1.41) |
| Current use of medications | |||
| MTX | NA | 0.77 (0.33–1.80) | 0.65 (0.26–1.62) |
| Corticosteroids | NA | 0.95 (0.41–2.20) | 0.94 (0.39–2.27) |
| Biologic agents | NA | 1.09 (0.47–2.56) | 0.89 (0.35–2.25) |
| NSAIDs | NA | 1.55 (0.64–3.71) | 1.48 (0.57–3.85) |
| Antihypertensives | NA | 1.61 (0.69–3.78) | 1.64 (0.66–4.03) |
| Antidiabetics | NA | 0.28 (0.05–1.50) | 0.32 (0.05–1.95) |
| Lipid lowering agents | NA | 9.90 (3.30–29.67) | 15.71 (4.33–57.01) |
>12th grade vs ≦12th grade.
Adjusted OR are calculated in a multivariate model including age, sex, race, and education. There was not enough power to model multivariate regressions for diabetes.
Among participants with hyperlipidemia, there were 50 (57%) in the RA group and 70 (50%) in the control group who achieved target goals (P = 0.41). Among patients with previously diagnosed hyperlipidemia, target LDL cholesterol goals were achieved in 78% RA and 70% non-RA subjects, respectively (P = 0.36). As expected, patients who were receiving lipid lowering drugs were more likely to have their LDL under appropriate control (OR = 9.90, 95% CI: 3.30, 29.67) (Appendix 2).
Among participants with diabetes, normal fasting glucose was present in 57% RA patients compared with only 8% of non-RA controls (P = 0.001). Although the number of previously underdiagnosed diabetics was higher in non-RA controls, when the analysis was restricted to individuals with previously diagnosed diabetes mellitus, the rate of achievement of normal glucose increased modestly to 62% in RA and 11% in non-RA subjects, but remained significantly different between groups (P = 0.006).
DISCUSSION
The novel findings of this study are that (1) the rates of undiagnosed hypertension, elevated LDL, and type II diabetes are high in patients with RA and (2) treatment target goals, as defined by current therapeutic guidelines, are achieved in only 40% of RA patients with hypertension, 57% of patients with elevated LDL, and 57% of patients with diabetes.
This is important because, although inflammation may account for a portion of the higher atherosclerosis burden seen in patients with RA, many traditional cardiovascular risk factors also play a major role in atherosclerosis (26,27) and, importantly, are amenable to intervention.
Hypertension, a major risk factor for cardiovascular disease, affects more than 58 million people in the US and its rate is increasing (28). In RA, hypertension doubled the risk of a composite cardiovascular outcome that included myocardial infarction, heart failure, and cardiovascular death (26), and estimations projected that untreated hypertension accounted for an attributable yearly increment of 1572 cases of ischemic heart disease in the US (29). Using a careful protocolized measurement of blood pressure, we found that hypertension was highly prevalent in patients with RA. Concordant with Panoulas and others (30), rates of hypertension were higher than in matched non-RA subjects.
Many mechanisms may contribute to the high prevalence of hypertension in patients with RA (31). First, systemic inflammation reduces the production of nitric oxide, resulting in vasoconstriction. Second, higher oxidative stress and activation of the angiotensin-converting enzyme may be shared pathways between the inflammatory process in RA and hypertension (32–34). In addition, medications commonly used in RA, such as corticosteroids and NSAIDs, are associated with hypertension (31). Our study did not address the first 2 hypotheses and did not find any association of prednisone nor NSAIDs with elevated blood pressure. This lack of association might be explained by the low doses of both prednisone and aspirin used by study participants.
The high prevalence of hypertension is of interest, but even more striking are our data about management. Current guidelines provide evidence-based indications for when to initiate therapeutic interventions and set target blood pressure goals. However, we found that only 40% of RA patients meeting criteria for hypertension were at target blood pressure goals. Although this rate is similar to that in the non-RA group (48%), it is nonetheless strikingly low.
Traditional modifiable risk factors other than hypertension contribute significantly to the risk for CHD events. Recent data from 51 countries showed that the odds of a myocardial infarction increases between 2.24 to 2.95 times if modifiable CV risk factors such as elevated BMI, diabetes mellitus, hypertension, or current smoking were present (35). Our data in patients with RA indicate that high LDL cholesterol is present in almost 1 of every 2 patients with RA. Furthermore, only 57% of RA patients with a diagnosis of elevated LDL exhibited guideline target LDL levels. These data are consistent with a study that evaluated the care and calculated quality scores for management of arthritis, comorbid conditions, and health care maintenance in patients with RA.
The prevalence of diabetes was similar in both groups, but the proportion of newly diagnosed patients was 7% in the RA group and the 31% in the control group. Although the numbers are small, this phenomenon might be explained by differences in health care utilization. It is possible that patients with RA visit their doctors more frequently. Furthermore, it is likely that routine laboratory monitoring for drug-related toxicities increases the chances of detecting hyperglycemia in patients with RA.
Evaluating the care provided for diabetes mellitus, congestive heart failure, myocardial infarction, coronary artery disease, and gastrointestinal bleeds, MacLean and colleagues estimated that the quality score (defined as the number of recommended processes performed divided by the numbers recommended in an eligibility period) for comorbid conditions was only 52% in patients with RA (36). The identification and treatment of traditional cardiovascular risk factors have a major impact on public health.
Interventions such as initiating aspirin in high-risk individuals, lowering blood pressure and LDL cholesterol, weight reduction, and smoking cessation are effective (37). Estimations from the general population suggest that the risk of myocardial infarction and stroke could be reduced by 63% and 31%, respectively, if they are implemented.
In the absence of specific guidelines for patients with RA, one would expect that current guidelines for the prevention, identification, and treatment of major modifiable traditional cardiovascular risk factors already developed in the general population would be followed. There are many reasons this may not happen. For example, patients with a chronic condition are likely to receive suboptimal care for additional unrelated disorders (38). Thus, in patients with RA, the attention may be focused primarily on the arthritis (36), especially if time needed by doctors to treat multiple medical conditions is coupled with decreasing reimbursement rates.
The risk of drug-to-drug interactions, side effects, and poor compliance are other potential barriers to therapeutic interventions in patients with multiple chronic conditions (39). All may contribute to the low rates of intervention and proper control of modifiable traditional cardiovascular risk factors in patients with RA. Furthermore, compliance and adherence decrease with more complex regimens (40) and current guidelines can be overly complicated (41). Adding supplementary medications, such as aspirin, to an older patient taking other nonsteroidal anti-inflammatory drugs and prednisone, can have deleterious effects. Finally, cost, high out-of-pocket copayments and lack of insurance, can be major limiting factors.
This study has several strengths. First, both groups are contemporary. Second, all clinical evaluations and the ascertainment of cardiovascular risk factors in both the RA and the control groups were done following exactly the same rigorous protocol. Third, the data on rates of underdiagnosis and proper management according to contemporaneous guidelines are novel.
Our study has also some limitations. The data about other potential interventions, including weight control and smoking cessation, were not available. For controlled diabetes the standard of care includes not only fasting glucose but also glycated hemoglobin, a measure that was not assessed in our study. The definition of prevalent cases of diabetes and hypertension included history of prior use of insulin, oral antidiabetic agents, and antihypertensives. While insulin and oral antidiabetic agents are very specific to diabetes; antihypertensives can have other indications. For example, ACE inhibitors are indicated in patients with congestive heart failure and beta-blockers in patients with prior myocardial infarction. However, given that patients with prior myocardial infarction or congestive heart failure were excluded from the study, we think that misclassification due to this definition is not significant.
Despite the recognized high risk of atherosclerosis in patients with RA, the rates of underdiagnosed hypertension, elevated LDL, and type II diabetes remain high. Furthermore, many RA patients with hypertension, elevated LDL, and diabetes do not achieve target goals as defined by current therapeutic guidelines. Further research is needed to better identify barriers to achieve cardiovascular risk control.
Acknowledgments
We are indebted to the ESCAPE RA staff, Marilyn Towns, Michelle Jones, Patricia Jones, Marissa Hildebrandt, and Shawn Franckowiak, and to the staffs of the Johns Hopkins Bayview Medical Center General Clinical Research Center and the field center of the Baltimore MESA cohort and the MESA Coordinating Center at the University of Washington, Seattle. We thank Drs Uzma Haque, Clifton Bingham III, Carol Ziminski, Jill Ratain, Ira Fine, Joyce Kopicky-Burd, David McGinnis, Andrea Marx, Howard Hauptman, Achini Perera, Peter Holt, Alan Matsumoto, Megan Clowse, Gordon Lam and others for recommending their patients for this study.
This work was supported by Grant Number AR 050026-01 (JMB) from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Contracts N01-HC-95159 through N01-HC-95166 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and the ACR/REF Ephraim P. Engleman Endowed Resident Research Preceptorship (CPC).
References
- 1.Cobb S, Anderson F, Bauer W. Length of life and cause of death in rheumatoid arthritis. N Engl J Med. 1953;249(14):553–6. doi: 10.1056/NEJM195310012491402. [DOI] [PubMed] [Google Scholar]
- 2.Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional decline, work disability and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27(8):864–72. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 3.Prior P, Symmons DP, Scott DL, Brown R, Hawkins CF. Cause of death in rheumatoid arthritis. Br J Rheumatol. 1984;23(2):92–9. doi: 10.1093/rheumatology/23.2.92. [DOI] [PubMed] [Google Scholar]
- 4.Mutru O, Laakso M, Isomaki H, Koota K. Cardiovascular mortality in patients with rheumatoid arthritis. Cardiology. 1989;76(1):71–7. doi: 10.1159/000174474. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(4):481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 6.Myllykangas-Luosujarvi R, Aho K, Kautianien H, Isomaki H. Cardiovascular mortality in women with rheumatoid arthritis. J Rheumatol. 1995;22(6):1065–7. [PubMed] [Google Scholar]
- 7.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Chung CP, Oeser A, Raggi P, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 10.Kremers HM, Crowson CS, Therneau TM, Roger VL, Gabriel SE. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58(8):2268–74. doi: 10.1002/art.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giles JT, Szklo M, Post W, et al. Coronary arterial calcification in rheumatoid arthritis: comparison with the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther. 2009;11(2):R36. doi: 10.1186/ar2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowson CS, Gabriel SE. Towards improving cardiovascular risk management in patients with rheumatoid arthritis: the need for accurate risk assessment. Ann Rheum Dis. 2011;70(5):719–21. doi: 10.1136/ard.2010.145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–31. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 14.Bartoloni E, Alunno A, Bistoni O, Gerli R. Cardiovascular risk in rheumatoid arthritis and systemic autoimmune rheumatic disorders: a suggested model of preventive strategy. Clin Rev Allergy Immunol. 2011 doi: 10.1007/s12016-010-8251-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111(10):1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Giles WH, Mokdad AH. The distribution of 10-Year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43(10):1791–6. doi: 10.1016/j.jacc.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe F. Which HAQ is best? A comparison of the HAQ, MHAQ and RA-HAQ, a difficult 8 item HAQ (DHAQ), and a rescored 20 item HAQ (HAQ20): analyses in 2,491 rheumatoid arthritis patients following leflunomide initiation. J Rheumatol. 2001;28(5):982–9. [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.Rosendorff C, Black HR, Cannon CP, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115(21):2761–88. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 22.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 24.Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106(3):388–91. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino S, Grundy S, Sullivan LM, Wilson P for the CHD Risk Prediction Group. Validation of the Framingham Coronary Heart Disease Prediction Scores: Results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez A, Maradit KH, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67(1):64–9. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 27.Chung CP, Oeser A, Avalos I, et al. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2006;8(6):R186. doi: 10.1186/ar2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajjar I, Kotchen TA. Trends in prevalence. Awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 29.Singh G, Miller JD, Huse DM, Pettitt D, D’Agostino RB, Russell MW. Consequences of increased systolic blood pressure in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2003;30(4):714–9. [PubMed] [Google Scholar]
- 30.Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46(9):1477–82. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 31.Panoulas VF, Metsios GS, Pace AV, et al. Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 2008;47(9):1286–98. doi: 10.1093/rheumatology/ken159. [DOI] [PubMed] [Google Scholar]
- 32.Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6(6):265–78. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minuz P, Patrignani P, Gaino S, et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation. 2002;106(22):2800–5. doi: 10.1161/01.cir.0000039528.49161.e9. [DOI] [PubMed] [Google Scholar]
- 34.Flammer AJ, Sudano I, Hermann F, et al. Angiotensin-converting enzyme inhibition improves vascular function in rheumatoid arthritis. Circulation. 2008;117(17):2262–9. doi: 10.1161/CIRCULATIONAHA.107.734384. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 36.MacLean CH, Louie R, Leake B, et al. Quality of care for patients with rheumatoid arthritis. JAMA. 2000;284(8):984–92. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 37.Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;118(5):576–85. doi: 10.1161/CIRCULATIONAHA.108.190186. [DOI] [PubMed] [Google Scholar]
- 38.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338(21):1516–20. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 39.Steinbrook R. Patients with multiple chronic conditions—how many medications are enough? N Engl J Med. 1998;338(21):1541–2. doi: 10.1056/NEJM199805213382111. [DOI] [PubMed] [Google Scholar]
- 40.Boers M, Dijkmans B, Gabriel S, Maradit-Kremers H, O’Dell J, Pincus T. Making an impact on mortality in rheumatoid arthritis: targeting cardiovascular comorbidity. Arthritis Rheum. 2004;50(6):1734–9. doi: 10.1002/art.20306. [DOI] [PubMed] [Google Scholar]
- 41.Gaziano JM, Gaziano TA. Simplifying the approach to the management of dyslipidemia. JAMA. 2009;302(19):2148–9. doi: 10.1001/jama.2009.1685. [DOI] [PubMed] [Google Scholar]