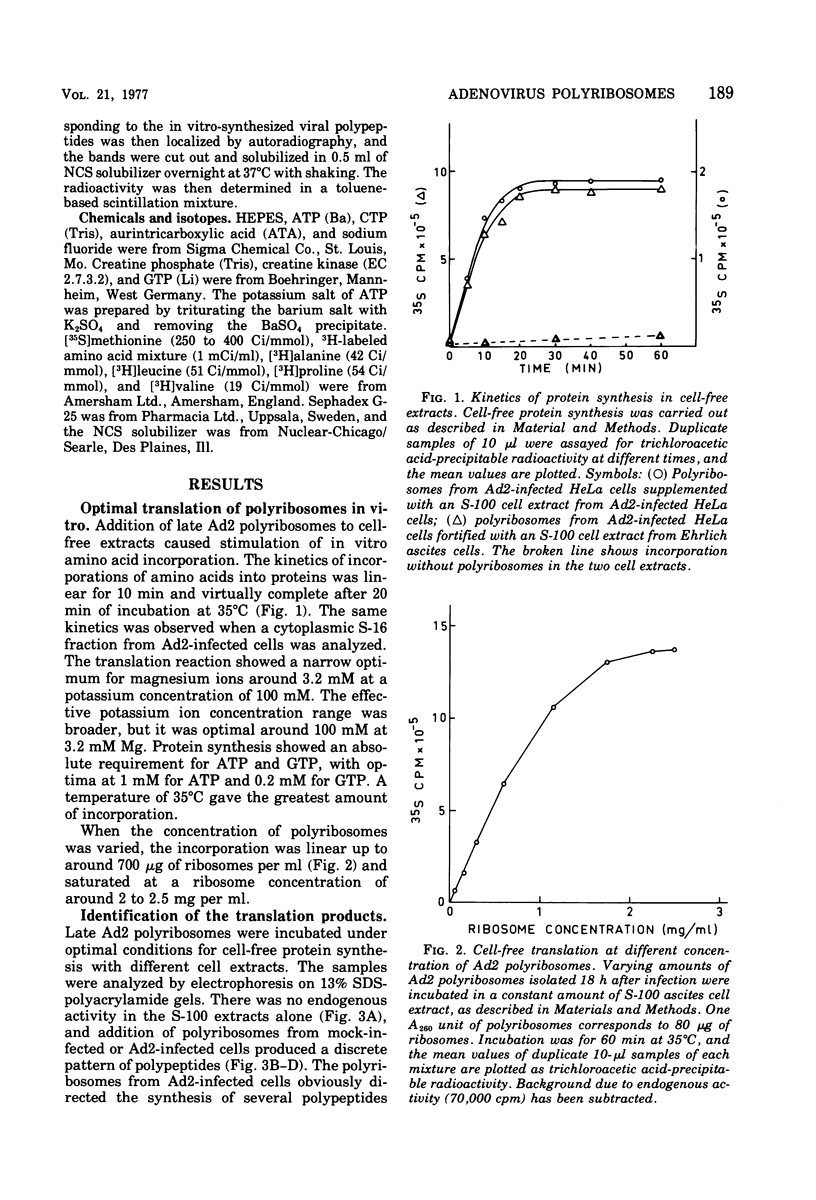
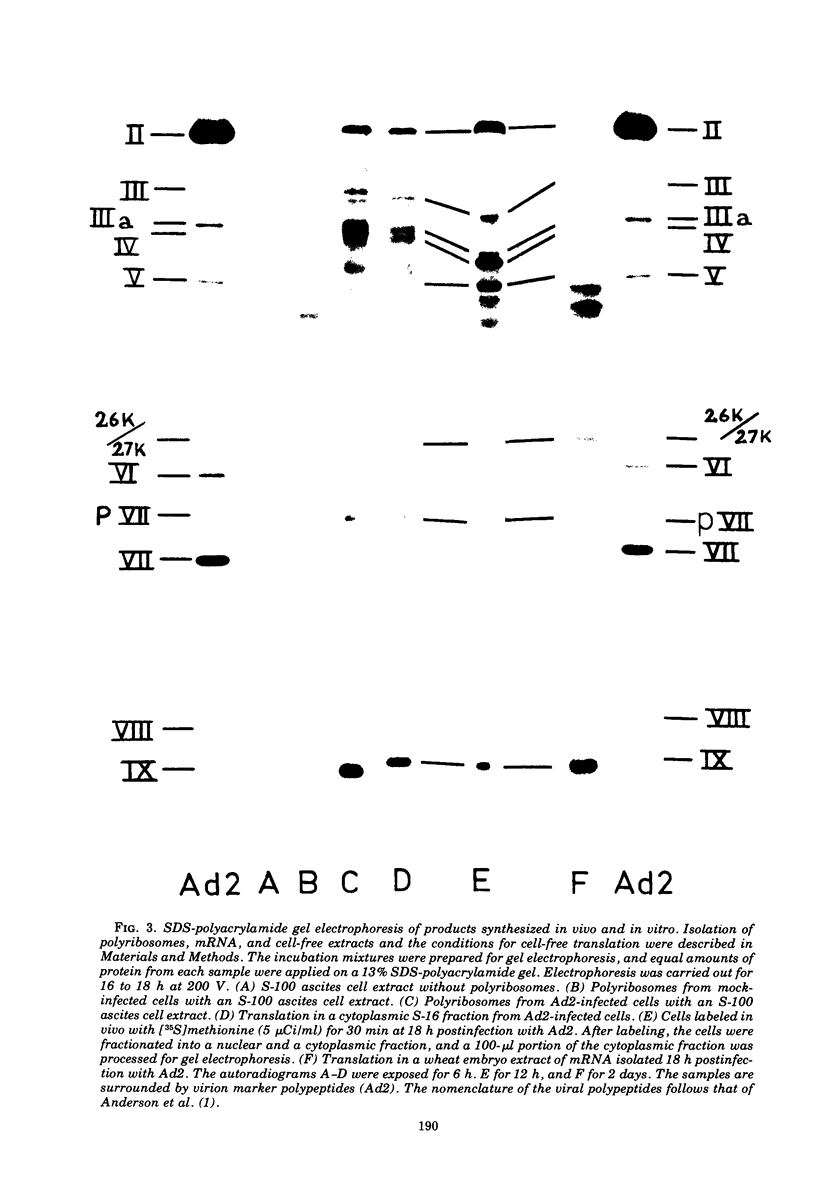
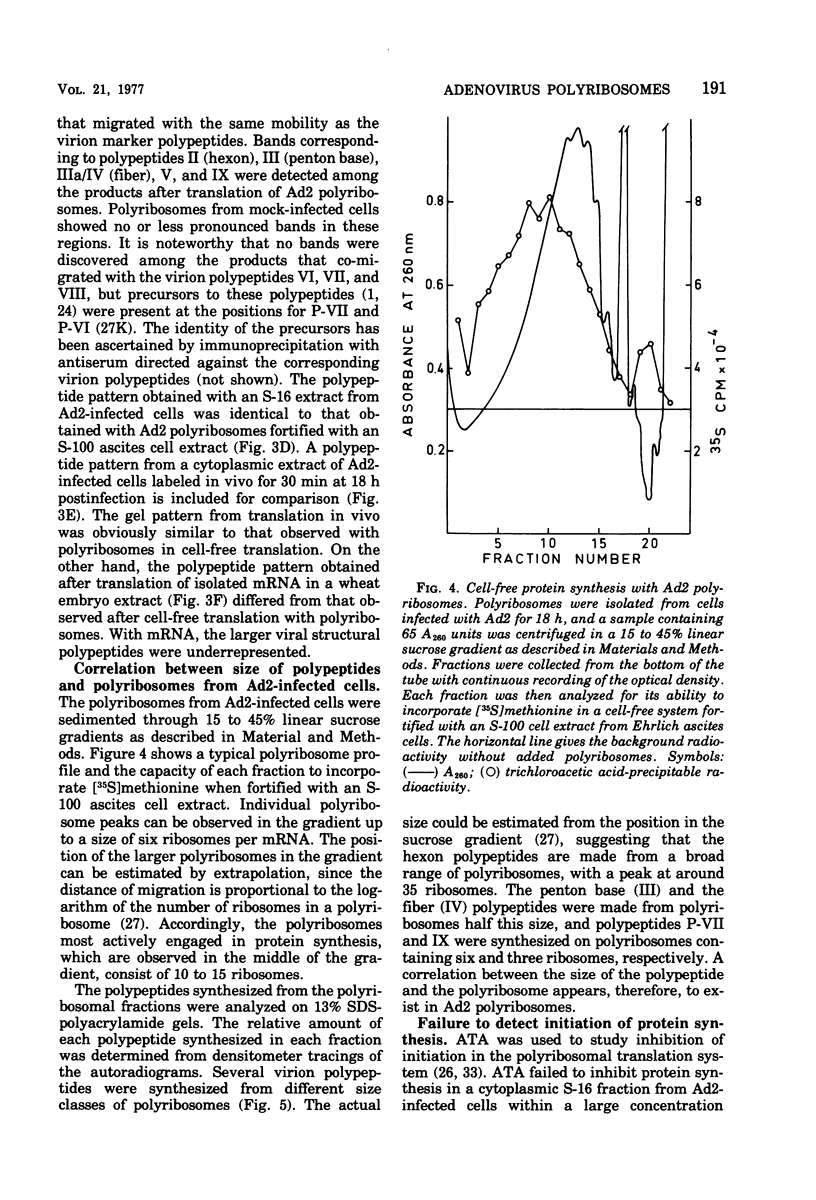
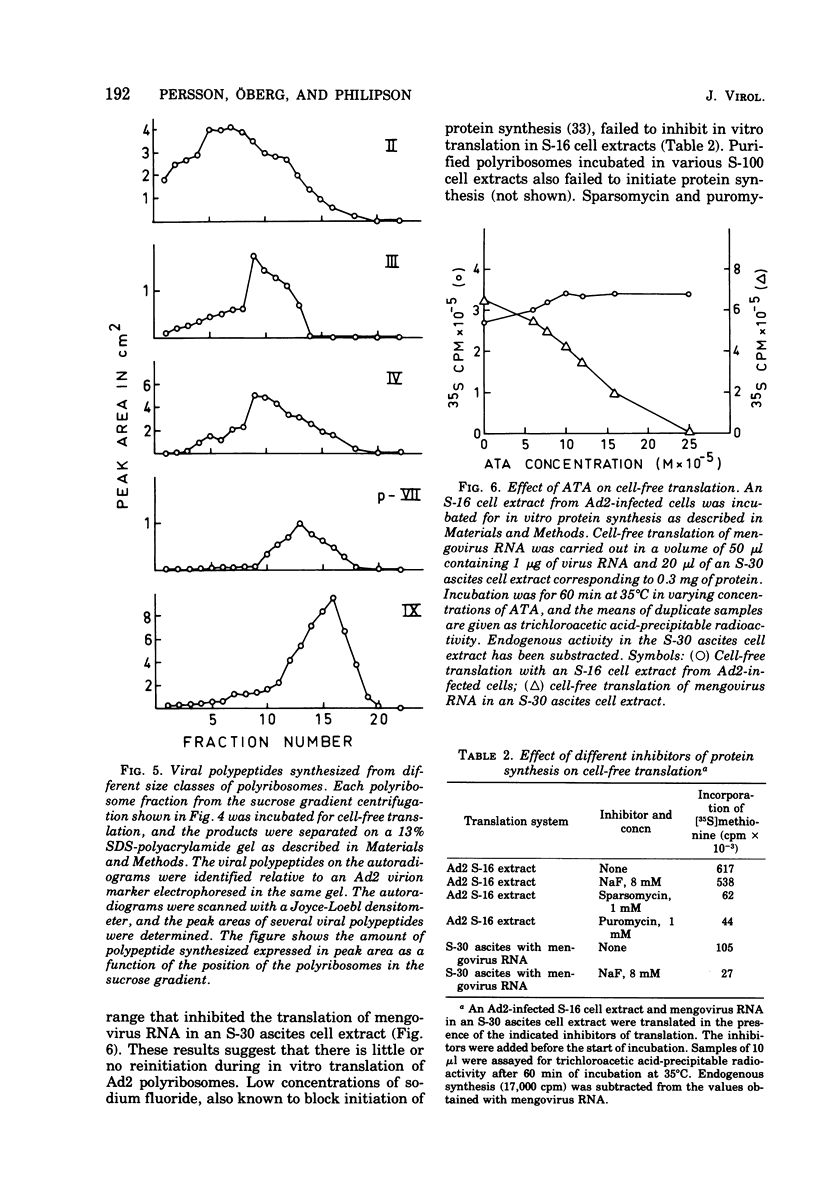
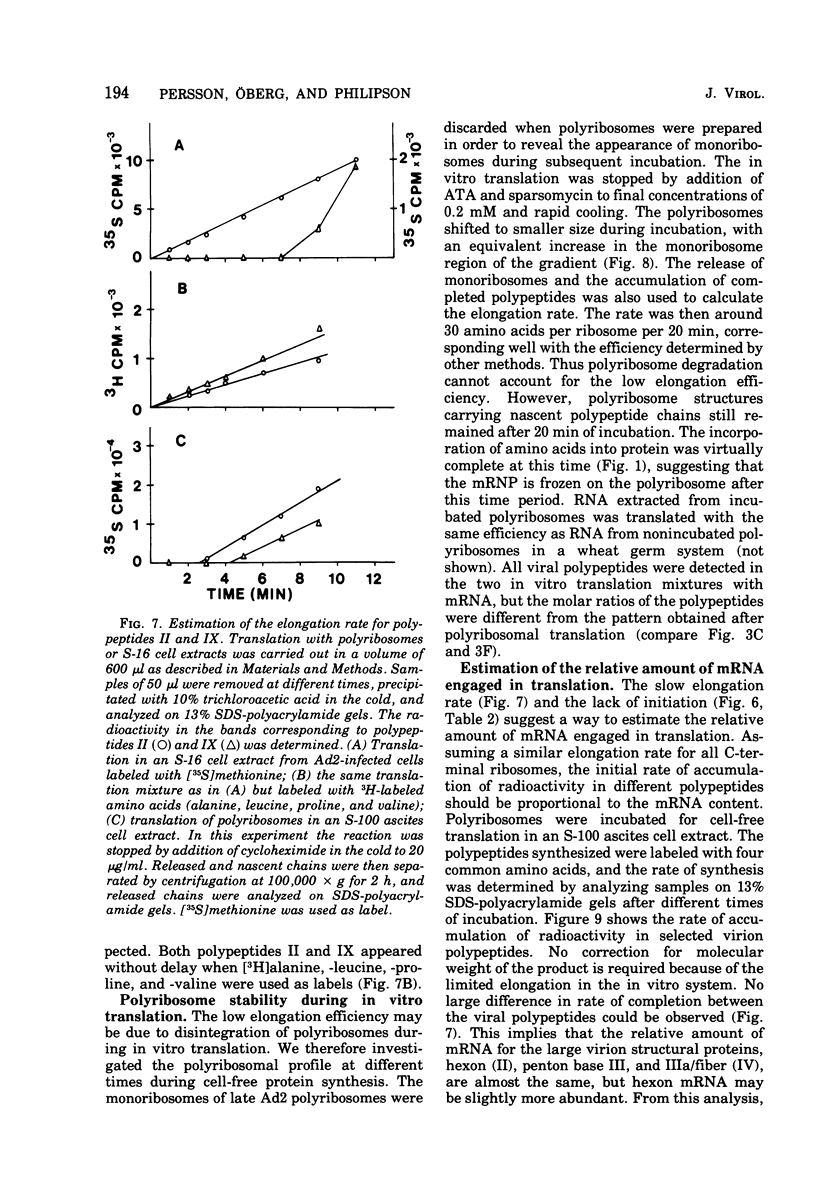
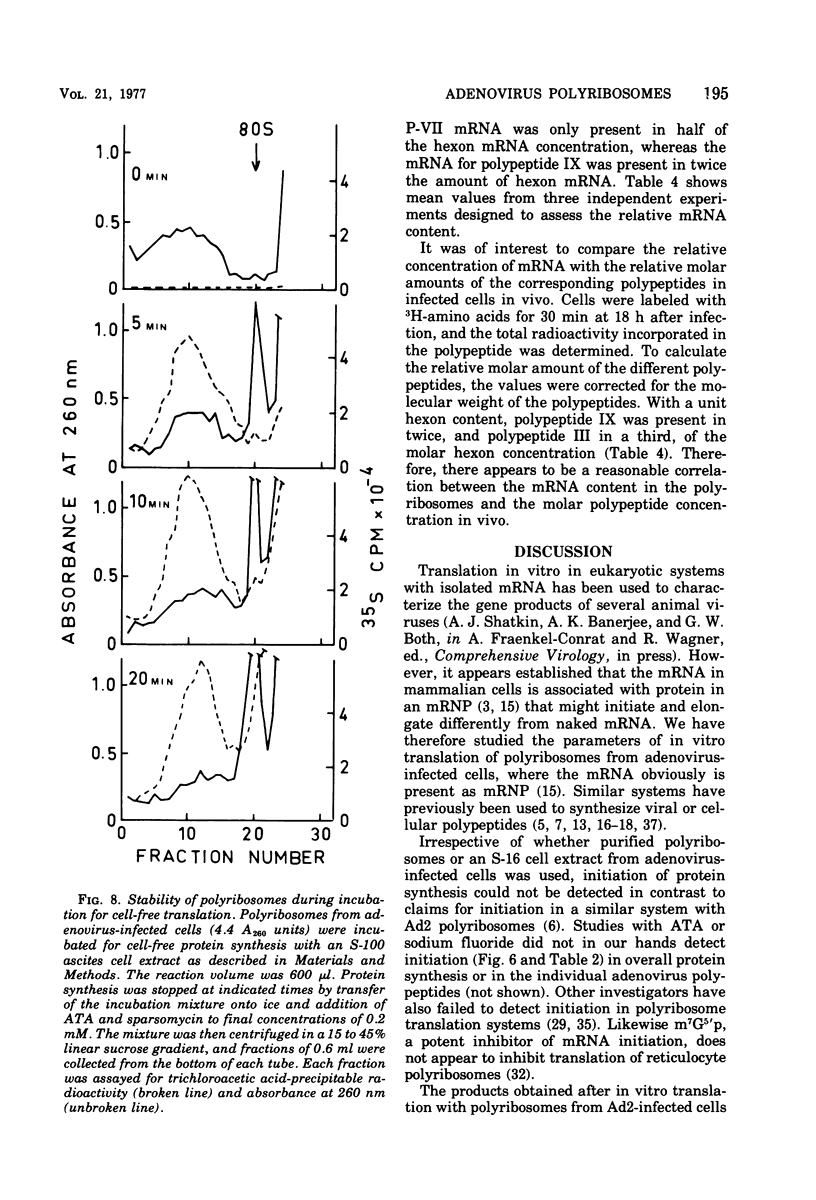
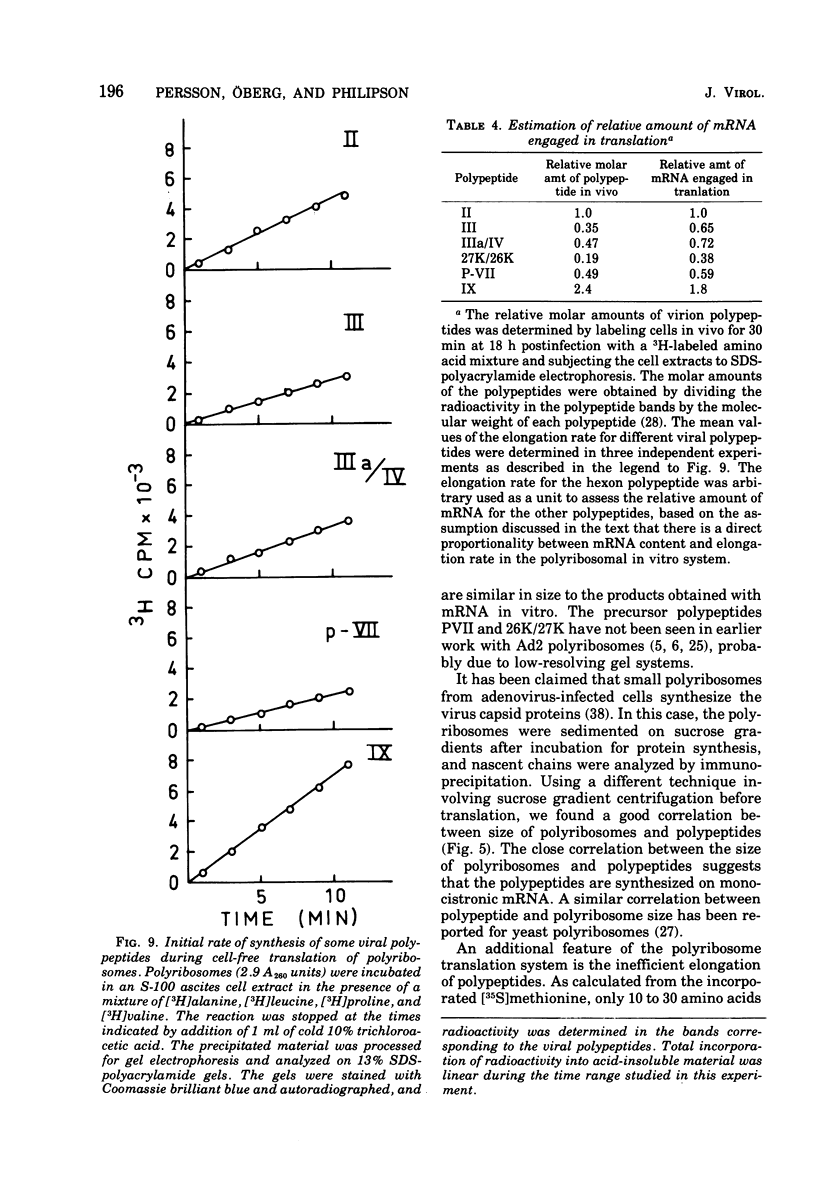
Abstract
Polyribosomes isolated from adenovirus type 2 (Ad2)-infected HeLa cells late in productive infection can be used for translation in cell-free systems. At least eight viral polypeptides are synthesized, including the precursors to virion polypeptides VI and VII. Separation of polyribosomes by zonal rate centrifugation followed by translation in a cell-free system reveals a correlation between the sizes of the polyribosomes and the polypeptides synthesized. The cell-free extracts incorporate amino acid linearly for only 10 min and show little or no capacity to reinitiate protein synthesis. The elongation efficiency measured as the number of amino acids incorporated per ribosome in 20 min is low, ranging from 10 to 100. The maximum chain elongation rate is estimated to be 10 to 20 amino acids per min. The limited elongation has been used to assess the relative concentration of mRNA's engaged in translation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Caffier H., Green M. Adenovirus proteins. 3. Cell-free synthesis of adenovirus proteins in cytoplasmic extracts of KB cells. Virology. 1971 Oct;46(1):98–105. doi: 10.1016/0042-6822(71)90009-2. [DOI] [PubMed] [Google Scholar]
- Caffier H., Raskas H. J., Parsons T. J., Green M. Initiation of mammalian viral protein synthesis. Nat New Biol. 1971 Feb 24;229(8):239–241. doi: 10.1038/newbio229239a0. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Translation of poliovirus RNA in vitro: detection of two different initiation sites. J Mol Biol. 1975 Nov 15;98(4):761–780. doi: 10.1016/s0022-2836(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Collins J. F., Crystal R. G. Characterization of cell-free synthesis of collagen by lung polysomes in a heterologous system. J Biol Chem. 1975 Sep 25;250(18):7332–7342. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Eggen K. L., Shatkin A. J. In vitro translation of cardiovirus ribonucleic acid by mammalian cell-free extracts. J Virol. 1972 Apr;9(4):636–645. doi: 10.1128/jvi.9.4.636-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Westphal H. Cell-free translation of highly purified adenovirus messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3385–3389. doi: 10.1073/pnas.71.9.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Philipson L. Structural proteins of adenoviruses. XI. Purification of three low molecular weight virion proteins of adenovirus type 2 and their synthesis during productive infection. Virology. 1974 Nov;62(1):253–269. doi: 10.1016/0042-6822(74)90320-1. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Summers D. F. In vitro protein-synthesizing activity of vesicular stomatitis virus-infected cell extracts. J Virol. 1973 Aug;12(2):265–274. doi: 10.1128/jvi.12.2.265-274.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Sundquist B. Isolation of messenger ribonucleoproteins from mammalian cells. J Mol Biol. 1974 Jun 25;86(2):451–468. doi: 10.1016/0022-2836(74)90030-8. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Mager W. H., Planta R. J. Yeast ribosomal proteins are synthesized on small polysomes. Eur J Biochem. 1976 Feb 2;62(1):193–197. doi: 10.1111/j.1432-1033.1976.tb10113.x. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Osborn M. The rate of polypeptide chain elongation in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1974 Mar 8;340(2):147–152. doi: 10.1016/0005-2787(74)90107-5. [DOI] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K. An in vitro protein synthesizing system from mouse L fibroblasts infected with reovirus. Virology. 1971 Sep;45(3):724–733. doi: 10.1016/0042-6822(71)90186-3. [DOI] [PubMed] [Google Scholar]
- Nwagwu M. Activity of polyribosomes from the muscle of normal and dystrophic mice in cell-free amino-acid incorporation. Eur J Biochem. 1975 Aug 1;56(1):123–127. doi: 10.1111/j.1432-1033.1975.tb02214.x. [DOI] [PubMed] [Google Scholar]
- Oberg B., Philipson L. Replication of poliovirus RNA studied by gel filtration and electrophoresis. Eur J Biochem. 1969 Dec;11(2):305–315. doi: 10.1111/j.1432-1033.1969.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Oberg B., Saborio J., Persson T., Everitt E., Philipson L. Identification of the in vitro translation products of adenovirus mRNA by immunoprecipitation. J Virol. 1975 Jan;15(1):199–207. doi: 10.1128/jvi.15.1.199-207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo C. K., Raskas H. J. A reconstituted system for in vitro synthesis of adenovirus 2 proteins. Virology. 1972 Feb;47(2):487–490. doi: 10.1016/0042-6822(72)90285-1. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., McLaughlin C. S. Monocistronic messenger RNA in yeast. J Mol Biol. 1973 Nov 25;81(1):33–45. doi: 10.1016/0022-2836(73)90245-3. [DOI] [PubMed] [Google Scholar]
- Reichman M., Penman S. Stimulation of polypeptide initiation in vitro after protein synthesis inhibition in vivo in HeLa cells. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2678–2682. doi: 10.1073/pnas.70.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. Gene order of the poliovirus capsid proteins. J Virol. 1972 Mar;9(3):479–487. doi: 10.1128/jvi.9.3.479-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Weinstein J. A., Safer B., Merrick W. C., Weber L. A., Hickey E. D., Baglioni C. Evidence for role of m7G5'-phosphate group in recognition of eukaryotic mRNA by initiation factor IF-M3. Nature. 1976 May 27;261(5558):291–294. doi: 10.1038/261291a0. [DOI] [PubMed] [Google Scholar]
- Vuust J., Piez K. A. A kinetic study of collagen biosynthesis. J Biol Chem. 1972 Feb 10;247(3):856–862. [PubMed] [Google Scholar]
- Weber L. A., Feman E. R., Baglioni C. A cell free system from HeLa cells active in initiation of protein synthesis. Biochemistry. 1975 Dec 2;14(24):5315–5321. doi: 10.1021/bi00695a015. [DOI] [PubMed] [Google Scholar]
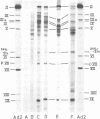
- White D. O., Scharff M. D., Maizel J. V., Jr The polypeptides of adenovirus. 3. Synthesis in infected cells. Virology. 1969 Jul;38(3):395–406. doi: 10.1016/0042-6822(69)90152-4. [DOI] [PubMed] [Google Scholar]
- Wiche G., Zomzely-Neurath C., Blume A. J. In vitro synthesis of mouse neuroblastoma tubulin. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1446–1450. doi: 10.1073/pnas.71.4.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J. M., Ginsberg H. S. Synthesis in vitro of type 5 adenovirus capsid proteins. J Virol. 1972 Jun;9(6):973–980. doi: 10.1128/jvi.9.6.973-980.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]