Abstract
Men who have sex with men (MSM) especially those who are HIV positive are at risk for HPV-associated anal cancer. We systematically reviewed studies with data on the prevalence of vaccine preventable anal HPV among men who have sex with men aged 25 or younger and identified 6 studies. None of these studies were specifically designed to determine the prevalence of HPV in this population. Available data, albeit limited, suggest many young MSM may not already be HPV infected. Further studies using representative sampling focused on teenage MSM are required to confirm this.
Keywords: Human papillomavirus (HPV), Men who have sex with men, Prevalence
Background
Genital human papillomavirus (HPV) infection is widespread [1-3] and usually asymptomatic [4]. HPV types 6 and 11 are the types most commonly associated with anogenital warts [5], while types 16 and 18 are associated with HPV related malignancies including anal and cervical cancer [1-3,6]. Studies demonstrate that the great majority of adult MSM are infected with multiple HPV types [7-9], with a substantial proportion infected with HPV 16 or 18, which together cause about 80% of anal cancers [10-12]. MSM especially those who are HIV positive are at increased risk for anal cancer [13].
The prophylactic quadrivalent HPV vaccine is effective in preventing infection with HPV types 6, 11, 16 and 18 and in reducing the incidence of anogenital warts and anal intraepithelial neoplasia in males [14,15]. However, to date no countries have introduced universal, free vaccination of boys. The extent to which young MSM are already infected with HPV at the age at which targeted vaccination is available is not well documented. We therefore undertook a review of studies examining the prevalence of HPV among MSM aged 25 and younger.
Methods
We systematically reviewed studies by searching MEDLINE and abstracts from the European Research Organisation on Genital Infection and Neoplasia (EUROGIN) Conferences and the International Papillomavirus (IPV) Conferences. Key words used in the search included: “men who have sex with men” or “MSM” or “homosexual” or “bisexual” or “gay” and “human papillomavirus” or “HPV”.
The following data were extracted: author, year of publication, study setting, number of MSM, number of MSM aged 25 or younger, sample collection method, HPV DNA testing method, and HPV type tested. HPV 6/11/16/18 prevalence were extracted, and where possible, HPV 6/11/16/18 prevalence stratified by age group (≤20 years old; 21–25 years old; ≤25 years old).
Results
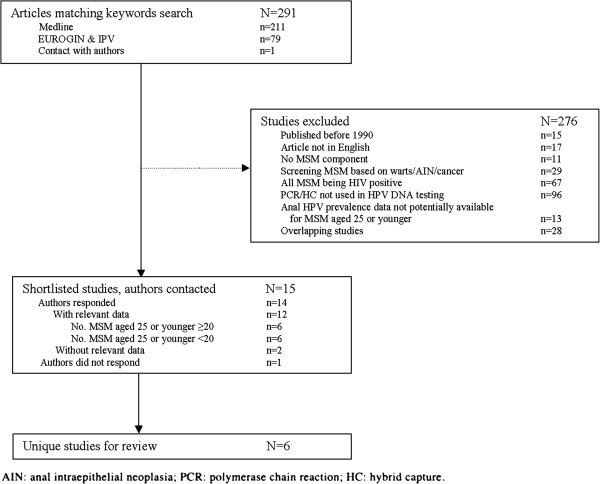
The outcome of the review is summarized in Figure 1. The study populations, selection criteria, testing methods for HPV and HPV types covered in the 6 studies included are presented in Table 1. Importantly, none of the studies specifically sought to determine the prevalence of HPV infection among a representative sample of young MSM [7,16-19].
Figure 1.
Selection procedure for studies of anal HPV prevalence among HIV-negative MSM25 years. AIN:anal intraepithelial neoplasia; PCR: polymerase chain reaction; hybrid capture.
Table 1.
Overview of studies with data on anal HPV detection among men who have sex with men aged ≤ 25 years
| Author, year | Study setting | Selection criteria | No. MSM | No. MSM <=25a | Sample collection method | HPV DNA testing method | HPV types tested |
|---|---|---|---|---|---|---|---|
| (i) Kiviat, 1993 [16]; (ii) Critchlow, 1998 [17] |
(i) Homosexual men attending an STD clinic in Seattle (ii) Homosexual men attending a community-based clinic for HIV screening in Seattle |
(i) Age 16–50; being homosexual or bisexual. (ii) Age ≥18; having sex with other men |
854 |
58b |
Clinician collected |
PCR; primers MY09 and MY11 |
PCR: 6,11,16,18,31 33, 35,39 and 45 |
| Moscicki, 2003 [18] |
Adolescents in primary care centres in 13 US cities who were at high risk of HIV infection |
Age 13–18; having high-risk behaviours and/or injecting drug use |
83 |
25c |
Clinician collected |
PCR, primers MY09, MY11 and HMB01 |
16,18,31,33,39,42, 44,45,51,52, 56 and 58 |
| Nyitray, 2011[7] |
MSM from the general population, universities, STD clinics and organized health care systems in Sao Paulo, Cuernavaca, Tampa. |
Age 18–70; having no prior penile or anal cancer or genital warts and no current HIV/STD diagnosis |
176 |
46 |
Clinician collected |
PCR;the QIAamp Media MDx kit (Qiagen); primers PGMY09/11 |
6,11,16,18,26,31,33, 35,39,40,42,45, 51–56,58,59,61,62,64, 66–73,81-84, IS39 and CP6108 |
| Gilbert, 2011 [20] |
MSM recruited from bars, festivals, associations, community events, bathhouses, and businesses in Vancouver |
Age ≥19; identifying as a man who has ever had sex with men |
178 |
30 |
Self collected |
PCR; PCR and Linear Array kit (Roche); primers PGMY. |
36 types including 6,11,16,18 |
| Goldstone, 2011[19] | MSM in a randomized, placebo-controlled, double-blinded trial of HPV vaccination in 14 countries | Age 16–26; having ≤ 5 lifetime male and/or female sexual partners; having had penetrative intercourse, including oral sex | 602 | 539 | Clinician collected | PCR; QIAamp DNA kit (Qiagen); primers based on published L1, E6 and E7 sequences. | 6,11,16,18, 31,33,35,39, 45,51,52, 56, 58 and 59 |
Note:
a Number of HIV negative MSM ≤ 25 years old for whom data on anal HPV DNA was provided by the authors.
b Combined data from two separate studies (i and ii) provided by the author.
c 58 men who were HIV positive were excluded.
The 6 studies included provided data on anal HPV for a total of 698 MSM aged ≤ 25. Among men aged ≤ 20, the prevalence of anal HPV 6, 11, 16 and 18 ranged from 0 to 17%, 0 to 17%, 0 to 14% and 0 to 20%, respectively. Among men aged 21 to 25, the prevalence of anal HPV 6, 11, 16 and 18 ranged from 8 to 19%, 0 to 8%, 0 to 18% and 3 to 8%, respectively.
Discussion
Through this review, we found that there have been no published studies that have been designed to specifically determine the prevalence of HPV among young MSM using a representative sample of young MSM. Of the six studies identified, all had potential biases because of the way the studies were designed. These could have led to either an overestimate or an underestimate of the true HPV prevalence among young MSM. Several studies recruited men from clinics including STD clinics [7,17,18] while others had selection criteria that may have led to higher risk or lower risk men being included [15,19]. Several studies recruited MSM across a wider age range leaving only a much smaller subset of younger MSM with data on HPV prevalence. Most data derive from a study that was designed to determine HPV vaccine efficacy in MSM rather than HPV prevalence [15]. A further limitation is that all studies only reported HPV detection at a single time point rather than defining infection by the presence of the same HPV type present at two separate time points.
Despite these important limitations, the available data suggest HPV infection may still be uncommon among teenage same sex attracted males, although the data are currently insufficient to draw this conclusion with confidence. A community-based, representative sample is needed to determine the prevalence of HPV infection, defined rigorously, among mainly teenage same sex attracted males.
Given that HPV 16 and 18 are responsible for around 80% of anal cancers [12] and HPV 6 and 11 account for most genital warts [21] such a study would help to ascertain whether targeted HPV vaccination of young MSM is likely to be effective in preventing anal cancer in this group of at-risk males. Ideally, HPV vaccination should occur prior to the onset of sexual activity – ahead of potential HPV acquisition. To date, however, no country has implemented universal HPV vaccination of school aged boys, although the Australian Government has recently announced it is planning to roll out the HPV vaccination program to 12 and 13 years old schoolboys from 2013. Targeted vaccination of MSM to prevent anal cancer would likely only be effective if, in addition to low existing HPV rates, young same sex attracted males are willing to disclose their sexuality to health care providers in order to obtain the HPV vaccine. Further studies to determine the acceptability and feasibility of targeted vaccination of young same sex attracted males are required.
Abbreviations
HPV: Human papillomavirus; MSM: Men who have sex with men; EUROGIN: European Research Organisation on Genital Infection and Neoplasia; IPV: The International Papillomavirus Society; STD: Sexually transmitted diseases.
Competing interests
CF has received honoraria from CSL Biotherapies and Merck and research funding from CSL Biotherapies. CF owns shares in CSL Biotherapies the manufacturer for Gardasil. JH has received an honorarium from CSL Biotherapies and is an investigator on an Australian Research Council funded project (LP0883831) that includes CSL Biotherapies as a research partner. AG has received honoraria and untied research funding from CSL biotherapies, and has received honoraria from Merck. SG has received advisory board fees and grant support from CSL and GlaxoSmithKline, and lecture fees from Merck, GSK and Sanofi Pasteur; in addition, she has received funding through her institution to conduct HPV vaccine studies for MSD and GSK. SG is a member of the Merck Global Advisory Board as well as the Merck Scientific Advisory Committee for HPV. None of this relates to this specific work. MC and HZ have no conflicts of interest.
Authors’ contributions
HZ and MC carried out the literature review and drafted the article. JH, SG, KF and AG participated in the coordination and editing of the article. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Huachun Zou, Email: rolfe1234@gmail.com.
Christopher K Fairley, Email: cfairley@mshc.org.au.
Jane S Hocking, Email: jhocking@unimelb.edu.au.
Suzanne M Garland, Email: suzanne.garland@thewomens.org.au.
Andrew E Grulich, Email: agrulich@kirby.unsw.edu.au.
Marcus Y Chen, Email: mchen@mshc.org.au.
Acknowledgements
We are grateful to the following people who kindly provided us with original data from their studies: Deoraj Caussy, Heidi Friedman, Elizabeth Garner, Mark Gilbert, Anna Giuliano, James Goedert, Stephen Goldstone, Richard Haupt, Stephen Hawes, Travis Salway Hottes, Nancy Kiviat, Yifei Ma, Jennifer Brooke Marshall, Anna Barbara Moscicki, Alan Nyitray, Gina Ogilvie, Joel Palesfky, Maria Alejandra Picconi, Claudia Rank, Eric van der Snoek, Claire Vajdic and Rianne Vriend. We would like to thank Lyle Gurrin, Minh Bui, Eric Chow, Nan Hu, Fengyi Jin, Dorothy Machalek and Lei Zhang for advice on data analysis.
References
- Misra S, Chaturvedi A, Misra NC. Penile carcinoma: a challenge for the developing world. Lancet Oncol. 2004;5(4):240–247. doi: 10.1016/S1470-2045(04)01427-5. [DOI] [PubMed] [Google Scholar]
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194(8):1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, Joura EA. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nyitray AG, da Silva RJ C, Baggio ML, Lu B, Smith D, Abrahamsen M, Papenfuss M, Villa LL, Lazcano-Ponce E, Giuliano AR. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study. J Infect Dis. 2011;203(1):49–57. doi: 10.1093/infdis/jiq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palefsky JM, Shiboski S, Moss A. Risk factors for anal human papillomavirus infection and anal cytologic abnormalities in HIV-positive and HIV-negative homosexual men. J Acquir Immune Defic Syndr. 1994;7(6):599–606. [PubMed] [Google Scholar]
- Vajdic CM, van Leeuwen MT, Jin F, Prestage G, Medley G, Hillman RJ, Stevens MP, Botes LP, Zablotska I, Tabrizi SN. et al. Anal human papillomavirus genotype diversity and co-infection in a community-based sample of homosexual men. Sex Transm Infect. 2009;85(5):330–335. doi: 10.1136/sti.2008.034744. [DOI] [PubMed] [Google Scholar]
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10(12):845–852. doi: 10.1016/S1473-3099(10)70219-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter A, Jesse M, Potthoff A, Brockmeyer NH, Gambichler T, Stucker M, Bechara FG, Pfister H, Wieland U. Expression of proliferative biomarkers in anal intraepithelial neoplasia of HIV-positive men. J Am Acad Dermatol. 2009;63(3):490–498. doi: 10.1016/j.jaad.2009.08.043. [DOI] [PubMed] [Google Scholar]
- Grulich AE, Jin F, Conway EL, Stein AN, Hocking J. Cancers attributable to human papillomavirus infection. Sex Health. 2010;7(3):244–252. doi: 10.1071/SH10020. [DOI] [PubMed] [Google Scholar]
- Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, Hillman RJ, Petoumenos K, Roberts J, Tabrizi SN. et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R. et al. Efficacy of Quadrivalent HPV Vaccine against HPV Infection and Disease in Males. N Engl J Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palefsky J. Quadrivalent HPV Vaccine Efficacy against High-Grade Anal Intraepithelial Neoplasia in Men Having Sex with Men. Lisbon, Portugal: Poster on EUROGIN 2011, May 2011; 2011. Poster number: MSS2-6. [Google Scholar]
- Kiviat NB, Critchlow CW, Holmes KK, Kuypers J, Sayer J, Dunphy C, Surawicz C, Kirby P, Wood R, Daling JR. Association of anal dysplasia and human papillomavirus with immunosuppression and HIV infection among homosexual men. AIDS. 1993;7(1):43–49. doi: 10.1097/00002030-199301000-00007. [DOI] [PubMed] [Google Scholar]
- Critchlow CW, Hawes SE, Kuypers JM, Goldbaum GM, Holmes KK, Surawicz CM, Kiviat NB. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12(10):1177–1184. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- Moscicki AB, Durako SJ, Houser J, Ma Y, Murphy DA, Darragh TM, Farhat S, Wilson CM. Human papillomavirus infection and abnormal cytology of the anus in HIV-infected and uninfected adolescents. AIDS. 2003;17(3):311–320. doi: 10.1097/00002030-200302140-00004. [DOI] [PubMed] [Google Scholar]
- Goldstone S, Palefsky JM, Giuliano AR, Moreira ED Jr, Aranda C, Jessen H, Hillman RJ, Ferris DG, Coutlee F, Liaw KL. et al. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J Infect Dis. 2011;203(1):66–74. doi: 10.1093/infdis/jiq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Kwag M, Mei W, Rank C, Kropp R. et al. Feasibility of incorporating self-collected rectal swabs into a community venue-based survey to measure the prevalence of HPV infection in men who have sex with men. Sex Transm Dis. 2011;38:964–969. doi: 10.1097/OLQ.0b013e318222899d. [DOI] [PubMed] [Google Scholar]
- Anic GM, Lee JH, Stockwell H, Rollison DE, Wu Y, Papenfuss MR, Villa LL, Lazcano-Ponce E, Gage C, Silva RJ. et al. Incidence and Human Papillomavirus (HPV) Type Distribution of Genital Warts in a Multinational Cohort of Men: The HPV in Men Study. J Infect Dis. 2011;204(12):1886–1892. doi: 10.1093/infdis/jir652. [DOI] [PMC free article] [PubMed] [Google Scholar]