Abstract
Objective
Repetitive, paired peripheral and transcranial stimulation targeting the cerebral cortex can increase cortical excitability, outlasting the stimulation period. It is unknown whether paired stimulation specifically targeting the spinal cord can modulate spinal excitability. We tested whether the H-reflex facilitation from a sub-threshold conditioning TMS pulse could modulate spinal excitability if delivered repetitively.
Method
In 13 healthy subjects, we delivered single-pulse TMS (80%RMT) for the right soleus muscle, 20ms prior to an electrical peripheral nerve stimulus delivered over the posterior tibial nerve on the same side at 0.1Hz during 15 minutes.
Results
PNS alone evoked an H-reflex of 0.25mV±0.06SEM, while pairing of TMS and PNS facilitated the H-reflex to 0.7±0.11mV. TMS-PNS pairs delivered at 0.1Hz for 15mins progressively increased in the evoked response to ~130% (r2=0.94) of the starting amplitude (normalized to 1st min). Post intervention, H-reflex threshold decreased (pre=12.9±1.7mA; post=11.6±1.6mA; p=0.04), as did the stimulus intensity at maximum H-reflex amplitude (pre=23.5±02.8mA; post=21.6±2.6mA; p=0.03), and recruitment curve width (pre=11.6±1.5mA; post=10.93±1.4mA; p=0.03). No such changes were observed with intervention of PNS or TMS alone.
Conclusion
Paired stimulation targeting spinal facilitatory interactions, when applied repetitively, can increase spinal excitability during and after the intervention.
Significance
Spinal associative stimulation may have potential for neuromodulation in spinal cord injury patients.
Keywords: Transcranial magnetic stimulation, afferent stimulation, conditioned H-reflex, spinal associative stimulation
INTRODUCTION
Numerous studies have investigated neuromodulatory non-invasive brain stimulation techniques to change cerebral cortex excitability with the aim of improving function (Bolognini et al., 2009; Edwards et al., 2008; Oliveri et al., 1999; Pascual-Leone et al., 1994; Siebner et al., 2004; Wassermann and Lisanby, 2001). These studies have largely used focal repetitive stimulation over an area of brain tissue of interest, yet a pairing of peripheral nerve stimulation (PNS) and transcranial magnetic stimulation (TMS), known as paired associative stimulation (PAS), can lead to similar changes in excitability when the two inputs are timed to coincide at the cortical level (Mrachacz-Kersting et al., 2007; Quartarone et al., 2006; Roy et al., 2007; Stefan et al., 2002; Stefan et al., 2000; Uy and Ridding, 2003). However, few studies have investigated whether the spinal cord can also be targeted in this way (Petersen et al., 2002).
The H-reflex has been considered the electrophysiological equivalent of the monosynaptic tendon tap reflex and changes in its size are thought to reflect segmental motor excitability changes in spinal motoneurons (Pierrot-Deseilligny and Mazevet, 2000; Wolpaw, 1987). Valls-Sole and co-workers demonstrated that a single sub-threshold conditioning TMS pulse delivered 10-20ms (early phase) or 70-90ms (late phase) before a PNS can facilitate the soleus H-reflex in healthy subjects and in patients with neurological lesions (Serranova et al., 2008; Valls-Solé et al., 1994; Valls-Sole and Valldeoriola, 2002). The site of interaction is thought to occur in the spinal cord based on conduction time rationale, and may be mediated by TMS disinhibiting afferent activity (Valls-Sole et al., 1994).
We hypothesized that TMS-induced facilitation of the H-reflex, if delivered repetitively, may form the basis for a spinal associative stimulation (SAS) technique to modulate spinal excitability that may outlast the stimulation period. To determine this, we measured spinal excitability (H-reflex) during and after an intervention comprising 15 minutes of SAS targeting the early phase of facilitation (TMS precedes PNS by 20ms), delivered at low repetition frequency (0.1Hz).
METHODS
Subjects and study design
We use a cross-sectional within-subjects design, to test changes in neurophysiologic measures before, during and after SAS (paired PNS and TMS), and two control protocols consisting of PNS or TMS alone. The SAS experiments were carried out in 13 healthy participants (4F, 24-37 years of age), PNS-alone in 8 participants (5F, 17-39 years of age), and TMS-alone in 8 participants (5F, 29-52 years of age). All participants had no history of neurological disease or contra-indications to PNS or TMS, and gave written informed consent prior to their inclusion in the study, which was approved by the Institutional Review Board (IRB) of the Burke Rehabilitation Hospital. The NIH guidelines for application of TMS were followed (Rossi et al., 2009; Wassermann, 1998).
Participant positioning and set-up
The participants were seated in a comfortable reclining armchair, with their head resting in a foam head support. To ensure muscle relaxation, both legs were supported with a cushion under the knee (to maintain slight flexion) and a band lightly fastened around both legs at the distal thigh to prevent the legs falling into external rotation and abduction (Knash et al., 2003). The distal leg was supported with the ankle joint free, and resting in a slightly plantar-flexed position. Figure 1a illustrates the experimental set-up with peripheral and central stimulation.
Fig 1a.
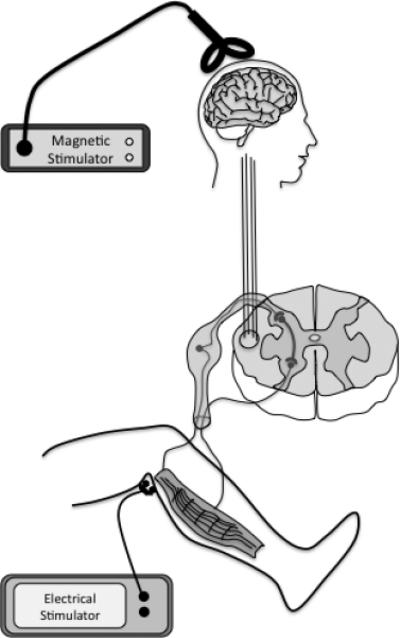
Schematic of the experimental set-up, a subthreshold, single pulse of TMS over the leg motor cortex, conditions segmentally the H-reflex, from a single supra-threshold PNS over the tibial nerve in the popliteal fossa. We proposed that this temporal association applied repetitively would leads to a Hebbian-like modulation of the spinal excitability.
Electromyographic (EMG) recording
Pre-amplified bipolar surface EMG electrodes (1cm diameter, 2cm inter-pole distance, x1000 gain, band-pass filter 20-400Hz; Biometrics Ltd, UK) were taped over the belly of the right soleus muscle, recording the evoked muscle response to TMS and peripheral nerve stimulation (M response and H reflex). Measurements were performed at rest and the responses were measured as the peak-to-peak amplitude of the non-rectified signal. During the experiments real-time EMG activity was continuously monitored with visual feedback to ensure muscle relaxation. EMG silence during the experiment was confirmed offline.
Transcranial Magnetic Stimulation
TMS was applied with a convex figure-of-eight-coil (DB-80 model) delivered by a MagPro X100 stimulator (MagVenture). The coil was fixed in a mechanical frame (Brainsight, Magstim Company Ltd, UK), with the handle posterior (aligned in the sagittal plane) so as to induce posterior-anterior currents in the brain, and positioned over the optimal site to obtain the maximum motor evoked potential (MEP) responses from the right soleus muscle, identified using exploration in the mid-sagittal plane at approximately the vertex with constant supra-threshold stimulus intensity. The Resting Motor Threshold (RMT) was determined as the lowest intensity required to elicit 50μV amplitude MEP in three of five trials in the relaxed soleus muscle.
Peripheral Nerve Stimulation
Electrical stimulation of the posterior tibial nerve was elicited with surface bipolar electrodes in the popliteal fossa of the right leg, to evoke an H-reflex in the right soleus muscle at rest. Electrical stimulation was performed using a Digitimer DS7AH constant-current stimulator (Digitimer Ltd, UK, maximal output 1A) with single 200μs rectangular pulses.
H-reflex recruitment curve
Unconditioned H-reflex recruitment curve were recorded at varying PNS intensities (0.2 to 2mA increments depending on rate of recruitment, 0.5Hz delivery), from sub-threshold to the intensity sufficient to abolish the H-reflex. Intensity was increased until the Maximum M-wave was recorded. Five stimuli were delivered at each PNS intensity. Recruitment curves were measured before and after the SAS and control interventions.
SAS and control protocols
For the SAS protocol, TMS intensity was set to 80%RMT and PNS intensity was set to elicit a conditioned H-reflex of 0.5-1mV peak-to-peak amplitude. TMS was delivered 20ms prior to PNS. SAS was carried out for 15 minutes, with TMS-PNS pairs delivered every 10 seconds (Fig. 1b).
Fig 1b.
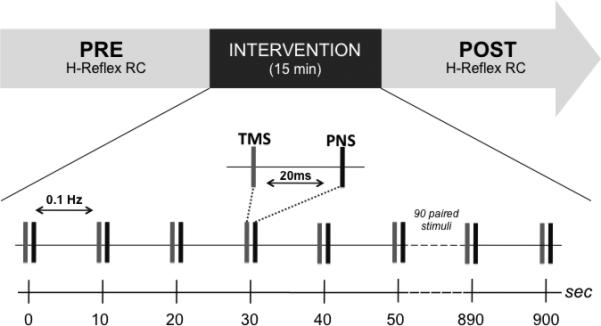
Experimental design and protocol. A within-subjects repeated-measures design was employed. An H-reflex recruitment curve (RC) was recorded at baseline then again following the intervention for each subject. The intervention involved a paired stimulation protocol, with sub-threshold TMS preceding PNS by 20ms (PNS intensity adjusted to elicit a conditioned H-reflex amplitude of 0.5-1.0mV) repeated at 0.1Hz for 15 minutes (90 stimulus pairs).
The PNS and TMS-alone protocols followed the same procedure however stimuli were not paired, and only PNS or TMS was delivered. For the PNS-alone experiments, intensity was adjusted to elicit an unconditioned H-reflex amplitude, comparable to that of the paired SAS intervention group (0.5-1mV).
Data Analysis
Peak-to-peak H and M amplitude was calculated on individual waveforms using Spike 2 software (Cambridge Electronic Design, Cambridge, UK). The mean conditioned H-reflex amplitude for each minute of the SAS intervention was calculated for each participant, then assessed for change over time on group data using linear regression analysis. The same analysis was performed for the unconditioned H-reflex amplitude for the PNS-alone protocol. A similar analysis was not performed during the TMS-alone protocol as intensity was sub-threshold.
The H-reflex recruitment curves pre and post intervention were averaged across each of the 5 stimuli and plotted against PNS intensity. For each participant a Gaussian function was fit to the H-reflex recruitment curve data using the equation y = a * e(-0.5 * (x-b)^2 / c^2), where y is the amplitude of the EMG response, x is PNS intensity, and the parameters a, b and c define the curve fit. The parameter ‘a’ defines the maximum H-reflex amplitude, and ‘b’ defines the intensity at which the curve reaches this maximum. H-reflex threshold was defined as the intensity at which the Gaussian reaches 10% of ‘a’. As an additional measure of threshold, the x-intercept of the tangent to the curve at the point at which the curve reaches 50% of ‘a’ was calculated, according to the method of Carroll et al. (2002), as well as the intensity at 50% of H-max and the slope of the curve at that point. A measure of the spread of the H-reflex curve was determined from the full-width half-maximum of the Gaussian, given by 2.355c. Group data for these parameters were tested for significant differences pre to post intervention using a two-tailed paired t-test and an alpha level of 0.05.
For illustrative purposes Gaussian fits were normalized using a transform that resulted in the pre-intervention fits having a=1, b=0, and c=1. The transformed post-Gaussian parameter ‘a’ was then given by a1/a0, ‘b’ by (b1-b0)/c0, and ‘c’ by c1/c0 ('1' = post, ‘0’ = pre parameters of the original Gaussians). All the parameters are expressed as mean±SEM, in the text and the figures.
RESULTS
The TMS-conditioned H-reflex amplitude increased almost 3-fold relative to the unconditioned H-reflex amplitude (mean unconditioned H-reflex=0.25mV±0.06mV; conditioned H-reflex=0.7±0.11mV; p<0.05; Fig. 2b). In the first minute of SAS protocol, conditioned H-reflex amplitude was 0.8±0.11mV, and this increased progressively during the 15 minutes of intervention (r2= 0.97) with a ~25% increase in amplitude by the end of the intervention, 1.03±0.15mV (Fig. 3). There were no changes in unconditioned H in the PNS-alone intervention (initial=0.72±0.09mV; final=0.71±0.14mV). Maximum M-wave amplitude (pre=2.6±0.04mV; post=2.6±0.03mV) and area (pre=9.30±0.72mVms; post=9.42±0.78mVms) were not significantly different before and after SAS. There was not detectable background EMG recorded during or after SAS.
Fig 2b.
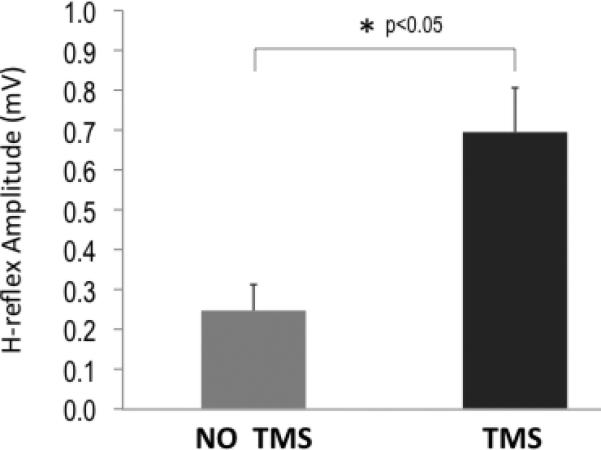
Group mean data showing the effect of subthreshold TMS conditioning on the H-reflex. Relative to unconditioned H-reflex amplitude, a conditioning TMS pulse significantly increased the amplitude of the H-reflex.
Fig 3.
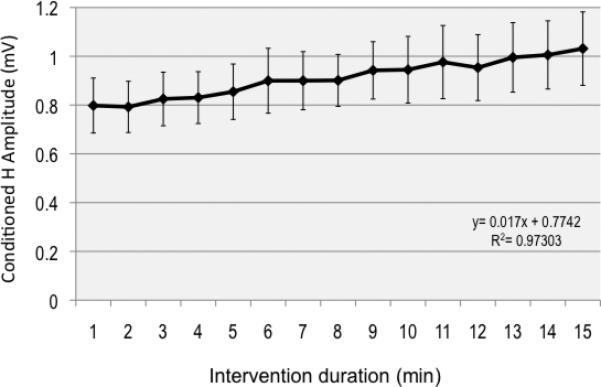
Group mean data of conditioned H-reflex amplitude for each minute of the intervention showing a progressive increase in amplitude (~25%) across the intervention period. The regression equation is displayed.
The H-recruitment fitted curves before and after SAS are presented in Figure 4a and 4b, and show that overall there was a leftward shift in the post-SAS curves and a reduction in maximal amplitude. Maximum H-reflex (parameter ‘a’) was reduced after the intervention (pre=1.51±0.18mV; post=1.38±0.15mV, p=0.01). H-threshold (derived from the 10% of ‘a’ measure) was significantly decreased after SAS (pre=12.9±1.7mA; post=11.6±1.6mA; p=0.04), as was the intensity required to achieve the maximum H-reflex (parameter ‘b’; pre=23.6±02.8mA; post=21.7±2.6mA; p=0.03). Threshold, as determined from the x-intercept of the tangent at half H-max, was also reduced after the intervention (pre=12.0±1.7mA; post=10.7±1.5mA; p<0.05), as was the intensity required to achieve half H-max (pre=14.8±1.9mA; post=13.4±1.8mA; p=0.04). There was no significant change in slope at that intensity (pre=0.40±0.11mV/mA; post=0.37±0.09mV/mA; p=0.22). The width of the curves was reduced post-SAS (derived from parameter ‘c’; pre=11.6±1.5mA; post=10.93±1.4mA; p=0.03) (Fig 5). There were no significant changes in the H-reflex curve-fit parameters pre and post for PNS-alone protocol (0.88±0.16 vs 0.86±0.18mV, p=0.9; 19.8±2.3 vs 19.8±2.6mA, p=0.99; 4.0±0.6 vs 3.9±0.4mA, p=0.83; ‘a’, ‘b’ and ‘c’ respectively for pre vs post PNS). Likewise there was no significant change for TMS-alone protocol (1.28±0.23 vs 1.19±0.26mV, p=0.33; 25.8±5.2 vs 25.4±5.0mA, p=0.54; 5.2±1.1 vs 5.2±1.2mA, p=0.87; ‘a’, ‘b’ and ‘c’ respectively for pre vs post TMS).
Fig 4a.
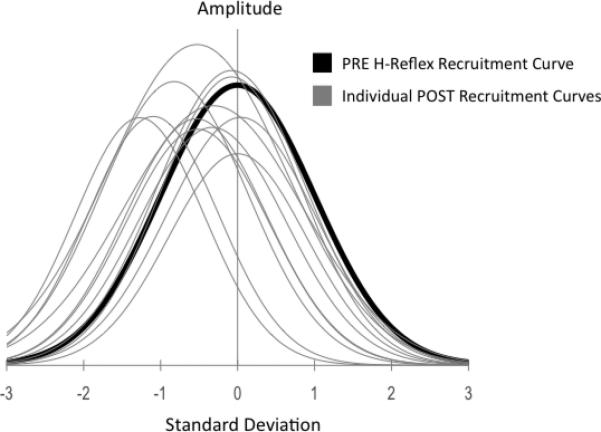
Pre-intervention H-reflex Recruitment Curve group data fit to a Gaussian curve, with overlaid group mean H-reflex recruitment curve data post intervention normalized to pre, illustrating the range of responses across subjects and the dominance of a left-hand shift, consistent across the group. A shift of the curve post intervention corresponds to decreased threshold, and larger evoked response relative to stimulus intensity.
Fig 4b.
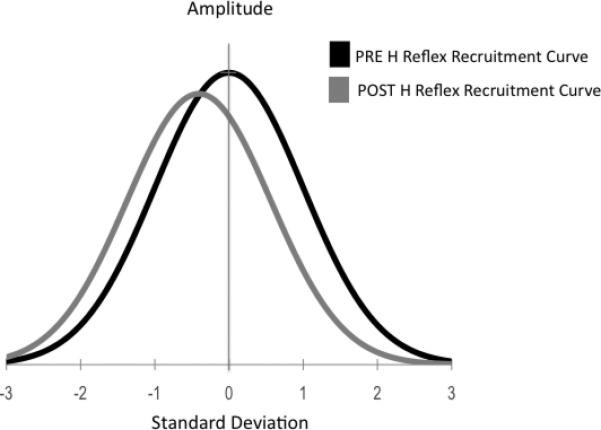
Group H-reflex recruitment curve data post-intervention normalized to pre-intervention, showing the shift to the left of the recruitment curve following the intervention.
Fig 5.
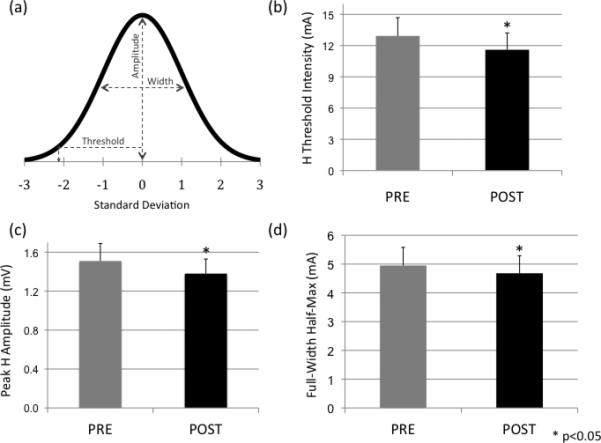
(a) Example Gaussian curve, illustrating the reported parameters for the subsequent graphs reporting group mean data: Peak amplitude (calculated as the maximum H value in the curve), threshold (calculated as the corresponding stimulus intensity at 10% of the maximum H amplitude), and width (calculated as the full-width intensity half-maximum of the Gaussian); (b) group data showing that the PNS intensity at threshold is significantly reduced post intervention (mean±SEM); (c) the peak H reflex amplitude is significantly reduced post intervention; and (d) the H-reflex recruitment curve width is significantly reduced post intervention, indicating a smaller range of stimulus intensities required to produce a full recruitment curve.
DISCUSSION
In this study we have shown that conditioning an H-reflex evoked from stimulation of the posterior tibial nerve, with a sub-threshold TMS at 20ms inter-stimulus interval, increases the H-reflex by almost 300%. Further we have shown that by repeatedly delivering stimulus pairs, the conditioned H-reflex progressively increases by a further ~25% by the end of 15 minutes of stimulation. Following the intervention, the unconditioned H-reflex threshold is decreased. There were no significant changes in any parameters before and after PNS or TMS alone protocols. We conclude that this repetitive conditioning paradigm induces short-term plastic changes in the excitability at spinal cord level, and use the term spinal associative stimulation (SAS) to reflect the interaction between activity in the afferent fibers of the posterior tibial nerve (TN) that produces the monosynaptic reflex, and efferent activity in the corticospinal tract stimulated by TMS.
PAS protocols involving the interaction of Ia afferent activity with TMS-evoked activity are reported in the literature to modify synaptic efficacy at the level of the human cortex (Klein et al., 2004; Kujirai et al., 2006; Litvak et al., 2007; Stefan et al., 2000). Both the immediate interaction of afferent activity with TMS, and the cumulative effects, are known to be timing-dependent. The progressive increase or decrease in excitability is thought to resemble the long-term potentiation or depression (LTP/LTD) mechanisms that have been intensively studied in cellular, animal and human models of learning and memory formation (Artola and Singer, 1993; Bailey et al., 2000; Bliss and Collingridge, 1993; Carvalho and Buonomano, 2009; Levy and Steward, 1983).
In a modification of previous PAS protocols, we used paired stimulation where TMS below motor threshold preceded the peripheral nerve stimulation. Using this paradigm, Valls-Sole and co-workers showed the greatest facilitation in H response at an ISI of ~20ms and proposed that the descending corticospinal activity interacts with the afferent peripheral activity at spinal cord level (Valls-Solé et al., 1994). Interactions at this level have been previously reported in the literature where presynaptic interneurones receive projections from peripheral and central pathways (Deletis et al., 1992; Deuschl et al., 1991; Meunier, 1999; Mrachacz-Kersting et al., 2007). The continuous flow of excitatory inputs carried by Ia afferent terminals to homonymous motoneurons is constantly regulated by presynaptic mechanisms (Lamy et al., 2010). The excitatory mono-synaptic H-reflex is regulated in this way by input from the same nerve, and may be blocked by subthreshold TMS, resulting in removal of inhibition and a heightened H-reflex (Valls-Sole et al., 1994). The paired association in the present protocol was designed to occur at this level (Meunier et al., 2007; Poon et al., 2008), although secondary post-synaptic or supraspinal effects could also be involved (Brooks et al., 1950).
In this conditioning paradigm the descending activity (generated by sub-threshold TMS) was insufficient to elicit an EMG response in the soleus muscle, but may be sufficient to generate post-synaptic effects by depolarizing a portion of the motoneuron pool at the spinal cord. Since there is insufficient time for this to occur before the depolarization caused by the afferent volley (Valls-Sole et al., 1994), it is unclear whether this is implicated in the cumulative change in spinal excitability observed in this study, although post-synaptic changes have been demonstrated using operant conditioning (learning) paradigms of the H-reflex in animals (Chen et al., 2006a; Chen et al., 1999; Chen et al., 2006b; Wolpaw and Carp, 2006). Other possibilities include changes in intrinsic motoneuron properties or alterations in post-activation depression (PAD), a short-term sustained decrease in post-synaptic excitability associated with repetitive stimulation (Crone and Nielsen, 1989).
As well as reduced threshold post-intervention we also observed smaller and narrower recruitment curves. We interpret this as a greater central effect of PNS-induced antidromic activity in peripheral motor axons post intervention. It is plausible that the progressive increase in conditioned H-reflex partly occurs through a concomitant increased and sustained depolarization of the post-synaptic cell. This could occur since it is well established that maximum H response reflects the interaction between orthodromic volleys along Ia afferents that excite the motoneuron, and the antidromic volleys along the motor axons that depolarize the cell body and place the motoneuron in a relative refractory period (Hultborn et al., 1996). The effect of antidromic activity may be greater since the post-synaptic membrane is partially depolarized. The implication of this finding is that reduced maximum H post intervention may result from the technique used to examine spinal excitability, because the other measures point to significantly heightened responses. The finding of a narrower recruitment curve post intervention could equally be explained by increased effect of antidromic activity. A greater effect of antidromic activity at progressively increasing PNS intensities would lead to a rapid loss of the H-reflex as a larger proportion of the motoneuron pool is placed in a refractory state. The narrowing in addition to the left-wards shift of the recruitment curve means that the loss of H-reflex (downward slope of RC) relative to pre-intervention is increased. As individual motoneurons draw closer to and cross firing threshold (depolarization) the relative number of remaining motoneurons available for a monosynaptic H response becomes less and might lead to a more rapid decline in the H response post-peak.
Human and animal models support the association of afferent activity with motor activity in primary cortex for the development of sustained changes in cortical excitability (Abbruzzese et al., 2001; Kujirai et al., 2006; Russmann et al., 2009; Siebner et al., 2004). These changes are thought to represent via long-term potentiation via spike-timing mechanisims (Classen et al., 2004; Litvak et al., 2007; Wolters et al., 2003; Wolters et al., 2005), and might be important components of change in motor behavior associated with repetitive activity (Di Lazzaro et al., 2009). The present findings showed that SAS, described as the repetitive spinal interaction between synchronous afferent volleys with descending corticospinal activity, led to excitability changes in spinal cord as indicated by H-reflex threshold decrease. Spinal plastic changes induced by our associative stimulation were developed gradually (across the 15 min intervention) and outlasted the intervention period (10 min). These characteristics are consistent with changes in synaptic efficacy involving long-term potentiation reported in animal and cellular models, suggesting that similar mechanisms may be involved in our conditioning H-reflex protocol (Carvalho and Buonomano, 2009).
CONCLUSION
Our findings show that paired peripheral and central stimulation can be used to target spinal cord in healthy subjects and to enhance spinal excitability. This finding has similarities to the emerging literature of neuromodulation using paired associative stimulation targeting the brain. The significance of the present findings of SAS-related changes in spinal excitability is that similar changes have been implicated in long-term adaptation during the acquisition of new motor skills (Thompson et al., 2009), and restoration of motor function in animal models with partial spinal cord injury (Chen et al., 2006b). It remains to be determined whether SAS can improve motor function in spinal cord injury or other neurological disorders.
Highlights.
Induction of spinal cord plasticity occurs with central and peripheral interactions
Repetitive paired intervention (TMS preceded by PNS) induces changes in H-reflex recruitment curve that doesn't occur when single stimulation protocols are used (PNS alone or TMS alone).
Spinal Associative Stimulation can effectively modulate spinal cord excitability
Fig 2a.
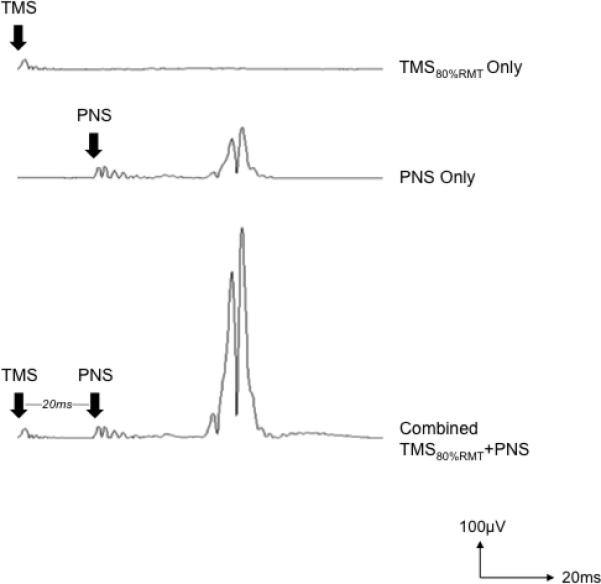
Average rectified EMG traces from one subject illustrating that subthreshold TMS alone (80%RMT) resulted in no EMG response (top trace), suprathreshold PNS stimulation alone elicits a small H-reflex (middle trace), and when the same subthreshold TMS pulse precedes the PNS pulse by 20ms, the H-reflex amplitude grows substantially.
Acknowledgements
This work was supported by NIH grant K24 RR018875 for APL.
The authors would like to acknowledge F Mastaglia, BT Volpe and P Huerta for helpful discussions with data interpretation.
REFERENCES
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124:537–545. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CM, Downman CB, Eccles JC. After-potentials and excitability of spinal motoneurones following antidromic activation. J Neurophysiol. 1950;13:9–38. doi: 10.1152/jn.1950.13.1.9. [DOI] [PubMed] [Google Scholar]
- Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron. 2009;61:774–785. doi: 10.1016/j.neuron.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Chen Y, Chen L, Tennissen AM, Wolpaw JR. Corticospinal tract transection permanently abolishes H-reflex down-conditioning in rats. J Neurotrauma. 2006a;23:1705–1712. doi: 10.1089/neu.2006.23.1705. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR, Jakeman LB, Stokes BT. Operant conditioning of H-reflex increase in spinal cord--injured rats. J Neurotrauma. 1999;16:175–186. doi: 10.1089/neu.1999.16.175. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci. 2006b;26:12537–12543. doi: 10.1523/JNEUROSCI.2198-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A, Kunesch E. Paired associative stimulation. Suppl Clin Neurophysiol. 2004;57:563–569. [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78:28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- Deletis V, Schild JH, Beric A, Dimitrijevic MR. Facilitation of motor evoked potentials by somatosensory afferent stimulation. Electroencephalogr Clin Neurophysiol. 1992;85:302–310. doi: 10.1016/0168-5597(92)90106-l. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Ebner A, Hammers R, Lucking CH. Differences of cortical activation in spontaneous and reflex myoclonias. Electroencephalogr Clin Neurophysiol. 1991;80:326–328. doi: 10.1016/0168-5597(91)90117-g. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Profice P, Pilato F, Oliviero A, Mazzone P, Di Iorio R, Capone F, Ranieri F, Florio L, et al. LTD-like plasticity induced by paired associative stimulation: direct evidence in humans. Exp Brain Res. 2009;194:661–664. doi: 10.1007/s00221-009-1774-9. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Talelli P, Rothwell JC. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 2008;7:827–840. doi: 10.1016/S1474-4422(08)70190-X. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Klein T, Magerl W, Hopf HC, Sandkuhler J, Treede RD. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J Neurosci. 2004;24:964–971. doi: 10.1523/JNEUROSCI.1222-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knash ME, Kido A, Gorassini M, Chan KM, Stein RB. Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res. 2003;153:366–377. doi: 10.1007/s00221-003-1628-9. [DOI] [PubMed] [Google Scholar]
- Kujirai K, Kujirai T, Sinkjaer T, Rothwell JC. Associative plasticity in human motor cortex during voluntary muscle contraction. J Neurophysiol. 2006;96:1337–1346. doi: 10.1152/jn.01140.2005. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Russmann H, Shamim EA, Meunier S, Hallett M. Paired associative stimulation induces change in presynaptic inhibition of Ia terminals in wrist flexors in humans. J Neurophysiol. 2010;104:755–764. doi: 10.1152/jn.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy WB, Steward O. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience. 1983;8:791–797. doi: 10.1016/0306-4522(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Litvak V, Zeller D, Oostenveld R, Maris E, Cohen A, Schramm A, Gentner R, Zaaroor M, Pratt H, Classen J. LTP-like changes induced by paired associative stimulation of the primary somatosensory cortex in humans: source analysis and associated changes in behaviour. Eur J Neurosci 2007 May. 2007;25(9):2862–74. doi: 10.1111/j.1460-9568.2007.05531.x. 25, - 74. [DOI] [PubMed] [Google Scholar]
- Meunier S. Modulation by corticospinal volleys of presynaptic inhibition to Ia afferents in man. J Physiol Paris. 1999;93:387–394. doi: 10.1016/s0928-4257(00)80066-2. [DOI] [PubMed] [Google Scholar]
- Meunier S, Russmann H, Simonetta-Moreau M, Hallett M. Changes in spinal excitability after PAS. J Neurophysiol. 2007;97:3131–3135. doi: 10.1152/jn.01086.2006. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Fong M, Murphy BA, Sinkjaer T. Changes in excitability of the cortical projections to the human tibialis anterior after paired associative stimulation. J Neurophysiol. 2007;97:1951–1958. doi: 10.1152/jn.01176.2006. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Traversa R, Cicinelli P, Filippi MM, Pasqualetti P, Tomaiuolo F, Caltagirone C. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain. 1999;122(Pt 9):1731–1739. doi: 10.1093/brain/122.9.1731. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Gandevia SC. The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii. J Physiol. 2002;544:277–284. doi: 10.1113/jphysiol.2002.024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Poon DE, Roy FD, Gorassini MA, Stein RB. Interaction of paired cortical and peripheral nerve stimulation on human motor neurons. 2008;188:21. doi: 10.1007/s00221-008-1334-8. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant'Angelo A, Girlanda P, Siebner HR. Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol. 2006 Sep 1;575(Pt 2):657–70. doi: 10.1113/jphysiol.2006.114025. Epub 2006 Jul 6 - 575, - 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy FD, Norton JA, Gorassini MA. Role of sustained excitability of the leg motor cortex after transcranial magnetic stimulation in associative plasticity. J Neurophysiol. 2007;98:657–667. doi: 10.1152/jn.00197.2007. [DOI] [PubMed] [Google Scholar]
- Russmann H, Lamy JC, Shamim EA, Meunier S, Hallett M. Associative plasticity in intracortical inhibitory circuits in human motor cortex. Clin Neurophysiol. 2009;120:1204–1212. doi: 10.1016/j.clinph.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serranova T, Valls-Sole J, Munoz E, Genis D, Jech R, Seeman P. Abnormal corticospinal tract modulation of the soleus H reflex in patients with pure spastic paraparesis. Neurosci Lett. 2008;437:15–19. doi: 10.1016/j.neulet.2008.03.068. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci. 2009;29:5784–5792. doi: 10.1523/JNEUROSCI.4326-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy J, Ridding MC. Increased cortical excitability induced by transcranial DC and peripheral nerve stimulation. J Neurosci Methods. 2003;127:193–197. doi: 10.1016/s0165-0270(03)00142-0. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Alvarez R, Tolosa ES. Vibration-induced presynaptic inhibition of the soleus H reflex is temporarily reduced by cortical magnetic stimulation in human subjects. Neurosci Lett. 1994;170:149–152. doi: 10.1016/0304-3940(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Alvarez R, Tolosa ES. Responses of the soleus muscle to transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:421–427. doi: 10.1016/0168-5597(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Valldeoriola F. Neurophysiological correlate of clinical signs in Parkinson's disease. Clin Neurophysiol. 2002;113:792–805. doi: 10.1016/s1388-2457(02)00080-9. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol. 1987;57:443–459. doi: 10.1152/jn.1987.57.2.443. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Carp JS. Plasticity from muscle to brain. Prog Neurobiol. 2006;78:233–263. doi: 10.1016/j.pneurobio.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, Benecke R, Reiners K, Classen J. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–1052. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]


