Abstract
Context
Youth with bipolar disorder (BD) and those with severe, non-episodic irritability (severe mood dysregulation, SMD) show amygdala dysfunction during face emotion processing. However, studies have not compared such patients to each other and to comparison subjects in neural responsiveness to subtle changes in face emotion; the ability to process such changes is important for social cognition. We employed a novel parametrically designed faces paradigm.
Objective
Using a parametrically morphed emotional faces task, we compared activation in the amygdala and across the brain in BD, SMD, and healthy volunteers (HV).
Design
Case-control study.
Setting
Government research institute.
Participants
57 youths (19 BD, 15 SMD, 23 HV).
Main Outcome Measure
Blood oxygenated level dependent (BOLD) data. Neutral faces were morphed with angry and happy faces in 25% intervals; static face stimuli appeared for 3000ms. Subjects performed hostility or non-emotional facial feature (i.e., nose width) ratings. Slope of BOLD activity was calculated across neutral-to-angry (N→A) and neutral-to-happy (N→H) face stimuli.
Results
In HV, but not BD or SMD, there was a positive association between left amygdala activity and anger on the face. In the N→H whole brain analysis, BD and SMD modulated parietal, temporal, and medial-frontal areas differently from each other and from HV; with increasing facial-happiness, SMD increased, while BD decreased, activity in parietal, temporal, and frontal regions.
Conclusions
Youth with BD or SMD differ from HV in modulation of amygdala activity in response to small changes in facial anger displays. In contrast, BD and SMD show distinct perturbations in regions mediating attention and face processing in association with changes in the emotional intensity of facial happiness displays. These findings demonstrate similarities and differences in the neural correlates of face emotion processing in BD and SMD, suggesting these distinct clinical presentations may reflect differing pathologies along a mood disorders spectrum.
INTRODUCTION
Pediatric bipolar disorder (BD) is diagnosed with increasing frequency,1 perhaps because some suggest that it presents as either severe, non-episodic irritability or episodic mania.2 This suggestion raises questions about clinical and pathophysiological differences between these two presentations. To evaluate this, the phenotype of severe, non-episodic irritability was operationalized as “severe mood dysregulation” (SMD).2 Family history3 and longitudinal data4, 5 suggest that BD and SMD are dissociable phenotypes, whereas behavioral data find similarities in face information processing.6–9 Imaging studies can elucidate similarities and differences in neural mediators of such information-processing findings.10, 11 Here, we compare blood oxygenated level dependent (BOLD) activation patterns during the presentation of parametrically morphed emotional faces in BD, SMD, and healthy volunteers (HV).
Emotional faces are salient nonverbal social cues. Appropriate identification of, and responses to, such cues are necessary for proper learning and behavior in social situations.12 Children with BD or SMD, but not those with other forms of psychopathology, show perturbed face-emotion labeling ability.6–10 On a task in which neutral faces morph into full emotional expressions, both BD and SMD youth, compared to HV youth, require more intense emotion to be displayed on the face before they can identify it.7 In addition, both SMD and BD patients perceive neutral faces as more fear-producing than do healthy subjects.10
However, while BD and SMD youth exhibit similar behavioral impairments on face-labeling tasks, the only study to compare face processing in the two phenotypes found that the neural correlates of the two phenotypes differ.10 Specifically, Brotman et al.10 found that SMD, relative to BD and HV youth, exhibited amygdala hypo-activation during explicit processing of neutral faces. Of note, most pediatric BD studies find amygdala hyperactivation during both implicit and explicit face-emotion processing, including hostility ratings of neutral faces.13–17 Indeed, a recent study found that amygdala dysfunction extends to youth at risk of BD,18 emphasizing the importance of the amygdala as a region of interest in BD. However, while literature on affect dysregulation suggests the amygdala is important, the amygdala functions in the service of affect regulation and dysregulation as part of a distributed circuit encompassing other regions (including DLPFC and VLPFC).19 Together, these studies suggest that amygdala dysfunction varies as a function of diagnosis. However, insufficient work compares SMD and BD directly, so the degree to which dysfunction in the amygdala or other regions is shared or disorder-specific in SMD and BD during face processing requires further investigation.
Here, we build on prior work showing that SMD and BD perform more poorly than HV on face emotion processing tasks. Specifically, BD or SMD patients require more intense emotional information than do HV before they can identify an emotion,7 and BD patients rate neutral faces as being more hostile than do control subjects.7 Both SMD and BD demonstrate amygdala dysfunction;10 here we used a parametric design to examine amygdala modulation in the context of an emotion processing task. We employed both an implicit and explicit emotion processing condition since previous work has demonstrated amygdala dysfunction in BD13–17 and SMD10 during explicit emotion processing, while evidence suggests that implicit emotion processing activates the amygdala more consistently in healthy populations20 and can also distinguish pediatric bipolar patients from controls.17 Therefore, questions remain on whether group differences in the current study would manifest in both or one specific attention condition. Specifically, we morphed faces parametrically from neutral to 100% happy or 100% angry in 25% increments and examined the degree to which each group exhibited a linear relationship between face emotion intensity and behavioral and neural measures. Thus, we examined the “dose-response” curve of neural activity changes with increases in emotion on a face. This approach is sensitive to subtle group differences; avoids problems with rapid amygdala habituation to identical emotional stimuli; and allows us to examine the neural underpinnings to the finding that, compared to HV, BD and SMD youths require more emotional intensity in order to label a face emotion accurately.7, 10
Given our interest in amygdala dysfunction, we conducted an ROI analysis of this region, in addition to a whole brain analysis. Based on the behavioral findings noted above, we hypothesized that both SMD and BD youth would differ from HV in amygdala responsiveness to subtle changes in emotional expression. However, the directionality of expected differences was unclear, given inconsistencies in prior findings. Finally, given the observed differences in the clinical presentation, longitudinal course, and pathophysiology of SMD and BD, we expected diagnosis-specific neural correlates in other brain regions, including the DLPFC and VLPFC, which have shown to be less active in youth with BD compared to controls in emotional face tasks.14, 15, 17, 21
METHODS
Participants
Usable fMRI data were acquired from 57 subjects, including BD (N = 19), SMD (N = 15), and HV (N = 23) (see Table 1); data from two controls, two BD, and three SMD were excluded for excessive motion. All participants, ages 8–18, were enrolled in an Institutional Review Board-approved study at the National Institute of Mental Health. Parents and youths gave written informed consent/assent. Patients and healthy subjects were recruited and assessed using methods described previously.10 Subjects were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL), including a module to ascertain SMD.22 Interviewers were master’s- and doctoral-level clinicians, with excellent interrater reliability (k>0.9 for all diagnoses, including differentiating BD from SMD). Diagnoses were based on best-estimate procedures generated in a consensus conference led by a psychiatrist. BD patients were “narrow phenotype,” with at least one full-duration (hypo)manic episode characterized by abnormally elevated mood and at least three DSM-IV “B” mania symptoms.2 SMD youth had non-episodic irritability, over-reactivity to negative emotional stimuli at least three times/week, and hyperarousal symptoms. Symptoms had to begin before age 12, be present for at least one year with no symptom-free periods exceeding two months, and cause severe impairment in at least one setting (i.e., home, school, peers), and mild impairment in another. Euphoric mood or distinct (hypo)manic episodes lasting more than one day were exclusionary.2 HV had no lifetime psychiatric diagnoses and, as ascertained by parent interview, no first-degree relatives with a mood disorder.
Table 1.
Demographic data
| BD (N = 19) | SMD (N = 15) | HV (N = 23) | |
|---|---|---|---|
|
| |||
| Mean± SD | Mean± SD | Mean± SD | |
|
|
|||
| Age a | 15.33 ± 2.13 | 14.53 ± 2.24 | 14.92 ± 1.99 |
| WASI IQb | 103.63 ± 15.34 | 109.00 ± 12.04 | 108.35 ± 13.53 |
| YMRS c | 6.58 ± 5.22 | --- | --- |
| CDRS d | 28.63 ± 7.70 | 22.50 ± 6.42 | --- |
| CGAS e | 52.11± 8.93 | 45.36 ± 18.61 | --- |
| Number of medications f | 3.16 ± 1.64 | 1.53 ± 1.55 | --- |
|
|
|||
| N (%) | N (%) | N (%) | |
|
|
|||
| Male g | 7 (37) | 11 (73) | 12 (52) |
| Bipolar I | 16 (84) | --- | --- |
| Bipolar II | 3 (16) | --- | --- |
| MoodState h | --- | ||
| Euthymic | 16 (84) | 15 (100) | --- |
| Depressed | 1 (5) | 0 (0) | --- |
| Hypomanic | 1 (5) | --- | --- |
| Mixed | 1 (5) | --- | --- |
| ComorbidConditions | --- | ||
| ADHD | 10 (53) | 13 (87) | --- |
| ODD or CD | 5 (26) | 12 (80) | --- |
| Anxiety | --- | ||
| GAD | 7 (37) | 4 (27) | --- |
| SAD | 3 (16) | 2 (13) | --- |
| Social Phobia | 4 (21) | 1 (7) | --- |
| Medication | |||
| Unmedicated | 2 (11) | 6 (40) | 23 (100) |
| Atypical Antipsychotic | 9 (47) | 5 (33) | --- |
| Lithium | 8 (42) | 1 (7) | --- |
| Antiepileptic | 11 (58) | 3 (20) | --- |
| Antidepressant | 7 (37) | 3 (20) | --- |
| Stimulants | 7 (37) | 7 (47) | --- |
NS
NS
Young Mania Rating Score.
Children’s Depression Rating Score; missing data from 1 SMD. p < .05.
Children’s Global Assessment Scale of the past 6 months; missing data from 1 SMD.
Number of medications at time of scan. p < .01
NS
Mood State indicated by CDRS, Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders (SIGH-SAD), and YMRS.
Exclusion criteria for all subjects were: IQ<70, history of head trauma, neurological disorder, pervasive developmental disorder, unstable medical illness, or substance abuse/dependence. HV were medication-free. Most BD and SMD youths were medicated.
Behavioral Paradigm
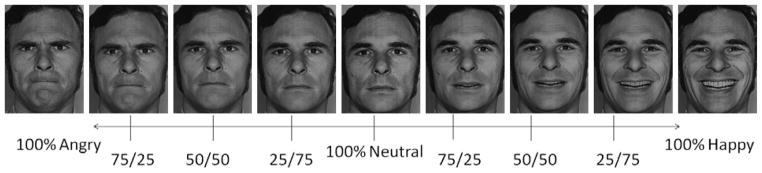
Faces were taken from the Ekman pictures of facial affect23 and were morphed between a 100% anger face and a neutral face, and between a 100% happy face and a neutral face (Figure 1). Face morphs were created using methods from LaBar et al.24 with MorphMan 2000 software (STOIK, Moscow, Russia). Five female faces and five male faces were included.
Figure 1.
Example of a male identity used for creating the 9 morphs in the current experiment. 100 Angry, 75/25 Angry/Neutral, 50/50 Angry/Neutral, 25/75 Angry/Neutral, Neutral, 25/75 Happy/Neutral, 50/50 Happy/Neutral, 75/25 Happy/Neutral, and 100 Happy.
Thee morphs were made at 25% increments, resulting in 9 face emotions [100 Angry (hereinafter called A100), 75/25 Angry/Neutral (A75), 50/50 Angry/Neutral (A50), 25/75 Angry/Neutral (A25), 100 Neutral (Neut), 25/75 Happy/Neutral (H25), 50/50 Happy/Neutral (H50), 75/25 Happy/Neutral (H75), and 100 Happy (H100)]. To have adequate statistical power while keeping the paradigm brief enough to be well-tolerated by children with severe psychopathology, we focused on only two emotions, one positive (happy) and one negative (angry). We chose these emotions because previous neuroimaging work has shown differences in neural activity between youths with BD and HV while processing them.14, 15, 17
There were two behavior conditions: nose width and hostility ratings. Ratings were on a 5 point scale: 1=least wide/least hostile, 5=most wide/most hostile. In each trial, faces appeared for 3000ms, during which subjects made ratings. The inter-trial interval varied from 750–1250ms (average 1000ms; Figure 2).
Figure 2.
Examples of the beginning of (A) hostility and (B) nose-width rating blocks. The event-related paradigm consisted of two runs of four blocks each, two hostility and two nose-width (47 trials/block). Attention state (nose vs. hostility) block order was randomized across subjects.
There were four blocks, each including 41 nose-width rating trials, 41 hostility rating trials, and 12 fixation trials to add jitter and provide a baseline. Stimuli and behavior conditions were presented randomly within each block. Since we were especially interested in neural activation while subjects viewed the morphs containing ambiguous emotion (i.e., A50, A25, Neut, H25, H50), these had more replicates than emotionally unambiguous faces (i.e., A100, A75, H75, H100). For the heavily weighted morphs, there were 6 trials per morph in each condition of each block (24 total), except that neutral had 5 trials per condition per block (20 total). For the less weighted morphs, there were 3 trials per morph in each condition of each block (12 total). Each block contained 94 trials at an average of 4000ms/trial and was approximately 6.3 minutes long, yielding a 25 minute experiment.
Image acquisition
Data were acquired on a 3T General Electric scanner. Structural images used T1-weighted axial acquisition [three-dimensional spoiled-gradient-recall acquisition in the steady state with inversion recovery prep pulse; 256 × 192 matrix; 124 1.2 mm axial slices; 22 cm field of view (FOV)] to allow normalization to standard space.25 Functional imaging was performed axially using a multi-slice gradient echo-planar sequence (24cm FOV, 96 × 96 matrix, 38 contiguous 2.6mm slices; TR=2300ms; TE=25ms).
Behavioral Data Analysis
Ratings and reaction time (RT) were compared in separate repeated-measures 3 (Group: SMD, BD, HV) × 2 (Behavior: hostility, nose-width ratings) ANOVAs on linear trend values that describe the data. We divided the analysis into neutral to 100% angry (N→A) and neutral to 100% happy (N→H). Tukey-corrected post-hoc analyses were conducted as needed.
Imaging Analysis
fMRI data were analyzed with Analysis of Functional NeuroImages (AFNI), using preprocessing methods described previously,26 with 6mm root mean square (RMS) blur. Data were scaled to a percentage of the voxel-wise mean, subject to a limit of 200 to avoid truncation artifacts. We censored multiple movement spikes greater than 1.5 mm and excluded participants with more than 5% censored time to repetitions (TRs). Regressors for each morph in each behavior condition were created by convolving stimulus times with a gamma-variate hemodynamic-response function. Linear regression modeling was performed per voxel, with 18 regressors, one for each stimulus condition (9 Morphs × 2 Behavior states), a third-order polynomial modeling the baseline drift, and 6 motion parameters. Blank fixation trials provided a baseline. Individual beta-coefficient maps were warped into standard space with a high-resolution anatomical image that had been manually normalized by identifying the anterior-posterior commissures, midsaggital plane, and outer boundary.
To model neural activity to the faces morphing from N→A and N→H, at the individual subject level two trend analyses were performed for the parametrically modeled stimulus classes, one each for N→A and N→H. The regressors were analyzed for linear trends between BOLD signal and face stimulus intensity.
For the amygdala ROI, we extracted average BOLD linear trend magnitudes for N→A and N→H in the right and left amygdala based on the Talairach-Tournoux Daemon.25 For each amygdala ROI a repeated-measures Group (BD, SMD, HV) x Behavior (hostility, nose-width) ANOVA was conducted on the linear trend magnitudes. If there was a main effect of Group, using SPSS, we evaluated if the slope value for each group differed significantly from zero using within-group t-tests, and conducted Tukey-corrected between-group post-hoc analyses.
At the whole-brain level, we examined linear trends for both N→A and N→H in a Group x Behavior ANOVA. We used AFNI’s AlphaSim Monte Carlo simulation to calculate the cluster size associated with a map-wise (entire brain mask) whole-brain false positive probability of p<.05. This threshold was applied to data initially passing a p<.005 threshold, yielding a smoothness of 8–9mm. With these features, k≥168 at a resolution of 2×2×2 emerged as the significance threshold. For clusters meeting this threshold, average signal was extracted, and post-hoc ANOVAs and Tukey-corrected post-hoc analyses were performed in SPSS. Anatomical locations were labeled using the Talairach-Tournoux Daemon.
Exploratory post-hoc ANOVAs were conducted to examine the impact of potentially confounding variables. For mood state, analyses were conducted in euthymic patients only and HV. To test the effect of comorbid anxiety disorders, ANOVAs compared non-anxious SMD, non-anxious BD, and HV. For medication, we conducted an ANOVA on BD and SMD separately with medication status (on vs. off) as the between-group variable. We additionally covaried medication status in the clusters where BD and SMD differed, and explored the potential impact of age, sex, pubertal status (Tanner score), and CDRS scores, which can be read in the Online Only Text.
RESULTS
Demographics
BD, SMD, and HV did not differ on age, gender, IQ, or the Children’s Global Assessment Scale (CGAS). BD had higher Child Depression Rating Scale (CDRS) scores than SMD, F(1, 31)=5.86, p<.03 (Table 1).
Behavior
Neutral → Angry
For the linear trends of angry morph ratings, there was no Group x Behavior interaction or Group main effect. There was a main effect of Behavior, with hostile ratings showing a steeper positive slope than nose-width ratings, F(1, 54)=117.68, p<.0001. This indicates that subjects used a broader range of the 1–5 scale for hostility ratings than for nose-width ratings.
Linear trend analyses on RT values showed no Group x Behavior interactions for angry morphs. To assess absolute RT differences between groups we also conducted a Group x Behavior x Morph ANOVA, and no group differences emerged.
Neutral → Happy
For the linear trends of happy ratings, the Group x Behavior interaction was significant, F(2, 54)=4. 36, p<.02. Follow-up ANOVAs demonstrated a group difference for hostile, but not nose-width, ratings of happy morphs, F(2, 54)=7.76, p<.001. Two Tukey HSD-corrected post-hoc analyses of the hostile ratings showed that the linear trend for BD differed from both SMD (p<.04) and HV (p<.001), with BD modulating their ratings less than the SMD and control youth.
Linear trend analyses on RT values showed no Group x Behavior interactions for happy morphs. To assess absolute RT differences between groups we also conducted a Group x Behavior x Morph ANOVA. No group differences emerged.
Imaging
ROI Analysis
Neutral → Angry
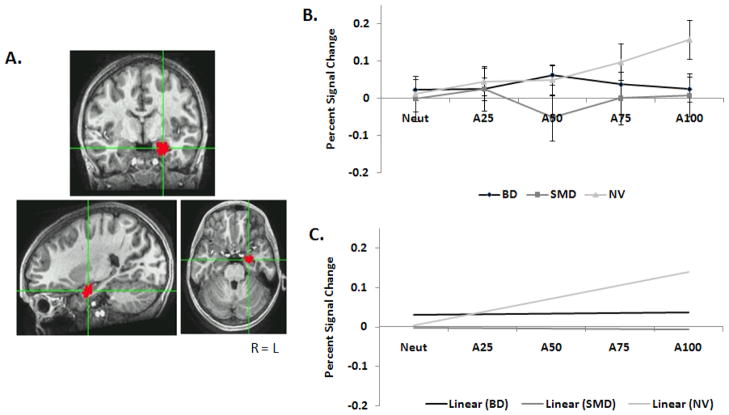
Separate Group x Behavior ANOVAs were conducted for right and left amygdala on the linear trend magnitudes for N→A. In the left amygdala for N→A, there was no Group x Behavior interaction and no main effect of Behavior. However, there was a main effect of Group, F(2, 54)=3.69, p<.04. Collapsed across the two task conditions, linear trend values (SD) for the groups were: BD=.003(.021), SMD=.001(.023), and HV=.069(.019), indicating stronger modulation by face-emotion features in healthy subjects than either patient group. Between-groups Tukey-corrected post hoc analyses revealed a trend, with NV differing from BD (p=.06) and SMD (p=.07). In addition, within-group contrasts were conducted with one-sample t-tests. Only healthy subjects had a significant slope across N→A, t(22)=3.3, p<.005 (Figure 3), with an increase in amygdala BOLD activity with increasing anger in the face. For BD and SMD, the slope did not differ from zero. In the R amygdala, there was no Group x Behavior interaction and no main effects.
Figure 3.
A) Example of the L amygdala ROI. B) Beta weights for each group along each N→A morph in the L amygdala. C) Linear trends for each group along N→A in the L amygdala.
Neutral → Happy
Separate Group x Behavior ANOVAs were conducted for right and left amygdala on the linear trend magnitudes for N→H. There was no Group x Behavior interaction and no main effects in either amygdala.
Whole-brain Analysis
Neutral → Angry
At the whole-brain level, no clusters showed a Group x Behavior interaction for N→A. Left posterior cingulate showed a main effect of Group, F(2, 54) = 11.50, p < .0001, Table 2a. Three Tukey-corrected post-hoc analyses showed that HV had a more positive slope than both BD (p<.0001) and SMD (p<.03).
Table 2.
Group main effects identified by whole-brain analysis
| Region1 | BA | Hemisphere | k | Coordinates | Post-Hoc Analysis | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A. | Neutral to Angry | |||||||
| Posterior Cingulate | 29/30 | L | 454 | −5 | −59 | 12 | BD vs. NV ***, SMD vs. NV* | |
|
| ||||||||
| B. | Neutral to Happy | |||||||
| Inferior Parietal Lobule | 40/7 | R | 407 | 52 | −57 | 40 | BD vs. SMD***, NV*, SMD vs. NV** | |
|
| ||||||||
| Middle Occipital Gyrus, Fusiform Gyrus | 37 | L | 368 | −37 | −61 | 0 | BD vs. SMD***, NV°, SMD vs. NV* | |
|
| ||||||||
| Middle Occipital Gyrus, Cuneus | 18/19 | R | 341 | 35 | −87 | 10 | BD vs. SMD***, NV*, SMD vs. NV* | |
|
| ||||||||
| Middle/Superior Frontal Gyrus | 6/8 | L | 254 | −33 | −12 | 48 | BD vs. SMD***, SMD vs. NV** | |
All regions significant at p < .05 Alphasim corrected
p ≤ .0001
p ≤ .01
p ≤ .05
p ≤ .07
Neutral → Happy
At the whole-brain level, no clusters showed a Group x Behavior interaction for N→H. However, four clusters showed a main effect of Group (Table 2b): R inferior parietal lobule (BA40/7), L Middle Occipital/Fusiform Gyrus (BA37), R Middle Occipital Gyrus/Cuneus (BA18/19), and L Middle/Superior Frontal Gyrus (BA6/8) (overall Fs>9.3, ps<.0001). In each region, Tukey-corrected post-hoc analyses showed that the between-group difference was driven by BD having a more negative slope than SMD (all ps<.0001) and HV (all ps<.05, except for L middle occipital gyrus: p=.07), and SMD having a more positive slope than HV (all ps<.05) (Figure 4).
Figure 4.
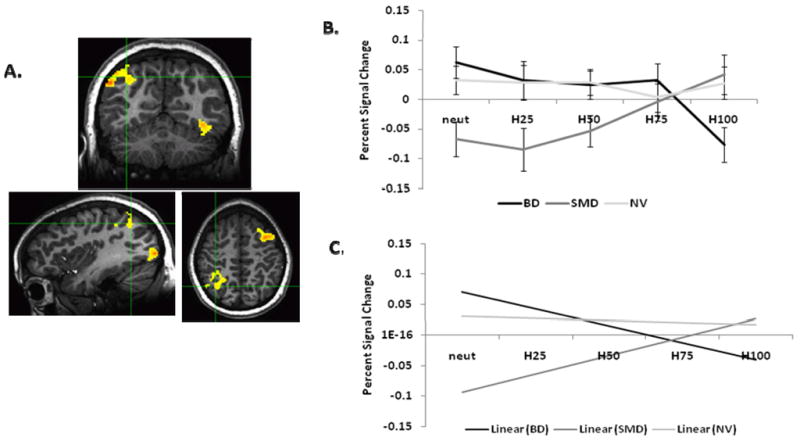
A) Example of the R inferior parietal lobule (BA 40) cluster. B) Beta weights for each group along each N→H morph in the R inferior parietal lobule (BA 40). C) Linear trends for each group along N→H in the R inferior parietal lobule (BA 40).
Effect of potential confounding variables
Effects of mood state
A post-hoc analysis in the L amygdala was conducted including only euthymic BD and SMD (N=16 BD, 15 SMD). There remained a Group effect F(2, 51)=3.29, p<.05, with Tukey-corrected post-hoc analyses showing HV differing from BD (trend, p=.06) and SMD (p=.03) for N→A. A similar analysis in the whole-brain clusters found significant between-group differences. Thus, these analyses revealed no effect of current mood state on our findings.
Effects of comorbid/co-occurring illness
An ANOVA comparing non-anxious SMD (N=8), non-anxious BD (N=11), and HV on the L amygdala N→A showed a Group effect, F(2, 39)=3.46, p<.05. Tukey-corrected post-hoc analyses showed a difference between non-anxious BD and HVs (p<.04), while non-anxious SMD did not differ from either group.
In an ANOVA comparing non-anxious SMD, non-anxious BD, and HV for N→A in the posterior cingulate, and in the clusters identified in the whole-brain N→H, most of the Group differences remained (see Online Only Text). Thus, excluding BD and SMD participants with comorbid anxiety disorder lessened some of the group differences, but this effect may be due to loss of statistical power.
Effects of medication
ANOVAs assessed medication effects on left amygdala N→A linear trends. For SMD, an ANOVA comparing unmedicated SMD (N=6) to medicated SMD (N=8) found no difference (p=.47). Only two BD subjects were unmedicated. However, correlations between number of medications and activation in all BDs on the amygdala and each whole-brain cluster were not significant. We also found no differences between SMDs on vs. off medication in any of the whole-brain clusters (all ps>.16).
DISCUSSION
Research shows that both SMD and BD youth have face emotion processing deficits6–9 and amygdala dysfunction on face-viewing tasks.10 However, studies have not explored amygdala modulation in response to varying face emotion intensity.13–17 Moreover, because only one prior brain-imaging study compared these two patient groups directly,10 questions remain regarding similarities and differences of face-elicited amygdala dysfunction in BD vs. SMD. Here, employing a novel parametric design, we compared BOLD activity in BD, SMD, and HV while participants made implicit or explicit ratings of emotional faces. We examined modulation of activity with changes in the degree of emotion across faces whose expressions ranged from neutral to 100% angry (N→A) or 100% happy (N→H), in 25% increments.
During both implicit and explicit ratings of the N→A morphs, only healthy subjects showed increasing amygdala activity with increasing anger intensity, as has been found using fear faces in healthy adults.27 The fact that neither BD nor SMD youth exhibited this relationship suggests similar amygdala dysfunction in SMD and BD youth. Of note, amygdala dysfunction was present during hostility and nose-width ratings combined, consistent with studies in BD demonstrating abnormalities during both implicit and explicit face emotion processing conditions.13, 15, 17, 21, 28 These data suggest that, in youth with SMD or BD vs. HV, there is diminished amygdala responsiveness to changes in face-emotion across several attentional conditions. This decreased amygdala responsiveness is consistent with behavioral data demonstrating that, compared to healthy subjects, both SMD and BD perform poorly on face emotion labeling tasks,6 requiring more intense emotional information before identifying face emotions.11
BD and SMD differed from each other, and from HV, in behavioral and neural responses to neutral faces as they morphed towards happy. HV youth, but not BD or SMD, exhibited increasing amygdala activation in response to increasing face emotion intensity. Also, compared to HV and SMD, BD patients perceived these faces to have a narrower range of hostility. A failure to interpret facial stimuli as having a broad range of hostility may have resulted in the lack of relationship between amygdala response and face emotion intensity. Alternatively, amygdala and perceived hostility ratings may be driven by an unknown third variable. While youths with SMD also exhibited a lack of amygdala modulation, they did not display a restricted range of hostility ratings. This may indicate different pathophysiological mechanisms between the two patient groups, warranting additional study.
In this parametric task, we varied the level of emotion depicted in face stimuli and examined between-group differences in the linear slope describing activation in response to each of the face morphs; this differs from more typical analyses that compare mean activation between groups. Since parametric designs model linear changes along a continuum, they may be more sensitive to subtle between-group differences in activation patterns. Our design, which includes different gradations of emotional expressions, may be particularly useful in research on amygdala function, since the amygdala habituates rapidly to repeated presentations of identical stimuli. However, our results cannot be compared directly to studies that report amygdala hyper-15, 17 or hypo-10 activation in response to faces displaying 100% emotion, or in response to a limited number of morphs. Nonetheless, our results are, broadly speaking, consistent with prior studies in youth and adults with BD that find amygdala dysfunction during face emotion processing,10, 14–17, 21, 29–35 as well as with a prior study in SMD youth.10 Additionally, our data need to be interpreted in light of the tasks used, ratings of hostility and nose width, not emotion labeling.
At the whole-brain level for N→A, only one cluster differentiated groups i.e., in L posterior cingulate (BA 29/30). The positive slope in HV indicates increased posterior cingulate activation in association with increased anger on the face, perhaps suggesting more effortful processing. In contrast, the negative slopes in BD and SMD suggest that increasing anger in a face is associated with relative disengagement. The posterior cingulate is activated by emotional stimuli;36 given its connections with amygdala, insula, orbito-frontal cortex, and parahippocampal regions,37 it is well-placed to modulate motivation and spatial attention.38 Data suggest posterior cingulate dysfunction in adult BD while viewing sad39 and happy faces.40 To our knowledge, this is the first study in pediatric BD to report dysfunction in posterior cingulated activity while viewing emotional faces. As in adult BD, such dysfunction in youth could be related to the emotional and attention dysregulation that is central to both BD and SMD.
For N→A morphs, BD and SMD demonstrated similar dysfunction relative to HV in the amygdala ROI and whole-brain analyses. In contrast, for N→H, across both implicit and explicit conditions, there were activation differences between BD and SMD in four regions important for attention and visual processing: R inferior parietal lobule, L middle occipital/fusiform gyrus, R middle occipital gyrus, and L middle/superior frontal gyrus. These findings are interesting given research demonstrating BD neural dysfunction elicited by happy face stimuli.32, 33, 41–43 As neutral faces morphed towards happy, each group showed a characteristic and unique pattern of activity across regions; specifically, BD decreased in activity (negative slope), SMD increased in activity (positive slope), and HV did not modulate activity. While mood states fluctuate in patients with BD, SMD is characterized by a persistent negative mood state; speculatively, the processing of positive stimuli may be particularly demanding in patients with chronic, severe irritability2 i.e., those with SMD.
Like the posterior cingulate findings, these results should be viewed with both caution and interest. Caution is warranted, given the lack of consistent prior face-viewing imaging work implicating these regions in SMD or BD. Interest is also warranted, given evidence implicating these structures in processes relevant to SMD and BD.10 Specifically, in healthy subjects, both inferior parietal lobule and middle occipital/fusiform gyrus are key in face processing.44–46 Additionally, the superior frontal gyrus is active during social emotion processing,47, 48 and the inferior parietal lobule mediates attention.49, 50
Depression in adult BD has been associated with dysfunction in anatomical and functional connectivity between the amygdala and frontal areas51 and between frontal areas.25 Here, depression scores were higher in BD vs. SMD, and BD and SMD showed opposite patterns of frontal activity on N→H with increasing amounts of happiness. Although the between-group differences remained in post-hoc analyses covarying CDRS scores (see Online Only Text), the impact of mood state on these findings merits further research.
Mood state, comorbidity, and medication all complicate research on BD and SMD. Most (84%) BD patients were euthymic, and results remained unchanged when the analysis included euthymic patients only. When only non-anxious BD (N=11) and SMD (N=8) were included, the differences between BD and HV remained but, perhaps because of Type II error, SMD did not differ from the other two groups. This demonstrates the need for a larger sample of non-anxious SMD patients. Since most patients were medicated, we cannot rule out the possibility that medication influenced the blunted amygdala response in N→A seen for BD and SMD. The 6 unmedicated vs. 8 medicated SMD did not differ in amygdala or whole-brain cluster slope values, although this could be due to insufficient power. When medication status was covaried from the four N→H clusters where BD and SMD differed, the groups remained statistically distinct from each other. Given the post-hoc nature of this analysis and the small sample sizes, replication in a larger sample is needed. While it would be ideal to study unmedicated patients, it is neither ethical nor feasible to test all patients medication-free. Since our paradigm included only angry and happy morphs, we cannot generalize beyond these two emotions e.g., to fear or sadness. Also, while prior behavioral studies demonstrate face emotion labeling deficits in BD and SMD, face emotion labeling was not assessed directly here. An additional limitation is that we did not systematically assess handedness.
In this novel parametric face paradigm with two attention conditions, we found that BD and SMD have both similarities (amygdala) and differences (parietal and medial frontal areas) in BOLD activity dysfunction versus healthy subjects. These data suggest that amygdala activity in both groups does not change in response to increasing amounts of anger. In contrast, in response to increasing amounts of happiness, BD and SMD have differing directions of dysfunction in frontal and parietal regions. The current work adds to data demonstrating that BD and SMD have clinical, neuropsychological and pathophysiological similarities as well as differences,10, 26 suggesting that SMD and BD may be manifestations of differing pathologies along a mood disorders spectrum. Future work is needed to examine the role of these neural correlates as they relate to differences in clinical outcomes.
While these data cannot be used for diagnostic purposes, they support the growing need to compare pediatric BD to children with SMD. SMD, like BD, is a severe mood disorder, but SMD patients differ from BD in that only BD patients experience manic episodes (presenting instead with non-episodic irritability), and, longitudinally, SMD patients develop depressive and anxiety disorders.4, 5, 43, 52 Future work can aid in distinguishing BD from SMD pathophysiologically, which has important diagnostic and treatment implications for children who present in community clinics with severe irritability and emotional dysregulation.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Mental Health.
References
- 1.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007 Sep;64(9):1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 2.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003 Mar;160(3):430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 3.Brotman MA, Kassem L, Reising MM, Guyer AE, Dickstein DP, Rich BA, Towbin KE, Pine DS, McMahon FJ, Leibenluft E. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. Am J Psychiatry. 2007 Aug;164(8):1238–1241. doi: 10.1176/appi.ajp.2007.06101619. [DOI] [PubMed] [Google Scholar]
- 4.Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, Egger HL, Angold A, Pine DS, Leibenluft E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006 Nov 1;60(9):991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Stringaris A, Baroni A, Haimm C, Brotman M, Lowe CH, Myers F, Rustgi E, Wheeler W, Kayser R, Towbin K, Leibenluft E. Pediatric bipolar disorder versus severe mood dysregulation: risk for manic episodes on follow-up. J Am Acad Child Adolesc Psychiatry. 2010 Apr;49(4):397–405. [PMC free article] [PubMed] [Google Scholar]
- 6.Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, Pine DS, Ernst M, Leibenluft E. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007 Sep;48(9):863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 7.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008 Spring;20(2):529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA. Facial emotion processing in acutely ill and euthymic patients with pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007 Aug;46(8):1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- 9.McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005 Sep;162(9):1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 10.Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010 Jan;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007 Feb;164(2):309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 12.Darwin C. The Expression of the Emotions in Man and Animals. Chicago: University of Chicago Press; 1872/1965. [Google Scholar]
- 13.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, Shah MP, Martin A, Constable RT, Blumberg HP. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009 Jun;48(6):636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 2011 Mar 10; doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007 Jul 15;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Pavuluri MN, Passarotti A. Neural bases of emotional processing in pediatric bipolar disorder. Expert Rev Neurother. 2008 Sep;8(9):1381–1387. doi: 10.1586/14737175.8.9.1381. [DOI] [PubMed] [Google Scholar]
- 17.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009 Mar;48(3):308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala hyperactivation during face emotion processing in bipolar disorder and children at familial risk. JOURNAL OF THE AMERICAN ACADEMY OF CHILD & ADOLESCENT PSYCHIATRY. doi: 10.1016/j.jaac.2011.12.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008 Sep;13(9):829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp. 2000 Feb;9(2):93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 2010;16(1):106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997 Jul;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Ekman P, Friesen W. Pictures of Facial Affect. Palo Alto: Consulting Psychologists; 1976. [Google Scholar]
- 24.LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cereb Cortex. 2003 Oct;13(10):1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- 25.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brian. New York: Thieme; 1988. [Google Scholar]
- 26.Adleman NE, Kayser R, Dickstein D, Blair RJ, Pine D, Leibenluft E. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2011 Nov;50(11):1173–1185. e1172. doi: 10.1016/j.jaac.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996 Oct 31;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 28.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006 Jun 6;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CH, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, Suckling J, Bullmore E. Explicit and implicit facial affect recognition in manic and depressed States of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006 Jan 1;59(1):31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008 Jan 15;162(1):27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004 Mar 15;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Surguladze SA, Marshall N, Schulze K, Hall MH, Walshe M, Bramon E, Phillips ML, Murray RM, McDonald C. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage. 2010 Oct 15;53(1):58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 33.Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR, Kupfer DJ, Phillips ML. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010 Mar 1;67(5):422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, Lee JH, Welge JA, DelBello MP, Fleck DE, Adler CM. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011 Feb 15;69(4):381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010 Mar 1;67(5):414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003 Jan;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris R, Paxinos G, Petrides M. Architectonic analysis of the human retrosplenial cortex. J Comp Neurol. 2000 May 22;421(1):14–28. doi: 10.1002/(sici)1096-9861(20000522)421:1<14::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 38.Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008 Nov;18(11):2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med. 2004 Jul;34(5):795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- 40.Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, Simmons A, Andrew C, Brammer M, David AS. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res. 1998 Sep 28;83(3):127–138. doi: 10.1016/s0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- 41.Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009 Sep 1;66(5):451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008 Dec;10(8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassel S, Almeida JR, Frank E, Versace A, Nau SA, Klein CR, Kupfer DJ, Phillips ML. Prefrontal cortical and striatal activity to happy and fear faces in bipolar disorder is associated with comorbid substance abuse and eating disorder. J Affect Disord. 2009 Nov;118(1–3):19–27. doi: 10.1016/j.jad.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radua J, Phillips ML, Russell T, Lawrence N, Marshall N, Kalidindi S, El-Hage W, McDonald C, Giampietro V, Brammer MJ, David AS, Surguladze SA. Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage. 2010 Jan 1;49(1):939–946. doi: 10.1016/j.neuroimage.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci. 1998 Oct 22;265(1409):1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanwisher N. Domain specificity in face perception. Nat Neurosci. 2000 Aug;3(8):759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- 47.Farrow TF, Zheng Y, Wilkinson ID, Spence SA, Deakin JF, Tarrier N, Griffiths PD, Woodruff PW. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001 Aug 8;12(11):2433–2438. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- 48.Beer JS, Ochsner KN. Social cognition: a multi level analysis. Brain Res. 2006 Mar 24;1079(1):98–105. doi: 10.1016/j.brainres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nat Rev Neurosci. 2000 Nov;1(2):91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- 50.Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004 Jun;8(6):261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Cerullo MA, Fleck DE, Eliassen JC, Smith MS, DelBello MP, Adler CM, Strakowski SM. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord. 2012 Mar;14(2):175–184. doi: 10.1111/j.1399-5618.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011 Feb;168(2):129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.