Abstract
Background
Little is known about time-varying effects of smoking quantity and nicotine dependence on the regularity of adolescent smoking behavior.
Methods
The sample was drawn from the Social and Emotional Contexts of Adolescent Smoking Patterns Study which followed adolescent smokers over 5 assessment waves spanning 48 months. Participants included former experimenters (smoked <100 cigarettes/lifetime but did not smoke in past 90 days), recent experimenters (smoked <100 cigarettes/lifetime and smoked in past 90 days), and current smokers (smoked >100 cigarettes/lifetime and smoked in past 30 days). Mixed-effects regression models were run to examine the time-varying effects of smoking quantity and nicotine dependence on regularity of smoking behavior, as measured by number of days smoked.
Results
Smoking quantity and nicotine dependence were each found to be significantly associated with regularity of adolescent smoking and the size of each effect exhibited significant variation over time. The effect of smoking quantity decreased across time for each smoking group, while the effect of nicotine dependence increased across time for former and recent experimenters. By the 48-month follow-up, the effects of smoking quantity and nicotine dependence had each stabilized across groups.
Conclusions
This study reveals that smoking quantity and nicotine dependence are not static risk factors for the development of more regular smoking patterns. At low levels of smoking when nicotine dependence symptoms are less common, smoking quantity is a stronger predictor of increased regularity of smoking, while for more experienced smokers, nicotine dependence predicts further increases in regularity.
Keywords: Adolescents, Mixed-effects regression model, Nicotine dependence, Smoking, Tobacco
1. Introduction
The most important factor currently believed to contribute to smoking persistence is nicotine dependence. Most commonly, nicotine dependence is characterized as physiological adaptations (i.e., tolerance, withdrawal) and other accommodating behaviors (e.g., time spent on obtaining/using nicotine and recovering from its effects, and forfeiting/reducing important social, occupational, or recreational activities) resulting from regular and frequent smoking. In fact, nicotine dependence and withdrawal have been consistently implicated in the maintenance of smoking behavior: nicotine dependence predicts smoking regularity and quantity (Dierker and Mermelstein, 2010; O’Loughlin et al., 2003; Shiffman and Sayette, 2005; Doubeni et al., 2010), failed quit attempts (DiFranza et al., 2002a; Haddock et al., 1999; Ip et al., 2011; Piper et al., 2011), and biochemical markers of nicotine exposure (Meneses-Gaya et al., 2009; Prokhorov et al., 2000; Carpenter et al., 2010).
Despite evidence for the central role of nicotine dependence in promoting the development of regular smoking (Dierker and Mermelstein, 2010), its association with smoking is reciprocal (Doubeni et al., 2010). That is, nicotine dependence symptoms both increase the regularity of smoking and are a consequence of it. The relative roles of nicotine dependence vs. smoking exposure in driving the development of regular smoking behavior have been difficult to disentangle, especially considering that these measures vary across time. While some studies have accounted for this variation over time by examining these variables as time-varying predictors of cessation or relapse (Kahler et al., 2010; Wileyto et al., 2004; Crittenden et al., 2007; Kandel et al., 2007; Van Zundert et al., 2010), their effects are typically estimated as an average. That is, time-varying measures of smoking behavior and nicotine dependence have produced only a single estimate of their average effect. Similarly, studies employing trajectory analyses have described time-varying smoking behavior (White et al., 2002; Karp et al., 2005; Brook et al., 2008) or nicotine dependence (Hu et al., 2008). In these analyses, however, only the outcome is permitted to be time-varying, thus limiting the association with the alternate construct to be either static or averaged across time.
The effects of smoking exposure and nicotine dependence on the regularity of smoking may in fact vary in magnitude as regular smoking behavior develops. To date, the possible variation of this relationship across time has not been investigated. It is possible, early in one’s smoking career, that smoking quantity may be more strongly associated with smoking regularity than are symptoms of dependence, while with continued exposure to cigarettes, nicotine dependence may begin to play a larger role in the development of smoking regularity. Characterizing the dynamics of these emerging relationships may be pertinent to designing interventions by guiding the decisions of the specific factors to target, and the optimal times to intervene on each factor, that are likely to most effectively reduce regular smoking behavior.
To address this hypothesis, the current study investigated the time-varying effects of both smoking quantity and nicotine dependence symptoms on regularity of smoking behavior, in a sample of adolescents followed over five assessment waves spanning 48 months. The aims of the study are to examine (1) whether nicotine dependence and smoking quantity have time-varying effects on smoking regularity, and if so, how exactly these relationships change over time, and (2) whether these results differ for adolescents who are at different stages in the development of regular smoking behavior. These questions were investigated while controlling for other factors known to be associated with smoking behavior and nicotine dependence, including parental smoking (Zagona and Zurcher, 1965; Bajda, 1964; Avenevoli and Merikangas, 2003; Kardia et al., 2003), other tobacco use (Gilpin and Pierce, 2003; Connolly et al., 1986), and gender (Bajda, 1964; Bohadana et al., 2003).
2. Methods
2.1. Participants
The sample was drawn from the Social and Emotional Contexts of Adolescent Smoking Patterns (SECASP) Study, which has been described elsewhere (e.g., Dierker and Mermelstein, 2010). High schools were selected for recruitment based on geographic location, size, ethnic/racial diversity, willingness to work with research staff in gaining parental consent, willingness to provide a school liaison to the study, and space for recruitment and data collection activities. Approximately 35 schools were invited to participate. All 9th and 10th grade students at the final sample of 16 Chicago-area high schools completed a brief screener survey of smoking behavior (N = 12,970). All students who reported (1) smoking in the past 90 days and smoking <100 cigarettes/lifetime, (2) smoking in the past 30 days and smoking >100 cigarettes/lifetime, or (3) smoking <100 cigarettes/lifetime, but not smoking in the past 90 days, were invited to participate, as were random samples of never-smokers. Of the 3654 students invited, 1344 agreed to participate (36.8%). Of these, 1263 (94.0%) completed the baseline measurement wave 2 months after screening.
Following the baseline assessment, 5 assessment waves to date have occurred at 6, 15, 24, 33, and 48 months following baseline. Retention at 48 months was 86.5% (N = 1092). Participants and non-participants at 48 months did not differ by gender, race/ethnicity, or age. However, nonparticipation was higher among youth whose parent did not complete the extensive parent questionnaire at baseline (X2 = 29.3, d.f. = 1), p < .0001. Non-completers reported higher 7-day smoking rates at baseline (M = 0.85 cigarettes/day, SD = 2.47 vs. M = 0.42, SD = 1.33), p = .025; a greater number of days smoked in the past 30 (M = 5.5 days, SD = 8.75 vs. M = 3.6, SD = 7.50), p = .007; and more cigarettes smoked on smoking days in the past 30 (M = 1.37 cigarettes, SD = 3.05 vs. M = 0.82, SD = 1.74), p = .022. Non-completers also had lower grade point averages at baseline (M = 3.50, SD = 0.68 vs. M = 3.77, SD = 0.76, p < .0001).
The present analyses focused on former experimenters (n = 304) who had smoked <100 cigarettes/lifetime at screening but had not smoked in the past 90 days, current experimenters (n = 594) who had smoked <100 cigarettes/lifetime and had smoked in the past 90 days, and current smokers (n = 152) who had smoked >100 cigarettes/lifetime, had smoked in the past 30 days, but were smoking ≤5 cigarettes/day at screening. Demographic and smoking characteristics are presented in Table 1. All groups had approximately the same mean age at baseline and reported a similar age of smoking initiation. The former and recent experimenters had an approximately equal composition of gender and ethnicity, while the current smokers had a higher percentage of males.
Table 1.
Demographic and smoking characteristics by subgroup.
| Baseline characteristicd | Former experimentersa N = 304 |
Recent experimentersb N = 594 |
Current Smokersc N = 152 |
|---|---|---|---|
| Female sex, n (%) | 170 (55.9) | 344 (57.9) | 71 (46.7) |
| Mean age, years, mean (SD) | 15.7 (0.62) | 15.6 (0.62) | 15.8 (0.63) |
| Race/ethnicity | |||
| Non-Hispanic White, n (%) | 151 (49.7) | 327 (55.1) | 107 (70.4) |
| Non-Hispanic Black, n (%) | 66 (21.7) | 96 (16.2) | 10 (6.6) |
| Hispanic, n (%) | 55 (18.1) | 117 (19.7) | 24 (15.8) |
| Asian/Pacific Islander, n (%) | 13 (4.3) | 17 (2.9) | 4 (2.6) |
| American Indian/Alaska Native, n (%) | 0(0) | 2 (0.3) | 0 (0) |
| Smoked in the past 24 h, n (%) | 7 (2.3) | 70 (11.8) | 93 (61.6) |
| Smoked in the past week, n (%) | 26 (8.6) | 230 (39.2) | 124 (83.2) |
| Other tobacco use in past 30 days, n (%) | 38 (12.5) | 189 (32.1) | 71 (46.7) |
| Ever smoked daily in lifetime, n (%) | 22 (7.3) | 90 (15.2) | 119 (79.3) |
| Smoked daily in the past month, n (%) | 0 (0) | 4 (0.7) | 45 (29.6) |
| Age of initiation in years, mean (SD) | 12.2 (2.13) | 12.7 (2.03) | 11.7 (1.91) |
| Mother’s smoking status, n (%) | |||
| Current smoker | 65 (22.9) | 139 (24.7) | 61 (41.5) |
| Ex-smoker | 69 (23.9) | 133 (23.6) | 35 (23.8) |
| Never-smoker | 151 (53.2) | 291 (51.7) | 51 (34.7) |
| Father’s smoking status, n (%) | |||
| Current smoker | 87 (36.3) | 157 (32.5) | 58 (43.9) |
| Ex-smoker | 66 (27.5) | 136 (28.2) | 27 (20.5) |
| Never-smoker | 87 (36.3) | 190 (39.3) | 47 (35.6) |
| Smoking regularitye, mean (SD) | 0.5 (1.48) | 3.2 (5.35) | 18.2 (11.06) |
| At 6-month assessment | 1.1 (3.46) | 4.1 (6.83) | 18.7 (11.90) |
| At 15-month assessment | 2.1 (5.89) | 5.7 (9.47) | 20.1 (12.32) |
| At 24-month assessment | 3.1 (7.78) | 7.2 (10.54) | 19.5 (12.21) |
| At 48-month assessment | 7.0 (11.13) | 10.5 (12.37) | 19.2 (12.78) |
| Smoking quantityf, mean (SD) | 0.2 (1.66) | 2.0 (5.58) | 19.4 (23.58) |
| At 6-month assessment | 0.6 (3.35) | 2.5 (7.74) | 24.1 (27.12) |
| At 15-month assessment | 1.9 (9.34) | 5.3 (15.12) | 32.6 (37.98) |
| At 24-month assessment | 2.9 (11.05) | 7.6 (12.30) | 25.8 (30.92) |
| At 48-month assessment | 9.3 (22.8) | 15.9 (31.5) | 38.3 (44.53) |
| NDSS score, mean (SD) of 10 items on a scale of 1–4 | 1.0 (0.27) | 1.2 (0.52) | 2.2 (0.86) |
| At 6-month assessment | 1.0 (0.35) | 1.3 (0.63) | 2.2 (0.87) |
| At 15-month assessment | 1.1 (0.50) | 1.4 (0.72) | 2.3 (0.89) |
| At 24-month assessment | 1.1 (0.58) | 1.4 (0.75) | 2.3 (0.89) |
| At 48-month assessment | 1.9 (0.84) | 2.1 (0.86) | 2.6 (0.86) |
At screening, youths who had smoked <100 cigarettes/lifetime but had not smoked in the past 90 days.
At screening, youths who had smoked <100 cigarettes/lifetime and had smoked in the past 90 days.
At the screening phase of the study, youths who had smoked >100 cigarettes/lifetime, had smoked in the past 30 days, but smoke ≤5 cigarettes/day.
Percentages based on valid responses.
Smoking regularity is defined as the number of days smoked in the past 30 days.
Smoking quantity is defined as the number of cigarettes smoked in the past 7 days.
2.2. Measures
2.2.1. Smoking
Smoking behavior was measured at the baseline, 6-, 15-, 24-, and 48-month assessment waves with two items. Participants were asked on how many days they smoked cigarettes in the past 30 days (smoking regularity) and how many cigarettes they smoked in the past 7 days (smoking quantity).
2.2.2. Nicotine dependence
Nicotine dependence was assessed at the baseline, 6-, 15-, 24-, and 48-month follow-up assessments with a shortened version of the nicotine dependence syndrome scale (NDSS; Shiffman et al., 2004), modified for use with adolescents. The full NDSS scale was reduced to 10 items based on psychometric analyses conducted on an adolescent sample (Sterling et al., 2009), retaining those items reflecting mainly drive and tolerance from the original NDSS. Research supports the reliability, stability, construct validity, and predictive validity of the NDSS for use with adolescents (Clark et al., 2005; Sledjeski et al., 2007), and the modified version demonstrated strong internal consistency with the current sample (coefficient alpha = .93). Items in the current study were answered on a four-point Likert-type scale, ranging from 1 (not at all true) to 4 (very true), and were averaged into a total NDSS score.
2.2.3. Other tobacco use
Other tobacco use was measured at baseline with the following questions. During the past 30 days, on how many days did you (a) use chewing tobacco, snuff or dip; (b) smoke cigars, cigarillos or little cigars; (c) smoked bidis or (d) smoked kreteks? Responses were dichotomized into any other tobacco use vs. no other tobacco use.
2.2.4. Parental smoking
Adolescents reported the current smoking status (current, ex-, or never-smoker) of each biological parent at baseline.
2.3. Analyses
Using smoking regularity as the outcome, main effects and time-varying effects of smoking quantity and nicotine dependence were examined, while controlling for mother’s smoking status, father’s smoking status, other tobacco use, and gender. Mixed-effects regression models, which include both fixed effects of variables and random effects to account for the repeated measurements of participants over time, were run using SAS’s PROC MIXED. Random effects included intercepts (allowing individual differences in baseline smoking regularity) and time trends (allowing individual differences in the rate of change in smoking regularity across follow-up waves), with an unstructured covariance structure. The model is of the form
yij = b0i + blitij + b2iXij + b3i(xij × tij) + εij
where Xij is the matrix of all covariates (fixed effects) in the model for subject i and time j, xij is the matrix of the subset of fixed effects for which time-varying effects will be investigated, b2i is the vector of coefficients for the fixed effects of Xij, b3i is the vector of coefficients for the fixed time-varying effects of xij, tij is the assessment wave, and εij is the error. The random effects in the above equation are described by
b0i = β0i + v0i
b1i = β1i + v1i
where β0i and β1i are the fixed intercept and slope, respectively, and v0i and v1i are the random intercepts and slopes, respectively. F statistics for all fixed effects in the model are calculated using standard Type 3 F tests.
Due to a change in measurement procedures at the 48-month assessment wave, NDSS items were not given to past-month nonsmokers and were coded as missing. Since observations containing missing data are excluded from the analysis, the 48-month assessment wave differs from the other waves in that it does not contain any participants who smoked 0 days out of the past-month. To rectify this inconsistency, past-month nonsmokers at all earlier assessment waves were also removed from the dataset prior to analysis; from baseline through the 24-month wave, this excluded 993/1216 observations from former experimenters, 1448/2376 observations from recent experimenters, and 204/608 observations from current smokers.
Smoking regularity (outcome), smoking quantity, and nicotine dependence were all time-varying, while mother’s smoking status, father’s smoking status, other tobacco use, and gender were static and derived from the baseline reports. A categorical time variable specified the assessment wave. Time-varying effects were investigated by including interactions between each variable and time, and coefficients that compared each time point to a reference time point were estimated. The model was run multiple times using sequential contrasts in order to directly compare each pair of neighboring assessment waves. The model was stratified by adolescent smoking status (former experimenter, current experimenter, or current smoker) to investigate group-specific effects.
Finally, effect sizes were calculated using Cohen’s f2 (Cohen, 1988) in order to compare the unique impacts of both smoking exposure and nicotine dependence on regularity of smoking. Cohen’s f2 values were calculated separately within each smoking group and assessment wave, as described elsewhere (Selya et al., 2012). 95% confidence intervals for Cohen’s f2 values were obtained by running 1000 bootstrap replications and determining the 2.5th and 97.5th percentiles of the resulting distribution.
3. Results
Baseline characteristics, smoking behavior, and nicotine dependence for each smoking group are shown in Table 1. Differences for these descriptive data over time (linear difference between baseline and 48 months) and between smoking groups are as follows. Smoking became more regular for experimenters between baseline and 48 months (former experimenters: M = 6.50, t = 9.49, p < .0001; recent experimenters: M = 7.27, t = 12.27, p < .0001). Smoking regularity at the 48-month follow-up differed by smoking group (F(2, 892) = 41.61, p < .0001), with the current smokers smoking more regularly than the recent experimenters (M = 8.61, p < .0001), who in turn smoked more regularly than the former experimenters (M = 3.44, p = .0005). Smoking quantity also reliably increased over time for all smoking groups (former experimenters: M = 9.02, t = 6.45, p < .0001; recent experimenters: M = 13.93, t = 9.81, p < .0001; current smokers: M = 18.90, t = 4.21, p < .0001). Finally, nicotine dependence also increased over time (former experimenters: M = 0.95, t = 12.07, p < .0001; recent experimenters: M = 0.85, t = 15.82, p < .0001; current smokers: M = 0.34, t = 3.05, p = .0026), and at the 48-month wave was highest for the current smokers (vs. former experimenters: M = 0.66, t = 5.70, p<.0001; vs. recent experimenters: M = 0.50, t = 5.02, p < .0001).
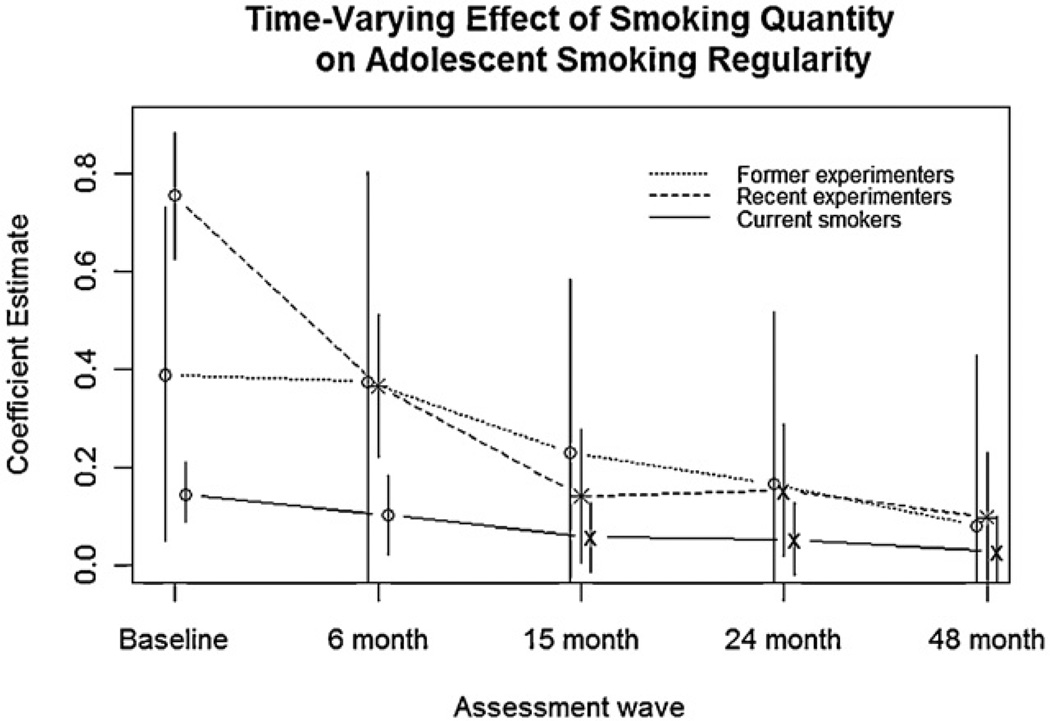
The results of the mixed-effects regression models are shown in Table 2. For all three groups of adolescent smokers, higher smoking quantity (former experimenters: b = 0.39; recent experimenters: b = 0.75; current smokers: b = 0.15) and higher nicotine dependence (former experimenters: b = −0.23; recent experimenters: b = 2.33; current smokers: b = 6.62) were significantly associated with more regular smoking (all p < .0001), after controlling for baseline measures of maternal and paternal smoking, other tobacco use, and gender. In addition to these main effects, smoking quantity showed a significant time-varying effect for all three smoking groups (former experimenters: F(4, 71) = 3.64, p = .0093; recent experimenters: F(4, 447) = 33.86, p < .0001; current smokers: F(4, 228) = 2.90, p = .0228). As Fig. 1 illustrates, this time-varying association between smoking quantity and smoking regularity progressively decreases over the course of 48 months, such that higher smoking quantity is associated with progressively reduced increases in smoking regularity. All three adolescent smoking groups showed this decline overall, though sequential contrasts between neighboring assessment waves did not always reach significance. Confirming this trend, effect sizes of smoking quantity (Table 3) were initially large and decreased over time, with some variation in the initial magnitude of effect size. Though this decline was not always significant due to small sample sizes causing large variation in the bootstrap samples, the trend confirms the findings from the mixed-effects model. Together, these results indicate that smoking quantity develops a weaker positive association with smoking regularity as time progresses.
Table 2.
Unstandardized fixed effects on adolescent smoking frequency, based on results of a mixed-effects regression model, stratified by adolescent smoking subgroup. Bold values indicate significance at p < .05.
| Effect | Former experimentersa F value (DF) |
Recent experimentersb F value (DF) |
Current smokersc F value (DF) |
|---|---|---|---|
| Smoking quantity | 31.22 (1/71)*** | 335.91 (1/447)*** | 42.30 (1/228)*** |
| NDSS score | 52.30 (1/71)*** | 337.18 (1/447)*** | 156.90 (1/228)*** |
| Maternal smoking status | 1.33 (2/71) | 1.18 (2/447) | 5.40 (2/228)** |
| Paternal smoking status | 0.33 (2/71) | 0.18 (2/447) | 2.39 (2/228) |
| Other tobacco use | 0.01 (1/71) | 0.27 (1/447) | 0.51 (1/228) |
| Gender | 0.30 (1/71) | 0.32 (1/447) | 0.92 (1/228) |
| Time | 1.87 (4/71) | 4.32 (4/447)*** | 1.90 (4/228) |
| Smoking quantity by time interaction | 3.64 (4/71)** | 33.86 (4/447)*** | 2.90 (4/228)* |
| NDSS score by time interaction | 3.07 (4/71)* | 13.08 (4/447)*** | 0.26 (4/228) |
At screening, youths who had smoked <100 cigarettes/lifetime but had not smoked in the past 90 days.
At screening, youths who had smoked <100 cigarettes/lifetime and had smoked in the past 90 days.
At the screening phase of the study, youths who had smoked >100 cigarettes/lifetime, had smoked in the past 30 days, but smoke ≤5 cigarettes/day.
p < .05.
p < .01.
p < .0001.
Fig. 1.
Coefficient estimates of smoking quantity from a mixed-effects regression model are shown for each assessment wave, and for each of the three subgroups of adolescent smokers defined at screening: former experimenters (smoked under 100 cigarettes/lifetime but did not smoke in the past 90 days), recent experimenters (smoked under 100 cigarettes/lifetime and smoked in the past 90 days), and current smokers (smoked over 100 cigarettes/lifetime, smoked in the past 30 days, but did not smoke more than 5 cigarettes/day). Smoking groups are slightly staggered around assessment wave to show differences. Coefficient estimates are based on the average fixed effect of smoking quantity across adolescents in each group. Asterisks indicate a significant difference for the corresponding wave, relative to the coefficient estimate of the previous wave, and ’×s indicate a significant difference relative to the baseline wave. Vertical bars indicate 95% confidence intervals.
Table 3.
Effect sizes of smoking quantity and nicotine dependence on the regularity of smoking, over 5 assessment waves. Cohen’s f2 (Cohen, 1988) is shown for smoking quantity (top half) and NDSS (bottom half) within each assessment wave and smoking group, along with 95% confidence intervals (based on 2.5th and 97.5th percentiles of the distribution from 1000 bootstrap replications). Conventionally, f2 values of .02, .15, and .35 are considered small, medium, and large effect sizes, respectively (Cohen, 1988).
| Former experimentersa f2 (95% CI) |
Recent experimentersb f2 (95% CI) |
Current smokersc f2 (95% CI) |
|
|---|---|---|---|
| Smoking quantity | |||
| Baseline | 0.653 (0.134–1.96) | 1.300 (0.682–2.212) | 0.337 (0.127–0.644) |
| 6-month | 0.533 (0.001–2.984) | 0.677 (0.435–1.02) | 0.092 (0.007–0.229) |
| 15-month | 0.485 (0.078–1.313) | 0.163 (0.062–0.324) | 0.097 (0.011–0.244) |
| 24-month | 0.257 (0.049–0.575) | 0.169 (0.072–0.310) | 0.115 (0.027–0.239) |
| 48-month | 0.235 (0.045–0.566) | 0.099 (0.034–0.189) | 0.046 (0.000–0.171) |
| Nicotine dependence | |||
| Baseline | 0.111 (0.000–0.462) | 0.109 (0.039–0.202) | 0.428 (0.126–0.881) |
| 6-month | 0.340 (0.014–0.916) | 0.296 (0.124–0.526) | 0.398 (0.147–0.814) |
| 15-month | 0.480 (0.033–0.1.69) | 0.680 (0.364–1.072) | 0.392 (0.139–0.793) |
| 24-month | 0.335 (0.081–0.816) | 0.357 (0.176–0.581) | 0.401 (0.120–0.885) |
| 48-month | 0.229 (0.055–0.517) | 0.377 (0.212–0.587) | 0.336 (0.096–0.787) |
At screening, youths who had smoked <100 cigarettes/lifetime but had not smoked in the past 90 days.
At screening, youths who had smoked <100 cigarettes/lifetime and had smoked in the past 90 days.
At the screening phase of the study, youths who had smoked >100 cigarettes/lifetime, had smoked in the past 30 days, but smoke ≤5 cigarettes/day.
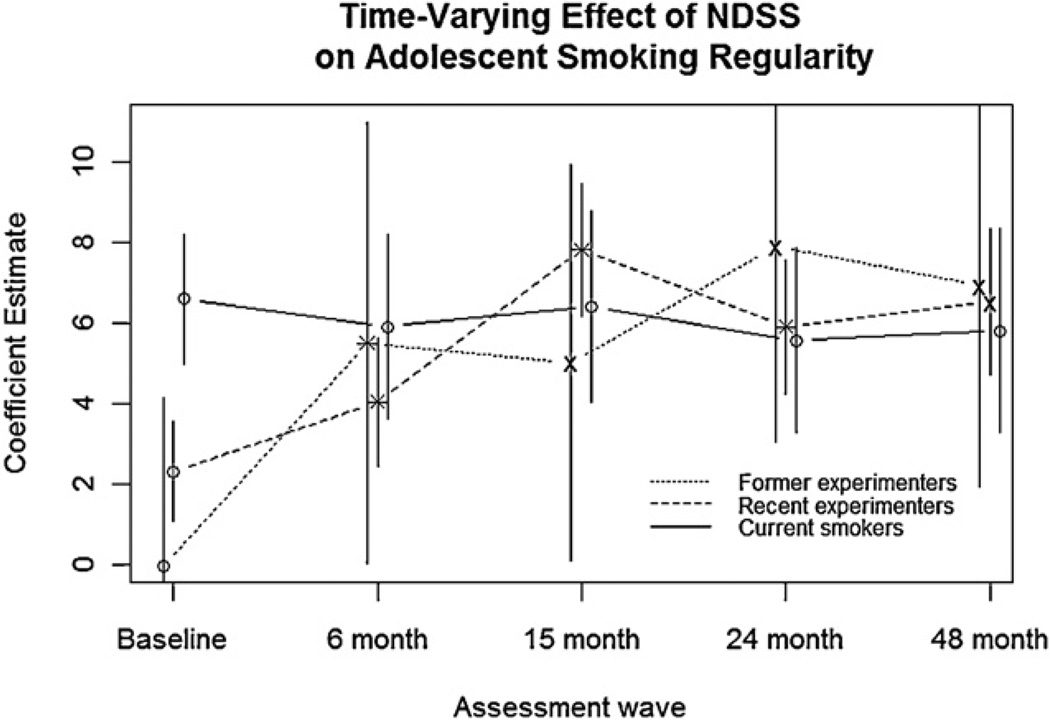
Nicotine dependence also showed a significant time-varying effect on regularity of smoking for former experimenters (F(4, 71) = 3.07, p = .0215) and recent experimenters (F(4, 447) = 13.08, p < .0001). As Fig. 2 illustrates, the time-varying association between nicotine dependence and smoking regularity progressively increases over 48 months for former and recent experimenters, but remains steady for current smokers. That is, higher nicotine dependence is associated with increasingly more regular smoking over time for former and recent experimenters. This trend is confirmed by increasing effect sizes over time for nicotine dependence (Table 3) in these two groups. For current smokers, the association between nicotine dependence and smoking regularity was large at baseline, and did not change over subsequent waves (Fig. 2). Similarly, the effect sizes of nicotine dependence (Table 3) were large at baseline for this group and remained large throughout the follow-up period.
Fig. 2.
Coefficient estimates of NDSS score from a mixed-effects regression model are shown for each assessment wave, and for each of the three subgroups of adolescent smokers defined at screening: former experimenters (smoked under 100 cigarettes/lifetime but did not smoke in the past 90 days), recent experimenters (smoked under 100 cigarettes/lifetime and smoked in the past 90 days), and current smokers (smoked over 100 cigarettes/lifetime, smoked in the past 30 days, but did not smoke more than 5 cigarettes/day). Smoking groups are slightly staggered around assessment wave to show differences. Coefficient estimates are based on the average fixed effect of NDSS across adolescents in each group. Asterisks indicate a significant difference for the corresponding wave, relative to the coefficient estimate of the previous wave, and ’×s indicate a significant difference relative to the baseline wave. Vertical bars indicate 95% confidence intervals.
4. Discussion
The present study examined the time-varying effects of smoking quantity and nicotine dependence on smoking regularity among three groups of adolescent smokers. While smoking quantity and nicotine dependence had strong cross-sectional associations with smoking regularity, the strength of these associations also showed significant variation over time. Mixed-effects regression models revealed that the effect of smoking quantity decreased over time, while that of nicotine dependence increased over time for all but the heaviest smokers at baseline. These findings are among the first to characterize the time-varying, reciprocal relationships between nicotine dependence, tobacco exposure, and regularity of smoking.
Smoking quantity was most strongly associated with regularity of smoking for the baseline assessment wave, and this relationship became consistently weaker over time. That is, smoking quantity was associated with more regular smoking, but over time became associated with weaker increases in smoking regularity. Smoking groups differed in the exact course of this decline: former and recent experimenters had a stronger initial association which declined steeply over time, while current smokers showed a smaller-magnitude decrease from an initially weaker association. By the 48-month follow-up assessment, the association between smoking quantity and regularity of smoking had declined to a similar level across all three smoking groups. Relative to nicotine dependence, tobacco exposure seems to be more relevant to increased smoking regularity during the earliest stages of smoking.
Nicotine dependence, in contrast, was weakly associated with smoking regularity at baseline, and this association became progressively stronger over time for the former and recent experimenters. That is, nicotine dependence initially had a negligible effect size on smoking regularity, but over time, higher nicotine dependence became associated with progressively more regular smoking. Though nicotine dependence was a significant cross-sectional predictor of smoking regularity at all assessment waves and for all smoking groups, its association with smoking regularity was not time-varying for the current smokers, for whom the effect of nicotine dependence was already strong at baseline. The increase over time in the effect of nicotine dependence observed in the former and recent experimenters resulted in all three groups having an equivalent effect size of nicotine dependence by the 48-month assessment. Thus, compared to tobacco exposure, which is a stronger predictor of smoking regularity at low levels of smoking when nicotine dependence symptoms are rare, nicotine dependence explains further increases in regularity once adolescents have developed relatively consistent smoking patterns.
Although not statistically significant, the effect of nicotine dependence seemed to peak at 15 months and 24 months following baseline for the recent and former experimenters, respectively, before subsequently declining. However, this decline approached significance (p < .10), possibly suggesting the importance of nicotine dependence during early stages of infrequent smoking among novice adolescent smokers, consistent with previous findings (Dierker and Mermelstein, 2010; DiFranza et al., 2002b, 2007; O’Loughlin et al., 2003). In particular, early-emerging nicotine dependence may increasingly explain smoking regularity at the earliest stages of smoking and eventually reach a point after which its effect slightly weakens and then stabilizes. The timing of this peak seems to be related to the stage of smoking behavior, since recent experimenters peaked slightly earlier than former experimenters, who are generally farther behind in smoking development. However, since the decline in the effect of nicotine dependence did not reach significance, future research is needed to more thoroughly investigate the presence or absence of this possible peak in the effect of nicotine dependence.
The current findings also suggest that an adolescent’s cumulative exposure to cigarettes plays a role in how strongly nicotine dependence and tobacco exposure explain smoking regularity. Specifically, the effects of nicotine dependence and tobacco exposure had varied strengths across the three smoking groups at baseline, but reached similar levels as more of the former and current experimenters became established smokers. Moreover, the effect sizes at baseline for the current smokers were similar to the effect sizes at the 48-month assessment wave for the former and recent experimenters, suggesting that the observed group differences mainly reflect different stages in smoking development and cumulative exposure to cigarettes. Together, these results suggest that the effects of nicotine dependence and tobacco exposure vary by stage of smoking development, and eventually converge to a stable level across smoking groups as regular smoking patterns are established.
Little remains known about the reciprocal relationship between nicotine dependence and smoking regularity. This and other recent studies have begun to address the reciprocal nature of these relationships. Doubeni et al. (2010) provided evidence for this positive feedback, in which nicotine dependence predicted future smoking frequency and vice versa over multiple assessment waves. The current study complements these results by evaluating cross-sectional associations over several years, providing a series of proximal measures of instantaneous associations between nicotine dependence, tobacco exposure, and smoking regularity. This approach allows an examination of how these reciprocal relationships become stronger or weaker over the development of smoking behavior, and thus augments the findings of Doubeni et al. (2010) showing the existence of this bidirectional relationship throughout the early stages of smoking. Future work combining the current methods with data collected within other time frames, such as ecological momentary assessment (EMA), may be promising. For example, analyzing individual episodes of smoking and craving/ withdrawal using time-varying effects models could address how daily experiences of dependence symptoms are associated with concurrent smoking behavior, and how this relationship evolves over time for smokers with different cumulative exposures to nicotine.
As a whole, the results presented here are consistent with theoretical accounts of addiction involving reinforcement. While positive reinforcement is hypothesized to play a role during initiation in that rewarding, hedonic effects of nicotine establish repeated self-administration (Koob, 1996; Wise, 1988), negative reinforcement is theorized to trigger and maintain nicotine dependence once smoking has been initiated (Koob, 1996; Watkins et al., 2000). This negative reinforcement process consists of some period of abstinence from nicotine, a resulting negative affective state, and, finally, smoking in order to relieve withdrawal symptoms (Watkins et al., 2000; Koob, 1996; Dani and Heinemann, 1996). This process may start with an increase in one’s hedonic set point, necessitating increased nicotine consumption to achieve the same level of positive reward (Watkins et al., 2000; Ahmed and Koob, 1998). Biologically, chronic nicotine exposure leads to desensitization, inactivation, and a compensatory upregulation of nicotinic acetylcholine receptors (Watkins et al., 2000; Dani and Heinemann, 1996). This tolerance to nicotine persists well into abstinence (Watkins et al., 2000), when withdrawal symptoms result from a now-excessive number of nicotinic receptors returning to active states and triggering non-reward pathways that result in negative affective states and increased stress response (Dani and Heinemann, 1996; Watkins et al., 2000; Koob, 1996). The findings presented here fit into this theoretical framework in that nicotine dependence is relatively uncommon in novice adolescent smokers, and thus its impact on their smoking regularity is small. Instead, early increases in smoking regularity are more likely to result from the positive reinforcement of nicotine’s hedonic qualities and the beginning of tolerance. Over time, and presumably after repeated tobacco exposure has altered the brain’s non-reward pathways, nicotine dependence becomes a much stronger contributor to increased smoking regularity. The current results are consistent with this theory that negative reinforcement, via increased nicotine dependence, takes over as the primary force in developing nicotine addiction.
The current findings should be considered within the context of study limitations. First, the need to remove past-month nonsmokers from analysis significantly reduced the sample size, limiting the ability to examine additional important correlates of smoking (e.g., detailed demographic information, secondhand smoke exposure, and detailed use of other tobacco products and other substances) while retaining sufficient power to characterize the basic time-varying effects presented here. Future research utilizing larger sample sizes is needed to investigate more complex models of smoking regularity. Additionally, these results are directly tied to the measurement of nicotine dependence according to the NDSS, and as such mainly reflect drive and tolerance dimensions. The optimal time frame for assessing these time-varying relationships is unclear, and these results may be augmented by future analyses of data with coarser- or finer-grained assessment intervals. Further, substantial individual variability exists within prominent group patterns, especially in terms of experienced nicotine dependence symptoms at a given level of smoking. Finally, the relationships between nicotine dependence and smoking behavior presented here are associational, and cannot inform about causation.
Despite these limitations, the current study has a number of strengths. The investigation of time-varying effects as a series of cross-sectional associations captures both proximal measures of concurrent associations with smoking regularity and their variability over 4 years. The SECASP sample allows a description of these time-varying effects for smokers at diverse stages in the development of regular smoking. By the 48-month follow-up, the majority of participants are young adults, allowing assessments of smoking behavior that are robust across a variety of factors that may influence regularity, such as ease of access to cigarettes and legality of purchasing cigarettes. This study is among the first to describe the time-varying nature of the bidirectional relationship between smoking behavior and nicotine dependence.
As the largest preventable cause of death in the US, cigarette smoking represents a significant public health burden (Pleis et al., 2009). The vast majority of smokers initiate smoking during adolescence (Escobedo et al., 1990; Rigotti et al., 2000; Schoenborn and Adams, 2010), and can become addicted for many decades (Russell, 1990). Thus, studying smoking behavior and nicotine dependence during this crucial time period is critical for developing intervention and treatment programs. The findings that tobacco exposure has a larger effect than does nicotine dependence on smoking regularity at early stages of smoking, and vice versa at later stages, have important implications for early intervention programs. For example, strategies to reduce the number of cigarettes smoked are likely to be most successful at the earliest stages of smoking development when nicotine dependence is less relevant. In contrast, adolescents who are beginning to show some regularity may be better served by programs that reduce or treat nicotine dependence symptoms. The current study suggests that additional consideration of specific stages in smoking development is critical to the success of intervention strategies.
Acknowledgments
Role of funding source
This research was supported by Project Grant P01 CA098262 from the National Cancer Institute, R01 DA022313 A2, R01 DA022313 S1, R21 DA029834-01, and R21 DA024260, P50 DA010075 from the National Institute on Drug Abuse, and Center Grant P50 DA010075 awarded to Penn State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NCI, or NIDA.
Footnotes
Contributors
Arielle S. Selya performed analyses and drafted the manuscript. Lisa C. Dierker and Jennifer S. Rose designed the study and revised the manuscript. Jennifer S. Rose, Donald Hedeker, Xianming Tan, and Runze Li supervised and assisted with data analyses, and revised the manuscript. Robin J. Mermelstein contributed to the study design and manuscript revisions. All authors contributed to and have approved the final manuscript.
Conflict of interest
No conflict declared.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Merikangas KR. Familial influences on adolescent smoking. Addiction. 2003;98(Suppl. 1):1–20. doi: 10.1046/j.1360-0443.98.s1.2.x. [DOI] [PubMed] [Google Scholar]
- Bajda J. A survey of the smoking habits of students of Newton High School—a cooperative project. Am. J. Public Health Nations Health. 1964;54:441–446. doi: 10.2105/ajph.54.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: influence of baseline smoking behavior. Nicotine Tob. Res. 2003;5:111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- Brook DW, Brook JS, Zhang C, Whiteman M, Cohen P, Finch SJ. Developmental trajectories of cigarette smoking from adolescence to the early thirties: personality and behavioral risk factors. Nicotine Tob. Res. 2008;10:1283–1291. doi: 10.1080/14622200802238993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Baker NL, Gray KM, Upadhyaya HP. Assessment of nicotine dependence among adolescent and young adult smokers: a comparison of measures. Addict. Behav. 2010;35:977–982. doi: 10.1016/j.addbeh.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Wood DS, Martin CS, Cornelius JR, Lynch KG, Shiffman S. Multidimensional assessment of nicotine dependence in adolescents. Drug Alcohol Depend. 2005;77:235–242. doi: 10.1016/j.drugalcdep.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Cohen JE. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- Connolly GN, Winn DM, Hecht SS, Henningfield JE, Walker B, Jr, Hoffmann D. The reemergence of smokeless tobacco. N. Engl. J. Med. 1986;314:1020–1027. doi: 10.1056/NEJM198604173141605. [DOI] [PubMed] [Google Scholar]
- Crittenden KS, Manfredi C, Cho YI, Dolecek TA. Smoking cessation processes in low-SES women: the impact of time-varying pregnancy status, health care messages, stress, and health concerns. Addict. Behav. 2007;32:1347–1366. doi: 10.1016/j.addbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Dierker L, Mermelstein R. Early emerging nicotine-dependence symptoms: a signal of propensity for chronic smoking behavior in adolescents. J. Pediatr. 2010;156:818–822. doi: 10.1016/j.jpeds.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, O’Loughlin J, Pbert L, Ockene JK, McNeill AD, Hazelton J, Friedman K, Dussault G, Wood C, Wellman RJ. Symptoms of tobacco dependence after brief intermittent use: the Development and Assessment of Nicotine Dependence in Youth-2 study. Arch. Pediatr. Adolesc. Med. 2007;161:704–710. doi: 10.1001/archpedi.161.7.704. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch. Pediatr. Adolesc. Med. 2002a;156:397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob. Control. 2002b;11:228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubeni CA, Reed G, Difranza JR. Early course of nicotine dependence in adolescent smokers. Pediatrics. 2010;125:1127–1133. doi: 10.1542/peds.2009-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo LG, Anda RF, Smith PF, Remington PL, Mast EE. Sociodemographic characteristics of cigarette smoking initiation in the United States. Implications for smoking prevention policy. JAMA. 1990;264:1550–1555. [PubMed] [Google Scholar]
- Gilpin EA, Pierce JP. Concurrent use of tobacco products by California adolescents. Prev. Med. 2003;36:575–584. doi: 10.1016/s0091-7435(02)00064-6. [DOI] [PubMed] [Google Scholar]
- Haddock CK, Lando H, Klesges RC, Talcott GW, Renaud EA. A study of the psychometric and predictive properties of the Fagerstrom Test for Nicotine Dependence in a population of young smokers. Nicotine Tob. Res. 1999;1:59–66. doi: 10.1080/14622299050011161. [DOI] [PubMed] [Google Scholar]
- Hu MC, Muthen B, Schaffran C, Griesler PC, Kandel DB. Developmental trajectories of criteria of nicotine dependence in adolescence. Drug Alcohol Depend. 2008;98:94–104. doi: 10.1016/j.drugalcdep.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip DT, Cohen JE, Bondy SJ, Chaiton MO, Selby P, Schwartz R, McDonald P, Garcia J, Ferrence R. Do components of current “hardcore smoker” definitions predict quitting behaviour? Addiction. 2011;107:434–440. doi: 10.1111/j.1360-0443.2011.03674.x. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob. Res. 2010;12:781–785. doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Hu MC, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug Alcohol Depend. 2007;91:26–39. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardia SL, Pomerleau CS, Rozek LS, Marks JL. Association of parental smoking history with nicotine dependence, smoking rate, and psychological cofactors in adult smokers. Addict. Behav. 2003;28:1447–1452. doi: 10.1016/s0306-4603(02)00245-9. [DOI] [PubMed] [Google Scholar]
- Karp I, O’Loughlin J, Paradis G, Hanley J, Difranza J. Smoking trajectories of adolescent novice smokers in a longitudinal study of tobacco use. Ann. Epidemiol. 2005;15:445–452. doi: 10.1016/j.annepidem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- Meneses-Gaya IC, Zuardi AW, Loureiro SR, Crippa JA. Psychometric properties of the Fagerstrom Test for Nicotine Dependence. J. Bras. Pneumol. 2009;35:73–82. doi: 10.1590/s1806-37132009000100011. [DOI] [PubMed] [Google Scholar]
- O’Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, Clarke PB, Hanley J, Paradis G. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am. J. Prev. Med. 2003;25:219–225. doi: 10.1016/s0749-3797(03)00198-3. [DOI] [PubMed] [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, Bolt DM, Kim SY, Kaye JT, Hefner KR, Baker TB. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berlin) 2011;216:569–578. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleis JR, Ward BW, Lucas JW. Summary Health Statistics for U.S Adults: National Health Interview Survey, 2009. Vital Health Stat. 10. 2009;249:1–217. [PubMed] [Google Scholar]
- Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu S. Validation of the modified Fagerstrom tolerance questionnaire with salivary cotinine among adolescents. Addict. Behav. 2000;25:429–433. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Lee JE, Wechsler H. US college students’ use of tobacco products: results of a national survey. JAMA. 2000;284:699–705. doi: 10.1001/jama.284.6.699. [DOI] [PubMed] [Google Scholar]
- Russell MA. The nicotine addiction trap: a 40-year sentence for four cigarettes. Br. J. Addict. 1990;85:293–300. doi: 10.1111/j.1360-0443.1990.tb03085.x. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA, Adams PE. Health behaviors of adults: United States, 2005–2007. Vital Health Stat. 10. 2010;245:1–132. [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A practical guide to calculating Cohen’s f(2), a measure of local effect size, from PROC MIXED. Front. Psychol. 2012;3:1–6. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Sayette MA. Validation of the nicotine dependence syndrome scale (NDSS): a criterion-group design contrasting chippers and regular smokers. Drug Alcohol Depend. 2005;79:45–52. doi: 10.1016/j.drugalcdep.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine Tob. Res. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Sledjeski EM, Dierker LC, Costello D, Shiffman S, Donny E, Flay BR. Predictive validity of four nicotine dependence measures in a college sample. Drug Alcohol Depend. 2007;87:10–19. doi: 10.1016/j.drugalcdep.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Sterling KL, Mermelstein R, Turner L, Diviak K, Flay B, Shiffman S. Examining the psychometric properties and predictive validity of a youth-specific version of the nicotine dependence syndrome scale (NDSS) among teens with varying levels of smoking. Addict. Behav. 2009;34:616–619. doi: 10.1016/j.addbeh.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zundert RM, Ferguson SG, Shiffman S, Engels RC. Dynamic effects of self-efficacy on smoking lapses and relapse among adolescents. Health Psychol. 2010;29:246–254. doi: 10.1037/a0018812. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob. Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- White HR, Pandina RJ, Chen PH. Developmental trajectories of cigarette use from early adolescence into young adulthood. Drug Alcohol Depend. 2002;65:167–178. doi: 10.1016/s0376-8716(01)00159-4. [DOI] [PubMed] [Google Scholar]
- Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, Hawk L, Lerman C. Do small lapses predict relapse to smoking behavior under bupropion treatment? Nicotine Tob. Res. 2004;6:357–366. doi: 10.1080/1462220042000202463. [DOI] [PubMed] [Google Scholar]
- Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J. Abnorm. Psychol. 1988;97:118–132. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- Zagona SV, Zurcher LA., Jr An analysis of some psycho-social variables associated with smoking behavior in a college sample. Psychol. Rep. 1965;17:967–978. doi: 10.2466/pr0.1965.17.3.967. [DOI] [PubMed] [Google Scholar]