Abstract
Rationale
Exceedingly little experimental research exists on the popular recreational drug mephedrone (4-methylmethcathinone) despite clinical reports concerning its behavioral and cardiovascular toxicity.
Objective
To characterize mephedrone preclinically by examining its capacity to: 1) serve as a discriminative stimulus, 2) disrupt the acquisition of response sequences, and 3) disrupt mean arterial pressure (MAP) and heart rate (HR).
Methods and Results
In one group of subjects that reliably discriminated 3.2 mg/kg of mephedrone from saline (n=9), substitution tests indicated that stimulants (cocaine, MDMA and methamphetamine) more closely approximated the mephedrone discriminative stimulus than non-stimulants (fenfluramine, morphine, and phencyclidine), although none fully substituted. In a second group (n=6), mephedrone (0.56–10 mg/kg, i.p.) dose-dependently decreased response rate and increased errors in both components of a procedure in which subjects either acquired a new response sequence each session (repeated acquisition) or completed the same response sequence each session (performance). Finally, in a third group (n=12), radio telemetry probes were used to measure the changes in MAP and HR elicited by mephedrone and then compared them to a known stimulant, methamphetamine. In these studies, mephedrone (0.01–9 mg/kg, i.v.) elicited increases in MAP and HR that were very similar to those elicited by methamphetamine (0.01–9 mg/kg, i.v.). The tachycardia and pressor responses to mephedrone (3 mg/kg) were blocked by the β-blocker atenolol (1 mg/kg, i.v.) and the α1, α2-blocker phentolamine (3 mg/kg, i.v.), respectively.
Conclusions
Mephedrone produces behavioral and cardiovascular responses that are similar to other stimulants; however, differences from the classical stimulants were also apparent.
Keywords: mephedrone, bath salts, drug discrimination, learning, blood pressure, heart rate, stimulants
Introduction
A new group of drugs illicitly marketed as “bath salts” or “plant food” has reached the United States and is creating a public-health crisis. Among these new drugs is mephedrone (1-(4-methylphenyl)-2-methylaminopropan-1-one), a semi-synthetic beta-keto derivative of phenethylamine that is structurally related to cathinone, a naturally-occurring psychomotor stimulant found in the khat plant in eastern Africa (Schifano et al. 2010). Until recently when mephedrone was classified as a Schedule I drug by the United States Drug Enforcement Agency, it was commonly known as a “legal high.” Legal highs refer to natural or synthetic products that have psychological and physiological effects similar to those of the already illicit hallucinogens, entactogens, and stimulants. Mephedrone is most often snorted, but can also be taken orally or injected (Motbey et al. 2012). Users report that the subjective effects of mephedrone resemble a cross between 3,4-methylenedioxymethamphetamine (MDMA) and cocaine (cf. Hadlock et al. 2011); however, there are also reports that this drug can produce auditory and visual hallucinations similar to more traditional serotonergic hallucinogens (Winstock et al. 2011). The implication by users that mephedrone may have unique subjective effects is also supported by the limited experimental data available. Based on its chemical structure alone, mephedrone should have effects similar to stimulants, yet repeated mephedrone administrations in rats produced persistent serotonergic deficits, not dopaminergic deficits (Hadlock et al. 2011; Angoa-Perez et al. 2012). These deficits would seem to make mephedrone more like MDMA than the classical stimulants methamphetamine or methcathinone (Hadlock et al. 2011; Baumann et al. 2012).
Coincident with the increase in the recreational use of mephedrone are increased reports of serious, sometimes fatal toxicity (Meng et al. 2012; Nicholson et al. 2010; Regan et al. 2010; Wood et al. 2010). From September to December of 2010 alone, the Louisiana Poison Control Center received 165 calls (85% from emergency rooms or physicians) regarding cases of extreme paranoia, hallucinations, delusions, agitation, suicidal ideation, suicide, hypertension, cardiac arrhythmia and chest pain attributed to this drug (Adkins 2011). Whether these toxicities were produced by mephedrone, impurities or adulterants, or interactions with other illicit agents is impossible to determine given the paucity of experimental data. The scant information that is available has been gleaned from emergency room reports and the personal accounts of users posted on internet websites and blogs (Regan et al. 2010; Schmidt et al. 2010; Wood et al. 2010).
Given the limited data available regarding mephedrone, the aims of this study were to characterize its behavioral and cardiovascular effects in rats by 1) establishing mephedrone as a discriminative stimulus to ascertain whether known stimulants and hallucinogens would substitute for mephedrone, 2) determining whether mephedrone is disruptive to the acquisition and performance of response sequences, and 3) investigating the cardiovascular responses elicited by mephedrone and comparing them to those of methamphetamine. The primary reason for establishing mephedrone as a discriminative stimulus was that this procedure provides the experimenter with a pharmacologically-specific behavioral model for studying the components of a drug’s actions and these actions generally reflect events at the neuronal level (Holtzman 1990; Balster and Prescott 1992). In addition, substitution tests under this procedure provide a ready means of comparing the subjective effects of mephedrone to those of other drugs of abuse. For example, in the present study, the discriminative effects of mephedrone were compared to those of methamphetamine, MDMA, cocaine, morphine, and phencyclidine (PCP). Given the likely role of dopamine in the behavioral effects of mephedrone (Kehr et al. 2011; Baumann et al. 2012; Lisek et al. 2012), the capacity of haloperidol to block the discriminative effects of mephedrone was also examined.
To assess the effects of mephedrone on complex behavioral processes such as learning, the effects of mephedrone in rats responding under a multiple schedule of repeated acquisition and performance behavior were determined. Repeated acquisition is a well-established technique for assessing the effects of drugs on learning, and when combined with a performance component under a multiple schedule, can determine the capacity of a drug to disrupt learning apart from it capacity to produce more general psychomotor effects (Moerschbaecher et al. 1979; Winsauer et al. 2002; Winsauer et al. 2003; Quinton et al. 2005). Finally, assessing the cardiovascular effects of mephedrone seemed essential given the reports of mephedrone-related cardiovascular events (Nicholson et al. 2010; Regan et al. 2010; Wood et al. 2010), and the well known association between stimulant use and cardiovascular and cardiac toxicity (Badon et al. 2002; Shenouda et al. 2010; Lord et al. 2010). An important aspect of the cardiovascular experiments was also determining the extent to which alpha and beta adrenergic receptors mediated the cardiovascular responses of mephedrone.
Methods
Animals
The behavioral studies were conducted using male Long-Evans hooded rats (245–265 g), whereas the cardiovascular studies were conducted using male Sprague-Dawley rats (289–353 g); all of the rats were obtained from the same vendor (Harlan Sprague Dawley, Indianapolis, IN). During these experiments, all of the rats were housed individually in temperature- and humidity-controlled rooms with a 12-h light/dark cycle. Subjects in the behavioral studies were maintained at approximately 90% of their free-feeding weight, while subjects in the cardiovascular studies had unlimited access to food and water. All procedures were conducted during the light cycle and were in accordance with National Institutes of Health guidelines for the care and use of experimental animals. The procedures were also approved by the Institutional Animal Care and Use Committee at Louisiana State University Health Sciences Center.
Mephedrone
Mephedrone was synthesized from 4-methylpropiophenone, prior to mephedrone being designated as a Schedule 1 drug. The purity and identity of the product was verified by mass spectrometry.
Apparatus
Nine (6 from MED Associates Inc., St. Albans, VT, and 3 BRS/Foringer, Beltsville, MD) operant-conditioning chambers enclosed within sound-attenuating cubicles were used to conduct the discrimination experiments. These chambers all had two response levers with stimulus lights located above each lever. Six operant conditioning chambers (Coulbourn Instruments, Whitehall, PA) were used for repeated-acquisition experiments, and these chambers had three horizontally-aligned response keys that could be transilluminated with one of three colored stimuli. Both sets of chambers also had a houselight, pellet trough, and pellet dispenser; white noise was present to mask extraneous noise and a fan provided ventilation. Data were collected using MED-PC for Windows, Version IV (MED Associates, Inc., St. Albans, VT).
Mephedrone Discrimination Procedure
The initial training sessions, which began shortly after postnatal day 75 for the 9 rats serving as subjects, was similar to that described previously (Winsauer et al. 2012). When responding on both levers stabilized under a FR-20 schedule, the stimuli over the levers were eliminated and injections of either saline or mephedrone were initiated. Saline or mephedrone was administered intraperitoneally (i.p.) before the start of the session, which began with a 15-min timeout. The 15-min timeout period was followed by 30-min period during which the houselight was illuminated and responding under the FR-20 schedule resulted in food presentation. Injections occurred in a fixed sequence that was repeated throughout the experiment (mephedrone [M], saline [S], S, M, S, M, M, S, M, S) and each injection served as the discriminative stimulus for the appropriate lever (i.e., saline served as the discriminative stimulus for responding on one lever, whereas mephedrone served as the discriminative stimulus for responding on the other lever). Lever designations were also counterbalanced across subjects. Responding on the incorrect lever had only one programmed consequence, which was resetting the ratio requirement on the correct lever.
In all subjects, the final training dose of mephedrone was 3.2 mg/kg; however, this dose was determined after initially starting with a mephedrone dose of 0.56 mg/kg and gradually incrementing this dose until all of the subjects met two training criteria on 9 of 10 consecutive days: 1) greater than 90% responding on the appropriate lever, and 2) less than 20 responses on the incorrect lever prior to the first reinforcement. Initially, 4 rats met the training criteria while discriminating 1.8 mg/kg of mephedrone. The average number of training sessions for these subjects was 48 (range: 41–53). However, the training dose for these subjects was eventually increased to 3.2 mg/kg, because either the training dose was not accurately discriminated under test conditions, or simply to ensure comparability in training dose among the entire group. For the subjects that did not meet the training criteria until 3.2 mg/kg of mephedrone was administered as the training dose (n=5), the average number of training sessions was 74 (range: 62–90).
After meeting these training criteria, test sessions with other doses of mephedrone or other drugs commenced. Test sessions differed from training sessions in that 20 responses on either lever resulted in presentation of a food pellet, and a cumulative-dosing procedure was utilized. The advantage of a cumulative-dosing procedure is that a complete dose-effect curve can be determined during a single session. In this study, the procedure was used during test sessions to 1) establish a dose-effect curve for mephedrone, 2) assess the capacity of other drugs to substitute for mephedrone, and 3) determine the effects of various pretreatments on the dose-effect curve for mephedrone. To accommodate the cumulative-dosing procedure, test sessions were comprised of four 10-minute components, each separated by a 15 min timeout period during which successive injections increased the cumulative dose by [1/4 or 1/2] log-unit. For example, 0.32 mg/kg of mephedrone was injected 15 min before the first test component, 0.68 mg/kg before the second component, 2.2 mg/kg before the third component, and 6.8 mg/kg before the fourth component, thereby producing a cumulative dose-effect curve of 0.32, 1, 3.2, and 10 mg/kg for mephedrone. The discriminative stimulus effects of the vehicles for mephedrone and the other drugs were also assessed routinely under test conditions. After test sessions with doses other than the training dose or other drugs, subjects were always required to meet the training criteria for 3 consecutive days.
Repeated-Acquisition Procedure
Six rats were also trained to respond under a multiple schedule of repeated acquisition and performance of response sequences as described previously (Gerak et al. 2004; Winsauer et al. 2011). Under terminal baseline conditions, sessions began with an acquisition component and alternated with a performance component after 40 reinforcers or 20 min, whichever occurred first. Sessions ended after 200 reinforcers or 80 min, whichever occurred first. Intraperitoneal mephedrone administration began after the following criteria were satisfied for 10 consecutive baseline sessions: response rates were within 20 responses/min of the mean and the percentage of errors was within 10% of the mean. In general, baseline sessions (no injections) occurred on Mondays and Wednesdays, drug sessions on Tuesdays and Fridays, and vehicle sessions on Thursdays.
Cardiovascular Procedure
Mean arterial pressure (MAP) and heart rate (HR) were recorded in 12 conscious, unrestrained rats using a radio telemetry system (Dataquest A.R.T. 2.3; Data Sciences International, St. Paul, MN). Under ketamine and xylazine (90/10 mg/kg) anesthesia, the pressure cannula of a radio telemetry probe was inserted into the left femoral artery. The body of the probe was placed in the lower abdominal cavity and secured to the abdominal musculature. A polyethylene cannula fused to a silastic catheter was inserted into the right jugular vein, and the free end tunneled subcutaneously to the nape of the neck and exteriorized. Catheters were filled with heparinized saline (250 U/ml).
Approximately 1 week after surgery, the home cage of each rat was placed over a telemetry receiver (250 Hz) that was connected to a personal computer for the recording of MAP and HR signals. For drug administration, the subjects were divided into two groups: one that received mephedrone (n=5 for 0.01–0.1 mg/kg and n=7 for 1–9 mg/kg), and one that received methamphetamine (n=7 for 0.01–9 mg/kg). Intravenous doses of drug or saline (0.9%) were then administered in a mixed order within each group, except for the 9-mg/kg dose, which was administered last. Each dose was determined once and a minimum of 24 hours separated each drug administration. Subjects were weighed daily and baseline MAP and HR were recorded for a minimum of 30 min prior to the administration of each dose, and then for 3 hr following injection. Mephedrone was administered i.v. to mimic nasal insufflations and i.v. drug use by humans. For studies using adrenergic receptor antagonists, baseline MAP and HR were recorded from the same two groups of rats for a minimum of 20 min prior and then one group received atenolol (1 mg/kg, i.v.) and the other received phentolamine (3 mg/kg, i.v.). Mephedrone (3 mg/kg) was then administered to both groups 10 min after each antagonist.
Data analyses
The dependent measures for the discrimination procedure were the percentage of responses on the lever for which mephedrone was serving as a discriminative stimulus (i.e., “mephedrone-lever” responding) and the overall response rate in responses per second. Group data for each variable were expressed as a grand mean and standard error of the mean (S.E.M.), which was tabulated by averaging the mean data for each subject. Typically, full substitution in drug discrimination studies is considered to be 80% drug-lever responding or more, whereas a 20% increase or decrease from control is considered to be an effect on response rate. The percentage of mephedrone-lever responses was not included in the analyses when the response rate was less than 0.08 responses/sec. Individual differences in potency for mephedrone and the comparison drugs also led to differences in the number of subjects represented by each data point, and this prevented a repeated-measures analysis of these data. A repeated-measures analysis was conducted on the antagonism data with haloperidol using a General Linear Model of analysis whereby the sum of squares is estimated to produce a marginal sum of squares (SigmaPlot Software, SYSTAT Software, Inc. Point Richmond, CA). One advantage of this model is that it does not eliminate the assumption of a possible interaction between factors. As an additional means of comparing the dose-effect curves for mephedrone-lever responding after haloperidol administration, the doses that generated a 50% increase in mephedrone-lever responding (ED50s) were determined by linear regression using two or more data points that reflected the ascending portion of each dose-effect curve (SigmaPlot Software, SYSTAT Software, Inc. Point Richmond, CA).
The dependent measures for the repeated-acquisition data were the overall response rate (response/min, excluding timeouts), percentage of errors [(errors/total responses) × 100], and number of errors per bin of 60 responses. When rates of responding fell below 5 responses per min, the percentage of errors was not analyzed because of the small number of responses involved. The data for each dependent measure were also analyzed using a two-way repeated-measures ANOVA with the component and dose serving as factors.
Cardiovascular data were obtained using Data Sciences Dataquest acquisition software. This software produced a data stream that was averaged every 2 seconds and displayed as a continuous histogram of BP or HR versus time. Averaging the data every 2 seconds (about 12 heart beats) reduced movement artifacts. The magnitude of the peak changes in MAP and HR elicited by the drugs were calculated off-line as the difference between baseline and peak drug responses using the Dataquest analysis program. Response durations (the onset of the response until its return to baseline) were calculated from the experimental traces. The MAP and HR responses elicited by mephedrone and methamphetamine were compared to baseline (saline) using a one-way ANOVA because of the different subject numbers used for mephedrone. MAP and HR responses elicited by mephedrone with or without phentolamine and atenolol were also analyzed using a one-way ANOVA. Significance was accepted at an α level of p< 0.05 for all statistical tests.
Results
Discriminative Stimulus Effects
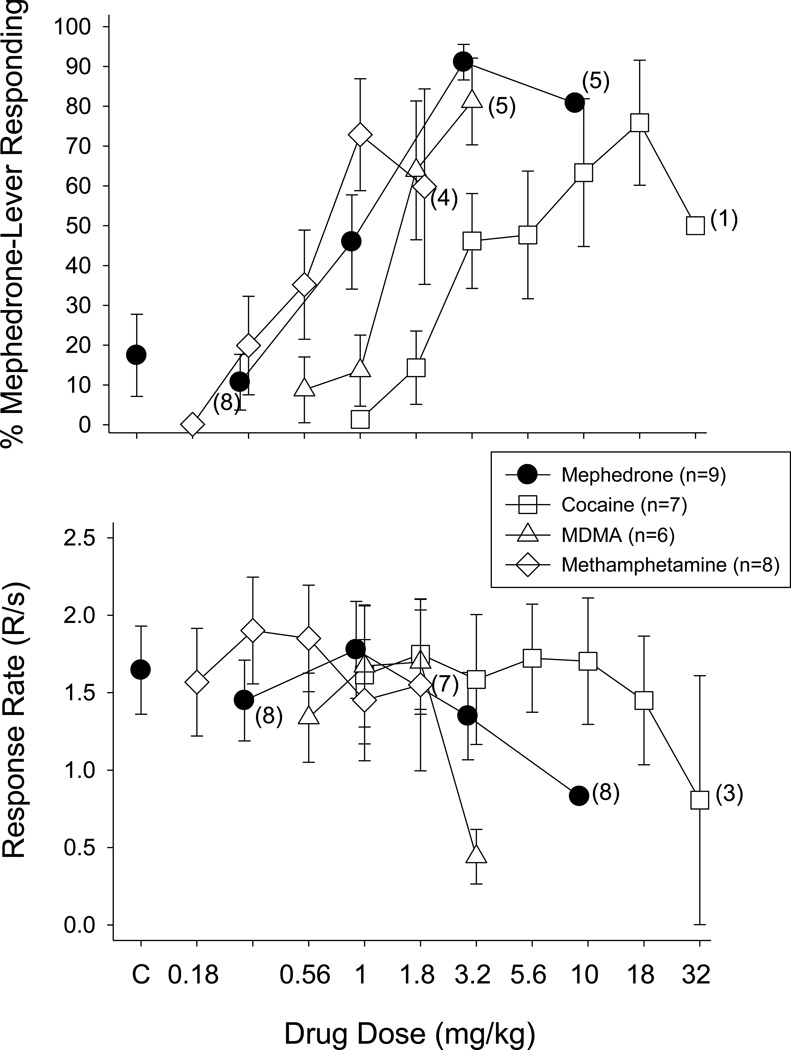
When saline (the vehicle for mephedrone) was administered during test sessions, subjects responded on the saline-appropriate lever during each of the four FR components and response rate during each component was similar to rates obtained when saline was administered under training sessions. For these reasons, the data from all four components of the test session were averaged and shown as a single data point (solid circles above C in Figure 1). With respect to response rate, the mean and SEM for all 9 rats after saline administration was 1.65 ± 0.28 responses/sec.
Fig. 1.
Effects of cumulative doses of mephedrone, cocaine, MDMA, and methamphetamine on the percentage of mephedrone-lever responding (upper panels) and overall response rate (lower panels) in 6–9 male rats. Doses of each drug were administered i.p. 15 min prior to the start of each 10-min FR component. Data points and vertical lines above “C” in the top and bottom panel represent the grand mean and SEM for 1 to 6 saline or vehicle determinations in each of the 9 rats. The data points and vertical lines in the dose-effect curves for each drug represent a grand mean and SEM, which was obtained from the means of 2–5 determinations of mephedrone, 2–3 determinations of cocaine or MDMA, and 1–5 determinations of methamphetamine. Numerical values in parentheses and adjacent to a data point indicate the number of subjects represented by that point when it differed from the total number of subjects for that drug group due to individual differences in the potency of that drug’s rate-decreasing effects. For example, 3.2 mg/kg of mephedrone (filled circles) eliminated responding in one rat, so only 8 of 9 rats received 10 mg/kg and the percentage of mephedrone-lever responding could only be calculated for 5 of the rats that received this dose.
In contrast to the administration of saline during test sessions, increasing cumulative doses of mephedrone prior to each of the four FR components dose-dependently increased mephedrone-lever responding in all subjects. As shown in Figure 1 (solid circles), doses larger than 1 mg/kg resulted in rats responding predominately on the mephedrone lever, with full substitution occurring at 3.2 mg/kg (i.e., the training dose). When rats received 3.2 mg/kg of mephedrone during training sessions, the mean response rate was 1.35 ± 0.28 responses/sec, with 10 mg/kg decreasing response rate to 61.5% of control.
The effects of substituting cocaine, MDMA, and methamphetamine are shown in Figure 1, whereas the effects of fenfluramine, morphine, and phencyclidine are shown in Figure 2. Among cocaine, MDMA, and methamphetamine, only 3.2 mg/kg of MDMA produced greater than 80% mephedrone-lever responding; however, this dose also decreased responding to 26.7% of control. Cocaine and methamphetamine produced 75.86% and 72.86% mephedrone-lever responding, respectively, but this did not constitute full substitution for mephedrone. Moreover, only 5 of 7 subjects that received cocaine, and 5 of 8 subjects that received methamphetamine, occasioned greater than 80% mephedrone-lever responding at doses that did not decrease the overall rate of responding. The decrease in response rate that occurred after 1.8 mg/kg of methamphetamine is not evident from the graph because 4 of the rats with the highest response rates responded at, or near, control levels, while the other half had response rates near zero. Therefore, the mean rate for the group was near control levels.
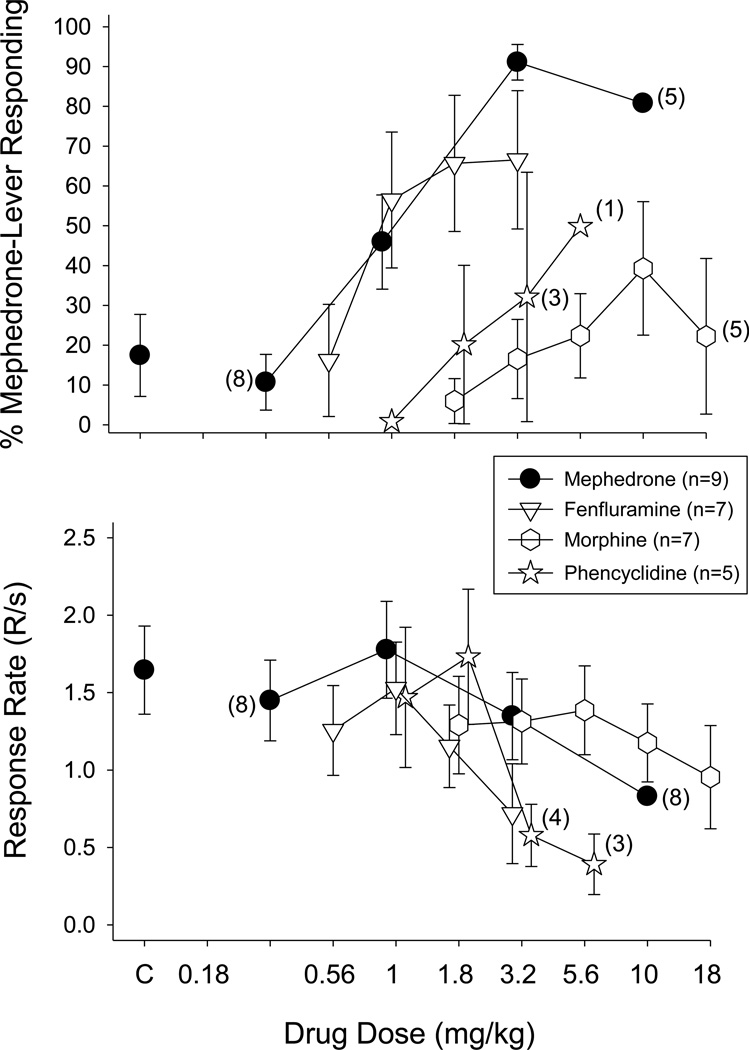
Fig. 2.
Effects of cumulative doses of mephedrone, fenfluramine, morphine, and phencyclidine on the percentage of mephedrone-lever responding (upper panels) and overall response rate (lower panels) in 5–9 male rats. Data points and vertical lines above “C” in the top and bottom panel represent the grand mean and SEM for 1 to 6 saline or vehicle determinations in each of the 9 rats. The data points and vertical lines in the dose-effect curves for each drug also represent a grand mean and SEM, which was obtained from the means of 2–5 determinations of mephedrone, 1–3 determinations of fenfluramine, 2 to 4 determinations of morphine, and 1–3 determinations of phencyclidine. Numerical values in parentheses and adjacent to a data point indicate the number of subjects represented by that point when it differed from the total number of subjects for that group due to individual differences in the potency of that drug’s rate-decreasing effects.
Of the drugs shown in Figure 2, only fenfluramine produced greater than 50% mephedrone-lever responding at a dose that did not markedly decrease response rate (e.g., 1 mg/kg). Unlike fenfluramine, both morphine and phencyclidine did not produce greater than 50% mephedrone-lever responding at any dose tested, including those that decreased response rate. For example, 5.6 mg/kg of phencyclidine decreased response rate to 23.6% of control, whereas the highest dose of morphine decreased response rate to 57.6% of control.
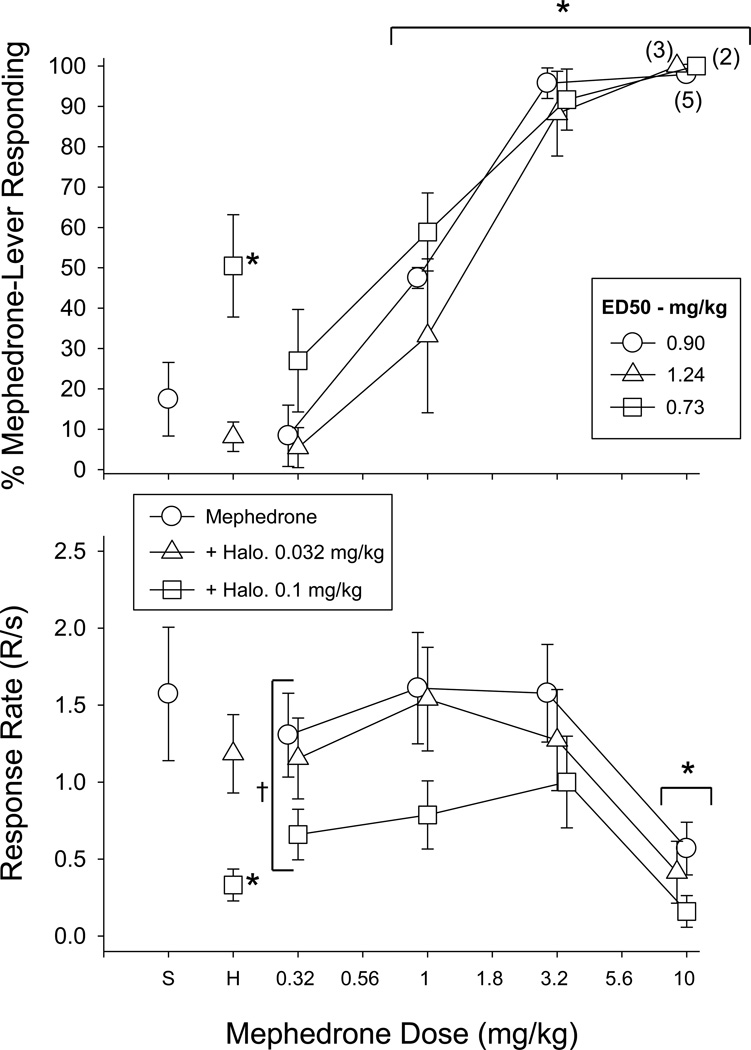
The dose-effect curves in Figure 3 show mephedrone-lever responding when cumulative doses of mephedrone followed an acute pretreatment with either 0.032 or 0.1 mg/kg of haloperidol. As shown, neither dose of haloperidol in combination with saline substituted for mephedrone (data above “H”) and neither dose of haloperidol in combination with mephedrone produced more than a two-fold shift in the mephedrone dose-effect curve (see ED50 values in Figure 3). These data were verified statistically in that a two-way ANOVA of the dose-effect curves indicated that there was only a main effect of dose (F(3,21)=56.95, p<0.001), whereas there was no main effect of haloperidol treatment (F(2,21)=1.43, p=0.27) and no interaction between treatment and dose (F(6,21)=0.86, p=0.54).
Fig. 3.
Effects of haloperidol alone and in combination with cumulative doses of mephedrone on the percentage of mephedrone-lever responding (upper panels) and overall response rate (lower panels) in 7 male rats. A single dose of haloperidol was administered 20 min prior to the first component, whereas cumulative doses of mephedrone were administered i.p. 15 min prior to the start of each 10-min FR component. Data points and vertical lines above “C” in each panel represent the grand mean and SEM for 1 to 2 saline or vehicle determinations in 6 of the 7 rats, as one rat did not receive vehicle and saline under test conditions. Data points and vertical lines above “H” in each panel represent the grand mean and SEM for 1–3 determinations of haloperidol alone in each of the 7 rats, whereas the data points and vertical lines in the dose-effect curves for each drug also represent a grand mean and SEM that was calculated from 1–4 determinations of mephedrone with each dose of haloperidol. Asterisks along with a bracket indicate doses of mephedrone that were significantly different from saline and vehicle administration (control). Asterisks without a bracket indicate doses of haloperidol that were significantly different from saline administration (p<0.05) as determined by a one-way ANOVA. A cross with a bracket indicates the only dependent measure for which there was a significant main effect of haloperidol pretreatment as determined by two-way ANOVA.
Unlike the administration of 0.032 mg/kg of haloperidol alone, which did not decrease response rates significantly compared to control rates (Figure 3, bottom panel), the 0.1-mg/kg dose markedly decreased response rates for the group when administered with saline (F(2,9)=6.96, p=0.02). Furthermore, the 0.1-mg/kg dose of haloperidol produced a significant effect of treatment when combined with mephedrone (F(2,30)=5.38, p=0.03) and shifted the response rate dose-effect curve for this combination downward significantly compared to that for mephedrone alone. A two-way ANOVA also indicated that there was a significant effect of mephedrone dose with and without haloperidol (F(3,30)=10.19, p<0.001), but no significant interaction between mephedrone dose and haloperidol pretreatment (F(6,30)=1.37, p>0.05). As shown, 10 mg/kg of mephedrone significantly decreased responding compared with the lowest dose of mephedrone (i.e., 0.32 mg/kg).
Effects on Acquisition and Performance
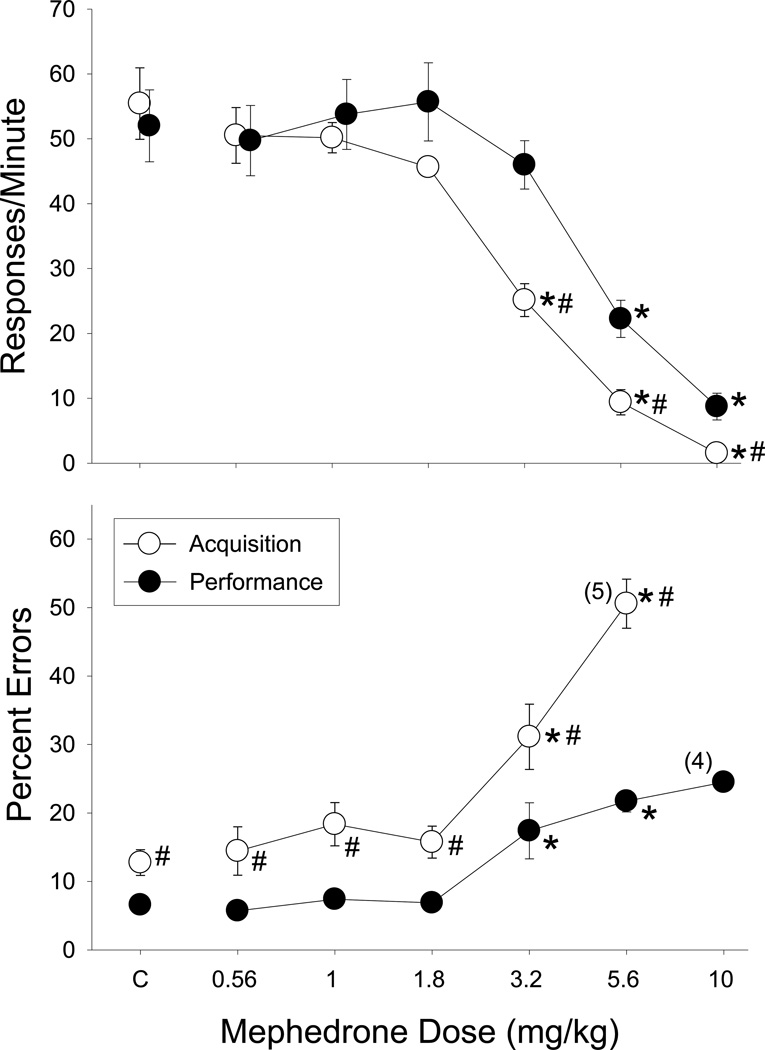
When the effects of mephedrone were determined in rats responding under the multiple schedule (Figure 4), rates of responding were comparable in the acquisition and performance components following saline administration (points above C, upper panels, Figure 4), whereas the percentage of errors was higher in the acquisition component than in the performance component (12.75 ± 1.88% in acquisition versus 6.58 ± 0.84% in performance; points above C, lower panels, Fig. 4). When compared to saline administration, mephedrone dose-dependently decreased overall rates of responding in the acquisition component (F(6,30)=44.99, p<0.001), and the performance component (F(6,30)=19.25, p<0.001). However, the response rate in acquisition was affected more potently than the response rate in performance, as indicated by the significant interaction (F(6,30)=7.27, p<0.001) and the subsequent one-way ANOVA tests. A similar component by dose interaction was also obtained after a two-way analysis of the data for percent errors (F(5,24)=11.69, p<0.001). More specifically, mephedrone significantly increased the percentage of errors in the acquisition component (F(5,24)=40.55, p<0.001) and in the performance component (F(5,24)=14.22, p<0.001). Thus, mephedrone produced dose-dependent rate-decreasing and error-increasing effects, with 3.2 mg/kg producing an equipotent disruption of errors in both the acquisition and performance components.
Fig. 4.
Effects of mephedrone on the overall response rate and percentage of errors in 6 male rats responding under a multiple schedule of repeated acquisition and performance of response sequences. The unfilled circles represent data from the acquisition components, whereas the filled circles depict represent data from the performance components. The data points and vertical lines above “C” (i.e., control) and in the dose-effect curves represent a grand mean and standard error of the mean (SEM), as all of the individual subjects received multiple determinations of both saline and each dose of mephedrone. Any points without vertical lines indicate instances in which the SEM is encompassed by the data point. Asterisks indicate effects of mephedrone that differed significantly from control injections. Pound symbols indicate significant differences between the acquisition and performance data. Numerical values in parentheses and adjacent to a data point indicate the number of subjects represented by that point when it differed from the total number of subjects in the group. The percentage of errors was not analyzed or plotted when response rate fell below 5 responses/min in either component due to the small number of responses emitted.
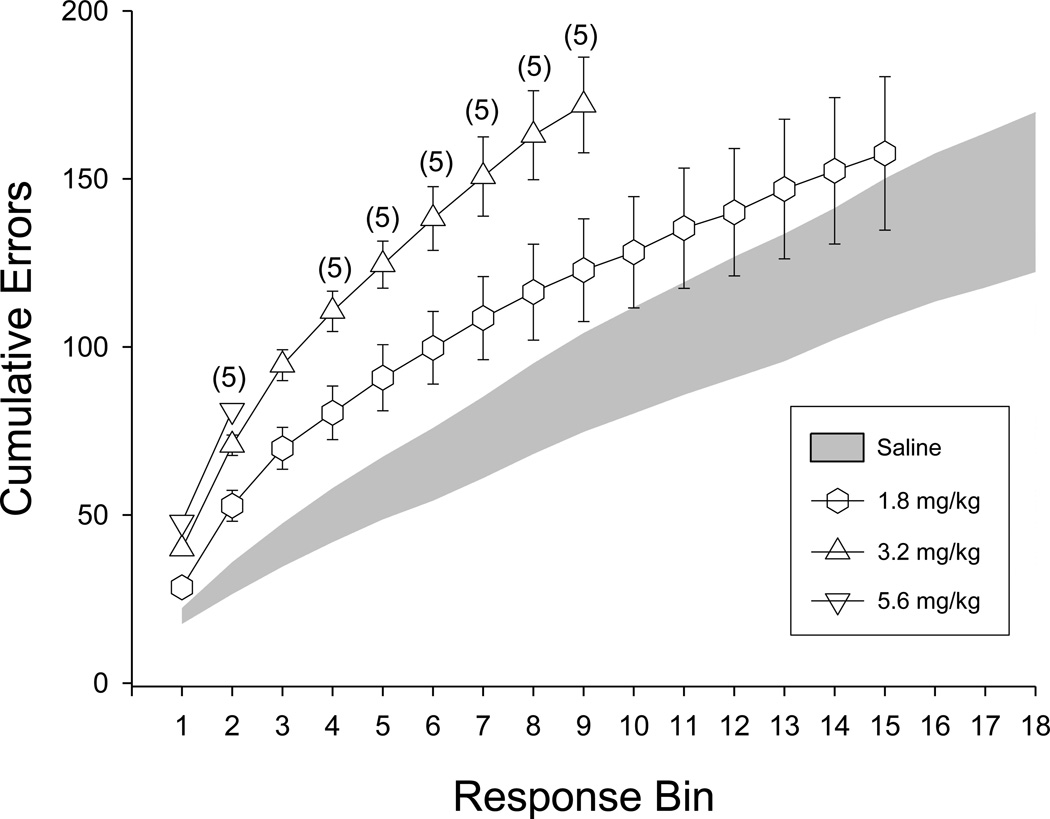
The error-increasing effects of mephedrone were also apparent in the within-session pattern of errors in acquisition (Figure 5). When vehicle was administered, the cumulative number of errors increased at the very beginning of the session and then gradually leveled off, indicating that acquisition had occurred. For example, an average of 50 ± 8.05 errors were emitted during the first four bins (i.e., 240 responses) following saline administration, whereas after the eighteenth bin (i.e., 1080 responses) the total number of errors was only 146 ± 23.80. Thus, over 34% of the total number of errors was emitted (committed) during the first 240 responses under control conditions. In contrast to control conditions, mephedrone dose-dependently disrupted acquisition, as illustrated by the linear increases in the number of errors throughout the session. For example, when 3.2 mg/kg was administered, rates of responding were decreased by more than 50% and this resulted in a decrease in the total number of responses emitted and a smaller number of response bins. Also of interest were the within-session data for 1.8 mg/kg, because this dose clearly increased errors during the early portions of the session, but did not significantly increase the overall percentage of errors for the group (Figure 4). This was likely due to the fact that the responding of all of the subjects generally recovered during the session after 3.2 mg/kg, and therefore, acquisition of the response sequence occurred by the end of the session.
Fig. 5.
Within-session pattern of errors in rats after either vehicle (shaded area) injections or injections with different doses of mephedrone (unfilled symbols). The data are plotted as the cumulative number of errors per bin of 60 consecutive responses.
Cardiovascular Effects
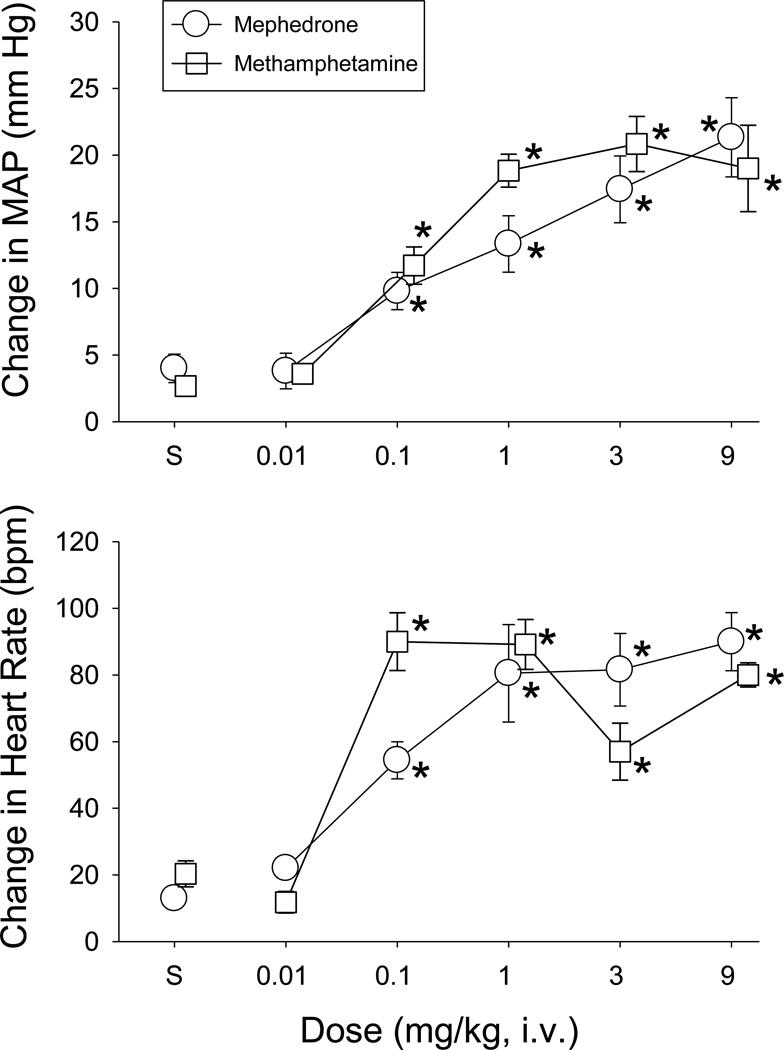
As shown in Figure 6, baseline values for MAP and HR in the mephedrone (118±4 mmHg, 379±10 bpm) and methamphetamine (116±3 mmHg, 381±12 bpm) groups were similar prior to drug administration. Following i.v. administration, MAP values were significantly increased by both mephedrone (F(2,8)=9.32, p=0.008; F(3,16)=8.33, p=0.001) and methamphetamine (F(5,24)=16.48, p<0.001) compared to control. For mephedrone, the increases in MAP peaked within 2–3 minutes and lasted from 0.09±0.02 hr (0.1 mg/kg, n=5) to 1.53±0.26 hr (9 mg/kg, n=6).
Fig. 6.
MAP and HR responses elicited in conscious rats by the intravenous injection of mephedrone (open circles, n=5–7) or methamphetamine (open squares, n=7). The data points and vertical lines above “S” (i.e., saline control) and in the dose-effect curves represent the mean of the peak changes in MAP and HR and standard error of the mean (SEM). Doses of mephedrone and methamphetamine were delivered once per day in mixed order, except for the 9-mg/kg dose, which was given on the final day. Asterisks indicate doses of mephedrone or methamphetamine that were significantly different from saline administration (p<0.05) as determined by a one-way ANOVA.
HR was also increased significantly by mephedrone (F(2,8)=33.01, p<0.001; F(3,16)=10.58, p<0.001) and methamphetamine (F(5,24)=24.83, p<0.001) after doses of 0.1 mg/kg or higher (lower panel, Figure 6). Peak levels occurred between 1 and 9 mg/kg for mephedrone, and 0.1 and 9 mg/kg for methamphetamine, although the magnitude of the increase was more variable for methamphetamine. For mephedrone, the tachycardic responses peaked within 2 to 5 minutes and lasted 0.3±0.23 hr (0.1 mg/kg, n=5) to 1.27±0.57 hr (9 mg/kg, n=6).
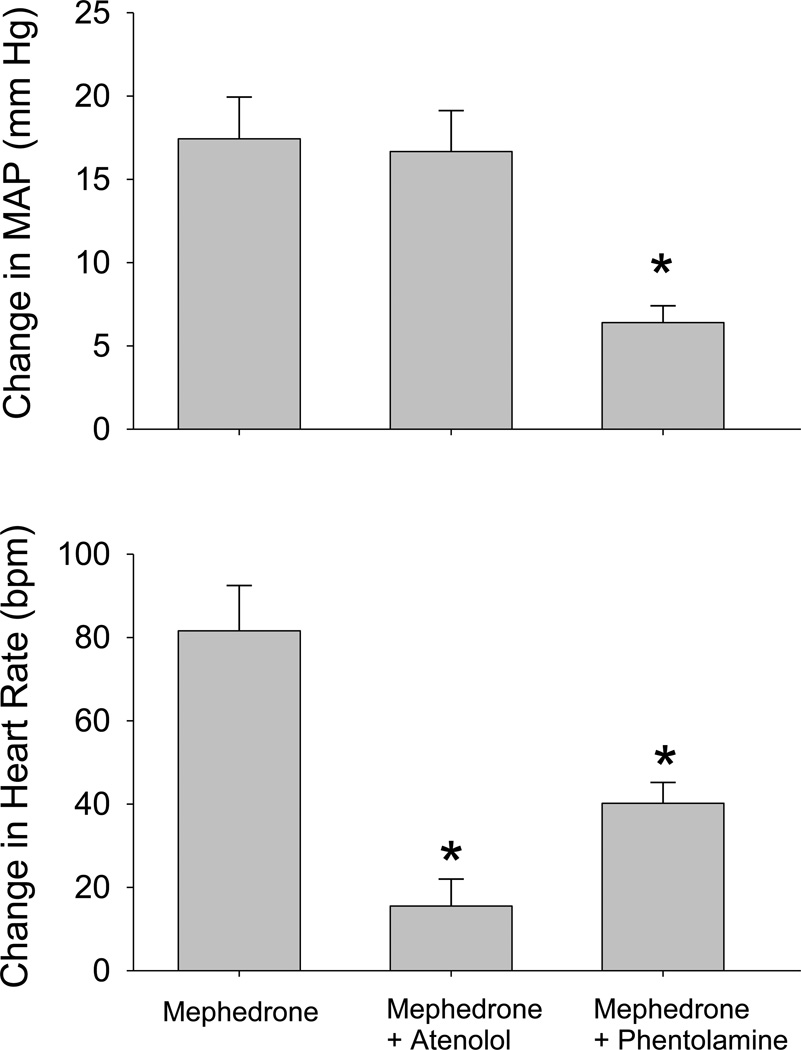
Figure 7 shows that pretreatment with the β1-adrenergic inhibitor atenolol (1 mg/kg) did not significantly alter the magnitude of the pressor response elicited by 3 mg/kg of mephedrone (p>0.05), but it did significantly attenuate the tachycardia produced by this dose (F(2,15)=13.72, p<0.001). Baseline MAP in the rats was largely unchanged as it was 124±4 mmHg before atenolol and 123±4 mmHg after atenolol; however, baseline HR changed from 384±10 to 352±8 bpm.
Fig. 7.
Comparison of the peak changes in MAP and HR elicited in conscious rats by mephedrone (3 mg/kg, i.v) alone and after blockade of β-adrenergic receptors with atenolol (1 mg/kg, i.v.) or α-adrenergic receptors with phentolamine (3 mg/kg, i.v.). Data for mephedrone alone are the same as in Figure 6. Separate groups of rats received atenolol and mephedrone (n=6), or phentolamine and mephedrone (n=5). Asterisks indicate significant difference from the peak response elicited by mephedrone alone (p<0.05) as determined by a one-way ANOVA.
In contrast to atenolol, pretreatment with phentolamine (3 mg/kg) significantly attenuated the pressor (F(2,15)=5.64, p=0.015) and tachycardic (p<0.05) responses to 3 mg/kg of mephedrone. Phentolamine decreased baseline MAP in the rats from 114±10 to 70±5 mmHg, and increased baseline HR from 363±3 to 435±24 bpm.
Discussion
In the present study, rats were successfully trained to discriminate mephedrone from saline, indicating that mephedrone produces discriminable subjective effects at doses that do not decrease response rate. In addition, these effects were very similar, but not identical, to the effects of MDMA, cocaine and methamphetamine; MDMA produced greater than 80% mephedrone-lever responding at a dose that substantially decreased response rate, whereas mephedrone-lever responding was increased to 75.86% and 72.86% after cocaine and methamphetamine administration, respectively. The fact that haloperidol was unable to antagonize the effects of mephedrone also indicated that the discriminative stimulus effects of mephedrone were not mediated predominately by dopamine type-2 (D2) receptors in rats. Across a similar range of doses (i.e., 0.32 –10 mg/kg, i.p.), mephedrone also disrupted the acquisition and performance of response sequences by decreasing response rate and increasing errors. Although mephedrone was equally potent in disrupting percent errors, response rate was disrupted more potently in the acquisition component than in the performance component. Interestingly, the dose of mephedrone that significantly increased errors in both the acquisition and performance components was also the dose that served as the training dose in the drug discrimination procedure (i.e., 3.2 mg/kg).
The lack of full substitution by methamphetamine and cocaine was intriguing given that mephedrone is a cathinone derivative and both amphetamine and cocaine substitute in rats discriminating cathinone from saline (Goudie et al. 1986; Schechter et al. 1984). The relationship between the discriminative stimulus effects of amphetamine and cathinone is also symmetrical (Glennon et al. 1984), and S(+)-methamphetamine fully substituted in rats trained to discriminate S(−)-methcathinone, a derivative very similar in structure to mephedrone (Young and Glennon 1998). Nevertheless, both cocaine and methamphetamine produced greater than 70% mephedrone-lever responding, which would indicate that these drugs have substantially overlapping, but not identical, discriminative stimulus effects. This conclusion is also supported by the fact that drugs such as morphine and phencyclidine did not substitute for mephedrone, and that some pharmacological specificity was established with the mephedrone stimulus. Our results also recapitulated findings from earlier studies indicating that fenfluramine does not substitute for drugs with CNS stimulant effects despite its structural similarity (Goudie et al. 1986; Young and Glennon 1998).
Another similarity between the discriminative stimulus effects of mephedrone and other CNS stimulants is the fact that they are not effectively antagonized by D2 receptor antagonists such as haloperidol. Though there is likely an important dopaminergic component of the discriminative stimulus effects of classical CNS stimulants (Murnane et al. 2009), blocking D2 receptors has rarely been shown to produce marked rightward shifts in the dose-effect curves for drug-lever responding (Goudie et al. 1986; Quinton et al. 2006). Moreover, in some of the studies in which haloperidol antagonized the discriminative stimulus effects of the CNS stimulants, the doses of haloperidol used were larger than those that produce rate-decreasing effects (e.g., Young and Glennon 1998). This may be important clinically, as the neuroleptic effects produced by many dopamine receptor antagonists (i.e., first generation antipsychotics) are often assumed to attenuate all of the effects that comprise drug-induced psychoses; however, data from a variety of studies suggests that they are often ineffective at attenuating the subjective effects. Likewise, haloperidol was ineffective at antagonizing the subjective effects produced by mephedrone.
Similar to many drugs of abuse administered to rats responding under non-spatial (Delatte et al. 2002; Leonard et al. 2009; Quinton et al. 2005) and spatial (Galizio et al. 2009) repeated-acquisition procedures, mephedrone produced both dose-dependent rate-decreasing and error-increasing effects. In the acquisition component, significant decreases in response rate occurred at doses that significantly increased percent errors (i.e., 3.2 mg/kg); however, within-session data clearly indicated that 1.8 mg/kg of mephedrone increased errors early in the session and thereby altered the “learning curve” compared to baseline or control sessions. In the performance component, significant decreases in response rate occurred less potently than significant increases in percent errors. Together, these data indicate that mephedrone does not selectively disrupt acquisition compared with performance, and that it disrupts behavior under both weak (acquisition) and strong (performance) stimulus control comparably. Similar effects have been reported for methylphenidate and methamphetamine in rats responding under a variation of the multiple schedule of repeated acquisition and performance used in this study. For example, in a procedure where rats nose-poked an array of stimuli on a touch-screen apparatus to receive reinforcement, Galizio et al. (2009) found that methylphenidate and methamphetamine significantly disrupted acquisition accuracy at doses that also disrupted performance. MDMA was also administered in that study, but it more potently disrupted both the percent correct (accuracy) and mean speed (rate) in the acquisition component than in the performance component. In contrast, using an almost identical procedure to that used in this study, Quinton et al. (2005) found that cocaine more potently increased percent errors in the acquisition component than in the performance component, but the increase in percent errors occurred at doses ½ to ¾ of a log unit lower than a dose that decreased response rate significantly. Thus, the effects of mephedrone on the two dependent measures were quite different from those of cocaine and more like methamphetamine and methylphenidate in rats despite the variation of the repeated acquisition procedure. These data, therefore, are also similar to the discrimination data in that mephedrone appears to have similar, but not identical, effects with several CNS stimulants that are abused.
In addition to mephedrone’s behavioral effects, i.v. administration of mephedrone also elicited dose-related increases in arterial pressure lasting upwards of 1.5 hrs. The pressor responses were accompanied by tachycardia that plateaued at a dose of 1 mg/kg, presumably due to pressor-mediated increases in baroreceptor reflex activity. The increases in MAP and HR observed after the injection of mephedrone are consistent with emergency room and internet reports that mephedrone can produce hypertension and tachycardia in humans (Regan et al. 2010; Wood et al. 2010). The effects of mephedrone on MAP and HR were remarkably similar to those elicited by i.v. administration of methamphetamine. Meng and colleagues (2012) recently reported that subcutaneous injection of mephedrone (3 or 15 mg/kg) significantly increases arterial pressure and HR in conscious rats. The responses elicited by subcutaneous injection had a much slower onset (peaking within 1–2 hours) and had a much longer duration (up to 5 hours) than the responses elicited by i.v. injection. The shorter response duration after i.v. administration may reflect rapid metabolism by the liver (Maurer 2010).
The pressor responses elicited by mephedrone were significantly attenuated by phentolamine, indicating that activation of peripheral α-adrenergic receptors plays an important role in mediating the MAP responses. Alpha-receptor blockade also blocks the increases in arterial pressure elicited by methamphetamine (Schindler et al. 1992). The tachycardic response to mephedrone was completely blocked by atenolol indicating that it was elicited by β-adrenergic receptor activation. The mechanism by which mephedrone produces its symapthomimetic cardiovascular responses is unknown. Given the structural similarities and the similar MAP and HR responses elicited by mephedrone and methamphetamine, it would be reasonable to suggest mephedrone causes the release of noreprinephrine from peripheral sympathetic nerves. In fact, mephadrone stimulates dopamine release from central synapses and blocks dopamine uptake into brain synaptasomes (Hadlock et al. 2011; Kehr et al. 2011). However, Meng and colleagues (2012) showed that mephedrone elicits pressor responses and tachycardia in reserpinized rats, indicating that the cardiovascular responses do not result from the release of norepinephrine from vesicular stores in peripheral sympathetic synapses (Meng et al. 2012). This is consistent with data from Baumann et al. (2012) indicating that mephedrone has substrate activity at norepinephrine transporters that could result in the release of cytoplasmic stores of norepinephrine.
In summary, the present study on the behavioral and cardiovascular effects of mephedrone replicates the small amount of data from controlled studies indicating that it has effects similar to other known stimulants with prominent abuse liabilities such as methamphetamine, MDMA, and to some extent cocaine. However, mephedrone also appears to have unique effects that distinguish it from each of these drugs (Hadlock et al. 2011; Motbey et al. 2012). For example, the discriminative stimulus effects of mephedrone were more similar to those of MDMA than cocaine or methamphetamine, whereas its effects on acquisition behavior and cardiovascular function were more similar to those of methamphetamine. Though more mechanistic studies will be required, these differences may relate to the capacity of each of these drugs (or their isomers) to differentially affect the serotonergic and dopaminergic systems (Murnane et al. 2009).
Acknowledgments
These studies were supported, in part, by grants awarded to Kurt Varner (K8P20GM103514 and 5P20RR018766).
References
- Adkins J. Bath Salts: Deadly New Designer Drug. Regional Organized Crime Information Center (ROCIC) Bath Salts Designer Drug Report. 2011 ROCIC Publications, 1–11. [Google Scholar]
- Angoa-Perez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn DM. Mephedrone, an abused psychoactive component of 'bath salts' and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badon LA, Hicks A, Lord K, Ogden BA, Meleg-Smith S, Varner KJ. Changes in cardiovascular responsiveness and cardiotoxicity elicited during binge administration of Ecstasy. J Pharmacol Exp Ther. 2002;302:898–907. doi: 10.1124/jpet.302.3.898. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte MS, Winsauer PJ, Moerschbaecher JM. Tolerance to the disruptive effects of Delta(9)-THC on learning in rats. Pharmacol Biochem Behav. 2002;74:129–140. doi: 10.1016/s0091-3057(02)00966-8. [DOI] [PubMed] [Google Scholar]
- Galizio M, McKinney P, Cerutti DT, Pitts RC. Effects of MDMA, methamphetamine and methylphenidate on repeated acquisition and performance in rats. Pharmacol Biochem Behav. 2009;94:305–311. doi: 10.1016/j.pbb.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Stevenson MW, Winsauer PJ, Moerschbaecher JM. Effects of pregnanolone alone and in combination with other positive GABAA modulators on complex behavior in rats. Psychopharmacology (Berl) 2004;173:195–202. doi: 10.1007/s00213-003-1717-2. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Hauck AE, McKenney JD. Structure-activity studies on amphetamine analogs using drug discrimination methodology. Pharmacol. Biochem. Behav. 1984;21:895–901. doi: 10.1016/s0091-3057(84)80071-4. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Atkinson J, West CR. Discriminative properties of the psychostimulant dlcathinone in a two lever operant task. Lack of evidence for dopaminergic mediation. Neuropharmacology. 1986;25:85–94. doi: 10.1016/0028-3908(86)90063-8. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of drugs: Relationship to potential for abuse. In: Adler MW, Cowan A, editors. Testing and Evaluation of Drugs of Abuse. New York: John Wiley & Sons; 1990. pp. 193–210. [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. Mephedrone ('bath salt') elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.04.021. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard ST, Gerak LR, Delatte MS, Moerschbaecher JM, Winsauer PJ. Relative potency and effectiveness of flunitrazepam, ethanol, and beta-CCE for disrupting the acquisition and retention of response sequences in rats. Behav Pharmacol. 2009;20:33–44. doi: 10.1097/FBP.0b013e3283242f2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord KC, Shenouda SK, McIlwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovasc Res. 2010;87:111–118. doi: 10.1093/cvr/cvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer HH. Chemistry, pharmacology, and metabolism of emerging drugs of abuse. Ther Drug Monit. 2012;32:544–549. doi: 10.1097/FTD.0b013e3181eea318. [DOI] [PubMed] [Google Scholar]
- Meng H, Cao J, Kang J, Ying X, Ji J, Reynolds W, Rampe D. Mephedrone, a new designer drug of abuse, produces acute hemodynamic effects in the rat. Toxicol Lett. 2012;208:62–68. doi: 10.1016/j.toxlet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Moerschbaecher JM, Boren JJ, Schrot J, Fontes JC. Effects of cocaine and d-amphetamine on the repeated acquisition and performance of conditional discriminations. J Exp Anal Behav. 1979;31:127–140. doi: 10.1901/jeab.1979.31-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, 'meow'): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict Biol. 2012;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Murai N, Howell LL, Fantegrossi WE. Discriminative stimulus effects of psychostimulants and hallucinogens in S(+)-3,4-methylenedioxymethamphetamine (MDMA) and R(−)-MDMA trained mice. J Pharmacol Exp Ther. 2009;331:717–723. doi: 10.1124/jpet.109.156174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: acute mephedrone 'meow' myocarditis. Heart. 2010;96:2051–2052. doi: 10.1136/hrt.2010.209338. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Gerak LR, Moerschbaecher JM, Winsauer PJ. Interaction of cocaine with positive GABAA modulators on the repeated acquisition and performance of response sequences in rats. Psychopharmacology (Berl) 2005;181:217–226. doi: 10.1007/s00213-005-2241-3. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Gerak LR, Moerschbaecher JM, Winsauer PJ. Effects of pregnanolone in rats discriminating cocaine. Pharmacol Biochem Behav. 2006;85:385–392. doi: 10.1016/j.pbb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan L, Mitchelson M, Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg Med J. 2011;28:1055–1058. doi: 10.1136/emj.2010.103093. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Rosecrans JA, Glennon RA. Comparison of behavioral effects of cathinone, amphetamine and apomorphine. Pharmacol Biochem Behav. 1984;20:181–184. doi: 10.1016/0091-3057(84)90238-7. [DOI] [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, Davey Z, Corkery J, Siemann H, Scherbaum N, Farre' M, Torrens M, Demetrovics Z, Ghodse AH. Mephedrone (4-methylmethcathinone; 'meow meow'): chemical, pharmacological and clinical issues. Psychopharmacology (Berl) 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Zheng JW, Tella SR, Goldberg SR. Pharmacological mechanisms in the cardiovascular effects of methamphetamine in conscious squirrel monkeys. Pharmacol. Biochem Behav. 1992;42:791–796. doi: 10.1016/0091-3057(92)90031-a. [DOI] [PubMed] [Google Scholar]
- Schmidt MM, Sharma A, Schifano F, Feinmann C. "Legal highs" on the net-Evaluation of UK-based Websites, products and product information. Forensic Sci Int. 2011;206:92–97. doi: 10.1016/j.forsciint.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Shenouda SK, Carvalho F, Varner KJ. The cardiovascular and cardiac actions of ecstasy and its metabolites. Curr Pharm Biotechnol. 2010;11:470–475. doi: 10.2174/138920110791591526. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Daniel JM, Filipeanu CM, Leonard ST, Hulst JL, Rodgers SP, Lassen-Greene CL, Sutton JL. Long-term behavioral and pharmacodynamic effects of delta-9-tetrahydrocannabinol in female rats depend on ovarian hormone status. Addict Biol. 2011;16:64–81. doi: 10.1111/j.1369-1600.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Filipeanu CM, Bailey EM, Hulst JL, Sutton JL. Ovarian hormones and chronic administration during adolescence modify the discriminative stimulus effects of delta-9-tetrahydrocannabinol (Delta(9)-THC) in adult female rats. Pharmacol Biochem Behav. 2012;102:442–449. doi: 10.1016/j.pbb.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Moerschbaecher JM, Brauner IN, Purcell JE, Lancaster JR, Jr, Bagby GJ, Nelson S. Alcohol unmasks simian immunodeficiency virus-induced cognitive impairments in rhesus monkeys. Alcohol Clin Exp Res. 2002;26:1846–1857. doi: 10.1097/01.ALC.0000042171.80435.F1. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Moerschbaecher JM, Molina PE, Roussell AM. Contingent and noncontingent cocaine administration in rhesus monkeys: a comparison of the effects on the acquisition and performance of response sequences. Behav.Pharmacol. 2003;14:295–306. doi: 10.1097/01.fbp.0000081785.35927.08. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Puchnarewicz M, Button J, Archer R, Ovaska H, Ramsey J, Lee T, Holt DW, Dargan PI. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J Med Toxicol. 2010;6:327–330. doi: 10.1007/s13181-010-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Glennon RA. Discriminative stimulus effects of S(−)-methcathinone (CAT): a potent stimulant drug of abuse. Psychopharmacology (Berl) 1998;140:250–256. doi: 10.1007/s002130050765. [DOI] [PubMed] [Google Scholar]